We studied the transduction of primary human B lymphocytes and myeloma cells with lentiviral vectors. In peripheral blood B cells that had been activated with helper T cells (murine thymoma EL-4 B5) and cytokines, multiply attenuated HIV-1–derived vectors pseudotyped with vesicular stomatitis virus (VSV) G-envelope protein achieved the expression of green fluorescence protein (GFP) in 27% ± 12% (mean ± 1 SD; median, 27%) of B cells in different experiments. When compared in parallel cultures, the transducibility of B cells from different donors exhibited little variation. The human cytomegalovirus (CMV) promoter gave 4- to 6-fold higher GFP expression than did the human elongation factor-1α promoter. A murine retroviral vector pseudotyped with VSV G protein proved inefficient even in mitotically active primary B cells. B cells freshly stimulated with Epstein-Barr virus were also transducible by HIV vectors (24% ± 9%), but B cells activated with CD40 ligand and cytokines resisted transduction. Thus, different culture systems gave different results. Freshly isolated, nondividing myeloma cells were efficiently transduced by HIV vectors; for 6 myelomas the range was 14% to 77% (median, 28%) GFP+ cells. HIV vectors with a mutant integrase led to no significant GFP signal in primary B or myeloma cells, suggesting that vector integration was required for high transduction. In conclusion, HIV vectors are promising tools for studies of gene functions in primary human B cells and myeloma cells for the purposes of research and the development of gene therapies.
Introduction
Efficient delivery of genes into primary human B lymphocytes would allow the investigation of gene functions in these cells for the purposes of research and the development of gene therapies. One could then test in mature B cells the promoters/genes potentially suitable for stem cell–based therapies for immunodeficiencies.1 Vectors achieving the efficient transfection of primary B cells would most likely also be suitable for the delivery of genes into freshly collected B tumor cells—for example, for the development of immune-based anti–B-tumor therapies.2 Various viral vectors are currently being studied for their ability to transduce hematopoietic cells.3-5
Retroviral vectors derived from murine leukemia virus (MLV)6,7 can transfer genes into immortal human B-cell lines, such as lymphoblastoid cells,8 and primary B precursors,9 but they are inefficient for mature human B cells.10,11 These simple retroviruses can transduce genes only into actively dividing cells,12 but a potent T-independent mitogen for human B cells in vitro, such as lipopolysaccharide (LPS) for murine B cells, has not been found.13 In addition, MLV vectors might not be well adapted for human B cells because of the host species difference. HIV-1 and HIV-derived pseudotyped lentiviral vectors efficiently integrate into human cells, irrespective of cell division.14-22 High transgene expression from such vectors in human T cells or total lymphocytes has been reported.23-25 Generally, productive HIV infection or lentivector-mediated transduction of truly quiescent lymphocytes has not been observed; activation, at least from G0 to G1, seems to be required.23,25-28 Efficient transduction of primary acute lymphoblastic leukemia cells with a bicistronic HIV vector, leading to the expression of a cytokine (granulocyte macrophage–colony-stimulating factor [GM-CSF]) and an immunostimulatory molecule (CD80), has also been achieved,29 indicating a potential use of such vectors in novel anti–B-tumor therapies.
In this study we investigated the transduction of peripheral blood B cells with multiply attenuated HIV vectors pseudotyped with vesicular stomatitis virus (VSV) G glycoprotein.21 Efficient transduction of such B cells occurred after their activation in a culture system using murine EL-4 B5 thymoma cells as helper T cells in conjunction with human cytokines.30-33 This system leads to proliferation and subsequent plasmocytic differentiation of all naive and memory human B subsets.33 Nondividing, freshly isolated multiple myeloma cells were also efficiently transduced by HIV vector. By contrast, an MLV vector pseudotyped with VSV G protein was inefficient even in dividing B cells.
Materials and methods
HIV-derived vectors
Vectors were produced by transient transfection into 293T cells as previously described,21 with the following modifications. A total of 2 × 106 293T cells were seeded in 10-cm–diameter dishes 24 hours before transfection in Dulbecco modified essential medium (DMEM) with 10% fetal bovine serum (FBS) in the presence of chloroquine (final concentration, 25 μM). Twenty micrograms plasmid DNA was used for the transfection of one 10-cm dish: 2.5 μg envelope-coding plasmid pMD.G, 7.5 μg packaging plasmid pCMVΔR8.91 (which expresses Gag, Pol, Tat, and Rev), and 10 μg transfer vector plasmid. The cells were washed with phosphate-buffered saline (PBS) after 14 to 16 hours and were incubated in serum-free EPISERF medium (Gibco, Invitrogen, Groningen, The Netherlands); the conditioned medium was collected after another 24 hours, cleared by low-speed centrifugation, and filtered through 0.45-μm pore-size polyvinylidene difluoride (PVDF) filters. The vector was concentrated 100-fold by one round of centrifugation at 50 000g for 90 minutes and resuspended for 30 minutes at room temperature in EPISERF medium. In some experiments we used as control a packaging construct (pCMVΔR8.2D64V)15 with a mutant integrase (mutation D64V).34
HIV vector plasmids were all derivatives of the original pHR′ backbone.21 GFP-expressing plasmids using either the cytomegalovirus (CMV), the murine phosphoglycerate kinase (PGK), or the human elongation factor-1α (EF1α) promoter were obtained as described.25 We also tested a vector with a compound promoter cassette composed of the CMV enhancer, chicken β-actin promoter, first exon, first intron, and 5′ untranslated part of the second intron, subcloned from plasmid pCAGGS35 (CAG compound promoter). MLV vector particles were produced using a human CMV early promoter–GagPol plasmid as packaging construct and a vector derived from pSLX, which expressed GFP from the CMV promoter, as described.25 Viral stocks were stored at −70°C. Titers of HIV vectors and MLV vector were determined by the transduction of HeLa cells by serial dilutions of supernatants and the counting of GFP+ HeLa cells by fluorescence-activated cell sorter (FACS). Titers were found to range from 107 to 108 HeLa-transducing units per milliliter. Stocks of integrase mutant vectors were normalized to stocks of wild-type integrase vectors on the basis of their reverse transcriptase activities, as described.34
B-cell cultures
Approval for research on human cells was obtained from the Medical Ethics Committee of the Geneva University Hospitals. Informed consent was provided for all cell samples according to the Declaration of Helsinki. B cells were isolated to more than 97% purity from buffy coats of virus-screened blood donations at the Geneva Transfusion Center using Ficoll-Hypaque centrifugation and anti-CD19 magnetic beads (Dynal, Oslo, Norway) as described.32 All cultures were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 10−5 M 2-mercaptoethanol (2-ME), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, and penicillin-streptomycin. Primary B cells were cultured in 96-well flat-bottom plates (200-μL cultures).32
B-cell activation with EL-4 B5 cells.
For the activation of B cells before transduction with vectors, 20 000 B cells per well were cocultured with 50 000 irradiated (5000 cGy) EL-4 B5 cells in the presence of 2 ng/mL interleukin-1β (IL-1β) (R&D Systems, Minneapolis, MN), 2 ng/mL tumor necrosis factor-α (TNF-α) (PeproTech EC, London, United Kingdom), 10 ng/mL IL-10 (Pepro Tech EC), and 50 ng/mL IL-2 (kind gift from the former Glaxo Institute for Molecular Biology, Geneva, Switzerland).33 IL-1 is required to induce cell contact–mediated helper activity in EL-4 B5 cells.30 For transduction, B cells were reisolated with anti-CD19 beads after different times and then cultured for 15 hours (25 000 cells/100 μL) with vector in fresh medium–FCS with cytokines, but no EL-4 cells. After 15 hours, fresh irradiated EL-4 cells were again added in 100 μL medium–FCS, without medium change for the B cells. This maintained vigorous B-cell proliferation until about 8 to 9 days of total culture time before leading to plasmocytic differentiation.
B-cell activation with CD40 ligand.
B cells were cultured (105 cells/200 μL) in the presence of IL-4 (10 ng/mL), IL-2 (50 ng/mL), IL-10 (10 ng/mL), and soluble oligomeric CD40 ligand (CD40L) (300 ng/mL; ACRP30-CD40L was kindly provided by Dr J. Tschopp and Dr P. Schneider, Institute of Biochemistry, University of Lausanne, Switzerland), as recently described.33 Vectors were added on different days (see “Results”).
B-cell activation with Epstein-Barr virus.
B cells were cultured (105 cells/200 μL) in the presence of Epstein-Barr virus (EBV)–containing culture supernatant, which was obtained from the virus producing B95-8 marmoset cell line after 1 week of culture, as described.36
Fresh myeloma B cells
Following institutional guidelines (see “B-cell cultures”), plasma cells from 6 patients with immunoglobulin G (IgG) multiple myeloma in relapse were isolated from the bone marrow aspirate using mouse anti-CD138 mAb (anti–syndecan-1; Immunotech, Marseilles, France) coupled to Dynabeads (CELLection Pan Mouse IgG Kit; Dynal). Plasma cells represented 35% to 70% of the total cellularity in bone marrow aspirates; their purity after isolation was 90% to 97% by 2-dimensional flow cytometry using fluorescein isothiocyanate (FITC)–anti-CD38 (DAKO, Glostrup, Denmark) and phycoerythrin (PE)–anti-CD138 (Immunoquality Products, Groningen, Netherlands). Plasma cells were cultured (105 cells/200 μL) in medium–FCS supplemented as above in the presence of 20 ng/mL IL-6 and 20 ng/mL GM-CSF (both from PeproTech). HIV vectors were added 24 hours after culture initiation.
Analyses of transduced cells
Flow cytometry for GFP expression.
Cells were treated with 200 μg/mL polyclonal mouse immunoglobulin (Sigma) for 10 minutes before staining with different antibodies. Viable, nonapoptotic cells were gated as 7-amino-actinomycin D (7-AAD)–low cells as described.33 Primary B cells were identified with phycoerythrin (PE)–coupled anti-CD19 mAb (DAKO), and myeloma B cells were identified with PE–anti-CD138 (Immunoquality Products), respectively. Cells were then analyzed using a FACScan analyzer and CellQuest software (Becton Dickinson, Mountain View, CA). Isotype-matched control stainings were performed in parallel.33
Immunoglobulin secretion and thymidine incorporation.
Measurements of immunoglobulin secretion by primary B cells, IgM, IgG, and IgA were performed using specific enzyme-linked immunosorbent assay (ELISA).33 Proliferation of various cells was measured at different times, following a pulse overnight with 1 μCi (0.037 MBq) [methyl 3H]-thymidine (Amersham Pharmacia Biotech).30 For myeloma cells, total peripheral blood lymphocytes (PBLs) obtained by Ficoll centrifugation, cultured (105 cells/200 μL) in medium–FCS in the absence or presence of 1 μg/mL phytohemagglutinin (PHA), served as negative and positive controls, respectively.
Cell cycle analysis
DNA cell-cycle profiles were obtained after staining of B cells in a hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100) in the presence of 50 μg/mL propidium iodide (Sigma), using the FACScan equipped with the Doublet Discrimination Module (Lysis II software; Becton Dickinson) for the exclusion of cell doublets and cell debris, as described.32 The mitosis inhibitor nocodazole (Sigma) was used at 50 ng/mL.
Results
Efficient transduction of T-cell–stimulated B cells with attenuated HIV-1–derived vectors
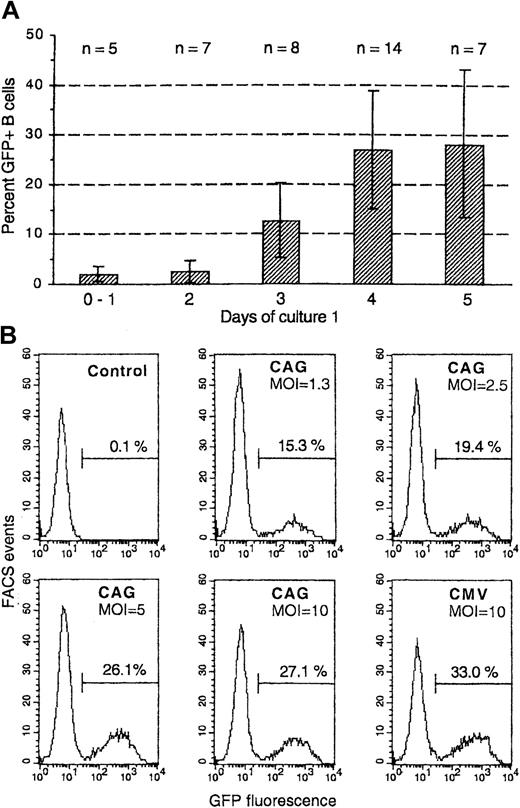
We studied the transduction of human B cells with attenuated HIV-1–derived vectors, produced with a packaging construct that expressed only Gag, Pol, Tat, and Rev and pseudotyped with VSV G protein.21 Nonstimulated peripheral blood B cells could not be transduced, but B cells that had been activated by cell contact with helper T cells (murine EL-4 B5 cells) in the presence of human cytokines30 33 were efficiently transduced. This was studied with vectors expressing GFP under the control of either the CMV promoter or a compound promoter cassette composed of the CMV enhancer and chicken β-actin promoter (CAG) or the human elongation factor-1α (EF1-α) promoter. CD19+ B cells were isolated after different times from primary culture with EL-4 T cells and were cultured with vector in medium–FCS with the above cytokines. After 15 hours, fresh irradiated EL-4 cells were again added. GFP expression was analyzed 4 days later (for transductions on days 2 to 5) or 6 days later (transductions on days 0 or 1), when the number of B cells had increased approximately 10-fold. We found that 4 or more days of primary culture led to maximal transduction (Figure1A shows data from 14 independent experiments in which transduction on 3 or more different days was compared). Of the B cells transduced on day 4, 26.8% ± 11.6% expressed GFP (range, 9%-52%; median, 27% GFP+ cells). Similar results were obtained for transduction on day 5 (and up to day 8; not shown). This data range was also representative for 24 other independent experiments in which B cells were transduced on a single day.
Transduction with HIV vectors of peripheral blood B cells activated by T cells and cytokines.
(A) Purified B cells were cultured with irradiated EL-4 T cells in the presence of IL-1β, TNF-α, IL-2, and IL-10 (see “Materials and methods”). At different times of this primary culture, the B cells were isolated and cultured in fresh medium/cytokines with HIV vectors expressing GFP under the control of the CMV, CAG, or EF1-α promoters (MOI, 10). After 15 hours, fresh irradiated EL-4 cells were again added (secondary culture). Shown are percentages of GFP+ cells among gated CD19+ 7-AADlow (nonapoptotic) cells detected after 4 days of secondary culture. Data are from 14 independent experiments in which transductions on 3 or more different days were compared; numbers of data for different days are indicated (n). The columns show means, and the bars indicate ± 1 SD. (B) Transduction was vector-dose dependent. B cells activated for 4 days in primary culture were transduced and then cultured and analyzed as above by using HIV-GFP vectors with the compound CAG or the CMV promoter in the same experiment; MOI is indicated in the figures. Shown are FACS histograms for GFP+ cells by gating on CD19+7-AADlow cells.
Transduction with HIV vectors of peripheral blood B cells activated by T cells and cytokines.
(A) Purified B cells were cultured with irradiated EL-4 T cells in the presence of IL-1β, TNF-α, IL-2, and IL-10 (see “Materials and methods”). At different times of this primary culture, the B cells were isolated and cultured in fresh medium/cytokines with HIV vectors expressing GFP under the control of the CMV, CAG, or EF1-α promoters (MOI, 10). After 15 hours, fresh irradiated EL-4 cells were again added (secondary culture). Shown are percentages of GFP+ cells among gated CD19+ 7-AADlow (nonapoptotic) cells detected after 4 days of secondary culture. Data are from 14 independent experiments in which transductions on 3 or more different days were compared; numbers of data for different days are indicated (n). The columns show means, and the bars indicate ± 1 SD. (B) Transduction was vector-dose dependent. B cells activated for 4 days in primary culture were transduced and then cultured and analyzed as above by using HIV-GFP vectors with the compound CAG or the CMV promoter in the same experiment; MOI is indicated in the figures. Shown are FACS histograms for GFP+ cells by gating on CD19+7-AADlow cells.
To study variations between B cells from different donors in the same experiment, we performed 2 experiments in which B cells from 4 and 5 donors, respectively, were transduced on day 4 in duplicate cultures (absolute differences between duplicates were less than 3.7% GFP+ B cells). Group means in the 2 experiments were 20.6% ± 0.9% (n = 4) and 27.4% ± 1.8% (n = 5) GFP+ B cells; the biggest difference between 2 donors in the same experiment was only 4.2% GFP+ B cells. Thus, there was little variation between different donors.
Transduction was vector-dose dependent, usually reaching a plateau at a multiplicity of infection (MOI) of 5 to 10 HeLa-transducing units per B cell. Figure 1B shows the effect of titration with a GFP-expressing vector using the CAG compound promoter; this promoter construct showed activity similar to that of the classical CMV promoter.
With B cells undergoing mock transduction on day 4, the secondary cultures with fresh irradiated EL-4 cells usually secreted from 10 to 40 μg/mL immunoglobulin within 7 days (with variable proportions of IgM, IgG, and IgA). With 6 tested vectors, which gave more than 20% GFP+ B cells, no or only modest inhibition (less than 17% inhibition) of immunoglobulin secretion was found at an MOI of 10, showing low vector toxicity for primary B cells.
Comparison of HIV vectors with different promoters and an MLV vector
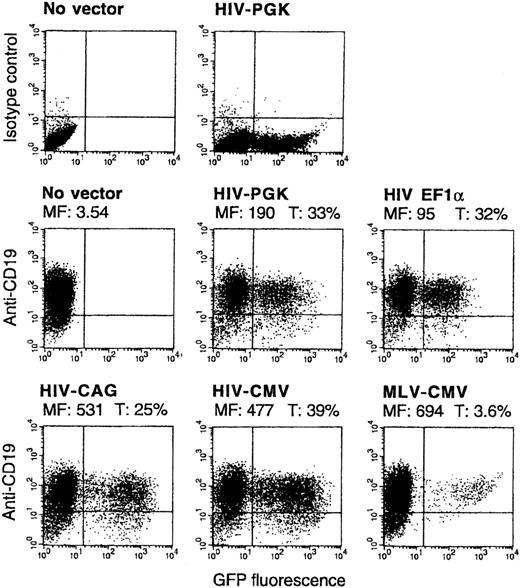
HIV vectors with 4 different internal promoters were compared—CMV, CAG, EF1-α, and murine phosphoglycerate kinase (PGK) promoters. All promoters could give high percentages of GFP+ B cells, but distinct levels of fluorescence intensity in transduced cells were found. In a representative experiment the CMV or CAG promoters led to 5-fold higher mean fluorescence intensity among GFP+ B cells than the EF1-α promoter. The PGK promoter showed intermediate activity (all vectors at an MOI of 10; Figure 2). All differences were reproducible in 3 or more other experiments. The CMV promoter gave from 4-fold to 6-fold higher mean fluorescence than the EF1-α promoter in 6 independent experiments in which both promoters were compared by using 2 different vector preparations for each promoter.
Comparison of HIV vectors expressing GFP under the control of PGK, EF1-α, CAG, or CMV promoters and of an MLV vector with the CMV promoter.
B cells were transduced and analyzed as in Figure 1B, in parallel with the indicated vectors (MOI, 10). In the FACS 2D plots, the y-axis represents cells stained by anti-CD19 or isotype control. Mean fluorescence of nontransduced cells or of the GFP+ fraction of transduced cells (MF) and percentages of GFP+ among CD19+ cells (T) are indicated. CD19low cells are usually more differentiated, plasmocytic cells in this culture system.
Comparison of HIV vectors expressing GFP under the control of PGK, EF1-α, CAG, or CMV promoters and of an MLV vector with the CMV promoter.
B cells were transduced and analyzed as in Figure 1B, in parallel with the indicated vectors (MOI, 10). In the FACS 2D plots, the y-axis represents cells stained by anti-CD19 or isotype control. Mean fluorescence of nontransduced cells or of the GFP+ fraction of transduced cells (MF) and percentages of GFP+ among CD19+ cells (T) are indicated. CD19low cells are usually more differentiated, plasmocytic cells in this culture system.
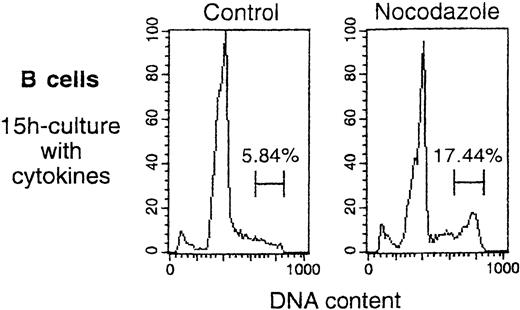
An MLV vector, also pseudotyped with VSV G protein and also containing the CMV internal promoter, led to high GFP expression in only 3.6% of B cells (also shown in Figure 2). This vector transduced HeLa and 239T adherent cells as efficiently as did HIV vectors. The fluorescence intensity in the GFPhigh B cells showed a distribution similar to that obtained with HIV-CMV vector (Figure2). Cell division analysis of B cells that had been cultured for 15 hours in medium/cytokines (as in the transduction protocol) in the presence or absence of a mitosis inhibitor showed that 11.6% of the B cells accumulated in late S/G2/M phases during this time (Figure 3). Thus, the low transduction efficiency of the MLV vector in human B cells was not caused by low mitotic activity of these targets.
B cells continued to divide under the conditions used for transduction.
B cells were activated for 4 days in primary culture, then isolated and cultured for 15 hours in medium/cytokines, as for the transduction in Figure 2. Shown are profiles for cellular DNA content obtained at the end of the 15-hour incubation, performed either in the absence (Control) or presence of mitosis inhibitor (Nocodazole, 50 ng/mL). Percentages of B cells in late S/G2/M are indicated. Peaks of cells with hypodiploid DNA content represent apoptotic cells.32
B cells continued to divide under the conditions used for transduction.
B cells were activated for 4 days in primary culture, then isolated and cultured for 15 hours in medium/cytokines, as for the transduction in Figure 2. Shown are profiles for cellular DNA content obtained at the end of the 15-hour incubation, performed either in the absence (Control) or presence of mitosis inhibitor (Nocodazole, 50 ng/mL). Percentages of B cells in late S/G2/M are indicated. Peaks of cells with hypodiploid DNA content represent apoptotic cells.32
Requirement for retroviral integrase for high B-cell transduction
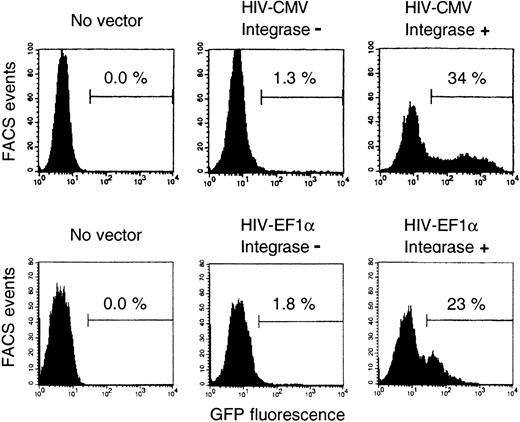
To study whether integrase was required for B-cell transduction by HIV vectors, we tested vectors with a mutant integrase. These vectors showed 2 distinct effects (Figure 4 shows 2 representative experiments using the CMV or the EF1-α promoter, respectively). On one hand, there was a slight shift of fluorescence (a 1.5- to 2-fold increase) of all exposed B cells. Most likely, this mainly reflected the passive transfer of GFP protein.37 In fact, this shift was also found in the GFP− cell fraction after treatment with the wild-type integrase vectors. In the various experiments reported above, the GFP+ cells were counted by FACS gating after the peak of the untransduced cells (Figures 1B, 2); thus, those results—particularly those obtained using the CMV promoter—were not significantly influenced by this phenomenon. On the other hand, there was higher GFP expression in a small fraction of the B cells (in less than 2% of B cells, according to 6 independent experiments performed with 3 preparations of integrase mutant vectors). This could reflect GFP synthesis from episomal DNA15,37and possibly residual integration of mutant vector.34Clearly, the high transduction of B cells was obtained only with vector particles containing fully functional integrase.
Comparison of vectors with or without integrase.
B cells were transduced with HIV-CMV vectors or HIV-EF1α vectors, and the vectors were packaged either with integrase mutant (integrase −) or wild-type (integrase +) plasmids. Vector amounts were normalized using reverse transcriptase activities, and they corresponded to an MOI of 10. Two independent experiments; same protocol for culture and cell analysis as in Figure 1; transductions on day 4 (HIV-CMV) or day 5 (HIV–EF1-α). Percentages of GFP+ cells among gated CD19+7-AADlow cells are indicated.
Comparison of vectors with or without integrase.
B cells were transduced with HIV-CMV vectors or HIV-EF1α vectors, and the vectors were packaged either with integrase mutant (integrase −) or wild-type (integrase +) plasmids. Vector amounts were normalized using reverse transcriptase activities, and they corresponded to an MOI of 10. Two independent experiments; same protocol for culture and cell analysis as in Figure 1; transductions on day 4 (HIV-CMV) or day 5 (HIV–EF1-α). Percentages of GFP+ cells among gated CD19+7-AADlow cells are indicated.
EBV-stimulated, but not CD40L-stimulated, B cells were transducible
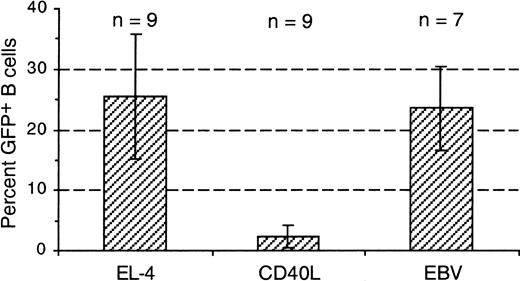
The transduction of B cells stimulated with CD40L and cytokines (IL-4, IL-2, and IL-10)33 was also investigated. In such cultures, HIV vectors with either the CMV or the EF1-α promoter gave only 2.3% ± 1.8% GFP+ B cells (mean ± 1 SD of 9 experiments; Figure 5). The CD40L-stimulated B cells strongly incorporated radiolabeled thymidine (50 000-75 000 cpm/culture after 5 days for 105 starting cells; see “Materials and methods”), but many cells died in culture; the viable cell number increased only approximately 1.5-fold in 5 days. In parallel cultures, B cells freshly stimulated with EBV showed similar thymidine incorporation and viability. However, these cells gave 24% ± 7% GFP+ B cells (7 experiments), similar to EL-4–activated B cells transduced with the same vectors (Figure 5). Thus, strikingly different results were obtained in different culture systems.
EBV-stimulated, but not CD40L-stimulated, primary B cells were transduced by HIV vectors.
B cells were cultured in parallel either in the EL-4 system (EL-4) or in the presence of CD40L, IL-4, IL-2, and IL-10 (CD40L) or with EBV containing B95-8 cell supernatant (EBV). B cells were transduced after 4 or 5 days (EL-4 system) or after 3 to 5 days (CD40L, EBV) with the same vector preparations, using CMV or EF1-α promoters. Columns show means of the percentages of GFP+ cells found among 1000 or more gated CD19+ 7-AADlow cells, 3 or 4 days later; numbers of experiments are indicated (n); bars indicate ± 1 SD.
EBV-stimulated, but not CD40L-stimulated, primary B cells were transduced by HIV vectors.
B cells were cultured in parallel either in the EL-4 system (EL-4) or in the presence of CD40L, IL-4, IL-2, and IL-10 (CD40L) or with EBV containing B95-8 cell supernatant (EBV). B cells were transduced after 4 or 5 days (EL-4 system) or after 3 to 5 days (CD40L, EBV) with the same vector preparations, using CMV or EF1-α promoters. Columns show means of the percentages of GFP+ cells found among 1000 or more gated CD19+ 7-AADlow cells, 3 or 4 days later; numbers of experiments are indicated (n); bars indicate ± 1 SD.
Transduction of nondividing myeloma B cells
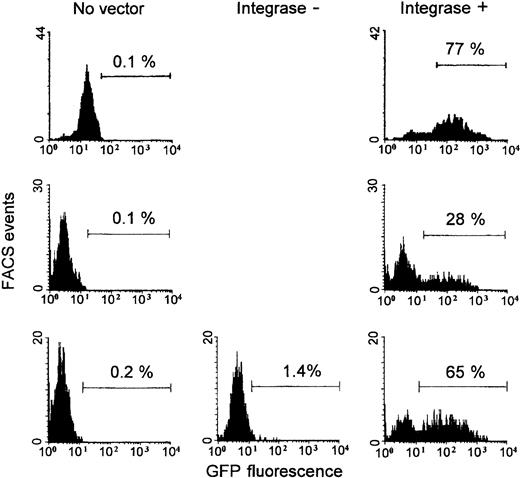
To explore possible applications of HIV vectors in lymphoma immunotherapy,2 we also studied the transduction of B tumor cells freshly isolated from bone marrow aspirate obtained from patients with multiple myeloma. Six different myeloma cells were cultured with IL-6 and GM-CSF. HIV vector with the CMV promoter was added after 24 hours, and GFP expression was measured 4 or 5 days later (Figure 6 shows the results obtained with 3 myelomas; the 3 other results were 14%, 24%, and 31% GFP+ cells; duplicate cultures, except for one myeloma). There was considerable variation of results between individual myelomas, but all 6 studied myelomas could be transduced, showing 39% ± 25% GFP+ cells (range, 14%-77%; median, 28%). Two myelomas transduced with HIV-CMV integrase mutant vectors gave very low GFP expression, similar to the results obtained with primary B cells (one experiment is also shown in Figure 6). With all these myeloma cells, the incorporation of radiolabeled thymidine, measured on days 3 or 4 in parallel cultures, was low (less than 1500 cpm, as was also obtained with unstimulated PBLs; PHA-stimulated PBLs gave 45 000-70 000 cpm). Thus, nondividing myeloma cells were efficiently transduced by HIV vectors.
Transduction of freshly isolated myeloma B cells.
Myeloma cells from 3 of the 6 patients (see “Results”) are shown. Cells were isolated and cultured with IL-6 and GM-CSF. HIV-CMV vector packaged with integrase wild-type (integrase +) or mutant (integrase −; one myeloma) plasmid was added 24 hours after culture initiation. Vector amounts were normalized using reverse transcriptase activities and corresponded to an MOI of 10. Percentages of GFP+ cells among gated CD138+7-AADlow cells were measured after 5 days (the myeloma shown at the top, single culture) or 4 days (the 2 other myeloma; duplicate cultures with integrase + vector gave 28%; 25% and 65%; 61% GFP+ cells).
Transduction of freshly isolated myeloma B cells.
Myeloma cells from 3 of the 6 patients (see “Results”) are shown. Cells were isolated and cultured with IL-6 and GM-CSF. HIV-CMV vector packaged with integrase wild-type (integrase +) or mutant (integrase −; one myeloma) plasmid was added 24 hours after culture initiation. Vector amounts were normalized using reverse transcriptase activities and corresponded to an MOI of 10. Percentages of GFP+ cells among gated CD138+7-AADlow cells were measured after 5 days (the myeloma shown at the top, single culture) or 4 days (the 2 other myeloma; duplicate cultures with integrase + vector gave 28%; 25% and 65%; 61% GFP+ cells).
Discussion
HIV-1–derived, attenuated lentiviral vectors efficiently delivered genes into primary human B cells activated with EL-4 T cells and cytokines. In accord with previous findings with T cells or total lymphocyte populations,23-25,28 quiescent peripheral blood B cells could not be transduced with such vectors. Activation during 4 days was required to obtain optimally transducible B cells that showed a median level of 27% GFP+ cells (range, 9%-52% GFP+ cells in different experiments). There was little variation of transducibility between B cells from different donors when tested in parallel cultures. Most of the variation between experiments seemed to have been caused by fluctuating B-helper activity of the EL-4 cells; the nature of this activity is still unknown. The activation time required for optimal transduction correlates with the time required to recruit all B cells into the active cell cycle in this system; cell activation is asynchronous because direct T–B cell contact is involved.32 It is unknown whether mitosis or a concomitant cell activation state is necessary for the transduction of primary B cells. It has been shown that after stimulation with certain cytokines, nondividing T cells become transducible with attenuated HIV vector.23 28
Surprisingly, B cells stimulated with CD40L and cytokines resisted transduction by the HIV vectors. B cells freshly stimulated with EBV—that is, the cells undergoing a short-term polyclonal response36—showed high transducibility, like EL-4–activated B cells. B cells stimulated with EL-4 T cells behave differently than CD40L-stimulated B cells—they show better viability and more plasmocytic differentiation.33 For CD40L- and EBV-stimulated B cells, viability is similar, but most EBV-stimulated B cells rapidly become infected by EBV.36 Thus, B cells are in different activation states in the various culture systems. Apparently this somehow strongly affects their permissiveness to transduction with HIV vectors.
Nondividing, freshly isolated myeloma B cells cultured with IL-6 and GM-CSF were also efficiently transduced by HIV vectors (range, 14%-77% GFP+ cells; 6 myelomas tested). Because myeloma cells were isolated and transduced in duplicate cultures, there appeared to be true variation of transducibility between different tumors. Finally, we recently found that B cells from 6 patients with chronic lymphocytic leukemia (B-CLL) resisted transduction with the HIV vectors. These B cells were cultured with CD40L, in the presence of either IL-4 or IL-2 and IL-10; 7-AADlowcells could be analyzed (T.M. et al, unpublished data, June 2002). Because of the results obtained with primary B cells under various culture conditions, it cannot be said at this time whether the B-CLL cells are transducible; other culture conditions must be investigated.
The low transduction efficiency of EL-4–activated B cells obtained with an MLV vector pseudotyped with VSV G protein (3.6%) was clearly lower than the proportion of B cells undergoing mitosis during 15-hour incubation in medium/cytokines, as performed in the transduction protocol (11.6%). Thus, mitosis was not the limiting factor for B transduction with this vector. This vector efficiently transduced adherent cells, and, in the B cells, the fluorescence intensity among the GFP+ cells was high, indicating that the vector construct was functional. Inefficient transduction of primary human B cells with murine retroviral vectors, as previously reported (5% or less),10 11 was thus also observed in the EL-4 culture system.
The HIV-1 long terminal repeat (LTR), which has 2 nuclear factor-κB binding sites,38 can act as an active promoter for the expression of gene constructs in B-lymphoblastoid cells.39Nevertheless, significantly different activities between attenuated HIV vectors with various internal promoters for GFP expression were now found in primary B cells. The EF1-α promoter, which we recently found to be greater than 10-fold more active than the CMV promoter in CD34+ cells,25 gave 4-fold to 6-fold lower mean fluorescence of GFP+ cells than the CMV promoter in the B cells. The PGK promoter had intermediate activity in B and in CD34+ cells. A compound promoter cassette composed of the CMV enhancer and the chicken β-actin promoter35 had similar, or slightly lower, activity than the CMV promoter in the B cells. These internal promoter activities are important regarding the development of lentiviral vectors with self-inactivating LTR modifications.25 40
Long-term gene expression is difficult to study in short-lived (in vitro) human B cells or in nondividing myeloma cells. Generally, lentiviral vectors are efficient for long-term expression.15-22 We found that vectors with a mutant integrase led to little GFP expression in such target cells, suggesting that genuine transduction caused the high GFP expression, as observed in other targets. Our results with the integrase mutant vectors are in accordance with the data reported in a previous study.37On one hand, there was a global shift to slightly higher GFP fluorescence in all exposed cells. This was also found in the GFP− fraction of cells treated with wild-type integrase vectors. In the previous study, testing of various vector particles showed that this mainly reflects the passive transfer of GFP protein.37 GFP is synthesized in the virus producer cells, and the vector preparations contain many defective viral particles that can contribute to such pseudotransduction. In particular with the CMV promoter, the GFP signals in the B cells obtained with integrase wild-type vectors were strong enough to clearly discriminate transduced and untransduced cells. On the other hand, a few cells (less than 2%) treated with integrase mutant vectors showed higher GFP fluorescence. Because the D64V integrase mutation does not abolish reverse transcriptase activity,34 some GFP synthesis from episomal DNA most likely occurred, as previously discussed.15,37 In addition, an HIV proviral clone with this mutation apparently still showed some integration.34 Although taken together our data suggest that integration occurred in primary B and myeloma cells transduced with the vectors with wild-type integrase, the possibility of significant GFP expression from unintegrated proviral DNA derived from such vectors has not been formally ruled out.
By measuring immunoglobulin secretion by B-cell populations exposed to HIV vectors, which also reflects the cell proliferation before plasmocytic differentiation in the EL-4 culture system, we found that such vectors exhibited remarkably low toxicity for primary B cells. By using such vectors it should now be possible to study gene functions in primary human B cells by performing various biologic assays that require high transduction. Such a system is urgently needed because various cellular activities seem to be differently regulated in human compared with murine B cells.13,41 Moreover, various aspects related to the development of gene therapies for immunodeficiencies can be addressed, such as the testing of B-cell–specific promoters or of promoters used for other cells that should not be active in B cells.42 This would facilitate the optimization of hematopoietic stem cell–based gene therapy protocols. In addition, there is reason to hope that the expression of costimulatory molecules in tumor cells may induce immune responses against modified and unmodified cells.2 43 Our finding that nondividing myeloma cells were efficiently transduced indicates that immune-based gene therapy for multiple myeloma could now also be investigated with lentiviral vectors.
K.K. is a member of the MD–PhD Program of the University of Pécs, Hungary.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2001-12-0249.
Supported by grants from the Swiss National Science Foundation (D.T., R.H.Z.), the European Community, and the Clayton Institute (D.T.).
F.B. and P.S. contributed equally to this work.
D.T. has declared a financial interest as consultant to Cell Genesis, a company whose potential product is related to the HIV-1 vectors used in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Zubler, Division of Hematology, University Hospital, 1211 Geneva-14, Switzerland; e-mail:rudolf.zubler@hcuge.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal