Changes in blood dendritic cell (BDC) counts (CD123hiBDC and CD11c+BDC) and expression of CD62L, CCR7, and CD49d were analyzed in healthy donors, multiple myeloma (MM), and non-Hodgkin lymphoma (NHL) patients, who received granulocyte-colony stimulating factor (G-CSF) containing peripheral blood stem cell (PBSC) mobilization protocols. Low-dose G-CSF in healthy donors (8-10 μg/kg/d subcutaneously) and high-dose G-CSF in patients (30 μg/kg/d) increased CD123hiBDC (2- to 22-fold, mean 3.7 × 106/L-17.7 × 106/L and 1.9 × 106/L-12.0 × 106/L) in healthy donors and MM but decreased CD11c+BDC (2- to 10-fold, mean 5.7 × 106/L-1.6 × 106/L) in NHL patients, on the day of apheresis, compared with steady state. After apheresis, CD123hiBDC counts remained high, whereas low CD11c+BDC counts tended to recover in the following 2-5 days. Down-regulation of CD62L and up-regulation of CCR7 on CD123hiBDC were found in most healthy donors and MM patients. CD49d expression was unchanged. Thus, PBSC mobilization may change BDC counts by altering molecules necessary for BDC homing from blood into tissues.
Introduction
Dendritic cells (DCs) initiate and direct primary immune responses and have attracted attention as cellular adjuvants for cancer immunotherapy. Two distinct subsets of blood dendritic cells (BDCs) have been defined, the CD123hiCD11c−BDC and CD123dimCD11c+BDC.1,2 They are produced in bone marrow and, upon release, migrate through the blood into body tissues. Adhesion molecules (CD62L, CD49d, CD44, CLA) and chemokine receptors (CCR7, CCR1, CCR5, CCR6, CXCR3) regulate their passage from bone marrow into blood and subsequent egress into the tissues.3-9 As a consequence, these molecules may contribute to BDC count regulation.
BDC counts can be increased in healthy donors by several stimuli, including surgical stress, exercise,10 and cytokines such as Flt3-ligand11,12 and granulocyte-colony stimulating factor (G-CSF).13 G-CSF combined with chemotherapy is used to mobilize peripheral blood stem cells (PBSCs) in patients undergoing PBSC transplantation. It is possible that BDC counts may be increased during PBSC mobilization, which could provide an opportunity for BDC harvesting for posttransplantation immunotherapy. Further data to support or refute this hypothesis is crucial to plan the integration of BDC harvesting with PBSC mobilization.14,15 An understanding of BDC homing molecule expression patterns may also help to further define mechanisms governing BDC trafficking and count regulation. We report new data detailing sequential BDC counts, omitted in previous studies,13 and changes in BDC homing molecules induced by PBSC mobilization in healthy donors, multiple myeloma (MM), and non-Hodgkin lymphoma (NHL) patients.
Study design
Blood samples
Donor characteristics and mobilization protocols are shown in Table 1. Blood samples were taken prior to commencement of the mobilization protocol (steady state, SS), on the day of apheresis (DA), and at various time points thereafter. Healthy donors underwent leukapheresis on days 4 and 5. MM and NHL patients were leukapheresed at the time of accelerated white cell recovery (white cell count > 4 × 109/L or CD34+cells > 20/μL; MM, days 5-18; NHL, days 9-13).
Donor characteristics
| . | Healthy donors . | Multiple myeloma . | Non-Hodgkin lymphoma . |
|---|---|---|---|
| No. of patients | 8 | 10 | 10 |
| Median age in years (range) | 38 (20-49) | 59 (52-68) | 51 (21-64) |
| Sex (male/female) | 4/4 | 5/5 | 6/4 |
| Mobilization regimen | |||
| G-CSF (8 μg/kg/day subcutaneously)† | 8 | 0 | 0 |
| G-CSF (30 μg/kg/day subcutaneously)* | 0 | 1 | 0 |
| CY (2 g/m2intravenously) + G-CSF (10 μg/kg/day subcutaneously)† | — | 3 | 3 |
| CY (2-4 g/m2intravenously) + G-CSF (30 μg/kg/day subcutaneously)* | — | 6 | 7 |
| Disease status at mobilization, no. (%) | |||
| Complete hematologic remission | — | 0 (0%) | 7 (70%) |
| Partial remission | — | 2 (20%) | 3 (30%) |
| Plateau | — | 5 (50%) | 0 (0%) |
| Progressive disease | — | 3 (30%) | 0 (0%) |
| Prior chemotherapy, no. (%)‡ | |||
| Untreated | — | 0 (0%) | 0 (0%) |
| 1-2 regimens of therapy | — | 9 (90%) | 10 (100%) |
| Prior radiotherapy, no. (%) | — | 3 (30%) | 2 (20%) |
| . | Healthy donors . | Multiple myeloma . | Non-Hodgkin lymphoma . |
|---|---|---|---|
| No. of patients | 8 | 10 | 10 |
| Median age in years (range) | 38 (20-49) | 59 (52-68) | 51 (21-64) |
| Sex (male/female) | 4/4 | 5/5 | 6/4 |
| Mobilization regimen | |||
| G-CSF (8 μg/kg/day subcutaneously)† | 8 | 0 | 0 |
| G-CSF (30 μg/kg/day subcutaneously)* | 0 | 1 | 0 |
| CY (2 g/m2intravenously) + G-CSF (10 μg/kg/day subcutaneously)† | — | 3 | 3 |
| CY (2-4 g/m2intravenously) + G-CSF (30 μg/kg/day subcutaneously)* | — | 6 | 7 |
| Disease status at mobilization, no. (%) | |||
| Complete hematologic remission | — | 0 (0%) | 7 (70%) |
| Partial remission | — | 2 (20%) | 3 (30%) |
| Plateau | — | 5 (50%) | 0 (0%) |
| Progressive disease | — | 3 (30%) | 0 (0%) |
| Prior chemotherapy, no. (%)‡ | |||
| Untreated | — | 0 (0%) | 0 (0%) |
| 1-2 regimens of therapy | — | 9 (90%) | 10 (100%) |
| Prior radiotherapy, no. (%) | — | 3 (30%) | 2 (20%) |
CY indicates cyclophosphamide (Endoxan-Astra, Parramatta, NSW, Australia); G-CSF, granulocyte-colony stimulating factor (Filgrastim; Amgen, Thousand Oaks, CA); —, not applicable.
No significant difference was noted in the intensity of chemotherapeutic regimens between the MM and NHL patient groups. Four NHL patients were treated with the anti-CD20 monoclonal antibody Mabthera (Roche Pharmaceuticals, Dee Why, NSW, Australia) concurrently with chemotherapy.
Flow cytometric analysis of BDCs
BDC subsets were identified among peripheral blood mononuclear cells (PBMCs) by 3-color flow cytometry as cells that lacked lineage and stem cell markers (Lin−; CD3−/CD14−/CD16−/CD19−/CD56−/CD34−cells) but expressed HLA-DR and the CD123 (CD123hiBDC) or CD11c (CD11c+BDC) marker. CD123hiBDC, CD11c+BDC, and CD34+ cell counts (×106/L) were determined by calculations based on our published technique.10 Homing molecule expression was analyzed in SS or on DA by 3-color flow cytometry on sorted Lin− cells followed by HLA-DR and CD123 labeling, in combination with CD62L, CCR7, and CD49d.
Results and discussion
Effect of G-CSF or cyclophosphamide (CY)/G-CSF treatment on BDC counts
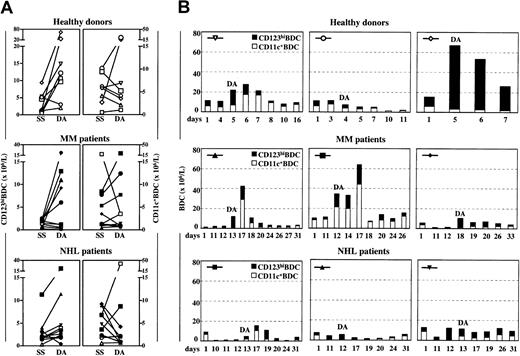
We compared BDC counts in SS with those on DA and at various time points thereafter in healthy donors, MM, and NHL patients. It should be emphasized that our assessment of changes in BDC counts is the first report to examine individually paired samples, which allows interindividual variability to be assessed. We also, for the first time, examined the kinetics of BDC counts after PBSC collection to establish the timing of peak CD123hiBDC or CD11c+BDC counts.
In healthy donors (7 of 8), low-dose G-CSF treatment increased CD123hiBDC counts (2- to 22-fold, from a mean of 3.7 × 106/L in SS to a mean of 17.7 × 106/L on DA) and occasionally increased CD11c+BDC counts on DA (Figure1A). Maximal CD123hiBDC counts coincided with peak CD34+ cell counts, predominantly on DA (data not shown). Declining counts were noted 1 to 2 days afterward. Maximal CD11c+BDC counts were found in SS or, in some cases, 1 to 2 days after apheresis (Figure 1B).
Changes in BDC counts after G-CSF or CY/G-CSF treatment.
CD123hiBDC and CD11c+BDC counts (× 106/L) were analyzed in steady state (SS; prior to commencement of the PBSC mobilization protocol), 1 day after commencement of the PBSC mobilization protocol (day 1), on the day of apheresis (DA), and at different time points thereafter. (A) Paired CD123hiBDC and CD11c+BDC counts are shown in healthy donors, MM, and NHL patients. (B) Kinetic data from 3 representative subjects in each group is shown. The symbols identify the patients shown in (A). Low-dose G-CSF (10 μg/kg/d subcutaneously) is indicated by open symbols, high-dose G-CSF (30 μg/kg/d subcutaneously) by filled symbols.
Changes in BDC counts after G-CSF or CY/G-CSF treatment.
CD123hiBDC and CD11c+BDC counts (× 106/L) were analyzed in steady state (SS; prior to commencement of the PBSC mobilization protocol), 1 day after commencement of the PBSC mobilization protocol (day 1), on the day of apheresis (DA), and at different time points thereafter. (A) Paired CD123hiBDC and CD11c+BDC counts are shown in healthy donors, MM, and NHL patients. (B) Kinetic data from 3 representative subjects in each group is shown. The symbols identify the patients shown in (A). Low-dose G-CSF (10 μg/kg/d subcutaneously) is indicated by open symbols, high-dose G-CSF (30 μg/kg/d subcutaneously) by filled symbols.
In MM patients, considerable interindividual variability in CD123hiBDC counts on DA was observed, most likely due to differences in mobilization regimen (Table 1). Only MM patients, who received high doses of G-CSF treatment, increased CD123hiBDC on DA compared with SS (5 of 7, 2- to 22-fold, from a mean of 1.9 × 106/L in SS to a mean of 12.0 × 106/L on DA, Figure 1A, filled symbols). The increase in CD123hiBDC counts also coincided with peak CD34+ cell counts (data not shown), with a rapid decline 2 to 5 days after apheresis. CY/G-CSF treatment in MM patients had a variable influence on CD11c+BDC counts. Maximal CD11c+BDC counts varied, occurring either in SS, on DA, or 4 to 5 days after apheresis (Figure 1A-B).
Despite receiving mobilization protocols of similar intensity, NHL patients produced lesser increases in BDC counts compared with MM patients. High CD123hiBDC counts were observed on DA in occasional NHL patients, with declining counts noted in the following 4 to 5 days (Figure 1B). In NHL patients, high doses of G-CSF treatment appeared to decrease CD11c+BDC counts to low levels on DA compared with SS (6 of 7, 2- to 10-fold, from a mean of 5.7 × 106/L in SS to a mean of 1.6 × 106/L on DA, Figure 1A, filled symbols). These counts tended to recover 4-5 days later (Figure 1B). Interestingly, another study, which defined BDC as CD80+/CD14+cells, suggested that a G-CSF-containing PBSC mobilization protocol produced minimal increases in BDC counts compared with granulocyte-macrophage colony-stimulating factor.16
The timing of CD123hiBDC harvesting appears to be optimal on the day of PBSC collection and for 2 to 5 days thereafter. It is striking that CD123hiBDCs were only increased in MM patients when high-dose G-CSF (30 μg/kg/d subcutaneously) was used. If CD11c+BDCs are considered the preferred cells for immunotherapy protocols, it may be prudent to harvest 4 to 5 days later, particularly in NHL patients.
These data suggest that it seems possible to combine PBSC mobilization with BDC harvesting for posttransplantation immunotherapy, particularly if BDC counts are carefully monitored.
Changes in the expression of CD62L, CCR7, and CD49d on BDC after G-CSF or CY/G-CSF treatment
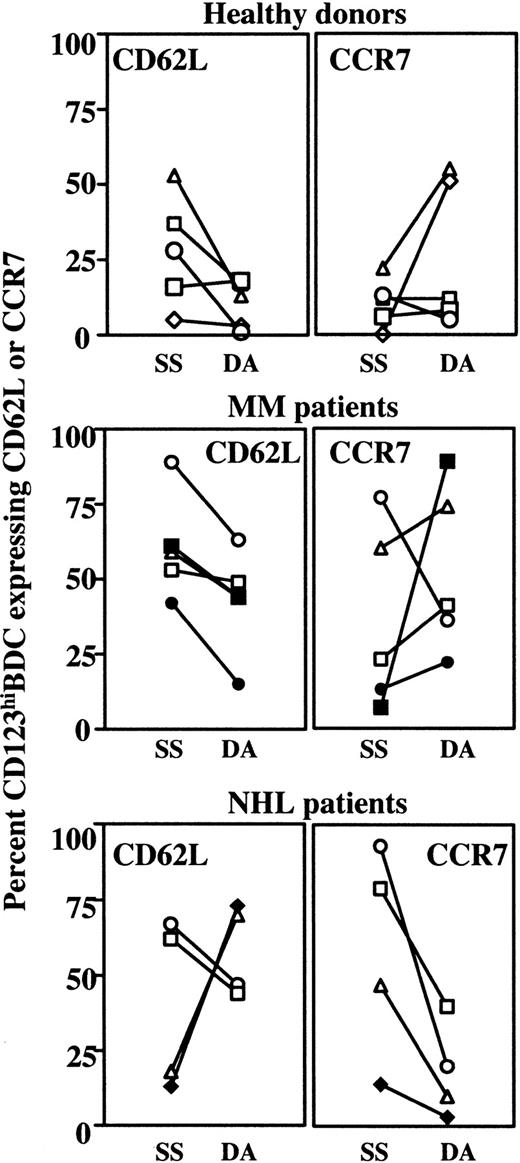
G-CSF may increase the blood count of several leukocyte populations, including CD34+ cells, by changing adhesion molecule expression17,18 or ligand interactions.19-23 Therefore, it is conceivable that G-CSF or CY/G-CSF treatment may contribute to changes in BDC counts by altering adhesion molecule and chemokine receptor expression. After G-CSF or CY/G-CSF treatment, considerable changes in expression of CD62L and CCR7 (but not CD49d) were noted on CD123hiBDCs, on DA compared with SS.
Healthy donors, all of whom had low-dose G-CSF treatment (open symbols) and the MM patients, who had high-dose G-CSF treatment (filled symbols) increased CD123hiBDCs (Figure 1A). The small number of study subjects precluded categoric conclusions, but changes in adhesion molecule (CD62L) and chemokine receptor expression (CCR7) on CD123hiBDCs appeared to be largely independent of G-CSF dose. However, in most cases, the study subjects down-regulated CD62L (2- to 28-fold) and up-regulated CCR7 (2- to 51-fold) (Figure2).
Changes in the expression of CD62L and CCR7 on CD123hiBDCs after G-CSF or CY/G-CSF treatment.
CD62L and CCR7 were analyzed on CD123hiBDCs in steady state (SS, prior to commencement of the PBSC mobilization protocol) and on the day of apheresis (DA). Data from individual healthy donors, MM, and NHL patients are represented. Open and filled symbols indicate therapy including low- and high-dose G-CSF, respectively, and correspond to the symbols in Figure 1 and Table 1.
Changes in the expression of CD62L and CCR7 on CD123hiBDCs after G-CSF or CY/G-CSF treatment.
CD62L and CCR7 were analyzed on CD123hiBDCs in steady state (SS, prior to commencement of the PBSC mobilization protocol) and on the day of apheresis (DA). Data from individual healthy donors, MM, and NHL patients are represented. Open and filled symbols indicate therapy including low- and high-dose G-CSF, respectively, and correspond to the symbols in Figure 1 and Table 1.
In NHL patients, most of whom failed to increase CD123hiBDC counts after CY/G-CSF treatment, changes in CD62L expression on CD123hiBDCs were variable (Figure 2). However, down-regulation of higher SS levels of CCR7 expression was prominent.
The apparent “mobilization” of CD123hiBDCs may be due to down-regulation of CD62L, with a subsequent inability of CD123hiBDCs to leave the blood and home to secondary lymphoid tissues.5,9 The association of “mobilized” CD123hiBDCs with CCR7 up-regulation in healthy donors and MM patients was unusual and not associated with BDC maturation.8 Of note, in MM and NHL patients, baseline levels of CCR7 expression were high, possibly as a result of disordered hematopoiesis due to prior marrow disease or cytotoxic therapy. The indirect contribution of G-CSF to the changes in CD62L and CCR7 on CD123hiBDC needs further investigation as in vitro exposure of BDCs to G-CSF did not induce the exact phenotype seen after in vivo G-CSF or CY/G-CSF treatment (data not shown).
G-CSF and CY/G-CSF treatment minimally affected CD62L, CCR7, and CD49d on CD11c+BDCs. Occasional up-regulation of CD62L was seen on CD11c+BDCs on DA in NHL patients (data not shown). Of note, in all groups tested, in SS and on DA, BDCs showed an immature phenotype without expression of CD80, CD86, and CD83 (data not shown).
These data may have implications for the therapeutic use of G-CSF and design of concurrent PBSC mobilization and BDC harvesting protocols.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-03-0973.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Derek N. J. Hart, Mater Medical Research Institute, Aubigny Place, Raymond Terrace, South Brisbane Qld 4101, Australia; e-mail: dhart@mmri.mater.org.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal