High-capacity adenoviral (HC-Ad) vectors expressing B-domain–deleted human or canine factor VIII from different liver-specific promoters were evaluated for gene therapy of hemophilia A. Intravenous administration of these vectors into hemophilic FVIII-deficient immunodeficient SCID mice (FVIIIKO-SCID) at a dose of 5 × 109 infectious units (IU) resulted in efficient hepatic gene delivery and long-term expression of supraphysiologic FVIII levels (exceeding 15 000 mU/mL), correcting the bleeding diathesis. Injection of only 5 × 107 IU still resulted in therapeutic FVIII levels. In immunocompetent hemophilic FVIII-deficient mice (FVIIIKO), FVIII expression levels peaked at 75 000 mU/mL but declined thereafter because of neutralizing anti-FVIII antibodies and a cellular immune response. Vector administration did not result in thrombocytopenia, anemia, or elevation of the proinflammatory cytokine interleukin-6 (IL-6) and caused no or only transient elevations in serum transaminases. Following transient in vivo depletion of macrophages before gene transfer, significantly higher and stable FVIII expression levels were observed. Injection of only 5 × 106 HC-Ad vectors after macrophage depletion resulted in long-term therapeutic FVIII levels in the FVIIIKO and FVIIIKO-SCID mice. Intravenous injection of an HC-Ad vector into a hemophilia A dog at a dose of 4.3 × 109 IU/kg led to transient therapeutic canine FVIII levels that partially corrected whole-blood clotting time. Inhibitory antibodies to canine FVIII could not be detected, and there were no signs of hepatotoxicity or of hematologic abnormalities. These results contribute to a better understanding of the safety and efficacy of HC-Ad vectors and suggest that the therapeutic window of HC-Ad vectors could be improved by minimizing the interaction between HC-Ad vectors and the innate immune system.
Introduction
Hemophilia A results from a deficiency in factor VIII (FVIII) that is characterized by spontaneous and prolonged bleeding in the joints, muscles, and internal organs.1Currently, hemophilia is treated with protein replacement therapy using either plasma-derived or recombinant FVIII. Although FVIII replacement markedly improves the life expectancy of patients with hemophilia, they are still at risk for severe bleeding episodes, chronic joint damage, and viral transmission from contaminated blood sources. Gene therapy could be an attractive alternative for the treatment of hemophilia A because the therapeutic window is relatively broad and levels slightly higher than 1% of normal physiologic levels are therapeutic.2 If successful, gene therapy could provide constant FVIII synthesis that may lead to a cure for this disease.
Different viral and nonviral gene therapy methods have been evaluated for the treatment of patients with hemophilia A.3,4 Adenoviral (Ad) vectors are the most efficient vectors for hepatic gene delivery.3,4 The adenoviral vector genome remains extrachromosomal, implying that the risk for neoplastic transformation from insertional mutagenesis is low. However, early-generation adenoviral vectors still contain most viral genes. Their expression contributes to inflammatory responses, toxicity, and short-term transgene expression.5-10
Previous studies have shown that injection of early-generation adenoviral vectors encoding canine or human FVIIIresulted in efficient liver transduction and in therapeutic levels of FVIII in hemophilic mice, dogs, and rhesus macaques.11-15 However, FVIII levels declined to baseline, possibly because of vector toxicity related to residual adenoviral gene expression. In the dog model, the induction of a humoral immune response against FVIII was associated with only transient correction of the bleeding diathesis.15 Inflammatory responses and vector toxicity further diminished therapeutic efficacy.15
To overcome the limitations of early-generation adenoviral vectors, novel adenoviral vectors have been developed, designated as high-capacity (HC) adenoviral vectors, devoid of all adenoviral genes. These HC-Ad vectors retain only the necessary cis-acting elements required for generating infectious vector particles during vector production, and they depend on the use of an E1-complementation cell line and a packaging-defective helper virus that provides the necessary viral functions in trans.16 17
In the present study, we evaluated whether HC-Ad vectors encoding FVIII could be used to cure hemophilia A in a clinically relevant animal model. HC-Ad vectors expressing human or canine B-domain–deletedFVIII genes from different liver-specific promoters were injected into FVIII-deficient mice with hemophilia A. Efficiency of liver gene transfer, biodistribution, biosafety, and specific or innate immune responses were analyzed. In immunodeficient FVIII-deficient mice, hepatic gene transfer resulted in long-term expression of human or canine FVIII at supraphysiologic levels, correcting the bleeding disorder. In immunocompetent mice, neutralizing antibodies were induced that were directed against FVIII. Following transient in vivo depletion of Kupffer cells and lymphoid (splenic) macrophages, stable FVIII expression and significantly higher FVIII levels were observed in most animals. Serum transaminase levels and cytokine profiles confirmed the safety of this new generation of adenoviral vectors. The use of canineFVIII genes was important in the preparation of efficacy and safety studies in hemophilic dogs. Subsequent injection of this HC-Ad vector into a hemophilia A dog resulted in therapeutic FVIII levels and confirmed the relative safety of these improved vectors in a clinically relevant large animal model.
Materials and methods
Construction of the B-domain deleted–FVIII cDNA
Human B-domain–deleted FVIII cDNA was generated by polymerase chain reaction (PCR) cloning starting from the pSP64-FVIII clone, originally obtained from the American Type Culture Collection (Rockville, MD), containing the full-length FVIII cDNA. This particular human B-domain–deleted FVIII cDNA had several unique features that were intended to increase gene expression. Kozak translational consensus was included to increase translational efficiency. A TAA stop codon was used instead of aTGA stop codon because of its greater efficiency. In addition, the 3′ untranslated region (UTR) was deleted because it may inhibit gene expression.18 The B-domain deletion started at amino acid Ser741, retaining the 740 thrombin cleavage site, until amino acid Ile1668, retaining the thrombin and Xa cleavage sites at position Arg1689 and the putative von Willebrand factor (VWF) binding site between Val1670 and Arg1689. This 4.3-kb B-domain–deleted FVIII was reported to have the same activity as plasma-derived FVIII.19 The 4.4-kb canine B-domain–deleted FVIII cDNA sequence has been described previously.20
Vector construction
The HC-Ad vectors Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII were constructed as infectious plasmids(pAd-AAT-cFVIII, pAd-AAT-hFVIII, andpAd-Apo-cFVIII). Schematic structures of the different HC-Ad vectors are shown in Figure 1. Details about the cloning procedure can be obtained on request. The Ad-AAT-cFVIII vector contains the Ad5 left terminus (nt 1-440), a 12-kb genomic fragment from the human α1-antitrypsin (AAT) locus (embedding the liver and macrophage-specific promoter, the first AAT exon and intron, and the splice acceptor of the second exon), the canine FVIII cDNA (B-domain–deleted), β-globin poly A, hypoxanthine phosphoribosyltransferase (HPRT) stuffer (nt 17855-14588, nt 8563-1799), and the Ad5 right terminus (nt 35818-35935) (Figure 1A). The Ad-AAT-hFVIII vector is comparable to that of Ad-AAT-cFVIII but contains the human (B-domain–deleted)FVIII cDNA instead. The Ad-Apo-cFVIII vector contains theAd5 left terminus, HPRT stuffer (nt 1799-21729),SV40 poly A, the canine FVIII cDNA (B-domain–deleted), the chimeric ApoE-ApoCII promoter(Apo), C346 stuffer (nt 13143-16750), and theAd5 right terminus. The AAT promoter corresponds to nt 1-7316 and a 5-kb upstream sequence derived from phage clone αNN containing the human AAT (hAAT) gene locus.21 The nucleotides refer to the sequences listed in GenBank. The ApoE enhancer and the ApoCIIpromoter were previously described.22 23
Design of HC-Ad vectors.
(A-B) The Ad-AAT-cFVIII and Ad-AAT-hFVIII vectors carry the human α1-antitrypsin promoter (AAT) to direct the expression of the canine or human B-domain–deleted FVIII cDNA, respectively. AnHPRT stuffer fragment was used to optimize vector size and to avoid vector rearrangements. (C) In the Ad-Apo-cFVIII vector, the B-domain–deleted FVIII cDNA was expressed from the hepatocyte-specific chimeric ApoE/ApoCII promoter (Apo). An additional stuffer sequence derived from the C346cosmid fragment was used in the Ad-Apo-cFVIII vector.
Design of HC-Ad vectors.
(A-B) The Ad-AAT-cFVIII and Ad-AAT-hFVIII vectors carry the human α1-antitrypsin promoter (AAT) to direct the expression of the canine or human B-domain–deleted FVIII cDNA, respectively. AnHPRT stuffer fragment was used to optimize vector size and to avoid vector rearrangements. (C) In the Ad-Apo-cFVIII vector, the B-domain–deleted FVIII cDNA was expressed from the hepatocyte-specific chimeric ApoE/ApoCII promoter (Apo). An additional stuffer sequence derived from the C346cosmid fragment was used in the Ad-Apo-cFVIII vector.
Rescue of HC-Ad vectors
Plasmids pAd-AAT-cFVIII, pAd-AAT-hFVIII, andpAd-Apo-cFVIII were digested with PmeI flanking the adenoviral termini, phenol/chloroform extracted, and precipitated with ethanol. Two micrograms each plasmid were transfected into 293-based Cre66 cells (G.S. et al, manuscript in preparation), which were coinfected with the loxP helper virus AdLC8cluc.17Subsequent amplification steps and large-scale preparation were performed as described previously.7 All vector preparations were purified twice by CsCl equilibrium density centrifugation, and the particle titers were evaluated by optical density measurements.
Animal studies
FVIII-deficient mice containing a disruption of the murineFVIII gene24 were bred to generate homozygous FVIII-deficient females and hemizygous FVIII-deficient males (designated as FVIIIKO). FVIIIKO mice were originally in a 129SV background backcrossed onto a C57Bl/6 background. To obtain immunodeficient hemophilic mice (designated as FVIIIKO-SCID), FVIII-deficient mice were crossed with SCID mice.25 These SCID mice are characterized by severe combined immune deficiency and are unable to mount a specific immune response to foreign antigens because of the lack of functional T and B cells. Genotyping and phenotypic characterization of the FVIIIKO and FVIIIKO-SCID offspring were performed as described,24 confirming that all FVIII-deficient mice used in this study contained the disrupted murineFVIII gene.
Two- to 3-month-old FVIIIKO or FVIIIKO-SCID mice were injected intravenously through the tail vein with the Ad-AAT-cFVIII, Ad-AAT-hFVIII, or Ad-Apo-cFVIII HC-Ad vector constructs at a dose of 5 × 109 infectious units (IU) per mouse. A dose-response study was performed with the Ad-AAT-cFVIII vector at doses of 5 × 109, 109, 5 × 108, 108, 5 × 107, and 5 × 106 IU per mouse. Similarly, FVIII-deficient littermates were injected with phosphate-buffered saline (PBS) as a control. Plasma samples were obtained from each mouse by retro-orbital bleeding in 20% 0.1 M sodium citrate. Mouse experiments were approved by the Animal Ethical Commission of the University of Leuven. A total vector dose of 5.6 × 1010 IU Ad-Apo-cFVIII, corresponding to 4.3 × 109 IU/kg, was administered through an in-dwelling cephalic vein catheter into a 6-year-old, mixed-breed dog with severe hemophilia A (body weight, 13 kg).26 The hemophilia dog was housed in facilities accredited by the Canadian Council for Animal Care, and experimental procedures were approved by the Queen's University Animal Care Committee.
Analysis of FVIII production and anti-FVIII antibodies
Biologically active human and canine FVIII was quantified in citrate-containing plasma samples from FVIIIKO or FVIIIKO-SCID mice by using FVIII COAtests (Chromogenix, Molndal, Sweden).27FVIII levels in the dog plasma were determined using the COAmatic assay (Chromogenix) with a human reference plasma as the standard. Plasma from FVIIIKO or FVIIIKO-SCID mice spiked with human plasma-derived FVIII (Octapharma, Langenfeld, Germany) of known activity was used as standard. One unit corresponded to 200 ng FVIII/mL (100% = 1000 mU). The sensitivity levels of the assay were less than 30 mU/mL and less than 5 mU/mL on diluted mouse and canine plasma, respectively. Physiologic FVIII concentrations were defined as 200 ng/mL. To further assess phenotypic correction of the clotting deficiency, plasma samples were subjected to a functional clotting assay (aPTT) that measures the activated partial thromboplastin time (SythASil APTT Reagent; Hemoliance, Lexington, MA). The whole blood clotting time in the hemophilia dog was measured following standard procedures. Inhibitory antibody titers in mice and in the dog were determined with Bethesda assays as described.28
Macrophage depletion study
Transient depletion of macrophages in livers and spleens of FVIIIKO and FVIIIKO-SCID mice was performed by intravenous injection of 0.2 mL clodronate-containing liposomes29 24 hours before injection of different doses of Ad-AAT-cFVIII vector, ranging from 108 IU to 5 × 106 IU. Clodronate was a gift of Roche Diagnostics GmbH (Mannheim, Germany). Phosphatidylcholine (Lipoid E PC) (Lipoid GmbH, Ludwigshafen, Germany) and cholesterol (Sigma, St Louis, MO) were used to prepare the liposomes. Littermates were injected with liposomes containing PBS and used as controls. To confirm the effectiveness of macrophage depletion, some mice were killed, and single-cell suspensions were prepared from livers and spleens that were subsequently analyzed by flow cytometry (FACScalibur, equipped with CellQuest software; Becton Dickinson, Sunnyvale, CA) using phycoerythrin (PE)–conjugated macrophage-specific antibodies including anti–major histocompatibility complex (anti-MHC) class 2 antibodies (BD PharMingen, Erembodegem, Belgium) and antibodies specific for the F4/80 macrophage marker (Serotec, Düsseldorf, Germany). Appropriate isotype-matched control antibodies were included in the fluorescence cell sorter (FACS) analysis.
Analysis of vector DNA and RNA in vivo
FVIIIKO mice were injected with 5 × 109 IU Ad-AAT-hFVIII. Genomic DNA from different organs was extracted by phenol/chloroform extraction as described previously.30Polymerase chain reaction (PCR) with human FVIII-specific primers 5′-CCATATAACATCTACCCTCA-3′ and 5′-GTTTCTCCTGAGAATGGGAA-3′) was used to detect HC-Ad FVIII DNA. PCR was performed using PlatinumTaq (Invitrogen, Merelbeke, Belgium) by denaturing for 2 minutes at 94°C, followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 55°C, 1 minute at 72°C), yielding a 0.6-kb fragment.30 A retrovirally transduced vector producer cell line with a known number of human FVIII gene copies served as the standard30,31 for comparison. For normalization, PCR with β-actin–specific primers was performed as described previously30 yielding a 0.2-kb fragment. Reverse transcription–PCR (RT-PCR) was performed as described previously,30 and the PCR conditions and primers were identical to those mentioned.
Toxicity studies
Measurements of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were performed on a Modular System (Roche/Hitachi) according to the manufacturer's instructions. Plasma samples were obtained from FVIIIKO-SCID mice (n = 3) injected with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII vectors or in FVIIIKO mice (n = 3) injected with 5 × 109 IU Ad-AAT-cFVIII. Plasma levels of the proinflammatory cytokine interleukin-6 (IL-6) were determined after injection of 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII vectors into FVIIIKO mice using sandwich enzyme-linked immunosorbent assay (ELISA) (Quantikine; R&D Systems, Minneapolis, MN). Platelet and red blood cell counts in FVIIIKO mice injected with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII vectors were measured in whole blood containing 10% EDTA (ethylenediaminetetraacetic acid) using a Cell-dyn 1300 blood counter (Abbott, IL). Toxicity assessment in the hemophilic dog was determined by measurement of serum ALT, AST, alkaline phosphatase (ALK P), bilirubin (Bili), urea, creatinine (Creat), creatinine kinase (CK), albumin (Alb), and iron levels according to standard procedures.15 Hematologic analysis was performed by quantifying platelets, red blood cells (RBCs), white blood cells (WBCs), hemoglobin (Hg), and mean corpuscular volume (MCV) according to standard procedures, along with fibrinogen (FGN) and fibrinogen-degradation products (FDPs).15
Results
Generation of HC-Ad vectors
Three HC-Ad vectors were compared in the present study (Figure 1). The Ad-AAT-cFVIII and Ad-AAT-hFVIII vectors express the canine or human B-domain–deleted FVIII cDNA, respectively, from the human α1-antitrypsin promoter (AAT) (Figure 1A-B). An HPRTstuffer fragment was used to optimize vector size for packaging and to avoid vector rearrangements.32
The rationale for using the AAT promoter was based on previous studies indicating that this promoter can lead to long-term, liver-specific expression of therapeutic proteins following transduction with HC-Ad vectors into immunocompetent mice and baboons.7,33,34 In an alternative vector design (Ad-Apo-cFVIII), the canine B-domain–deleted FVIII cDNA was under control of the hepatocyte-specific chimericApoE/ApoCII promoter (Apo). In this vector an additional fragment from the C346 cosmid was included (Figure 1C). Average particle titers obtained with the Cre66 packaging cell line were comparable for the different vectors: 9.2 × 1011particles/mL for Ad-AAT-cFVIII, 9.3 × 1011 particles/mL for Ad-AAT-hFVIII, and 8.4 × 1011 particles/mL for Ad-Apo-cFVIII. Average infectious titers, as determined by slot blot analyses,55 were 2.8 × 1010 IU/mL for Ad-AAT-cFVIII, 2.7 × 1010 IU/mL for Ad-AAT-hFVIII, and 1 × 1010 IU/mL for Ad-Apo-cFVIII. DNAs from CsCl-purified vectors were analyzed by restriction digestion and did not show any rearrangements.
HC-Ad–mediated FVIII gene delivery in immunodeficient hemophilic mice
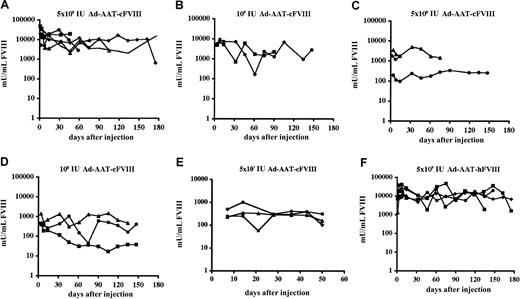
Adult hemophilic FVIIIKO-SCID mice were intravenously injected with the Ad-AAT-cFVIII vector at doses of 5 × 109 IU, 109 IU, 5 × 108 IU, 108 IU, 5 × 107 IU (Figure 2A-E), and 5 × 106 IU. FVIII-deficient littermates were injected with PBS as control. Immunodeficient hemophilic mice were chosen to assess the long-term expression potential of this nonintegrating vector type in the absence of confounding immune responses against the human or canine FVIII xenoproteins. FVIII production in the plasma of recipient mice was determined using a functional FVIII chromogenic assay.
FVIII expression levels were dose dependent, with the 3 highest doses showing supraphysiologic FVIII levels (greater than 1000 mU/mL) (Figure2). At the highest vector dose (5 × 109 IU) supraphysiologic canine FVIII levels were achieved for at least 6 months, stabilizing above the 15 000 mU/mL level (Figure 2A). These levels are at least 15-fold higher than the FVIII concentration in healthy human plasma. Peak FVIII production levels corresponding to nearly 50 000 mU/mL were observed. Even at a 100-fold lower vector dose (5 × 107 IU) (Figure 2E), therapeutic FVIII concentrations were still observed in all recipient mice (ie, 100-1000 mU/mL). No FVIII expression (less than 30 mU/mL) could be detected after injecting 5 × 106 IU Ad-AAT-cFVIII. Control animals injected with PBS had no detectable FVIII activity (less than 30 mU/mL).
Functional FVIII expression kinetics in adult hemophilic FVIIIKO-SCID mice following HC-Ad gene transfer.
Mice were injected intravenously with Ad-AAT-cFVIII at a dose of 5 × 109 (A), 109 (B), 5 × 108 (C), 108 IU (D), and 5 × 107 IU (E) or with Ad-AAT-hFVIII at a dose of 5 × 109 IU (F). FVIII-deficient littermates were injected with PBS as control.
Functional FVIII expression kinetics in adult hemophilic FVIIIKO-SCID mice following HC-Ad gene transfer.
Mice were injected intravenously with Ad-AAT-cFVIII at a dose of 5 × 109 (A), 109 (B), 5 × 108 (C), 108 IU (D), and 5 × 107 IU (E) or with Ad-AAT-hFVIII at a dose of 5 × 109 IU (F). FVIII-deficient littermates were injected with PBS as control.
In parallel, adult hemophilic FVIIIKO-SCID mice were intravenously injected with 5 × 109 IU of Ad-AAT-hFVIII (n = 5) (Figure 2F). Supraphysiologic human FVIII levels (exceeding 15 000 mU/mL) were obtained for at least 6 months, consistent with the results obtained with the Ad-AAT-cFVIII vector. Similarly, long-term expression of supraphysiologic human or canine FVIII levels were achieved in NOD-SCID mice (data not shown).
To further assess phenotypic correction of the clotting deficiency, plasma samples were subjected to a functional clotting assay (aPTT) that measures the activated partial thromboplastin time. Clotting times were significantly reduced (t test, P < .001) in plasma obtained from hemophilic FVIIIKO-SCID mice that were injected with 5 × 109 IU Ad-AAT-cFVIII (22 ± 8 seconds) and Ad-AAT-hFVIII vectors (40 ± 7 seconds) compared with the clotting time of plasma from untreated hemophilic FVIIIKO-SCID mice (90 ± 10 seconds) (n = 3). The clotting time of plasma from hemophilic animals injected with the FVIII vectors was similar or even lower than that of plasma from nonhemophilic mice (40 ± 4 seconds). Hence, gene therapy using HC-Ad vectors stably corrected the bleeding diathesis of these hemophilic mice.
HC-Ad–mediated FVIII gene delivery in immunocompetent hemophilic mice
To evaluate FVIII expression kinetics in immunocompetent animals, adult hemophilic FVIIIKO mice were intravenously injected with Ad-AAT-cFVIII and Ad-AAT-hFVIII at a dose of 5 × 109 IU (n = 6 for each vector). FVIII peak expression levels were observed within 5 to 8 days after injection (Figure 3A-B). These peak levels corresponded to 50 000 mU/mL for Ad-AAT-cFVIII and 75 000 mU/mL for Ad-AAT-hFVIII. FVIII levels had fallen to baseline 3 to 4 weeks after injection. This decrease in FVIII expression coincided with the induction of neutralizing antibodies directed against the xenogeneic FVIII protein (2-20 BU/mL).
Comparison of ApoE/ApoCII hepatocyte-specific promoter with AAT promoter
Previous studies had suggested that the inadvertent expression of transgenes in antigen-presenting cells might lead to the formation of neutralizing antibodies against the transgene product.35Because the AAT promoter is likely active in macrophagelike cells, possibly including Kupffer cells,7 it was important to rule out that the formation of neutralizing antibodies is attributed to the use of the AAT promoter that controls FVIII expression. An alternative HC-Ad was therefore evaluated that drives the canine B-domain–deleted FVIII gene from a chimericApoE/ApoCII hepatocyte-specific promoter (Ad-Apo-cFVIII). Intravenous injection of FVIIIKO mice with 5 × 109 IU Ad-Apo-cFVIII led to a supraphysiologic FVIII plasma concentration (up to 40 000 mU/mL) approximately 5 to 8 days after injection (Figure3C). Within 3 to 4 weeks after injection, expression declined to basal levels resembling the FVIII expression kinetics of the Ad-AAT-cFVIII and Ad-AAT-hFVIII vectors. This decline correlated with the induction of neutralizing antibodies against canine FVIII (4 BU/mL). Thus, the decline in human or canine FVIII expression in FVIIIKO mice was not inherent in the use of the AAT promoter.
Functional FVIII expression kinetics in adult hemophilic FVIIIKO mice following HC-Ad gene transfer.
Mice were injected intravenously with Ad-AAT-cFVIII (A), Ad-AAT-hFVIII (B), and Ad-Apo-cFVIII (C) at 5 × 109 IU per mouse (n = 6 for each vector).
Functional FVIII expression kinetics in adult hemophilic FVIIIKO mice following HC-Ad gene transfer.
Mice were injected intravenously with Ad-AAT-cFVIII (A), Ad-AAT-hFVIII (B), and Ad-Apo-cFVIII (C) at 5 × 109 IU per mouse (n = 6 for each vector).
After the injection of Ad-Apo-cFVIII into FVIIIKO-SCID (Figure 2) and NOD-SCID mice (data not shown), continuous FVIII expression (longer than 3 months) in the supraphysiologic range (exceeding 15 000 mU/mL) was observed, consistent with the expression profiles of Ad-AAT-cFVIII and Ad-AAT-hFVIII. Gene therapy using the Ad-Apo-cFVIII vector stably corrected the bleeding diathesis of these hemophilic mice. The clotting time based on aPTT assays was significantly reduced (t test,P < .001) in plasma obtained from hemophilic FVIIIKO-SCID mice injected with 5 × 109 IU Ad-Apo-cFVIII (27 ± 5 seconds) compared with that of plasma from untreated hemophilic FVIIIKO-SCID mice (90 ± 10 seconds) (n = 3).
Effect of transient macrophage depletion on FVIII expression in FVIIIKO and FVIIIKO-SCID mice transduced with HC-Ad vectors
The influence of cells of the innate immune system with regard to gene transfer efficiency using HC-Ad vectors has not been investigated. Therefore, the effect of transient depletion of tissue macrophages by clodronate liposome injection on FVIII expression was evaluated in FVIIIKO and FVIIIKO-SCID mice. Littermates were injected with PBS-containing liposomes as controls. Injection of liposomes containing clodronate resulted in a significant decrease in liver and spleen macrophages compared with controls, as confirmed by cytofluorometric analysis using macrophage-specific markers (MHC class II and F4/80) (data not shown). One day after liposome injection FVIIIKO-SCID and FVIIIKO mice were injected with Ad-AAT-cFVIII at doses of 5 × 109, 108, 5 × 107, 107, and 5 × 106 IU (Figures4-5).
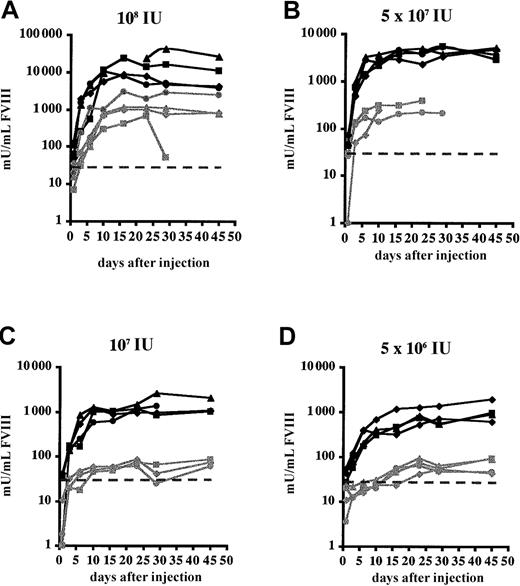
Functional FVIII expression in FVIIIKO-SCID mice following transient macrophage depletion.
Experimental mice were injected with clodronate liposomes (black lines) 1 day before injection with Ad-AAT-cFVIII at different doses: 108 (A), 5 × 107 (B), 107 (C), and 5 × 106 IU (D). Littermates were injected with PBS-containing liposomes as controls (gray lines). The detection limit of the assay corresponds to 30 mU/mL and was indicated on the graph by a dotted line.
Functional FVIII expression in FVIIIKO-SCID mice following transient macrophage depletion.
Experimental mice were injected with clodronate liposomes (black lines) 1 day before injection with Ad-AAT-cFVIII at different doses: 108 (A), 5 × 107 (B), 107 (C), and 5 × 106 IU (D). Littermates were injected with PBS-containing liposomes as controls (gray lines). The detection limit of the assay corresponds to 30 mU/mL and was indicated on the graph by a dotted line.
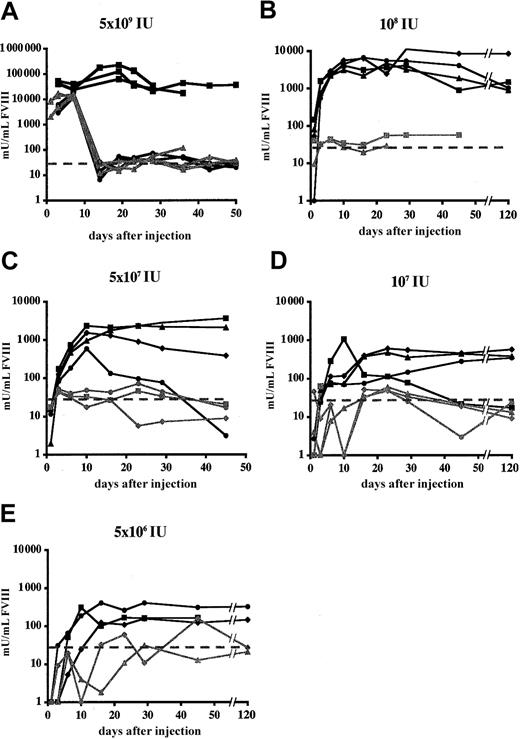
Functional FVIII expression in FVIIIKO mice following transient macrophage depletion.
Mice were treated with clodronate liposomes (black lines) 1 day before the injection of Ad-AAT-cFVIII vectors at different doses: 5 × 109 (A), 108 (B), 5 × 107(C), 107 (D), and 5 × 106 IU (E). Littermates were injected with PBS-containing liposomes as controls. Littermates were injected with PBS-containing liposomes as controls (gray lines). The detection limit of the assay corresponds to 30 mU/mL and was indicated on the graph by a dotted line.
Functional FVIII expression in FVIIIKO mice following transient macrophage depletion.
Mice were treated with clodronate liposomes (black lines) 1 day before the injection of Ad-AAT-cFVIII vectors at different doses: 5 × 109 (A), 108 (B), 5 × 107(C), 107 (D), and 5 × 106 IU (E). Littermates were injected with PBS-containing liposomes as controls. Littermates were injected with PBS-containing liposomes as controls (gray lines). The detection limit of the assay corresponds to 30 mU/mL and was indicated on the graph by a dotted line.
In FVIIIKO-SCID mice, treatment with clodronate liposomes resulted in a 10-fold increase of FVIII expression (Figure 4). Consequently, physiologic FVIII levels (1000 mU/mL) could still be achieved in the FVIIIKO-SCID mice using the lowest vector dose (5 × 106IU), whereas FVIII production was near background levels in FVIIIKO-SCID mice injected with PBS liposomes (Figure 4C-D). FVIII expression was stable in FVIIIKO-SCID mice treated with either clodronate or PBS liposomes, consistent with the results presented in Figure 2.
In clodronate-liposome–treated FVIIIKO animals, dose-dependent and significantly increased FVIII expression levels were observed after Ad-AAT-cFVIII injection (Figure 5). This is consistent with the results obtained in FVIIIKO-SCID mice (Figure 4). Supraphysiologic and relatively stable FVIII levels exceeding 10 000 mU/mL were achieved that lasted for at least 8 weeks, following the administration of 5 × 109 IU Ad-AAT-cFVIII in clodronate liposome-treated FVIIIKO mice. This was in contrast to the lower FVIII peak levels and the short-term FVIII expression in control FVIIIKO mice that received either PBS or PBS liposomes before injection with 5 × 109 IU Ad-AAT-cFVIII. The lower FVIII expression levels and the limited duration of FVIII expression correlated with the induction of neutralizing antibodies to cFVIII, as confirmed in Bethesda assays (7-17 BU/mL). Similarly, at lower vector doses, FVIII expression was higher and more lasting in the FVIIIKO mice that received the clodronate liposomes than in PBS liposome-treated controls. FVIII expression was near background levels (less than 30 mU/mL) in these animals (Figure 5B-E), which again correlated with the induction of neutralizing antibodies to cFVIII (10-20 BU/mL). The salutary effect of clodronate is long-lasting (longer than 6 months) in most recipient mice. In those few mice that showed a decline in FVIII expression, neutralizing antibodies to FVIII could be detected (2-11 BU/mL). Hence, a dual effect of clodronate-liposome treatment was apparent in FVIIIKO mice: not only was increased FVIII production observed, significantly prolonged FVIII gene expression was also observed.
Biodistribution, transduction efficiency, and specificity of FVIII expression following HC-Ad–mediated FVIII vector administration
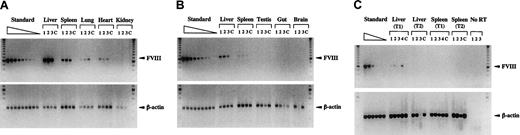
Biodistribution and gene transfer efficiency of HC-Ad vectors was determined in FVIIIKO mice injected with 5 × 109 IU Ad-AAT-hFVIII. Semiquantitative PCR using humanFVIII-specific primers indicated that gene transfer occurred predominantly into liver, spleen, lungs, and heart. Vector-specific PCR fragments were not detected in testis, gut, brain, or kidney (Figure 6A-B). Gene transfer was more efficient in the liver than in any other organ. In addition, gene transfer in the spleen was more efficient than in the heart and lungs (n = 3). Five days after injection, there were more transduced cells in the livers and spleens of recipient FVIIIKO mice than at a later time point (1 month after injection; Figure 6A-B). As expected, human FVIII gene-specific bands were not detectable in control animals injected with PBS.
To evaluate which transduced tissue expressed FVIII mRNA, semiquantitative RT-PCR analysis was performed using humanFVIII-specific primers. Human FVIII mRNA expression was detected in the liver but not in the other transduced organs—spleen (Figure 6C), lungs, or heart (data not shown). The apparent decrease of gene-marked cells in the liver (Figure 6A-B) correlated with a concomitant loss of hepatic human FVIII mRNA (Figure6C). As expected, endogenous β-actin mRNA could be detected by RT-PCR in spleen and liver (Figure 6C), lungs, and heart tissue (data not shown) of experimental (Figure 6C) and negative control animals (data not shown).
Biodistribution of HC-Ad vectors, gene transfer efficiency, and FVIII mRNA expression analysis.
FVIIIKO mice were injected with 5 × 109 IU Ad-AAT-hFVIII. Semiquantitative PCR (A-B) or RT-PCR (C) using humanFVIII-specific and β-actin–specific primers (n = 3 mice) at an early time point (5 days after injection, T1) in liver, spleen, lung, heart, and kidney (A) and testis, gut, and brain (B). PCR analysis of liver and spleen at a late time point (1 month after injection, T2) is shown in (B). Mice injected with PBS were used as controls (C). For RT-PCR analysis, controls included samples without reverse transcriptase (no RT) to exclude genomic DNA contamination. Molecular weight (MW) marker was the 100-bp ladder. FVIII PCR generated a 0.6-kb fragment, and β-actin PCR generated a 0.2-kb fragment. Standard corresponds to 2-fold serially diluted DNA with a known number of FVIII copies (ranging from 4 to 0 FVIII gene copies per diploid genomic equivalent).
Biodistribution of HC-Ad vectors, gene transfer efficiency, and FVIII mRNA expression analysis.
FVIIIKO mice were injected with 5 × 109 IU Ad-AAT-hFVIII. Semiquantitative PCR (A-B) or RT-PCR (C) using humanFVIII-specific and β-actin–specific primers (n = 3 mice) at an early time point (5 days after injection, T1) in liver, spleen, lung, heart, and kidney (A) and testis, gut, and brain (B). PCR analysis of liver and spleen at a late time point (1 month after injection, T2) is shown in (B). Mice injected with PBS were used as controls (C). For RT-PCR analysis, controls included samples without reverse transcriptase (no RT) to exclude genomic DNA contamination. Molecular weight (MW) marker was the 100-bp ladder. FVIII PCR generated a 0.6-kb fragment, and β-actin PCR generated a 0.2-kb fragment. Standard corresponds to 2-fold serially diluted DNA with a known number of FVIII copies (ranging from 4 to 0 FVIII gene copies per diploid genomic equivalent).
Safety assessment of HC-Ad vectors
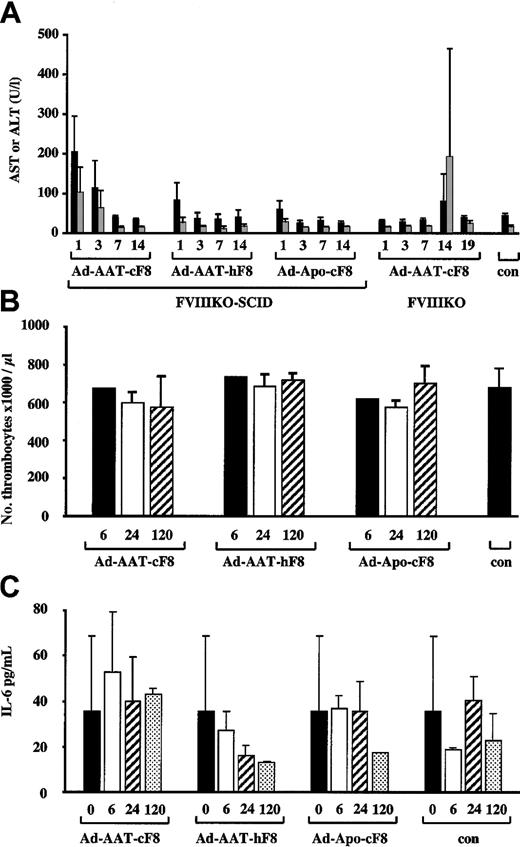
To evaluate potential hepatotoxicity associated with the use of HC-Ad vectors, liver transaminase levels (AST, ALT)36 were determined in the plasma at different intervals following injection of FVIIIKO-SCID mice with the highest dose of 5 × 109 IU of the Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII vectors (Figure7A). Ad-AAT-hFVIII and Ad-Apo-cFVIII vector preparations did not lead to a significant increase (P > .05) in AST or ALT level in the plasma, whereas a slight, yet significant (P < .001), 3- to 4-fold increase in AST and ALT levels was apparent 1 day after Ad-AAT-cFVIII transduction compared with PBS-injected controls. Nevertheless, after a few days, AST and ALT levels returned to basal levels indistinguishable from normal physiologic levels. The exact reason for the differences among the different vector batches is unclear but might reflect differences in vector purity. Injection of 5 × 109 IU Ad-AAT-cFVIII into FVIIIKO mice did not trigger any AST and ALT elevations during the first week after injection (Figure 7A). This confirms that direct hepatotoxicity of HC-Ad vector particles is limited or absent in this mouse model. However, at 2 weeks after injection, a transient yet significant increase in AST and ALT levels was apparent, consistent with the induction of a cellular immune response that eliminated the FVIII-transduced hepatocytes; this is in agreement with the PCR data (Figure 6).
Toxicity analysis of HC-Ad vectors.
AST (black bars) and ALT (gray bars) levels (A) in plasma from FVIIIKO-SCID mice injected intravenously with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII. Alternatively, 5 × 109 IU Ad-AAT-cFVIII was injected intravenously into FVIIIKO mice. (A) Platelet counts (B) and IL-6 levels (C) in plasma from FVIIIKO-SCID mice intravenously injected with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII.
Toxicity analysis of HC-Ad vectors.
AST (black bars) and ALT (gray bars) levels (A) in plasma from FVIIIKO-SCID mice injected intravenously with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII. Alternatively, 5 × 109 IU Ad-AAT-cFVIII was injected intravenously into FVIIIKO mice. (A) Platelet counts (B) and IL-6 levels (C) in plasma from FVIIIKO-SCID mice intravenously injected with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII.
Because the administration of early-generation adenoviral vectors is known to result in thrombocytopenia,37 platelet counts were performed in FVIIIKO mice that had received 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII. No significant differences in platelet counts were observed in mice that received the different HC-Ad vectors compared with PBS-injected FVIII-KO controls (Figure 7B). In addition, there was no evidence of anemia or erythroblastosis because erythrocyte counts were normal following HC-Ad vector administration in FVIIIKO mice (data not shown).
Finally, it has been shown that early-generation adenoviral vectors trigger a rapid induction of proinflammatory cytokines, particularly IL-6, associated with an acute-phase inflammatory response that contributes to vector toxicity.38 To determine whether the vectors used in this study were associated with an increase in IL-6, FVIIIKO mice were injected with 5 × 109 IU Ad-AAT-cFVIII, Ad-AAT-hFVIII, and Ad-Apo-cFVIII (Figure 7C). As in control animals, there were no significant increases (one-way analysis of variance [ANOVA]; P > .05) in plasma IL-6 levels after injection of the various HC-Ad vectors. In conclusion, these results underscore the relative safety of HC-Ad vectors for gene therapy of hemophilia A.
HC-Ad–mediated FVIII gene delivery in a hemophilia A dog
The Ad-Apo-cFVIII vector was injected into an adult dog with severe hemophilia A at a dose of 4.3 × 109 IU/kg or 3 × 1011 vp/kg (total vector dose per dog, 5.6 × 1010 IU or 4 × 1012 vp; dog weight, 13 kg). Transient therapeutic FVIII levels could be achieved to a maximum of 3.5% of normal human FVIII levels (35 mU/mL) at 3 days after injection (Figure 8), which correlated with a significant shortening of the whole blood clotting time (WBCT) from 14 minutes (baseline) to 8 minutes at 3 days after injection. Inhibitory antibodies to canine FVIII were absent, as confirmed by Bethesda assays. Most important, the HC-Ad vector was not associated with any detectable hepatotoxicity, nephrotoxicity (no significant changes in serum ALT, AST, ALKP, bilirubin, urea, creatinine, albumin, and iron levels, Table1), or hematologic abnormalities (no significant changes in platelet, RBC, WBC, HMCT, Hg, or MCV counts; Table 1). In addition, we found no evidence of abnormal levels of FGN (Table 1) or FDP (Table 1).
Functional FVIII expression kinetics in an adult hemophilia A dog following HC-Ad gene transfer.
The dog was injected intravenously with 5.6 × 1010 IU (4 × 1012 vector particles [vp]) Ad-Apo-cFVIII vector.
HC-Ad safety evaluation in hemophilia A dog model
| . | Serum chemistry values . | Hematology . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT, U/L . | AST, U/L . | ALK P, U/L . | Bili, μM . | Urea, mM . | Creat, μM . | CK, U/L . | Alb, g/L . | Iron, μM . | Platelet, × 109/L . | RBC, × 1012/L . | WBC, × 109/L . | Hg, g/L . | MCV, fL . | FGN, g/L . | FDP, ng/mL . | |
| Baseline | 54 | 28 | 56 | 3 | 4.9 | 49 | 244 | — | — | 304 | 7.7 | 10 | 180 | 67.6 | — | Normal |
| Preinjection | 50 | 22 | 44 | 3 | 2.8 | 50 | 122 | 34 | 21 | 233 | 7.1 | 7.3 | 164 | 68.1 | 1.7 | Normal |
| 5 h | 53 | 26 | 51 | 3 | 5.0 | 44 | 145 | 36 | 17 | 253 | 7.2 | 14.5 | 170 | 68.1 | 1.9 | Normal |
| 1 d | 51 | 24 | 61 | 3 | 2.5 | 48 | 148 | 34 | 24 | 220 | 6.5 | 4.9 | 151 | 68.7 | 2.6 | Normal |
| 2 d | 56 | 24 | 56 | 2 | 2.8 | 47 | 102 | 34 | 32 | 219 | 7.4 | 6.9 | 174 | 69.0 | 2.2 | Normal |
| 3 d | 51 | 25 | 49 | 3 | 3.2 | 46 | 106 | 33 | 26 | 221 | 6.9 | 7.9 | 160 | 67.8 | 1.7 | Normal |
| 7 d | 45 | 20 | 44 | 2 | 4.3 | 50 | 94 | 34 | 28 | 220 | 6.7 | 8.4 | 155 | 69.2 | 1.9 | Normal |
| 10 d | 48 | 22 | 46 | 2 | 4.1 | 52 | 105 | 35 | 31 | 251 | 7.0 | 8.8 | 165 | 69.2 | 1.9 | Normal |
| 14 d | 56 | 27 | 53 | — | 2.7 | 42 | 104 | 35 | 33 | 257 | 7.7 | 7.4 | 178 | 68.8 | 2.2 | Normal |
| 21 d | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1.8 | Normal |
| 28 d | 64 | 24 | 48 | — | 3.3 | 43 | 125 | 36 | 25 | 264 | 7.8 | 6.7 | 180 | 69.0 | 1.4 | Normal |
| 35 d | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| . | Serum chemistry values . | Hematology . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT, U/L . | AST, U/L . | ALK P, U/L . | Bili, μM . | Urea, mM . | Creat, μM . | CK, U/L . | Alb, g/L . | Iron, μM . | Platelet, × 109/L . | RBC, × 1012/L . | WBC, × 109/L . | Hg, g/L . | MCV, fL . | FGN, g/L . | FDP, ng/mL . | |
| Baseline | 54 | 28 | 56 | 3 | 4.9 | 49 | 244 | — | — | 304 | 7.7 | 10 | 180 | 67.6 | — | Normal |
| Preinjection | 50 | 22 | 44 | 3 | 2.8 | 50 | 122 | 34 | 21 | 233 | 7.1 | 7.3 | 164 | 68.1 | 1.7 | Normal |
| 5 h | 53 | 26 | 51 | 3 | 5.0 | 44 | 145 | 36 | 17 | 253 | 7.2 | 14.5 | 170 | 68.1 | 1.9 | Normal |
| 1 d | 51 | 24 | 61 | 3 | 2.5 | 48 | 148 | 34 | 24 | 220 | 6.5 | 4.9 | 151 | 68.7 | 2.6 | Normal |
| 2 d | 56 | 24 | 56 | 2 | 2.8 | 47 | 102 | 34 | 32 | 219 | 7.4 | 6.9 | 174 | 69.0 | 2.2 | Normal |
| 3 d | 51 | 25 | 49 | 3 | 3.2 | 46 | 106 | 33 | 26 | 221 | 6.9 | 7.9 | 160 | 67.8 | 1.7 | Normal |
| 7 d | 45 | 20 | 44 | 2 | 4.3 | 50 | 94 | 34 | 28 | 220 | 6.7 | 8.4 | 155 | 69.2 | 1.9 | Normal |
| 10 d | 48 | 22 | 46 | 2 | 4.1 | 52 | 105 | 35 | 31 | 251 | 7.0 | 8.8 | 165 | 69.2 | 1.9 | Normal |
| 14 d | 56 | 27 | 53 | — | 2.7 | 42 | 104 | 35 | 33 | 257 | 7.7 | 7.4 | 178 | 68.8 | 2.2 | Normal |
| 21 d | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1.8 | Normal |
| 28 d | 64 | 24 | 48 | — | 3.3 | 43 | 125 | 36 | 25 | 264 | 7.8 | 6.7 | 180 | 69.0 | 1.4 | Normal |
| 35 d | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
— indicates not determined.
Discussion
The present study showed that unprecedentedly high levels of human or canine B-domain–deleted factor VIII were produced in adult hemophilic mice following gene therapy with the latest generation HC-Ad vector technology. Peak FVIII levels of 7500% could be achieved in hemophilic animals using a vector dose (5 × 109IU/mouse) that was relatively nontoxic in mice. In particular, the HC-Ad vectors used in the present study appear to be at least 20- to 40-fold better than those used in a previous study based on HC-Ad vectors using comparable vector doses.39 Most important, the injection of a relatively low vector dose (108IU/mouse) still resulted in physiologic FVIII levels using the present HC-Ad vector, whereas in previously published studies no FVIII levels could be detected at these low vector doses using an HC-Ad vector currently in clinical trials.39 Furthermore, using the current HC-Ad vector design, therapeutic FVIII levels could still be obtained at a 100-fold lower dose (ie, 5 × 107 IU). Hence, this level of improvement may have important consequences for clinical trials.
The use of HC-Ad vectors resulted in FVIII levels in mice that were 1000- to 10 000-fold higher than the therapeutic threshold concentration of 1% needed to convert severe to moderate hemophilia. These levels are several orders of magnitude higher than the FVIII expression levels that could be achieved using current AAV40 (20%-30%) or lentiviral41 (10%-20%, after partial hepatectomy only) vectors in adult mice. In addition, many previous studies reported a lack of FVIII or short-term FVIII expression following gene therapy in animal models.42,43It is likely that the use of the HC-Ad vector technology and the optimization of the FVIII expression cassettes were responsible for the high FVIII levels unparalleled in any other study so far. This is consistent with the high factor IX levels that were recently obtained using HC-Ad vectors.56 The particular combination of FVIII transgenes, promoters, and stuffer fragments used in this study had not been tested previously. The use of the B-domain–deleted instead of the full-length FVIII cDNA might have boosted FVIII production because the B domain is known to suppress FVIII expression.44 45 The use of potent liver-specific promoters such as the chimeric ApoEenhancer/ApoCII promoter or the AAT promoter might have further contributed to these high FVIII expression levels. Finally, it is noteworthy that there was no apparent thrombotic toxicity associated with these extraordinarily high plasma FVIII levels.
The stable, long-term expression of supraphysiologic FVIII levels in FVIIIKO-SCID mice following HC-Ad–mediated gene delivery is consistent with previous reports.7,16,33,39,46 This prolonged transgene expression likely results from the lack of residual adenoviral gene expression, the reduced toxicity, and the reduced inflammatory properties of HC-Ad vectors compared with early-generation adenoviral vectors.15,36 In the present study, no thrombocytopenia, anemia, erythroblastosis, or elevation of proinflammatory cytokine IL-6 were apparent after HC-Ad vector administration in mice, which further underscores the relative safety of these improved adenoviral vectors in contrast to early-generation vectors.37 In addition, HC-Ad vector preparations triggered no or only limited and transient elevations in serum transaminases (AST and ALT). However, Wilson and colleagues47,48 have shown that mice are relatively resistant to toxicity from early-generation adenoviral vectors compared with nonhuman primates, which show significant morbidity and mortality at doses (1013 vp/kg) comparable to those used in our present study. Similarly, mice injected with an early-generation adenoviral vector encoding FVIII displayed significantly fewer toxic effects than did hemophilia A dogs.15,18 In addition, one patient with ortinithine carbamyl transferase (OTC) deficiency recently died after intrahepatic artery injection of 6 × 1011 vp/kg of an early-generation E1/E3/E4-deleted vector.49 It appears therefore that there may be significant differences in adenoviral toxicity among species and perhaps also between individuals. This makes it particularly difficult to extrapolate safety data from mice to larger animal models or human patients. However, these adenoviral toxicity studies were performed largely using early-generation adenoviral vectors that, by virtue of the residual adenoviral gene expression, are more toxic and trigger more potent inflammatory responses than HC-Ad vectors. The favorable safety profile of the HC-Ad vectors in the canine model, as shown in the present study, is therefore consistent with the safety profile in hemophilic mice and is in contrast to the toxicity of early-generation adenoviral vectors in large animal models, such as hemophilia A dogs or primates.15 50
Human and canine FVIII production was transient in immunocompetent FVIIIKO mice, in contrast to the long-term gene expression that was observed in immunodeficient hemophilic mice. Short-term FVIII expression in immunocompetent FVIIIKO mice was caused by an immune mechanism. This was confirmed by showing that neutralizing antibodies were induced that were associated with a decrease in FVIII expression. PCR analysis confirmed that FVIII-transduced cells disappeared over time (Figure 6), indicating that a cellular immune mechanism contributed to the loss of FVIII expression in FVIIIKO mice.
The induction of neutralizing antibodies against xenogenic FVIII proteins in mice has also been observed using HC-Ad vectors that express full-length human FVIII cDNA,39 though the onset was more rapid in the present study, perhaps because of higher FVIII expression levels. In addition, AAV,40lentiviral,41 and retroviral30vector-mediated FVIII gene delivery also resulted in the induction of neutralizing antibodies against human FVIII. These results indicate that human (and canine) FVIII proteins are highly immunogenic xenoantigens in (hemophilic) mice. However, in some previous studies using adenoviral vectors, no FVIII-specific antibodies were detected, a finding that was explained by genetic influences of the mice strain used.11
Expression of canine FVIII is also transient in the hemophilia A dog model. In contrast to transient human or canine FVIII expression in immunocompetent hemophilic mice, the decline in transgene expression following HC-Ad gene transfer in the dog does not correlate with the induction of neutralizing antibodies to canine FVIII, as measured using Bethesda assays. Despite the fact that Bethesda assay findings were negative, it cannot be excluded that a nonneutralizing anti-FVIII antibody could have resulted in the accelerated clearance of FVIII. Antibodies from hemophilia A patients are frequently directed toward epitopes of FVIII that are not directly involved in the function of the molecule and therefore escape detection in the Bethesda method,51 but these nonneutralizing antibodies are sometimes associated with increased FVIII clearance from the circulation (K. Peerlinck, C. Van Geet, personal communication, August 2002). Alternatively, the decline in FVIII expression may reflect some loss of FVIII transgenes or a transcriptional effect. Morral et al33 recently demonstrated that the duration and level of transgene expression (ie, hAAT) is variable in baboons injected with HC-Ad vectors: hAAT transgene expression was short term in one of the baboons, which correlated with the presence of anti-hAAT antibodies, whereas in the other 2 baboons, no anti-hAAT antibodies could be detected and long-term hAAT expression could be achieved. Hence, it is possible that if more dogs are tested, some may show prolonged expression. Future studies in new cohorts will be necessary to further address these issues.
Limited information is available on the role of the innate immune system with regard to gene transfer using HC-Ad vectors. To evaluate the contribution of the innate immune system on HC-Ad vector, macrophages were eliminated using clodronate liposomes. Clodronate liposomes are being evaluated in clinical trials by local administration in patients to transiently eliminate tissue macrophages.52 The effects of clodronate liposomes typically last for a few days at the maximum. Given the fact that depleted resident macrophages are replaced by new ones, differentiating from bone marrow–derived monocytes, the transient nature of the depletion is obvious. After the depletion of Kupffer cells in the liver, new Kupffer cells start to reappear within 1 week, and the mice then become capable of mounting an immune response to other neoantigens.53
The present results demonstrate that the transient depletion of macrophages using clodronate liposomes resulted in increased FVIII production levels in FVIIIKO and FVIIIKO-SCID mice. Most important, significantly prolonged FVIII gene expression was achieved in these FVIIIKO mice, indicating that the innate immune compartment limits the therapeutic efficacy of HC-Ad vectors. This reduced therapeutic efficacy is likely the combined result of a direct uptake of HC-Ad vector particles by antigen-presenting cells in conjunction with the induction of a specific humoral immune response.
The present results clearly indicate that the therapeutic window of HC-Ad vectors could be improved further by minimizing the interaction between HC-Ad vectors and the innate immune system. This proof-of-concept study justifies exploring alternative and clinically approved agents, such as gamma globulins, that act in a manner similar to that of clodronate liposomes.54 Alternatively, the development of targeted HC-Ad vectors that selectively transduce hepatocytes while bypassing the innate immune compartment is warranted.
To our knowledge, no previous publications report the use of HC-Ad expressing an autologous canine FVIII transgene in dogs with hemophilia A. Transient therapeutic canine FVIII expression levels were obtained in the present study that partially corrected a bleeding diathesis in a dog with hemophilia A. Inhibitory antibodies to canine FVIII could not be detected, unlike when first-generation adenoviral vectors are used to deliver canine FVIII to hemophilia A dogs.15 HC-Ad vector was not associated with any detectable hepatotoxicity or nephrotoxicity or with hematologic abnormalities in the hemophilia A dog, consistent with the safety profile of HC-Ad in hemophilic mice but in contrast to the toxicity of first-generation adenoviral vectors in large animal models.15,50 In addition, there was no evidence of a consumptive coagulation process, unlike when first-generation adenoviral vectors are used.50
The HC-Ad vector seems less efficacious in hemophilic dogs than in mice, based on FVIII expression levels, when comparable vector doses per kilogram are used (Table 1 vs Figure 2). It is therefore possible that the threshold dose is higher in dogs than in mice. This may reflect differences in receptor expression levels in dogs versus mice, which may influence the threshold effect. Other factors, including differences in vector biodistribution, may contribute to these differences between different species.
The present study contributes to a better understanding of the safety and efficacy of HC-Ad vectors in clinically relevant hemophilia animal models and suggests that the therapeutic window of HC-Ad vectors could be improved by minimizing the interaction between HC-Ad vectors and the innate immune system. This preclinical study has important implications for clinical trials based on HC-Ad vectors.
We thank Dr Kazazian (University of Pennsylvania) and Dr Saint-Remy (University of Leuven) for the FVIII-KO mice and immunodeficient FVIIIKO-SCID and Dr De Geest (University of Leuven) for providing us with the ApoE/ApoCII promoter. We also thank Mrs Vanslembrouck and Mrs Vangoidsenhoven for technical assistance, Dr Arnout and Mrs Van Russelt for their help with the aPTT assays, and Dr Vermylen (University of Leuven) for reviewing the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-03-0823.
Supported by the Flemish Fund for Scientific Research (FWO) (G.0110.00), the Flemish government, Canadian Institutes of Health Research (grant MOP10912), the German Federal Ministry of Education and Research (FKZ: 01KS9502), and the Center for Molecular Medicine Cologne (ZMMK) (S.K.). D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis. L.T. is an Instituut voor de aanmoediging van innovatie door Wetenschap en Technologie in Vlaanderen (I.W.T.) doctoral fellow.
M.K.L.C. and G.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thierry VandenDriessche, Center for Transgene Technology and Gene Therapy; Flanders Interuniversity Institute for Biotechnology, University of Leuven, 49 Herestraat B-3000 Leuven, Belgium; e-mail:thierry.vandendriessche@med.kuleuven.ac.be.



![Fig. 8. Functional FVIII expression kinetics in an adult hemophilia A dog following HC-Ad gene transfer. / The dog was injected intravenously with 5.6 × 1010 IU (4 × 1012 vector particles [vp]) Ad-Apo-cFVIII vector.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-03-0823/3/m_h80533939008.jpeg?Expires=1769131180&Signature=KtYMHwxi3Jaka7~GPwobbR0FoJ-ms3lTTjtZiZCLylf4k1fBDVWE8aqdZuqaluoXediRcVGxUdWMQHk-UbeaMMgCcq2ioUgV4tx5R-nPXlJdlOCxKZzZrjR-zCwd~dCIR3ZtO3tj8S3j9s0b9WNEHnWkzj5vJdWFxdUCd6wFK80xz2GLbK0pNM3srjUPbeD7AZ5fuf6y7MWVOth7ENINsvaDEEHgeFB6fzbIa1xxzDeBNMvl8pZjtwnk2j266nUJFJUxMJHBFux4EAiC4rkafVIuFIVjsSne0oNFGvfD6W77771xAkekX5Mzkl8BVsTd-QLRpdPB2AMDWACbUPZCyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal