Collagen-induced activation of platelets in suspension leads to αIIbβ3-mediated outside-in signaling, granule release, thromboxane A2 (TxA2) production, and aggregation. Although much is known about collagen-induced platelet signaling, the roles of TxA2 production, adenosine diphosphate (ADP) and dense-granule secretion, and αIIbβ3-mediated outside-in signaling in this process are unclear. Here, we demonstrate that TxA2 and ADP are required for collagen-induced platelet activation in response to a low, but not a high, level of collagen and that αIIbβ3-mediated outside-in signaling is required, at least in part, for this TxA2 production and ADP secretion. A high level of collagen can activate platelets deficient in PLCγ2, Gαq, or TxA2 receptors, as well as platelets treated with a protein kinase C inhibitor, Ro31-8220. Thus, activation of αIIbβ3 in response to a high level of collagen does not require these signaling proteins. Furthermore, a high level of collagen can cause weak TxA2 and ADP-independent aggregation, but maximal aggregation induced by a high level of collagen requires TxA2 or secretion.
Introduction
Normal hemostasis depends in part on platelet signal transduction initiated by the adherence of platelets to collagen fibers exposed at sites of blood vessel injury. Because of the central role of collagen/platelet interactions in hemostasis, collagen-induced platelet aggregation has been studied extensively. Studies using platelets from patients with collagen-related bleeding disorders, along with characterization of platelet signaling and aggregation in response to a variety of snake venom proteins and the analysis of platelets from knockout mice, have provided much of the foundation for our understanding of collagen-induced platelet signaling.1,2Together, these studies indicate that the integrin α2β1 is the major receptor for platelet adhesion to collagen and that glycoprotein VI (GPVI) is the primary, but not exclusive, signaling receptor for collagen-induced platelet signal transduction.2,3 However, the role of α2β1 in collagen-induced signal transduction is controversial and unresolved.4-6 Nonetheless, it is clear that α2β1 contributes to this signaling but is unable to generate all the signaling required to induce aggregation.5 While collagen-related peptide (CRP) specifically induces GPVI signaling, collagen has the advantage of being a physiologic agonist for studying platelet activation. Collagen activation of platelets induces tyrosine phosphorylation of the signaling molecules Fc receptor γ-chain (FcRγ-chain), Syk, phospholipase C γ2 (PLC γ2), and the adapter molecule SLP-76. These signaling molecules have been reported to be required for collagen-induced platelet aggregation and dense-body secretion.7-10 In addition, the adapter molecule LAT (linker for activation of T cells) is tyrosine phosphorylated in response to collagen treatment of platelets11 and is required for irreversible platelet aggregation in response to a low, but not a high, level of collagen.12,13 Furthermore, LAT is required for thromboxane A2 (TxA2) production in response to all levels of collagen.12
Despite the fact that a great deal is known about the participation of the above-mentioned signal transduction and adapter molecules in collagen-induced platelet signal transduction, the roles of TxA2 production, adenosine diphosphate (ADP) secretion, and αIIbβ3-mediated outside-in signaling in collagen-induced platelet activation have not been clarified. For example, a variety of studies support the view that TxA2 or ADP is absolutely required for collagen-induced human and murine platelet aggregation,14-16 but platelets from mice lacking TxA2 receptors17 or the ADP receptors P2Y118,19 or P2Y1220 aggregate in response to collagen. Additionally, the results of other studies suggest that platelet aggregation and therefore possibly the activation of αIIbβ3require collagen-induced secretion that is dependent on the FcRγ-chain,7 PLC γ2,10 and the function of Gαq.16 Although it is correct that activation of the FcRγ-chain is required for collagen-induced platelet aggregation and secretion, the results presented here demonstrate that platelet aggregation induced by a high level of collagen is not dependent on TxA2 and dense-granule secretion. The data presented here resolve the contradictions presented by the results cited above14-20and in so doing demonstrate that collagen activation of αIIbβ3 is not dependent on PLCγ2, Gαq, the thromboxane A2 receptor, or dense-body secretion. These results provide new insights into the signaling mechanisms underlying collagen-induced platelet aggregation and demonstrate that there are at least 2 distinct signaling pathways activated by collagen stimulation of platelets.
Materials and methods
Reagents
Collagen reagent Horm (native collagen fibrils from equine tendons) was purchased from Nycomed Arzneimittel (Munchen, Germany). Tp−/− mice were derived as described.17Hamster control immunoglobulin G (IgG) was from Jackson ImmunoResearch Laboratories (West Grove, PA). 1B5 (hamster antimouse αIIbβ3 antibody) was a generous gift from Dr Barry Coller (Rockefeller University, NY). Apyrase, A3P5P, Ro31-8220, and mepacrine were purchased from Sigma-Aldrich (St Louis, MO), and AR-C69931MX was a generous gift from Astra-Zeneca (Loughborough, England).
Platelet aggregation
Blood was collected from the abdominal aorta of isofluorane-anesthetized mice into syringes containing 2% (vol/vol) heparin. Platelet-rich plasma (PRP) was prepared by differential centrifugation. Aggregation tests were performed in a Chronolog aggregometer (CHRONO-LOG, Havertown, PA) using PRP (300 μL) adjusted to approximately 106 platelets/μL with plasma. In cases where antibody was used, washed platelets were prepared by differential centrifugation and resuspension into Tyrode buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1% bovine serum albumen [BSA]), pH 7.4). Washed platelets were incubated with an antibody for 10 minutes at room temperature prior to stimulation by collagen.
Measurement of ATP secretion
Adenosine trisphosphate (ATP) secretion was measured using CHRONO-LUME reagent (CHRONO-LOG) according to the manufacturer's protocol, with minor variations. After 4 to 6 minutes of platelet activation with stirring (1200 rpm), 10 μL luciferase-luciferin was added directly into the cuvettes. The luminescence intensity was measured at a luminescence setting of × 0.001.
Measurement of TxA2 production
TxA2 was assayed as TxB2 in the plasma after the platelets were removed at the end of the 4- to 6-minute aggregation period. The TxB2 EIA kit (Assay Designs, Ann Arbor, MI) was used according to the manufacturer's protocol to indirectly measure free TxA2. The plasma samples were diluted 1:50 with the supplied assay buffer for analysis. For the purpose of discussion, it is assumed that TxB2 levels reflect TxA2 levels.
Mepacrine uptake and secretion
Mepacrine is taken up into dense granules in platelets. The procedure for mepacrine uptake was followed as previously described.21 Washed platelets were incubated in modified Tyrode solution containing 10 μM mepacrine (quinacrine; Sigma Chemical, St Louis, MO) for 30 minutes at 37°C. After incubation, the platelets were removed from excess mepacrine by centrifugation and suspension of the platelets in modified Tyrode solution containing 5 mM EDTA (ethylenediaminetetraacetic acid). Resting platelets and platelets activated with 50 μg/mL collagen were stirred in the aggregometer for 4 minutes. After stirring, the platelets were washed twice and suspended in modified Tyrode solution containing 5 mM EDTA. Secretion of dense-body constituents was evaluated by measuring the fluorescence remaining in the platelets after stirring. For each measurement, 200 μL platelets were diluted 15-fold with modified Tyrode solution. Fluorescence intensity was measured in a Varian Cary Eclipse Fluorescence spectrophotometer (Walnut Creek, CA). The excitation wavelength for mepacrine is 488 nm; the emission wavelength selected was 530 nm. The fluorescence remaining in the control platelets was set as 100%. The fluorescence remaining in the platelets was assumed to represent unsecreted mepacrine. The loss of fluorescence by the collagen-treated platelets represents collagen-induced mepacrine secretion in the absence of platelet aggregation.
Results
ADP and TxA2 are required for aggregation induced by a low, but not a high, concentration of collagen
The studies that demonstrated the requirement for ADP signaling through P2Y1 and P2Y12 and TxA2 receptor signaling in collagen-induced platelet aggregation and secretion did not investigate these effects in response to high concentrations of collagen. Here, the relationship between secreted ADP, TxA2, collagen-induced αIIbβ3 activation, and platelet aggregation was investigated by treatment of thromboxane A2 receptor (Tp)–deficient platelets with a combination of apyrase, A3P5P, and AR-C69931MX to completely inhibit all signaling through TxA2 and ADP receptors in response to collagen. This study was done in 2 parts. Tp-deficient mouse platelets were stimulated with 2.5 or 50 μg/mL of collagen in the presence or absence of the combination of the inhibitors apyrase, A3P5P, and AR-C69931MX (the latter are antagonists for the ADP receptors P2Y1 and P2Y12, respectively). In the first part, platelet activation was characterized in response to a low level of collagen. In contrast to the wild-type platelets (Figure1A), Tp-deficient platelets aggregated less extensively and reversibly in response to a low level of collagen in the absence of inhibitors of ADP receptor signaling, indicating that TxA2 receptor signaling is required to cause irreversible aggregation in response to a low level of collagen. Tp-deficient platelets secreted about 66% less ADP and produced about 50% less TxA2 (measured as TxB2) than the wild-type platelets (Figure 1B) at a low collagen concentration without inhibitors. The diminished level of secretion and TxA2 production may be due to the diminished aggregation of Tp-deficient platelets, the lack of TxA2 receptor signaling, or both. Treatment with apyrase and the ADP receptor antagonists totally inhibited aggregation in response to a low level of collagen in both Tp-deficient and wild-type platelets (Figure 1C), indicating that ADP-induced signaling is absolutely required to cause aggregation in response to a low level of collagen. No ADP secretion was detected because of the treatment with apyrase, an ADP scavenger, but TxA2 production was measured. Tp-deficient and wild-type platelets in the presence of the aggregation inhibitors produced similar levels of TxA2 (Figure 1D), but the level of TxA2 production was less than the levels produced in the absence of inhibitors (Figure 1B,D). The results obtained (see Figure 1) in the presence of the ADP receptor antagonists and apyrase were no different from those obtained in the presence of the antagonists without apyrase (data not shown), indicating that any adenosine monophosphate (AMP) generated by apyrase had no significant effect on the results.
ADP- and TxA2-induced signaling is required to cause aggregation in response to a low concentration of collagen.
Wild-type (WT) or Tp-deficient (Tp−/−) platelets in plasma were activated with a low concentration (2.5 μg/mL) of collagen. Tp-deficient platelet aggregation was diminished and reversible (A), and ATP secretion and TxA2 production levels were substantially diminished in response to a low concentration of collagen (B). After preincubation with apyrase (10 U/mL), A3P5P (2mM), and AR-C69931MX (2 μM) for 3 minutes, wild-type and Tp-deficient platelets were activated with a low concentration (2.5 μg/mL) of collagen. Aggregation of both Tp-deficient and wild-type platelets was inhibited in response to a low concentration of collagen (C). TxA2 production from both Tp-deficient and wild-type platelets treated with apyrase, A3P5P, and AR-C69931MX were similar in response to a low concentration of collagen (D), although the level of TxA2 production was substantially less than in the platelets untreated with apyrase and the ADP receptor antagonists (B,D). Data were obtained from 6 tests. Bars represent means ± SEM.
ADP- and TxA2-induced signaling is required to cause aggregation in response to a low concentration of collagen.
Wild-type (WT) or Tp-deficient (Tp−/−) platelets in plasma were activated with a low concentration (2.5 μg/mL) of collagen. Tp-deficient platelet aggregation was diminished and reversible (A), and ATP secretion and TxA2 production levels were substantially diminished in response to a low concentration of collagen (B). After preincubation with apyrase (10 U/mL), A3P5P (2mM), and AR-C69931MX (2 μM) for 3 minutes, wild-type and Tp-deficient platelets were activated with a low concentration (2.5 μg/mL) of collagen. Aggregation of both Tp-deficient and wild-type platelets was inhibited in response to a low concentration of collagen (C). TxA2 production from both Tp-deficient and wild-type platelets treated with apyrase, A3P5P, and AR-C69931MX were similar in response to a low concentration of collagen (D), although the level of TxA2 production was substantially less than in the platelets untreated with apyrase and the ADP receptor antagonists (B,D). Data were obtained from 6 tests. Bars represent means ± SEM.
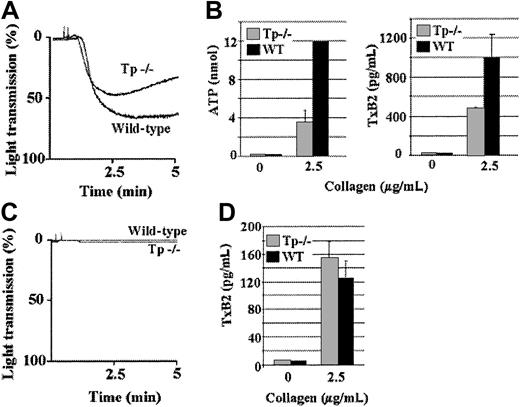
In the second part of this study, Tp-deficient platelets were treated with a high level of collagen in the presence and absence of the inhibitors. When Tp-deficient platelets were activated with a high level of collagen, they aggregated (Figure2A), secreted ADP, and produced TxA2 to similar extents as wild-type platelets (Figure 2B), indicating that TxA2 receptor signaling is not necessary for aggregation in response to a high level of collagen. Furthermore, wild-type platelets that were treated with apyrase, A3P5P, and AR-C69931MX aggregated to a similar level as platelets that were untreated (Figure 2A,C). In contrast, Tp-deficient platelets treated with apyrase, A3P5P, and AR-C69931MX aggregated slowly and only about 38% as extensively as the wild-type platelets in response to a high level of collagen (Figure 2C), even though the level of TxA2 production was similar to that of the wild-type platelets treated with apyrase and the ADP receptor antagonists (Figure 2D). So, the lack of both ADP and TxA2 receptor signaling results in diminished aggregation, and, in contrast to the situation in response to a low level of collagen, either ADP or TxA2 suffices to cause maximum aggregation in response to a high level of collagen. Moreover, αIIbβ3 activation in response to a high level of collagen does not require ADP or TxA2, because platelets that lack the TxA2 receptor and P2Y1- and P2Y12-propagated signaling can undergo a low level of aggregation in response to a high concentration of collagen. That apparent aggregation was not simply collagen-mediated clumping or α2β1-mediated adhesion because it was inhibited by the antimurine αIIbβ3 monoclonal antibody (mAb) 1B5 (Figure 2E), which inhibits aggregation of mouse platelets by preventing the binding of fibrinogen to its receptors.22
Aggregation of platelets from Tp-deficient mice is diminished in response to a high concentration of collagen in the presence of apyrase, A3P5P, and AR-C69931MX.
Platelets in plasma from Tp-deficient mice aggregated similarly to the wild-type platelets when activated with a high concentration (50 μg/mL) of collagen (A). ATP secretion and TxA2 production levels were undiminished from the Tp-deficient platelets in response to a high concentration of collagen (B). Tp-deficient and wild-type platelets were preincubated for 3 minutes with stirring in the presence of apyrase (10 U/mL), A3P5P (2 mM), and AR-C69931MX (2 μM) prior to stimulation with a high level of collagen. This treatment caused diminished aggregation of Tp-deficient platelets when compared to that of the wild-type platelets (C), but TxA2 production from Tp-deficient platelets was undiminished and similar to the wild-type platelets under the same conditions (D). Data were obtained from 6 tests. Bars represent means ± SEM. Aggregation of washed Tp-deficient platelets preincubated with the inhibitors (apyrase [10 U/mL], A3P5P [2 mM], and AR-C69931MX [2 μM]) and stimulated with 50 μg/mL collagen was inhibited by 10μg/mL hamster antimouse αIIbβ3 monoclonal antibody 1B5, but not by 10μg/mL hamster control IgG (E).
Aggregation of platelets from Tp-deficient mice is diminished in response to a high concentration of collagen in the presence of apyrase, A3P5P, and AR-C69931MX.
Platelets in plasma from Tp-deficient mice aggregated similarly to the wild-type platelets when activated with a high concentration (50 μg/mL) of collagen (A). ATP secretion and TxA2 production levels were undiminished from the Tp-deficient platelets in response to a high concentration of collagen (B). Tp-deficient and wild-type platelets were preincubated for 3 minutes with stirring in the presence of apyrase (10 U/mL), A3P5P (2 mM), and AR-C69931MX (2 μM) prior to stimulation with a high level of collagen. This treatment caused diminished aggregation of Tp-deficient platelets when compared to that of the wild-type platelets (C), but TxA2 production from Tp-deficient platelets was undiminished and similar to the wild-type platelets under the same conditions (D). Data were obtained from 6 tests. Bars represent means ± SEM. Aggregation of washed Tp-deficient platelets preincubated with the inhibitors (apyrase [10 U/mL], A3P5P [2 mM], and AR-C69931MX [2 μM]) and stimulated with 50 μg/mL collagen was inhibited by 10μg/mL hamster antimouse αIIbβ3 monoclonal antibody 1B5, but not by 10μg/mL hamster control IgG (E).
Protein kinase C–dependent secretion is not required for aggregation induced by a high level of collagen
Although Tp-deficient platelets treated with apyrase, A3P5P, and AR-C69931MX underwent a low level of aggregation in response to a high level of collagen, those results do not necessarily mean that collagen can induce platelet aggregation in the absence of dense-granule secretion. This issue was addressed by treating the platelets with Ro31-8220, an inhibitor of protein kinase C (PKC) and secretion. Tp-deficient platelets in plasma were treated with Ro31-8220 and tested for aggregation, ATP secretion, and TxA2 production in response to 50 μg/mL collagen. Tp-deficient platelets aggregated slowly (Figure3A) and only to an extent similar to that of the Tp-deficient platelets treated with apyrase and ADP receptor antagonists (Figure 2C). As expected, Tp-deficient platelets treated with 3 μg/mL collagen in the presence of Ro31-8220 did not aggregate (data not shown). Furthermore, even though treatment with Ro31-8220 prevented ATP secretion (Figure 3C), the Tp-deficient platelets produced an undiminished level of TxA2 (Figure 3D). These results indicate that collagen-induced signaling can cause aggregation, αIIbβ3 activation, and TxA2 production in the absence of demonstrable dense-granule secretion and sufficient PKC activation to cause ADP secretion. The aggregation caused by a high level of collagen in the presence of Ro31-8220 reflects activation of αIIbβ3 because that aggregation was inhibited by the mAb 1B5, but not nonimmune IgG (Figure 3B).
A high concentration of collagen can induce secretion-independent aggregation.
Tp-deficient platelets were stimulated with a high concentration (50 μg/mL) of collagen in the presence of 10 μM Ro31-8220 (a PKC inhibitor that prevents secretion) or dimethyl sulfoxide (DMSO) as a control. Ro31-8220 did not prevent aggregation induced by a high level of collagen but did affect the maximal aggregation response (A). Collagen (50 μg/mL)–induced aggregation of Ro31-8220–treated Tp-deficient platelets was inhibited by incubating the platelets with 10μg/mL hamster monoclonal antimouse αIIbβ3 antibody 1B5, but not by 10μg/mL hamster control IgG (B). Treatment of Tp-deficient platelets with Ro31-8220 prevents collagen-induced ATP secretion (C) but does not affect TxA2 production (D). Thus, Ro31-8220 affects only PKC activation and secretion and does not affect collagen-induced TxA2 production. Data were obtained from 3 tests. Bars represent means ± SEM.
A high concentration of collagen can induce secretion-independent aggregation.
Tp-deficient platelets were stimulated with a high concentration (50 μg/mL) of collagen in the presence of 10 μM Ro31-8220 (a PKC inhibitor that prevents secretion) or dimethyl sulfoxide (DMSO) as a control. Ro31-8220 did not prevent aggregation induced by a high level of collagen but did affect the maximal aggregation response (A). Collagen (50 μg/mL)–induced aggregation of Ro31-8220–treated Tp-deficient platelets was inhibited by incubating the platelets with 10μg/mL hamster monoclonal antimouse αIIbβ3 antibody 1B5, but not by 10μg/mL hamster control IgG (B). Treatment of Tp-deficient platelets with Ro31-8220 prevents collagen-induced ATP secretion (C) but does not affect TxA2 production (D). Thus, Ro31-8220 affects only PKC activation and secretion and does not affect collagen-induced TxA2 production. Data were obtained from 3 tests. Bars represent means ± SEM.
Aggregation induced by a high concentration of collagen does not require PLCγ2 or Gαq
As shown in Figure 2, TxA2 and dense-body secretion are not required to induce a low level of platelet aggregation in response to a high level of collagen. Contrary to the published data, these results support the view that platelets that lack PLCγ210 or Gαq16 would be expected to aggregate to a high, but not a low, level of collagen, assuming that the reported absence of aggregation is due to the lack of secretion and only if PLCγ2 or Gαq are not required to directly activate αIIbβ3. These predictions were tested by characterizing the aggregation response of platelets from both PLCγ2- and Gαq-deficient mice treated with a high concentration of collagen. As predicted, PLCγ2-deficient platelets aggregated in response to a high level of collagen (Figure 4A), although the aggregation was slow and the level was diminished in comparison with the wild-type platelets. This low level of aggregation was αIIbβ3-dependent aggregation because it was inhibited by mAb 1B5, but not IgG (Figure 4B). As expected, the extent of aggregation of the PLCγ2-deficient platelets was similar to that of the Tp-deficient platelets treated with Ro31-8220 (Figure 3A). Furthermore, the presence of apyrase, A3P5P, AR-C69931MX, and indomethacin did not decrease the extent or rate of the aggregation of PLCγ2-deficient platelets in response to a high level of collagen (Figure 4C), confirming that the aggregation was ADP and TxA2 independent. Likewise, treatment of wild-type platelets with the inhibitors rendered their aggregation in response to a high level of collagen similar to that of the PLCγ2-deficient platelets with or without inhibitors (Figure 4D). Therefore, the diminished aggregation of the PLCγ2-deficient platelets demonstrates that secretion and TxA2 are not absolutely required for a high level of collagen to cause αIIbβ3 activation and aggregation, although maximum aggregation requires secretion and/or TxA2.
A high concentration of collagen induces PLCγ2-independent aggregation.
PLCγ2-deficient (PLCγ2−/−) and wild-type (WT) platelets were stimulated with a high level (50 μg/mL) of collagen. PLCγ2-deficient platelets aggregated in response to a high level of collagen, but the extent of aggregation was diminished in comparison to wild-type platelets (A). Aggregation of PLCγ2-deficient platelets preincubated with inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) and stimulated with 50 μg/mL collagen was inhibited by 10μg/mL hamster antimouse αIIbβ3 monoclonal antibody 1B5, but not by 10μg/mL hamster control IgG (B). PLCγ2-deficient platelets with and without inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) aggregated in response to a high level of collagen; the extents of aggregation were similar (C). PLCγ2-deficient platelets in the absence of inhibitors and wild-type platelets plus inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) aggregated to similar extents in response to a high level of collagen (D). Wild-type platelets treated with 4 inhibitors and PLCγ2-deficient platelets with and without inhibitors did not secrete TxA2 (E) even in response to a high concentration of collagen. Data were obtained from 6 tests. Bars represent means ± SEM.
A high concentration of collagen induces PLCγ2-independent aggregation.
PLCγ2-deficient (PLCγ2−/−) and wild-type (WT) platelets were stimulated with a high level (50 μg/mL) of collagen. PLCγ2-deficient platelets aggregated in response to a high level of collagen, but the extent of aggregation was diminished in comparison to wild-type platelets (A). Aggregation of PLCγ2-deficient platelets preincubated with inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) and stimulated with 50 μg/mL collagen was inhibited by 10μg/mL hamster antimouse αIIbβ3 monoclonal antibody 1B5, but not by 10μg/mL hamster control IgG (B). PLCγ2-deficient platelets with and without inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) aggregated in response to a high level of collagen; the extents of aggregation were similar (C). PLCγ2-deficient platelets in the absence of inhibitors and wild-type platelets plus inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) aggregated to similar extents in response to a high level of collagen (D). Wild-type platelets treated with 4 inhibitors and PLCγ2-deficient platelets with and without inhibitors did not secrete TxA2 (E) even in response to a high concentration of collagen. Data were obtained from 6 tests. Bars represent means ± SEM.
In contrast to the PLCγ2-deficient platelets, Gαq-deficient platelets released normal levels of ADP (Figure5B) and TxA2 (Figure 5C) and aggregated to an extent similar to that of the control platelets from the littermate mice (Figure 5A) in response to a high level of collagen. Therefore, Gαq signaling is not required for aggregation induced by a high level of collagen. Thus, collagen-induced platelet aggregation is dependent on PLCγ2 and Gαq only in response to a low level of collagen. Specifically, collagen-induced platelet aggregation appears to be dependent on PLCγ2 and Gαq only in response to levels of collagen that cause secretion-dependent aggregation. Nonetheless, even though TxA2 and secretion are absolutely required only for irreversible aggregation in response to a low level of collagen, they enhance collagen-induced aggregation regardless of the level of collagen used to stimulate the platelets. Furthermore, PLCγ2- or Gαq-elicited signaling are not required for collagen-induced αIIbβ3 activation, because aggregation induced by a high level of collagen is TxA2 and secretion independent.
A high concentration of collagen induces Gαq-independent aggregation.
Gαq-deficient (Gαq−/−) and wild-type (WT) platelets were stimulated with a high level (50 μg/mL) of collagen. Gαq-deficient platelets aggregated in response to a high level of collagen, and the extent of aggregation was undiminished in comparison to wild-type platelets (A). Gαq-deficient platelets secrete ATP (B) and produce TxA2 (C) to a level similar to that of the wild-type platelets. Data were obtained from 2 tests. Bars represent means ± SEM.
A high concentration of collagen induces Gαq-independent aggregation.
Gαq-deficient (Gαq−/−) and wild-type (WT) platelets were stimulated with a high level (50 μg/mL) of collagen. Gαq-deficient platelets aggregated in response to a high level of collagen, and the extent of aggregation was undiminished in comparison to wild-type platelets (A). Gαq-deficient platelets secrete ATP (B) and produce TxA2 (C) to a level similar to that of the wild-type platelets. Data were obtained from 2 tests. Bars represent means ± SEM.
Secretion and TxA2 production in response to a low level of collagen is induced by αIIbβ3-mediated outside-in signaling
The observations that Tp-deficient platelets produced the same amount of TxA2 and secreted a similar amount of ADP as the control platelets in response to a high concentration of collagen but produced less TxA2 and secreted less ADP than the control platelets in response to a low level of collagen (Figure 3) support the hypothesis that TxA2 production and possibly ADP secretion are driven, at least in part, by aggregation in response to a low level of collagen. This hypothesis was tested by treating control platelets with the antimouse αIIbβ3 mAb 1B5 (Figure6A), which inhibits platelet aggregation by preventing fibrinogen binding.22 Platelets treated with a low level of collagen in the presence of the mAb 1B5 secreted and produced about 80% less ADP (Figure 6B) and TxA2 (Figure 6C), respectively, than platelets treated with control IgG. Thus under certain conditions, αIIbβ3-mediated outside-in signal transduction plays an important role in collagen-induced secretion and platelet aggregation.
A low concentration of collagen-induced, αIIbβ3-mediated aggregation drives ADP secretion and TxA2 production.
Wild-type platelets were incubated with 1B5, a hamster monoclonal antimouse αIIbβ3 antibody (10 μg/mL), or control hamster IgG (10 μg/mL) for 10 minutes before stimulation with a low concentration (2.5 μg/mL) of collagen. 1B5 inhibits fibrinogen binding to αIIbβ3, thereby preventing aggregation (A). Inhibition of aggregation substantially diminished ATP secretion (B) and TxA2 production (C). Therefore, αIIbβ3-mediated aggregation is required for maximum ATP secretion and TxA2 production in response to a low level of collagen. Data were obtained from 6 tests. Bars represent means ± SEM.
A low concentration of collagen-induced, αIIbβ3-mediated aggregation drives ADP secretion and TxA2 production.
Wild-type platelets were incubated with 1B5, a hamster monoclonal antimouse αIIbβ3 antibody (10 μg/mL), or control hamster IgG (10 μg/mL) for 10 minutes before stimulation with a low concentration (2.5 μg/mL) of collagen. 1B5 inhibits fibrinogen binding to αIIbβ3, thereby preventing aggregation (A). Inhibition of aggregation substantially diminished ATP secretion (B) and TxA2 production (C). Therefore, αIIbβ3-mediated aggregation is required for maximum ATP secretion and TxA2 production in response to a low level of collagen. Data were obtained from 6 tests. Bars represent means ± SEM.
Although αIIbβ3 outside-in signaling is required for maximum TxA2 production and ADP secretion in response to a low level of collagen, the following results demonstrate that this signaling is not absolutely required for those functions in response to high levels of collagen. Because 1B5 could not completely prevent platelet aggregation to a high level of collagen (50 μg/mL), EDTA was used to prevent aggregation under these conditions (Figure7A). Platelets treated with 50 μg/mL collagen in the presence of EDTA produced about 30% of the amount of TxA2 produced in its absence (Figure 7B). The cause of the diminished production of TxA2 is not known; but regardless of the cause, 50 μg/mL of collagen caused a substantial level of TxA2 production in the absence of platelet aggregation. The significance of these results is that TxA2 production occurred in the absence of aggregation (despite the ability of EDTA to interfere with the binding of collagen to α2β1). Dense-body secretion was measured using mepacrine21 because EDTA precludes using CHRONO-LUME to measure ATP secretion. As with TxA2 production, a high level of collagen appeared to have caused secretion of about 55% of the dense granule mepacrine (Figure 7C), which is inhibited by Ro31-8220, in the absence of aggregation (not shown). These results support the conclusion that treatment of platelets with high levels of collagen can cause a significant level of TxA2 production and ADP secretion in the absence of αIIbβ3-mediated outside-in signaling. Thus, collagen treatment of platelets can result in both aggregation-dependent and aggregation-independent TxA2 production and ADP secretion, demonstrating the occurrence of 2 distinct signaling mechanisms in response to collagen.
A high concentration of collagen induces ADP secretion and TxA2 production in the absence of aggregation.
Wild-type platelets were stimulated with a high level (50 μg/mL) of collagen in the presence or absence of EDTA (5 mM). EDTA inhibited platelet aggregation that 1B5 was unable to prevent in response to a high level of collagen (A). Furthermore, a high level of collagen induced TxA2 production in the absence of aggregation (B). Data were obtained from 3 tests. Bars represent means ± SEM. Since EDTA precludes the measurement of ATP secretion by luciferase assay, dense-granule secretion was evaluated by measuring a loss of mepacrine fluorescence following activation by collagen, using a spectrophotometer as described in “Materials and methods” (C). Blood was drawn from wild-type mice on 3 occasions. Washed platelets were prepared as described and the experiment was repeated 5 times using platelets from each set of mice, for a total of 15 repetitions. The data were pooled. Bars represent means ± SEM.
A high concentration of collagen induces ADP secretion and TxA2 production in the absence of aggregation.
Wild-type platelets were stimulated with a high level (50 μg/mL) of collagen in the presence or absence of EDTA (5 mM). EDTA inhibited platelet aggregation that 1B5 was unable to prevent in response to a high level of collagen (A). Furthermore, a high level of collagen induced TxA2 production in the absence of aggregation (B). Data were obtained from 3 tests. Bars represent means ± SEM. Since EDTA precludes the measurement of ATP secretion by luciferase assay, dense-granule secretion was evaluated by measuring a loss of mepacrine fluorescence following activation by collagen, using a spectrophotometer as described in “Materials and methods” (C). Blood was drawn from wild-type mice on 3 occasions. Washed platelets were prepared as described and the experiment was repeated 5 times using platelets from each set of mice, for a total of 15 repetitions. The data were pooled. Bars represent means ± SEM.
Discussion
The investigation of the cross-talk between receptors and signaling molecules in response to collagen activation of platelets described here has led to the conclusion that ADP and TxA2 signaling are not required for activation of αIIbβ3and aggregation in response to a high level of collagen. Contrary to the published data using platelets from mice deficient in PLCγ210 or Gαq,16 our results demonstrate that PLCγ2- or Gαq-deficient platelets aggregate to a high level of collagen, indicating that neither PLCγ2 nor Gαq are required for αIIbβ3 activation and aggregation under those conditions. The diminished level of PLCγ2-deficient platelet aggregation may be explained by the fact that no secretion or TxA2 production occurred, supporting the idea that there is cross-talk between collagen receptors with receptors of secreted products. Thus, αIIbβ3 activation induced by a level of collagen that causes secretion-independent aggregation is not dependent on PLCγ2, Gαq, dense-granule secretion, and the TxA2 receptor.
These results confirm the view that there are at least 2 distinct signaling pathways that can mediate platelet activation in response to collagen. Our data do not identify the mechanism underlying the selection between these pathways, but do demonstrate that selection between these pathways appears to be a function of the concentration of collagen used to activate the platelets. Interestingly, the pathway utilized to cause secretion-dependent aggregation (low levels of collagen) uses 2 phospholipases to cause discernible aggregation. In contrast, the signaling pathway activated by exposure to a high level of collagen that causes secretion-independent aggregation is not dependent on phospholipase activity. Secretion/Gαq-dependent aggregation induced by a low concentration of collagen is thought to use PLCβ to activate platelets.16 However, as reported elsewhere,10 aggregation induced by a low level of collagen is also dependent on PLCγ2.
The requirement for 2 distinct phospholipases that presumably have the same enzymatic function for platelet activation in response to collagen requires explanation. The relationship between the 2 phospholipases is revealed by the fact that PLCγ2-deficient platelets do not produce TxA2 or release ATP in response to any level of collagen (Figure 4), whereas the Gαq-deficient platelets release TxA2 and ATP in response to a high level, but not a low level, of collagen (Figure 5). Thus, the function of Gαq and consequently PLCβ in response to collagen is subordinate to PLCγ2. The significance of this relationship appears to be that Gαq amplifies the signal initiated by PLCγ2. So, the data presented here and elsewhere10 support the view that PLCγ2 function is required for activation of Gαq, and Gαq function is required for signal amplification to drive extensive platelet aggregation in response to a low level of collagen.16 Presumably, activation of PLCγ2 by a low level of collagen results in TxA2 and ADP release, which in turn activates Gαq via their receptors. Then Gαq activates PLCβ, which causes the release of TxA2 and ADP by platelets, thereby causing signal amplification and the recruitment of more platelets into growing aggregates.
The signaling pathway used by platelets treated with a high level of collagen causes secretion-independent aggregation. This pathway requires the function of GPVI/FcR γ-chain, but not LAT,12,13 PLCγ2 (Figure 4), SLP-76,13 or Gαq (Figure 5). Since platelets deficient in Fyn/Lyn,23Gαq (Figure 5), or LAT12 secrete dense granules in response to high concentrations of collagen, it is not known whether those signaling molecules are required for secretion-independent aggregation, because platelets from those knockout mice have not been tested in response to a high level of collagen in the presence of the required combination of inhibitors. However, platelets can activate αIIbβ3 in the absence of Fyn/Lyn, Gαq, or LAT, indicating that collagen-stimulated platelets can cause Fyn/Lyn-, Gαq-, or LAT-independent αIIbβ3activation. Furthermore, the absolute requirement of PLCγ2 for collagen-induced secretion and TxA2 production, but not for aggregation (Figure 4), demonstrates that PLCγ2 is not required for collagen-induced activation of αIIbβ3 and subsequent secretion-independent aggregation. It is also known that SLP-76 is not required for aggregation induced by a high level of collagen.13 The observations of others rule out the requirement for α-granule or lysosomal secretion for activation of αIIbβ3 in response to collagen, since it is well known that GPVI/FcRγ-chain deficient platelets do not secrete P-selectin or CD63 (granulophysin) in response to collagen but do activate αIIbβ3.5,24 The signaling pathway that is utilized in response to a low level of collagen requires FcR γ-chain, P2Y1 and P2Y12 (Figure 1), Gαq,16 PLC γ2,10 SLP-76,9and PKC (Figure 3) for αIIbβ3 activation in response to low concentrations of collagen. The function of these proteins is that they facilitate TxA2 production and dense-granule secretion, since the lack of or inhibition of these proteins substantially decreases TxA2 production and secretion.
The results presented here show that aggregation is required to cause maximal TxA2 production and dense-granule secretion (Figure 6), and that TxA2 and secretion seem to be required for maximal aggregation (Figure 2). Previous reports also provide evidence that coordinated signaling events between αIIbβ3-mediated outside-in signaling and P2Y1 and P2Y12-mediated signaling are essential for TxA2 production.25 Furthermore, cross-talk between GPVI and Gi-coupled receptors during collagen-induced platelet aggregation has been established.26 Thus, it appears that aggregation drives TxA2 production and secretion and the released agonists can induce aggregation, making it seem likely that these events participate in positive feedback induced by collagen throughout the aggregation process.
We thank Dr Stefan Offermanns for providing us with the Gαq knockout mice and Dr Barry Coller for providing us with the anti–mouse αIIbβ3 antibody 1B5.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-05-1363.
Supported in part by grants HL56369 and HL63216 from the National Heart, Lung, and Blood Institute; Cancer Center Support grants P30CA21765 and P01CA20180 from the National Cancer Institute, US Public Health Service; the American Lebanese Syrian Associated Charities; and the W. Harry Feinstone Center for Genomic Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
T. Kent Gartner, Department of Microbiology and Molecular Cell Sciences, University of Memphis, Memphis, TN 38152; e-mail:tgartner@memphis.edu.

![Fig. 2. Aggregation of platelets from Tp-deficient mice is diminished in response to a high concentration of collagen in the presence of apyrase, A3P5P, and AR-C69931MX. / Platelets in plasma from Tp-deficient mice aggregated similarly to the wild-type platelets when activated with a high concentration (50 μg/mL) of collagen (A). ATP secretion and TxA2 production levels were undiminished from the Tp-deficient platelets in response to a high concentration of collagen (B). Tp-deficient and wild-type platelets were preincubated for 3 minutes with stirring in the presence of apyrase (10 U/mL), A3P5P (2 mM), and AR-C69931MX (2 μM) prior to stimulation with a high level of collagen. This treatment caused diminished aggregation of Tp-deficient platelets when compared to that of the wild-type platelets (C), but TxA2 production from Tp-deficient platelets was undiminished and similar to the wild-type platelets under the same conditions (D). Data were obtained from 6 tests. Bars represent means ± SEM. Aggregation of washed Tp-deficient platelets preincubated with the inhibitors (apyrase [10 U/mL], A3P5P [2 mM], and AR-C69931MX [2 μM]) and stimulated with 50 μg/mL collagen was inhibited by 10μg/mL hamster antimouse αIIbβ3 monoclonal antibody 1B5, but not by 10μg/mL hamster control IgG (E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-05-1363/3/m_h80734073002.jpeg?Expires=1766016062&Signature=ZEUGNOGtxdunZpVHnqdL-R7-QnEl1xzkWHEXJ2HsXNiRllmeRKmoU9pzx0Zs05be4a8x0WUWkm-IqiRjoiUIlZPaN83KMxoEDBLI4Z6p~fH2x9SX3R~5juXlCS0drXvUR1aY~AGFZ~WMyxsgvo1tr8ZYFhHCRATrZ~hzBs~2DfAC9eYq7Glj7m3Wt~zNvEiGHqkWUctOfEpSS3Z5RDDpi9LDriJHgB-sMfRZHWgbsSwdsl8iYXz622xOo1W8NMLOHSrDOTvfUadcOOUXfApgl45Cue7U~i7etCWZrwy586XdzAbdAz3Mr61~IKtuKtQcZ3NNLoAvCq7~75yJxtnZtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. A high concentration of collagen induces PLCγ2-independent aggregation. / PLCγ2-deficient (PLCγ2−/−) and wild-type (WT) platelets were stimulated with a high level (50 μg/mL) of collagen. PLCγ2-deficient platelets aggregated in response to a high level of collagen, but the extent of aggregation was diminished in comparison to wild-type platelets (A). Aggregation of PLCγ2-deficient platelets preincubated with inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) and stimulated with 50 μg/mL collagen was inhibited by 10μg/mL hamster antimouse αIIbβ3 monoclonal antibody 1B5, but not by 10μg/mL hamster control IgG (B). PLCγ2-deficient platelets with and without inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) aggregated in response to a high level of collagen; the extents of aggregation were similar (C). PLCγ2-deficient platelets in the absence of inhibitors and wild-type platelets plus inhibitors (apyrase [10 U/mL], A3P5P [2 mM], AR-C69931MX [2 μM], and indomethacin [25μM]) aggregated to similar extents in response to a high level of collagen (D). Wild-type platelets treated with 4 inhibitors and PLCγ2-deficient platelets with and without inhibitors did not secrete TxA2 (E) even in response to a high concentration of collagen. Data were obtained from 6 tests. Bars represent means ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-05-1363/3/m_h80734073004.jpeg?Expires=1766016062&Signature=aZwi71C08pn~om5TAw6g1Pai3UyT22zcf9xVB0wmeDq~4EzQBVAbMcigrZ~xEt9Qn7E8R-ORMd2sIsx2SwfjRXNjVmvdcbV73azlhzTyAIKszNATENHCufELqDOqS0Pf02LKuXNLhY9Q5xS~YOg~yMqL7vo84quxNub1MMmewPsLUYc3u9MOjPRIp9Thoa7zY8GKqC4ADb3Hj5e-Q5TKQG0bNdM5AraSWeGoav8tmFPVIaXVhVdVD1hVRVV-TFVSPVjC10JWTYG4TANCEwWQNScX8-560Hv8MT0IrWQr8ffgc1g-7G834aXmVeR2Yl0Yhe4DRWrP3AJcn~SlcowpHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal