Programmed cell death of granulocytes is one of the mechanisms that limit inflammatory responses. Members of the Bcl-2 protein family are essential regulators of apoptosis induced by growth factor withdrawal or cytotoxic stress. We have used gene-targeted and transgenic mice to investigate the roles of the prosurvival molecules Bcl-2 and Bcl-w and their proapoptotic relatives Bax and Bim in spontaneous and stress-induced apoptosis of granulocytes from bone marrow or the peritoneum. Bim deficiency, like Bcl-2 overexpression, rendered granulocytes resistant to cytokine withdrawal and cytotoxic drugs, but absence of Bax alone had no protective effect. Loss of Bcl-2 or Bcl-w did not increase the sensitivity of granulocytes to any of these apoptotic stimuli, but Bcl-2 was essential for the in vitro survival of myeloid progenitors under conditions of cytokine withdrawal where cell death was mediated, in part, by Bim. Granulocyte colony-stimulating factor (G-CSF), a key survival factor for granulocytes, enhanced viability of cells lacking bcl-2, bcl-w, bax, orbim, indicating that none of these genes alone is the essential target of this cytokine's prosurvival function. Expression analysis of proapoptotic Bcl-2 family members in granulocytes revealed that the BH3-only protein Bmf is induced upon cytokine withdrawal. These results indicate that the BH3-only protein Bim and possibly also Bmf are critical initiators of spontaneous and drug-induced apoptosis of granulocytes, whereas Bcl-2, Bcl-w, and Bax act in a redundant manner in regulating granulocyte survival and death, respectively.
Introduction
In the bone marrow, extracellular regulatory factors, such as granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage CSF (GM-CSF), promote production and differentiation of myeloid progenitors into mature granulocytes or monocytes.1 Mature granulocytes enter the bloodstream, where they have a short life span unless they are stimulated by inflammatory cytokines.2 Activated granulocytes have the ability to ingest bacteria and infiltrate tissues. Inflammatory responses are kept in check, at least in part, by the apoptotic death of granulocytes, followed by their engulfment by macrophages to avoid release of histotoxic substances.3Life and death of granulocytes must be tightly regulated because excessive granulocyte apoptosis increases susceptibility to bacterial infections,2,4 whereas prolonged granulocyte survival is associated with inflammatory diseases5 and may predispose to leukemogenesis.6
Considerable insight into the control of programmed cell death (apoptosis) has emerged from genetic and biochemical analyses in mammals and Caenorhabditis elegans.7 The effector phase of apoptosis requires aspartate-specific cysteine proteases, termed caspases. Caspases are synthesized as zymogens with low enzymatic activity and, to become functional, must be cleaved at caspase recognition sites, either by adaptor protein–induced autocatalysis or by already active caspases.7 Mammals have 2 distinct apoptosis-signaling pathways for activating caspases. One is initiated when ligation of death receptors (members of the tumor necrosis factor receptor [TNF-R] family with an intracellular death domain) causes formation of a death-inducing signaling complex (DISC) in which Fas-associated death domain (FADD) adaptor proteins promote oligomerization and autocatalytic activation of caspase-8.7 The other pathway is triggered by growth factor deprivation or various stress conditions and is regulated by the interplay of proapoptotic and antiapoptotic members of the Bcl-2 family.7
The mechanisms that control the granulocyte life span are still unclear, but Bcl-2–regulated apoptosis signaling and death receptor signaling have both been implicated in this process. The expression and function of several cell death regulators can be regulated by proinflammatory cytokines, such as G-CSF and GM-CSF, or by bacterial products (eg, lipopolysaccharide [LPS]).8-10Bcl-2 mRNA and protein were reported to be expressed at low levels in mouse granulocytes6,11 but seem to be barely detectable in granulocytes from human blood.8,12 We and others have previously shown that expression of a bcl-2 transgene protects granulocytes from spontaneous and stress-induced cell death in culture but does not render them resistant to Fas ligand (FasL).11,13 The prosurvival Bcl-2 homolog Bcl-w is expressed at readily detectable levels in myeloid cells.11,14 When overexpressed, Bcl-w has similar effects to Bcl-2 and protects hematopoietic cell lines against apoptosis induced by cytokine withdrawal or drug treatment.15Analysis of mice lacking bcl-w demonstrated that it is required for spermatogenesis but dispensable for the development of other cell types, including those that are hematopoietic.16 Expression of the Bcl-2 family members Mcl-1 and A1 can be increased in myeloid cells by stimulation with G-CSF or GM-CSF.8,17 Granulocytes from mice lacking one of the genes for A1, A1a, undergo abnormally accelerated spontaneous death in culture.18 In contrast, mice lacking the proapoptotic BH3-only Bcl-2 family member Bim have an approximately 2-fold increase in granulocytes,19 and granulocytes from patients with certain inflammatory diseases were reported to have abnormally low levels of Bax.5 Bim is sequestered to microtubules in healthy cells and released in response to certain apoptotic stimuli, such as the anitcancer drug taxol, allowing its binding to and inactivation of Bcl-2–like molecules at inner membranes and mitochondria.20 Transfection experiments indicate that Bim acts upstream of Bax and Bak.21
To define the role of individual Bcl-2 family members in granulocyte survival, we investigated the impact of absence of the prosurvival molecules bcl-2 and bcl-w or loss of their proapoptotic relatives bax and bim on granulocyte survival. As a control, we also studied the effects caused by Bcl-2 overexpression or absence of the death receptor Fas/APO-1/CD95. Our results indicate that Bim and possibly other BH3-only proteins play an essential role in programmed death of granulocytes, whereas Bcl-2, Bcl-w, and Bax have redundant functions in the control of granulocyte apoptosis.
Materials and methods
Mice
The generation and genotyping of the bim-deficient mice (266/266del),19bcl-w–deficient mice,16 and vav-bcl-2-69 transgenic mice,22 expressing a human bcl-2 cDNA under control of the vav promoter at high levels in all hematopoetic cell types, have been described. Fas-deficientlpr mutant mice were provided by our institute's breeding facility. The bax+/− mice23 were purchased from the Jackson Laboratory, andbcl-2+/− mice were a kind gift from Dr D. Loh (Washington University, St Louis, MO).24 All mouse strains were on an inbred C57BL/6J genetic background or had been backcrossed for more than 8 generations onto this background. Becausebcl-2−/− mice do not survive beyond 6 weeks after birth due to severe polycystic kidney disease,24 25we generated chimeric mice by hematopoietic reconstitution of lethally irradiated (2 × 5.5 Gy [2 × 550 rad], 3-hour interval) C57BL/6-Ly5.1 mice with 2 × 106 fetal liver cells from E14 bcl-2−/− or (as a control) from wild-type (wt) embryos produced from intercrosses ofbcl-2+/− (C57BL/6-Ly5.2) animals. Granulocytes from chimeric animals were analyzed 10 to 16 weeks after reconstitution.
Cell culture and reagents
Granulocytes were isolated from the bone marrow of untreated mice or were collected by lavage from the peritoneal cavity of mice that had been injected intraperitoneally 3 hours earlier with 2 mL of a 0.5% casein/phosphate-buffered saline (PBS) solution. Purified granulocytes were cultured in the high-glucose version of Dulbecco modified Eagle medium (DMEM) supplemented with 13 μM folic acid, 250 μM l-asparagine, 50 μM 2-mercaptoethanol, and 10% fetal calf serum (FCS) (TRACE). Recombinant human G-CSF (rhG-CSF) (AMRAD, Melbourne, Australia) was used at 100 U/mL. Flag epitope-tagged FasL (Alexis, Lausen, Switzerland) was used at 1 to 100 ng/mL together with cross-linking M2 anti-Flag antibody (Sigma, St Louis, MO) at 1 μg/mL. Ionomycin (Sigma) was used at 200 ng/mL, VP-16 (etoposide) (David Bull Laboratories) was used at 10 or 20 μg/mL, and taxol (Sigma) at 1 μM.
Immunoblotting
Western blotting was performed as previously described.11 Membranes were probed with rabbit polyclonal anti-Bim and anti-Bad antibodies (Stressgen, San Diego, CA) or Bmf (Alexis), mouse monoclonal antibodies to Bid (Transduction Laboratories, Lexington, KY), and goat-polyclonal anti-Noxa (SC19; Santa Cruz Biotechnology, CA). A rabbit anti-Puma antiserum was generated by immunizing New Zealand White rabbits with a human GST-PUMA fusion protein. Membranes were probed with rabbit polyclonal anti-Bim antibodies (Stressgen) or Bmf26(Alexis) or mouse monoclonal antibodies to Bid (Transduction Laboratories). Horseradish peroxidase (HRP)-conjugated sheep antirabbit or rabbit antimouse immunoglobulin (Ig) antibodies (Silenus, Melbourne, Australia) served as secondary reagents, and the enhanced chemiluminescence (ECL) system was used for detection. To demonstrate equal protein loading, membranes were probed with a mouse monoclonal antibody to heat-shock protein Hsp70 (a gift from Dr R. Anderson, Peter MacCallum Cancer Institute, Melbourne, Australia). Membranes were stripped prior reprobing in 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate (SDS), 62.5 mM Tris (tris(hydroxymethyl)aminomethane) HCl, pH 6.7, for 30 minutes at 55°C.
Cell sorting and immunofluorescence analysis
Granulocytes were isolated by staining cell suspensions from bone marrow or peritoneal lavage with fluorescein isothiocyanate (FITC)- or cyanine 5 (Cy5)-conjugated rat anti-Gr-1 monoclonal antibody (mAb) RB6-8C5 (2 μg/mL) in PBS/10% FCS for 30 minutes on ice and cell sorting on a MoFlo high-speed flow cytometry instrument (Cytomation, Boulder, CO). Gating on the basis of forward (FSC) and side (SSC) light scatter and staining with the vital dye propidium iodide (1 μg/mL) was used to exclude dead cells. Only Gr-1high cells with large FSC/SSC profile were collected to minimize contamination with macrophages. In the case ofbcl-2−/− granulocytes, which were isolated from chimeric mice generated by reconstitution of lethally irradiated C57BL/6-Ly5.1 mice with fetal liver cells from C57BL/6-Ly5.2 E14bcl-2−/− embryos, recipient-derived cells were excluded by staining with FITC-conjugated rat anti-Ly5.1 mAb (A201.7). Alternatively, granulocytes were sorted by excluding cells stained with FITC-labeled RA3-6B2 anti-B220, T24.3.2.1 anti-Thy-1, 53.9.2 anti-Ter-119, and anti-F4/80. Sorted granulocytes were more than 98% positive for Gr-1 and Mac-1 but negative for the B-cell marker B220, the T-cell marker Thy-1, the erythroid cell marker TER-119, and the macrophage marker F4/80.
Absolute numbers of T cells, B cells, macrophages, and granulocytes in blood, bone marrow, or spleen were determined by cell counting and multiplying this number by the percentage of the cell type, determined by staining with surface marker–specific monoclonal antibodies and flow cytometric analysis. In the indicated mice reconstituted withbcl-2−/− or wt stem cell mice (see “Mice”), host and donor-derived cells were distinguished by staining with an FITC-conjugated mAb recognizing Ly5.2 (5.430-15.2), and generally more than 95% of the myeloid cells were donor derived in these chimeric mice.
Cell death assays
The percentage of viable cells in culture was determined by staining with 2 μg/mL propidium iodide plus FITC-coupled annexin V and analyzing the samples in a FACScan (Becton Dickinson, Sunnyvale, CA). Alternatively, cells were stained with trypan blue (0.1% in PBS) and analyzed in a hemocytometer.
RT-PCR analysis
Total RNA was isolated from granulocytes derived from the bone marrow of wt mice immediately after cell sorting or after culture in the presence or absence of G-CSF (100 U/mL) using Trizol reagents (Gibco, Carlsbad, CA) according to the manufacturer's recommendations. An amount of 250 ng of total RNA was transcribed into cDNA using avian myeloblastosis virus (AMV)–reverse transcriptase (AMV-RT) and oligo dT primers (Roche, Basel, Switzerland). Five-fold dilutions of cDNA templates (1:1, 1:5, 1:25) were amplified by polymerase chain reaction (PCR) using Taq polymerase and oligos specific for the BH3-only genes bim, bid, bad, blk, hrk/DP5, bmf, puma/bbc3, and noxa. Cycling conditions were chosen as follows: 94°C for 2 minutes; 5 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; followed by 25 cycles of 94°C for 15 seconds, 55°C plus 0.2°C per cycle for 30 seconds, and 72°C for 30 seconds; and a final synthesis step for 10 minutes at 72°C. Identity of PCR products was confirmed by Southern blotting and sequence-specific internal oligonucleotides.blk fwd. 5′ATGTCGGAGGCGAGACTTATG3′, rev. 5′CCCTGCCCCAGCTGCACTTCACTG3′, int. 5′CACTCAGGCGCCAGGAGTCAAGAC3′;hrk/dp5 fwd. 5′CCGGACCGAGCAACAGGTTAGC3′, rev. 5′GCTTCGGCCCAGTCCCCTCTAC3′, int. 5′CTCTCCCCTGCCACCCTAGACATTACG3′;bim fwd. 5′TTGCCATCGTCGCCGTCAC3′, rev. 5′CAGTTGTAAGATAACCATTTGAGGGTGG3′, int. 5′CAGCTCCTGTGCAATCCGTATCTC3′; bid fwd. 5′ATGGACTCTGAGG3′, rev. 5′TTAGTCCATCTCGTTTCTAACCAAG3′, int. 5′CAGTGTGGGCTGGATGTTGTGG3′; bad fwd. 5′TTCCAGATCCCAGAGTTTG3′, rev. 5′GGAGATCACTGGGAGGGGGTGG3′, int. 5′GCGCCTCCATGATGACTGTTGG3′; noxa fwd. 5′CGTCGGAACGCGCCAGTGAACCC3′, rev. 5′TCCTTCCTGGGAGGTCCCTTCTTGC3′, int. 5′AACCCGGGTGCCAGCAGACTTG3′; puma/bbc3 fwd. 5′CCTCAGCCCTCCCTGTCACCAG3′ rev. 5′CCGCCGCTCGTACTGCGCGTTG3′, int. 5′CGGCGGATGGCGGACGACCTC3′; bmf fwd. 5′CCCTTGGGGAGCAGCCCCCTG3′, rev. 5′CAAGACAGTATCTGTCCTCCCAGAC3′, int. 5′CATACGCAACAACACCAGCAG3′. PCR products were separated on 2% agarose gels in TBE buffer and transferred onto nylon membranes overnight in 0.4 M NaOH. Membranes were washed in 2 × SSC for 5 minutes, prehybridized at 42°C in CHURCH buffer, and hybridized overnight with 2 × 106 disintegrations per minute (dpm) per milliliter of internal oligonucleotide labeled with 32P γ-adenosine triphosphate (γ-ATP) and T4 polynucleotide kinase. Filters were washed in 40 mM Na2HPO4/1% SDS at 42°C for 20 minutes, dried, and exposed to x-ray film (Kodak, Emeryville, CA).
Progenitor cell assays
Assays for bone marrow progenitor cells in bone marrow from mice reconstituted with fetal liver cells from wt orbcl-2−/− mice or wt,bim−/−, and vav-bcl-2 transgenic mice were performed by culturing 2.5 × 104 nucleated cells in 0.3% semisolid agar (1 mL) in Dulbecco modified Eagle medium (DMEM) supplemented with 20% newborn calf serum (Hyclone, Logan, UT). Cells were plated in triplicate or quadruplicate and stimulated by a combination of murine stem cell factor (mSCF; produced by expression in Pichia pastoris) at 100 ng/mL and murine interleukin-3 (mIL-3; Peprotech, Rocky Hill, NJ) at 2500 U/mL. Cytokines were added at the time of plating or after a delay of 12, 18, 24, 48, or 72 hours. After an additional 7 days of incubation at 37°C, colonies were counted under a microscope, fixed, stained, and identified as described before.22 The number of colonies that grew when cytokines were added at the time of plating was considered 100% to allow calculation of relative colony survival of the cells that had been starved of cytokines for variable amounts of time.
Statistical analysis
Statistical analysis was performed by using the Fischer protected least significant difference (PLSD) test and Stat-view 4.1 software. P values of less than .04 were considered to indicate statistically significant differences.
Results
Bim is an essential inducer of programmed death of granulocytes
Granulocytes were isolated by immunofluorescent staining with surface marker–specific antibodies and flow cytometric sorting from the bone marrow of wt mice, chimeric mice lacking Bcl-2 in the hematopoietic system (see “Materials and methods”), mice deficient for Bcl-w, Bax, the BH3-only protein Bim, functional Fas/APO-1/CD95 (lpr mutant mice), or from animals expressing abcl-2 transgene in all hematopoietic cells.22Sorted cells were cultured in simple medium or treated with the cytotoxic drugs VP-16 (10 μg/mL), taxol (1 μM), the calcium ionophore ionomycin (250 ng/mL), or stimulated with Fas ligand (FasL) at 100 ng/mL. After 24, 48, or 72 hours in culture, cell survival was determined by staining with annexin V–FITC plus propidium iodide followed by flow cytometric analysis. Absence of the BH3-only protein Bim or Bcl-2 overexpression protected granulocytes from spontaneous or drug-induced cell death (Figure1A,C). For example, after 72 hours in culture, on average only 19% wt granulocytes remained alive, whereas 41% of bim−/− and 69% ofvav-bcl-2 trangenic cells had survived (Figure1A). In contrast, loss of Bim or Bcl-2 overexpression had no impact on FasL-induced killing (Figure 1C), whereas Fas-deficient lprmutant granulocytes were resistant to FasL but died normally in response to cytokine withdrawal or treatment with cytotoxic drugs and ionomycin (Figure 1B,D). Absence of the prosurvival proteins Bcl-2, Bcl-w, or their proapoptotic relative Bax did not increase the sensitivity of granulocytes to any of these death stimuli (Figure1B,D).
Bim deficiency and
bcl-2 transgene expression delay spontaneous, chemotherapeutic drug-induced, and stress-induced apoptosis of granulocytes. Granulocytes from bone marrow (A-D) of wt,vav-bcl-2 transgenic, bim−/−,bax−/−,bcl-w−/−, lpr mice, andbcl-2−/− reconstituted mice (see “Materials and methods”) were sorted by flow cytometry and cultured in simple medium.(A-B) Survival under conditions of cytokine withdrawal was determined after 24, 48, and 72 hours by staining with annexin V plus propidium iodide and flow cytometric analysis. (C-D) Granulocytes of the indicated genotypes were either cultured in simple medium, the presence of FasL (100 ng/mL) multimerized with M2 anti-Flag mAb (1 μg/mL), the cytotoxic drugs VP-16 (10 μg/mL), taxol (1 μM), or the calcium ionophore ionomycin (200 ng/mL). Survival was assessed after 48 hours of incubation as mentioned in panel A. Data shown represent arithmetic means ± SDs of 4 independent experiments. Analysis was performed in duplicate on 4 to 8 animals of each genotype. Analysis of mobilized granulocytes from the peritoneal cavity (E-F). Mice of the genotypes indicated in panel A were injected intraperitoneally with 0.5% casein in PBS (2 mL). Peritoneal exudate cells were harvested by lavage 3 hours later, and granulocytes were sorted by flow cytometry as indicated in panel A. (E-F) Survival under conditions of cytokine withdrawal was determined after 16, 24, and 48 hours as described in panel A. (G-J) Granulocytes of the indicated genotypes were cultured in simple medium or treated with FasL (100 ng/mL) multimerized with M2 anti-Flag mAb (1 μg/mL) or the cytotoxic drug VP-16 (20 μg/mL). Survival was assessed after 8 (G-H) or 16 (I-J) hours of incubation as described in panel A. Data shown represent arithmetic means ± SDs of 2 to 3 independent experiments performed in duplicate on 2 to 5 animals of each genotype.
Bim deficiency and
bcl-2 transgene expression delay spontaneous, chemotherapeutic drug-induced, and stress-induced apoptosis of granulocytes. Granulocytes from bone marrow (A-D) of wt,vav-bcl-2 transgenic, bim−/−,bax−/−,bcl-w−/−, lpr mice, andbcl-2−/− reconstituted mice (see “Materials and methods”) were sorted by flow cytometry and cultured in simple medium.(A-B) Survival under conditions of cytokine withdrawal was determined after 24, 48, and 72 hours by staining with annexin V plus propidium iodide and flow cytometric analysis. (C-D) Granulocytes of the indicated genotypes were either cultured in simple medium, the presence of FasL (100 ng/mL) multimerized with M2 anti-Flag mAb (1 μg/mL), the cytotoxic drugs VP-16 (10 μg/mL), taxol (1 μM), or the calcium ionophore ionomycin (200 ng/mL). Survival was assessed after 48 hours of incubation as mentioned in panel A. Data shown represent arithmetic means ± SDs of 4 independent experiments. Analysis was performed in duplicate on 4 to 8 animals of each genotype. Analysis of mobilized granulocytes from the peritoneal cavity (E-F). Mice of the genotypes indicated in panel A were injected intraperitoneally with 0.5% casein in PBS (2 mL). Peritoneal exudate cells were harvested by lavage 3 hours later, and granulocytes were sorted by flow cytometry as indicated in panel A. (E-F) Survival under conditions of cytokine withdrawal was determined after 16, 24, and 48 hours as described in panel A. (G-J) Granulocytes of the indicated genotypes were cultured in simple medium or treated with FasL (100 ng/mL) multimerized with M2 anti-Flag mAb (1 μg/mL) or the cytotoxic drug VP-16 (20 μg/mL). Survival was assessed after 8 (G-H) or 16 (I-J) hours of incubation as described in panel A. Data shown represent arithmetic means ± SDs of 2 to 3 independent experiments performed in duplicate on 2 to 5 animals of each genotype.
Recruitment to a site of inflammation has been reported to alter the responsiveness of granulocytes to certain apoptotic stimuli.27 28 We therefore challenged wt,bcl-2−/− hematopoietic chimeric animals,bcl-w−/−, bax−/−,bim−/−, and vav-bcl-2 transgenic mice by intraperitoneal injection of casein and 3 hours later harvested their peritoneal fluid to purify the mobilized granulocytes by immunofluorescent staining and cell sorting. Sorted cells were cultured in simple medium or treated with the cytotoxic drug VP-16 (20 μg/mL) or FasL (100 ng/mL). Mobilized wt granulocytes underwent spontaneous death in culture more rapidly than bone marrow–derived wt granulocytes (compare Figure 1A with 1E). Chemotherapeutic drug treatment or Fas activation therefore could only marginally accelerate apoptosis of mobilized granulocytes in culture. As shown for the bone marrow–derived granulocytes (Figure 1A), absence of Bim or Bcl-2 overexpression delayed spontaneous as well as drug-induced cell death, whereas Bax deficiency had no protective effect (Figure 1E,G,I). After 24 hours of culture in simple medium, only about 20% of wt granulocytes survived, but on average 59% ofbim−/− and 69% of the vav-bcl-2transgenic cells retained viability (Figure 1E). Loss of Bim or Bcl-2 overexpression had no impact on FasL-induced killing (Figure 1G). Mobilized granulocytes lacking Bcl-2 or Bcl-w did not differ from wt cells in their responsiveness to apoptotic stimuli (Figure 1F,H,J), whereas these granulocytes from lpr mutant mice were resistant to FasL but were otherwise indistinguishable from wt cells in their response to all other apoptotic stimuli (Figure 1F,H,J).
Bcl-2 influences the sensitivity of early myeloid progenitors to cytokine withdrawal
The role of Bcl-2 in determining the life span of cells from different hematopoietic lineages in vivo was examined by comparing the numbers of lymphoid and myeloid cells between lethally irradiated mice that had been reconstituted with wt orbcl-2−/− fetal liver–derived stem cells. In agreement with previous findings,29 mice reconstituted with bcl-2−/− stem cells had considerably lower numbers of B and T cells in bone marrow, peripheral blood, and spleen compared with animals reconstituted with wt stem cells (Figure2A,B and data not shown). The bcl-2−/−reconstituted mice (analyzed 8 to 16 weeks after reconstitution, more than 95% donor-derived myeloid cells) also had abnormally low numbers of macrophages in spleen (p < 0006) and peripheral blood (p < 0001) but roughly normal numbers of granulocytes (Figure2C,D).
Bcl-2 is a critical determinant of the sensitivity of early myeloid progenitors to cytokine withdrawal.
Chimeric mice with a bcl-2−/− or wt hematopoietic system were generated by reconstitution of lethally irradiated C57BL/6-Ly5.1 mice with 2 × 106 fetal liver cells from E14 bcl-2−/− or wt embryos (C57BL/6-Ly5.2) produced from intercrosses ofbcl-2+/− animals. Absolute numbers of T cells, B cells, macrophages, and granulocytes in blood (A,C) or spleen (B,D) of reconstituted mice were determined by cell counting and flow cytometric analysis staining cells with surface marker–specific mAbs (anti-B220, anti–Thy-1, anti–Gr-1, or anti–Mac-1). Host and donor-derived cells were distinguished by staining with an FITC-conjugated mAb to Ly5.2. Data shown for the peripheral blood represent arithmetic means ± SEs of 9bcl-2−/− reconstituted and 5 wt reconstituted animals that were analyzed 10 weeks after fetal liver stem cell transplantation. Data shown for the spleen represent arithmetic means ± SEs of 6 bcl-2−/− and 4 wt reconstituted animals analyzed 10 to 16 weeks after reconstitution. (E-F) Survival analysis of bone marrow–derived myeloid progenitors. Cells (2.5 × 104) from bone marrow of (E) wt mice, lethally irradiated wt mice reconstituted with either wt or bcl-2−/− fetal liver stem cells, and from bone marrow of (F) wt, bim−/−, andbcl-2 transgenic mice were cultured in 0.3% agar in triplicate or quadruplicate and stimulated with mSCF (100 ng/mL) and mIL-3 (2500 U/mL). Cytokines were added at the time of plating or after a delay of 12, 18, 24, 48, or 72 hours. After 7 days of stimulation with cytokines at 37°C, colonies were counted using a dissection microscope. Data shown represent arithmetic means ± SDs of 2 to 3 independent experiments performed using 4 to 6 animals of each genotype.
Bcl-2 is a critical determinant of the sensitivity of early myeloid progenitors to cytokine withdrawal.
Chimeric mice with a bcl-2−/− or wt hematopoietic system were generated by reconstitution of lethally irradiated C57BL/6-Ly5.1 mice with 2 × 106 fetal liver cells from E14 bcl-2−/− or wt embryos (C57BL/6-Ly5.2) produced from intercrosses ofbcl-2+/− animals. Absolute numbers of T cells, B cells, macrophages, and granulocytes in blood (A,C) or spleen (B,D) of reconstituted mice were determined by cell counting and flow cytometric analysis staining cells with surface marker–specific mAbs (anti-B220, anti–Thy-1, anti–Gr-1, or anti–Mac-1). Host and donor-derived cells were distinguished by staining with an FITC-conjugated mAb to Ly5.2. Data shown for the peripheral blood represent arithmetic means ± SEs of 9bcl-2−/− reconstituted and 5 wt reconstituted animals that were analyzed 10 weeks after fetal liver stem cell transplantation. Data shown for the spleen represent arithmetic means ± SEs of 6 bcl-2−/− and 4 wt reconstituted animals analyzed 10 to 16 weeks after reconstitution. (E-F) Survival analysis of bone marrow–derived myeloid progenitors. Cells (2.5 × 104) from bone marrow of (E) wt mice, lethally irradiated wt mice reconstituted with either wt or bcl-2−/− fetal liver stem cells, and from bone marrow of (F) wt, bim−/−, andbcl-2 transgenic mice were cultured in 0.3% agar in triplicate or quadruplicate and stimulated with mSCF (100 ng/mL) and mIL-3 (2500 U/mL). Cytokines were added at the time of plating or after a delay of 12, 18, 24, 48, or 72 hours. After 7 days of stimulation with cytokines at 37°C, colonies were counted using a dissection microscope. Data shown represent arithmetic means ± SDs of 2 to 3 independent experiments performed using 4 to 6 animals of each genotype.
We also investigated the influence of Bcl-2 deficiency on the survival of early myeloid progenitors under conditions of cytokine deprivation and compared it with the effect caused by Bim deficiency or expression of a bcl-2 transgene. Bone marrow-derived myeloid progenitors from normal mice, animals lacking Bcl-2, Bim, or fromvav-bcl-2 transgenic mice were grown in soft agar. Stem cell factor (SCF) and interleukin-3 (IL-3), cytokines that promote proliferation and differentiation of myeloid progenitors, were added either at the time of plating or after a delay of 12, 18, 24, 48, and 72 hours. When cultured from the start of the experiment in the presence of cytokines, bone marrow cells frombcl-2−/− reconstituted mice produced significantly fewer myeloid colonies compared with bone marrow cells from wild-type reconstituted mice (bcl-2−/−35 ± 9 vs wt 58 ± 8 colonies per 25 000 nucleated bone marrow cells; p.0006). Moreover, when cytokine addition was delayed, even by a short time, only few or no colonies arose frombcl-2–deficient bone marrow, whereas wild-type progenitors could still form significant numbers of colonies. For example, growth factor deprivation for 12 hours killed more than 90% ofbcl-2−/− colony-forming cells but only 25% of the wt cells (Figure 2E). As previously described,22 expression of a bcl-2 transgene rendered colony-forming cells refractory to cytokine withdrawal, and absence of Bim had a smaller but reproducible protective effect (Figure2F). Staining of colonies with hematoxylin after fixation and examination by light microscopy revealed that the relative frequencies of granulocyte, granulocyte-macrophage, macrophage, blast, and megakaryocytic colonies were similar in cultures of bone marrow from wt, bim−/−, as well asbcl-2−/− or wt chimeric mice (data not shown).
Expression of the BH3-only protein Bmf increases in cultured granulocytes
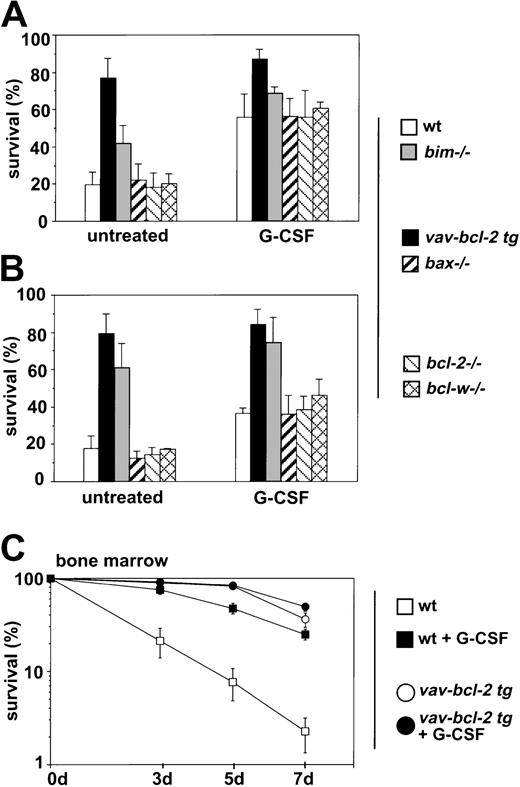
The effector molecules of the antiapoptotic response mediated by G-CSF in granulocytes are not clearly defined. We analyzed whether G-CSF receptor stimulation could modulate the expression of BH3-only proteins in granulocytes and tested whether loss of Bcl-2, Bcl-w, Bax, or Bim or overexpression of Bcl-2 had an effect on the ability of G-CSF to promote granulocyte survival in culture. Granulocytes from the bone marrow or mobilized granulocytes from the peritoneal cavity of casein-injected wt mice or chimeric mice reconstituted with abcl-2−/− hemopoietic system,vav-bcl-2 transgenic mice, or from animals lacking Bim, Bax, or Bcl-w were cultured in the presence or absence of G-CSF for 24 hours or 72 hours, and cell survival was assessed by propidium iodide staining with or without addition of annexin V and flow cytometric analysis. Both methods gave comparable results, and data of both types of experiments have therefore been pooled (Figure 3A,B). Granulocytes lacking Bcl-2, Bcl-w, or Bax responded to G-CSF–like normal cells, and survival of bone marrow–derived bim−/−granulocytes, which are partially protected from cytokine withdrawal, could still be augmented by G-CSF treatment (p.0114) (Figure 3A,B). These results demonstrate that none of these molecules alone is the key target for the prosurvival effect of G-CSF. Culturing of bone marrow–derived granulocytes expressing a bcl-2 transgene with G-CSF did not provide any additional survival benefit over a period of 7 days (Figure 3C), demonstrating that Bcl-2 can efficiently compensate for G-CSF receptor signaling.
Influence of G-CSF on granulocyte survival.
Granulocytes from mice of the indicated genotypes were isolated from (A, C) bone marrow or (B) the peritoneal cavity after injection with casein. Granulocytes were cultured in the absence or presence of rhG-CSF (100 U/mL). Cell survival was assessed after 72 hours (A; bone marrow–derived granulocytes) or 24 hours (B; mobilized peritoneal granulocytes) or at time points (C; bone marrow) by staining with propidium iodide plus or minus annexin V staining and flow cytometric analysis. Both methods gave comparable results, and data were therefore pooled. Bars represent means ± SDs of 3 to 6 independent experiments performed in duplicate on 4 to 8 animals of each genotype.
Influence of G-CSF on granulocyte survival.
Granulocytes from mice of the indicated genotypes were isolated from (A, C) bone marrow or (B) the peritoneal cavity after injection with casein. Granulocytes were cultured in the absence or presence of rhG-CSF (100 U/mL). Cell survival was assessed after 72 hours (A; bone marrow–derived granulocytes) or 24 hours (B; mobilized peritoneal granulocytes) or at time points (C; bone marrow) by staining with propidium iodide plus or minus annexin V staining and flow cytometric analysis. Both methods gave comparable results, and data were therefore pooled. Bars represent means ± SDs of 3 to 6 independent experiments performed in duplicate on 4 to 8 animals of each genotype.
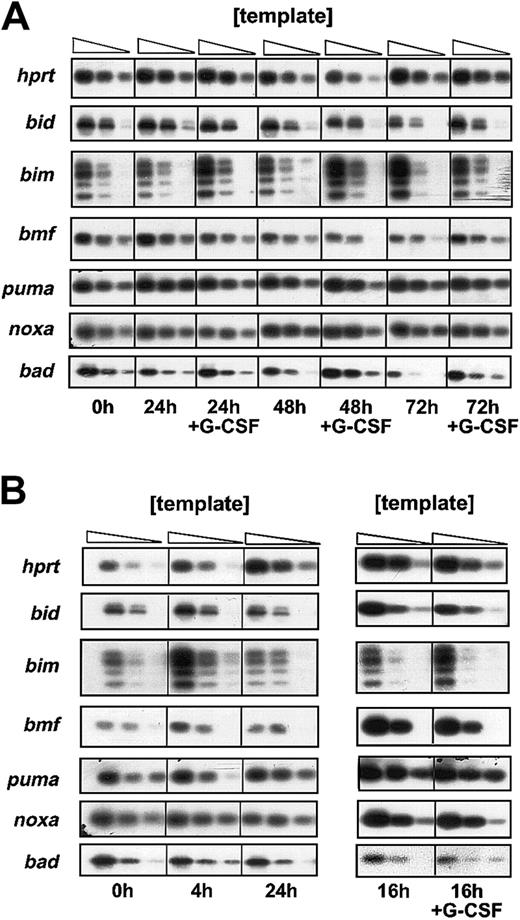
Bim-deficient granulocytes were partially protected from cytokine withdrawal–induced apoptosis in culture, and bone marrow–derivedbim−/− granulocytes still responded with enhanced survival to G-CSF (Figure 3A). Expression of abcl-2 transgene protected granulocytes from cytokine withdrawal–induced death more potently than loss of Bim (Figure 1A). Thus, G-CSF may confer its prosurvival effect in part via regulation of Bim expression or function and perhaps also through inhibitory effects on other proapoptotic Bcl-2 family members. We therefore analyzed expression of BH3-only genes at the mRNA and protein level in bone marrow–derived as well as mobilized peritoneal granulocytes that were freshly isolated or cultured in the presence or absence of G-CSF. Semiquantitative RT-PCR and Southern blotting demonstrated mRNA expression for the BH3-only genes bad, bid, bim(primers were designed to amplify all 4 known BH3-containing splice variants), blk, bmf, noxa, and PUMA/bbc3but not hrk/DP5, which has previously been reported to be expressed exclusively in neuronal cells.30
Expression levels of the mRNA of these genes did not change significantly over time, whether cells were cultured in the presence or absence of G-CSF (Figure 4). Moreover, the basal expression of these genes did not differ markedly between resting and activated granulocytes (Figure 4B). Thebim mRNA levels appeared to be increased under conditions of growth factor deprivation in mobilized but not in bone marrow–derived granulocytes (Figure 4B). This was not reflected by an increase in Bim protein level (data not shown) but, interestingly, loss of Bim protects activated granulocytes from spontaneous death more potently than bone marrow–derived resting granulocytes (Figure 1A vs 1E). Expression ofblk mRNA was found to be very low in all granulocyte populations tested (data not shown).
Analysis of mRNA expression of BH3-only genes.
To profile mRNA expression of BH3-only genes by semiquantitative RT-PCR, granulocytes were derived from (A) bone marrow or (B) the peritoneal cavity of C57BL/6 mice, sorted by flow cytometry, and cultured in the presence or absence of rh G-CSF (100 U/mL). RNA was extracted at the indicated time points and reverse transcribed into cDNA. Limiting dilutions of cDNAs were amplified by PCR. The PCR products obtained using BH3-only gene sequence–specific primers were separated on 2% agarose gels and the identity of the PCR products confirmed by Southern blotting using sequence-specific internal oligonucleotides as a probe. Results shown are representative of 2 independent experiments.
Analysis of mRNA expression of BH3-only genes.
To profile mRNA expression of BH3-only genes by semiquantitative RT-PCR, granulocytes were derived from (A) bone marrow or (B) the peritoneal cavity of C57BL/6 mice, sorted by flow cytometry, and cultured in the presence or absence of rh G-CSF (100 U/mL). RNA was extracted at the indicated time points and reverse transcribed into cDNA. Limiting dilutions of cDNAs were amplified by PCR. The PCR products obtained using BH3-only gene sequence–specific primers were separated on 2% agarose gels and the identity of the PCR products confirmed by Southern blotting using sequence-specific internal oligonucleotides as a probe. Results shown are representative of 2 independent experiments.
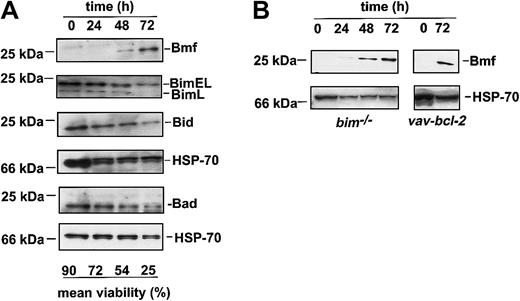
Surprisingly, protein expression analysis by Western blotting of lysates from sorted granulocytes undergoing spontaneous apoptosis in culture revealed that the BH3-only protein Bmf increased substantially, whereas levels of Bim, Bad, or Bid remained unchanged (Figure5A). Expression of the proteins PUMA/bbc3 or Noxa were undetectable under these conditions (data not shown). Accumulation of Bmf was also observed in cultured granulocytes frombim−/− or bcl-2 transgenic mice, indicating that this accumulation is not merely a consequence of cell death (Figure 5B). Bmf protein levels did not increase in thymocytes undergoing cytokine withdrawal–induced death, indicating that this increase may be specific for granulocytes (data not shown).
The BH3-only protein Bmf accumulates in granulocytes in culture.
(A) Sorted granulocytes from the bone marrow of C57BL/6 mice were cultured in simple medium for 24, 48, or 72 hours. Survival was assessed by trypan blue exclusion in a hemocytometer. Cells were harvested, lysed, and proteins subjected to Western blot analysis using antibodies specific for the BH3-only proteins Bim, Bid, or Bmf. Analysis of Hsp70 expression was performed as a loading control. Filters were sequentially probed, stripped, and reprobed (first Bmf, then Bim, then Bid, then Hsp70 or Puma, then Noxa, then Bad, then Hsp70). Data show 1 representative of 3 independent experiments. (B) Sorted granulocytes from Bim-deficient andbcl-2 transgenic mice were cultured in simple medium, and Bmf protein expression was analyzed as in panel A. Data show 1 of 2 independent experiments.
The BH3-only protein Bmf accumulates in granulocytes in culture.
(A) Sorted granulocytes from the bone marrow of C57BL/6 mice were cultured in simple medium for 24, 48, or 72 hours. Survival was assessed by trypan blue exclusion in a hemocytometer. Cells were harvested, lysed, and proteins subjected to Western blot analysis using antibodies specific for the BH3-only proteins Bim, Bid, or Bmf. Analysis of Hsp70 expression was performed as a loading control. Filters were sequentially probed, stripped, and reprobed (first Bmf, then Bim, then Bid, then Hsp70 or Puma, then Noxa, then Bad, then Hsp70). Data show 1 representative of 3 independent experiments. (B) Sorted granulocytes from Bim-deficient andbcl-2 transgenic mice were cultured in simple medium, and Bmf protein expression was analyzed as in panel A. Data show 1 of 2 independent experiments.
Discussion
We investigated the role of several proapoptotic and antiapoptotic Bcl-2 family members on the survival of bone marrow–derived and mobilized peritoneal granulocytes. We observed that absence of the BH3-only protein Bim, but not loss of proapoptotic Bax, rendered granulocytes refractory to apoptosis induced by cytokine withdrawal (Figure 1A,E), chemotherapeutic drugs, or the calcium ionophore ionomycin, the latter only inducing cell death in mouse but not human granulocytes31 (Figure 1C,G,I). In contrast, killing by FasL was not impaired in the absence of Bim or Bax (Figure 1C,G,I). Our results are consistent with the previous observations thatbim−/− thymocytes19,32 as well asbcl-2 transgenic lymphocytes33 and granulocytes11 are resistant to cell death induced by cytokine withdrawal or cytotoxic drugs but remain normally sensitive to FasL. The resistance of Bim-deficient granulocytes to spontaneous death in culture (Figure 1A,E) is consistent with abnormal granulocyte accumulation observed in bim−/−mice19 and indicates that this is due to a cell intrinsic defect and not a consequence of increased cytokine levels or a phagocytosis defect in the bim−/− animals. Bim-deficient granulocytes are less resistant to spontaneous death in culture than those overexpressing Bcl-2 (Figure 1A,E), indicating that Bcl-2–inhibitable death inducers in addition to Bim are involved in programmed death of granulocytes (see “Discussion”). Bax expression levels have been reported to be abnormally low in granulocytes from patients with certain inflammatory diseases, such as cystic fibrosis or acute pneumonia.5 Our findings, however, suggest that absence of Bax alone is not sufficient to extend granulocyte survival (Figure 1A,E), and this is consistent with the observation that bax−/− mice have no obvious increase in granulocytes (Lindsten et al34 and our data, not shown). In contrast,bax−/−/bak−/−double-deficient animals have an abnormal accumulation of granulocytes,34 indicating that these 2 multi-BH domain proapoptotic Bcl-2 family members have overlapping functions in programmed death of myeloid cells, probably acting downstream of BH3-only proteins.
Loss of Bcl-2 or Bcl-w does not sensitize granulocytes from bone marrow or peritoneal cavity to spontaneous (Figure 1B,F) or stress-induced apoptosis (Figure 1D,H,J), but Bcl-2 overexpression prolongs their survival. This indicates that Bcl-2 and Bcl-w act in a redundant manner in regulating granulocyte survival or that they may even be dispensable. Mice lacking A1a show a small but significant acceleration of spontaneous granulocyte apoptosis in culture.18 Because mice have 3 closely relatedA1 genes, all of which appear to be expressed in granulocytes,18 it is possible that combined loss of allA1 genes would evoke extensive granulocyte apoptosis and severe neutropenia. Another prosurvival Bcl-2 family member, Mcl-1, is induced in granulocytes by cytokine receptor stimulation,8and expression of a Mcl-1 transgene inhibits apoptosis of hematopoietic cells.35 The early embryonic lethality of Mcl-1–deficient mice precluded investigation of the function of this protein in myeloid cells,36 but it is possible that a critical role in granulocyte survival will emerge from studies with mice in which the gene can be deleted selectively in this cell type.
Because loss of Bcl-2 did not influence the survival of mature granulocytes in culture (Figure 1) but expression of a bcl-2transgene was reported to protect granulocytes as well as myeloid precursors against growth factor withdrawal in vitro,22 we assessed whether absence of Bcl-2 has an impact on the survival of early myeloid progenitors and compared this effect with the one caused by absence of the BH3-only protein Bim. Analysis of wt animals reconstituted with a Bcl-2–deficient hemopoietic system confirmed previous observations of strongly reduced numbers of lymphocytes in all hemopoietic compartments analyzed (ie, spleen, bone marrow, and peripheral blood)29 but also indicates a previously unrecognized role for Bcl-2 in the survival of mature macrophages, which were significantly reduced in numbers in peripheral blood and spleen when compared with animals reconstituted with wt stem cells (Figure 2A-D). Although granulocyte numbers were comparable in wt andbcl-2−/− reconstituted animals, analysis of the colony-forming potential of early myeloid progenitors in soft agar revealed that Bcl-2 is essential for the survival of all types of myeloid precursors under conditions of cytokine withdrawal (Figure 2E). Absence of Bim had a less pronounced but reproducible protective effect on the survival of cytokine-deprived myeloid progenitors (Figure 2F). The frequency of Bim-deficient myeloid precursors was comparable to that found in wt bone marrow. Expression of a bcl-2transgene proved most potent in protecting myeloid progenitors from the effects of delayed cytokine addition (Figure 2F and Ogilvy et al22). In agreement with previous results, the frequency ofbcl-2 transgenic myeloid precursors was comparable to that found in wt bone marrow with the exception of a previously described minor reduction of macrophage colonies.22 These observations are in line with data demonstrating that Bcl-2 protects hematopoietic stem cells and increases their number and repopulation potential in vivo.37 Furthermore, our findings are consistent with the notion that bcl-2−/− stem cells perform poorly in serial transplantation assays29 and demonstrate that although Bcl-2 is not essential for the survival of mature granulocytes, it is required to protect myeloid progenitors under conditions of cytokine withdrawal. Collectively, these results indicate that cytokine deprivation–induced death of myeloid progenitors is regulated by Bcl-2 and that Bim contributes to this process, although to a lower degree than in mature granulocytes (Figure1A,E).
G-CSF promotes maturation and proliferation of granulocytes in the bone marrow through activation of signal transducer and activator of transcription (STAT) 3 and STAT 5 as well as mitogen-activated protein kinase (MAPK).38 Little, however, is known about the mechanism by which G-CSF regulates granulocyte survival. Down-regulation of Bax expression levels5 as well as the prevention of a conformational change, required to allow Bax to insert into inner membranes and subsequent caspase-3 activation,39 were reported to contribute to the prosurvival effect of G-CSF. Our experiments analyzing the ability of G-CSF to promote survival of granulocytes lacking Bim, Bax, Bcl-2, or Bcl-w indicate that none of these molecules alone is the key target for the prosurvival effect of G-CSF. In cells expressing a bcl-2transgene, G-CSF failed to further enhance survival, demonstrating that Bcl-2 can efficiently compensate for G-CSF receptor signaling in these cells (Figure 4A,B). This indicates that Bcl-2–like prosurvival molecules, such as Mcl-1 or A1, may be the key targets of the antiapoptotic signals induced by G-CSF. Alternatively, because absence of Bim partially protects granulocytes under conditions of growth factor withdrawal (Figure 1A,E), G-CSF may modulate the function and/or expression levels of BH3-only proteins. We found no evidence that G-CSF would modulate mRNA expression levels of BH3-only proteins in granulocytes derived from bone marrow or the peritoneal cavity under conditions of growth factor withdrawal (Figure 4A,B).
Analyzing protein expression levels of BH3-only proteins, we observed accumulation of Bmf in cytokine-deprived bone marrow–derived cells (Figure 5A). This accumulation was also observed in granulocytes from Bim-deficient or bcl-2 transgenic animals (Figure 5B), which indicates that this accumulation is not merely a consequence of cell death but may actively contribute to apoptosis of granulocytes. The proapoptotic activity of Bim and Bmf can be controlled posttranslationally by sequestration to distinct subcellular compartments.20,26 This does not, however, exclude that transcriptional control can also regulate their proapoptotic function. Upon cytokine deprivation, Bim expression is induced by the forkhead-related transcription factor FKHR-L1 in the BaF3 B cell line40 and by AP-1 in neuronal cells.41 42However, because bmf mRNA levels did not increase upon cytokine deprivation (Figure 4A,B), Bmf accumulation appears to be due to a posttranslational mechanism, perhaps caused by enhanced protein stability.
In conclusion, our results demonstrate that the BH3-only protein Bim and possibly also its relative Bmf are key initiators of growth factor withdrawal and stress-induced apoptosis of granulocytes, whereas the function of the proapoptotic multi-BH domain protein Bax appears to be redundant. To allow efficient production but rapid turnover of granulocytes, the prosurvival function of Bcl-2 or Bcl-w may be more prominent in progenitors than in mature cells. Alternatively, Bcl-2 and Bcl-w or other more distantly related Bcl-2–like prosurvival molecules, such as Mcl-1 or A1, may act in a redundant manner in mature granulocytes.
We thank Drs A. Harris, J. Adams, and S. Cory for supplyingvav-bcl-2 transgenic and bcl-w−/−mice and Dr D. Loh for bcl-2+/−mice. We are grateful to S. Mifsud and Drs D. Metcalf and N. Nicola for gifts of cytokines and Dr R. Anderson for anti–HSP-70 antibody. We thank C. Tilbrook and A. Naughton for mouse care and injections and Dr F. Battye, V. Lapatis, J. Chang, C. Tarlinton, and D. Kaminaris for cell sorting.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-07-2132.
Supported by fellowships from the Human Frontiers Science Program (HFSP), the Leukemia Research Foundation, the National Health and Medical Research Council (NHMRC), and Royal Australian College of Physicians (RACP) and grants from the NHMRC (Canberra, registered key 973002), the Dr Josef Steiner Cancer Research Foundation (Bern), the National Cancer Institute (CA43540 and CA80188), and the Leukemia and Lymphoma Society of America (grant 7015-02).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Strasser, The Walter and Eliza Hall Institute of Medical Research, PO Royal Melbourne Hospital, Victoria, 3050, Australia; e-mail: strasser@wehi.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal