The endoplasmic reticulum (ER)–resident heat shock protein Gp96 is involved in protein folding and is released into the extracellular space after necrotic cell death. In this context, Gp96 has immunostimulatory properties: it activates dendritic cells or macrophages and delivers associated peptides into the antigen presentation pathway, resulting in the induction of specific T-cell responses. The inflammatory response after necrotic tissue damage leads to the recruitment of polymorphonuclear neutrophils (PMNs) and monocytes, allowing them to make their first encounter with Gp96. We therefore investigated whether PMNs and monocytes interact with Gp96. We were able to show that PMNs and monocytes specifically bind fluorescein isothiocyanate (FITC)–conjugated Gp96. The binding of Gp96-FITC was competed by lipopolysaccharide (LPS) or fucoidan, a known inhibitor of scavenger receptors. Interestingly, the binding of LPS-FITC was also competed not only by fucoidan, but by Gp96, suggesting that LPS and Gp96 share a common receptor on PMNs. One important effector function of PMNs is the clearance of an inflammatory site by phagocytosis. We therefore assessed the influence of Gp96 on phagocytic activity using fluorochrome-labeled polystyrene beads. We found a marked enhancement of phagocytosis in the presence of Gp96 and concluded that PMNs not only bind Gp96, but are also activated by it. Additionally, Gp96-stimulated PMNs and especially monocytes release large amounts of interleukin-8, a potent neutrophil-attracting chemokine. In conclusion, we demonstrate that Gp96 specifically binds to and activates PMNs and monocytes, extending the function of Gp96 as a danger signal to additional members of the innate immune system.
Introduction
The heat shock protein (HSP) Gp96 is localized in the endoplasmic reticulum (ER) and has a variety of roles in mammalian organisms. As a chaperone, it is involved in protein folding to prevent aggregation of partially unfolded proteins in the ER.1Gp96 also associates with intracellular peptides representative of the cellular protein content and is able to shuttle these peptides into the major histocompatibility (MHC) class I presentation pathway.2-4
After severe tissue damage, necrotic cell death induced by freeze-thaw cycles,5 or virus infection,6 Gp96 is released into the extracellular space where it reveals its immunostimulatory effects: Gp96 preparations from tumor cells have been shown to elicit protective and therapeutic responses against the tumor from which the HSP had been purified.7,8 The specificity of this immune response is attributed to tumor-derived peptides associated with Gp96.7 After receptor-mediated endocytosis of Gp96 by professional antigen-presenting cells (APCs),9these peptides are presented on MHC class I molecules,2,3a process usually called cross-presentation10,11 and one which might represent a key event during the priming of naive T cells.12 In addition, Gp96 mediates APC activation that results in the up-regulation of costimulatory molecules and the release of the proinflammatory and TH1-promoting cytokines, tumor necrosis factor α (TNF-α), and interleukin-12 (IL-12).5,13 The release of the anti-inflammatory and TH2-promoting cytokine IL-10 is only slightly stimulated. This cytokine milieu is an important prerequisite for the triggering of a cytotoxic T-cell response. To mediate its effects, Gp96 interacts specifically with several receptors on the surface of APCs. Representation of HSP-associated peptides was shown to be mediated by CD91-dependent14 and -independent pathways.15Activation of APCs is dependent on the interaction of HSPs with CD3616 or Toll-like receptor 4 (TLR4).17 The relevance of the latter pathway is supported by the fact that Gp96 also interacts physically with TLR1, 2, and 4 inside the ER.18
Receptor-mediated interactions with Gp96 have been demonstrated for only professional APCs, including dendritic cells (DCs), macrophages, and B cells. In addition to these cells, we were recently able to show that activated platelets, in particular, also specifically bind Gp96 resulting in the reduced activation of DCs.19
However, additional cell types can be expected to make contact with Gp96. Necrotic cell death after virus infection or severe tissue damage is followed by inflammatory reactions resulting in the recruitment of cells from the blood. The majority of these cells are not professional APCs, such as DCs or macrophages, but polymorphonuclear neutrophils (PMNs) and monocytes.20 These cells will make contact with Gp96 because of the fact that necrotic cell death results in the release of HSPs.5,6 PMNs and monocytes play an important part in the first line of defense against microbial pathogens by contributing substantially to the innate host defense with their ability to rapidly extravasate into inflamed tissue and their use of potent effector mechanisms (ie, phagocytosis; production of reactive oxygen species; and release of mediators and antimicrobial substances).21-23
By taking the immunostimulatory capacity of Gp96 and the role of PMNs and monocytes as potent effector cells of innate host defense mechanisms into consideration, we assessed whether these cells may also be able to interact with Gp96. Here we present for the first time evidence that PMNs and monocytes are able to specifically bind Gp96 and respond to Gp96 contact by up-regulation of effector functions.
Materials and methods
Materials
Purified mouse Gp96, fluorescein isothiocyanate (FITC)–labeled Gp96, and flanking fractions of Gp96 preparations were provided by Immatics Biotechnologies (Tübingen, Germany). Gp96 lots had a purity higher than 95% by silver staining and were tested endotoxin free (< 0.1 endotoxin units (EU)/μg protein) with a limulus amebocyte lysate assay (LAL, QCL-1000; BioWhittaker, Verviers, Belgium). FITC-labeled and unlabeled bacterial lipopolysaccharide (LPS) from Salmonella typhimurium and phorbol-12-myristate-13-acetate (PMA) were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Purification of cells
PMNs and mononuclear cells (MNCs) were separated from heparinized blood of healthy volunteer donors by dextran sedimentation using Polymorphprep (Nycomed, Oslo, Norway) according to the manufacturer's instructions and as described previously.24 Contaminating red blood cells were removed by an in-house lysis buffer (150 mM ammonium chloride, 1 mM potassium bicarbonate, 0.1 mM ethylene diamine tetra acetate [all from Sigma-Aldrich] in distilled water, pH 7.3). Purity of the cell preparation was assessed by cytofluorometry with CD66b as marker for PMNs. Usually 95% to 98% of cells were CD66b+. For binding assays, peripheral blood leukocytes (PBLs) were used by pooling separated MNCs and PMNs in a 1:1 ratio.
Stimulation of cells
Purified PMNs or MNCs were cultivated in RPMI 1640 (PAN Biotech, Aidenbach, Germany) with 3% (vol/vol) heat-inactivated (30 minutes at 56°C) fetal bovine serum (PAN Biotech) and various stimuli as indicated. Cells were suspended in a concentration of 2 × 106 cells/mL and cultivated at 37°C with 7.5% CO2 in air.
Antibodies and stainings for flow cytometry
The following antibodies were used for analysis by fluorescence-activated cell sorter (FACS): FITC-labeled monoclonal antibodies (mAbs) against CD14 (BD Pharmingen, Hamburg, Germany) or CD66b (Coulter Immunotech, Hamburg, Germany); R-phycoerythrin (PE)–labeled mAbs against CD11b, CD91, CD36, and IL-8 (all purchased from BD Pharmingen). For FACS analysis, aliquots of 2 × 105 cells were incubated with labeled antibodies or protein in (FACS buffer phosphate-buffered saline [PBS] supplemented with 1% [wt/vol] bovine serum albumin and 0.05% sodium azide [all from Sigma-Aldrich]) on ice, then washed twice with FACS buffer, and finally resuspended in FACS buffer. For competition assays, fucoidan from Fucus vesiculosus(Sigma; contained < 0.1 EU/μg by LAL assay) or mAbs against the 85-kDa subunit of CD91 (immunoglobulin G1 [IgG1], clone 5A6; Progen, Heidelberg, Germany), CD36 (clone SMO; Sigma-Aldrich), CD62L (clone TC1; Coulter Immunotech), or isotype controls (IgG1 or IgG2b; BD Pharmingen) were added 10 minutes prior to labeled Gp96. For intracellular stainings, the Cytofix/Cytoperm kit (BD Pharmingen) was used according to the manufacturer's instructions.
All analyses were performed by FACScalibur and CellQuest (both from Becton Dickinson, Heidelberg, Germany) software.
Phagocytosis
For analysis of phagocytic activity, ingestion of PE-labeled polystyrene microspheres (diameter, 1 μm; Fluoresbrite Plain Microspheres PCRed, Polysciences, Warrington, PA) was evaluated as described elsewhere.25 Briefly, aliquots of 2 × 105 freshly purified PMNs were incubated in the presence of stimuli as indicated and 5 × 106 microbeads (E/T ratio, 1:25) for 45 to 120 minutes at 37°C, then kept on ice, washed twice in FACS buffer, and fixed in 1% paraformaldehyde in PBS. Analysis was performed by FACS.
Detection of IL-8 production
For analysis by enzyme-linked immunosorbent assay (ELISA), supernatants were derived from stimulation with either purified PMNs, PMNs with 5% MNCs added, or MNCs after 6 hours, and frozen at −20°C until required. Supernatants were analyzed by ELISA for IL-8 (OptEIA; BD Pharmingen) according to the manufacturer's instructions.
For intracellular cytokine stainings, PMNs or MNCs were incubated with indicated stimuli in the presence of brefeldin A (Sigma) or monensin (GolgiStop, BD Pharmingen), respectively, and subsequently labeled with FITC-conjugated anti-CD66b or CD14 and PE-labeled anti–IL-8 as described in “Antibodies and stainings for flow cytometry.”
Results
Interaction of Gp96-FITC with PMNs and monocytes
We first investigated whether PMNs and monocytes are able to bind Gp96 specifically. Freshly isolated PBLs were incubated with FITC-labeled Gp96 with or without excess of unlabeled Gp96 or an irrelevant protein. FACS analysis showed that both PMNs and monocytes are able to bind FITC-labeled Gp96 (Figure1). The specificity of Gp96-FITC binding was indicated by competition of binding in the presence of excess of unlabeled Gp96 (Figure 1A or C), but not of an irrelevant protein (Figure 1B or D). In addition, we found that the presence of unlabeled Gp96 or LPS as well as fucoidan, a known ligand for selectins or class A scavenger receptors,26 27 interfered with LPS-FITC binding on PMNs (Figure 1E). Binding of Gp96-FITC could be also competed by unlabeled LPS or fucoidan, but not by antibodies against CD36 or CD91 (data not shown), suggesting that other receptors are involved.
Binding of FITC-labeled Gp96 to PMNs and monocytes.
PBLs (2 × 105) were incubated with Gp96-FITC (50 μg/mL) and analyzed by FACS gating on PMNs (CD66b+) or monocytes (CD14+). Gp96-FITC binding was competed in the presence of 5 times excess of unlabeled Gp96 on PMNs (A) or monocytes (C), but not by IgG2b as irrelevant protein (PMNs [B] or monocytes [D]). (E) The summarized results of normalized LPS-FITC or Gp96-FITC binding to PMNs from 4 independent experiments are presented with standard deviation. LPS-FITC or Gp96-FITC binding was competed using fucoidan (1 mg/mL), unlabeled LPS (5-fold), or Gp96 (5-fold) (*P < .004; **P < .05, by Student t test).
Binding of FITC-labeled Gp96 to PMNs and monocytes.
PBLs (2 × 105) were incubated with Gp96-FITC (50 μg/mL) and analyzed by FACS gating on PMNs (CD66b+) or monocytes (CD14+). Gp96-FITC binding was competed in the presence of 5 times excess of unlabeled Gp96 on PMNs (A) or monocytes (C), but not by IgG2b as irrelevant protein (PMNs [B] or monocytes [D]). (E) The summarized results of normalized LPS-FITC or Gp96-FITC binding to PMNs from 4 independent experiments are presented with standard deviation. LPS-FITC or Gp96-FITC binding was competed using fucoidan (1 mg/mL), unlabeled LPS (5-fold), or Gp96 (5-fold) (*P < .004; **P < .05, by Student t test).
PMNs pretreated with phorbolester showed an increased binding capacity for Gp96-FITC or LPS-FITC compared with unstimulated PMNs (Figure2), indicating that the receptors for both, Gp96 or LPS, are up-regulated (n = 5; for Gp96-FITC at 50 μg/mL, P < .05; for LPS-FITC at 30 μg/mL,P < .001, by Student t test). Stimulating PMNs with phorbolester resulted in the enhanced surface expression of CD66b and CD11b concurrent with a complete loss of L-selectin (CD62L). CD14 expression on PMNs was not altered (data not shown). This suggests that neither L-selectin nor CD14 are the main receptors for Gp96 or LPS on PMNs.
Enhanced binding of Gp96-FITC or LPS-FITC by activated PMNs.
Unstimulated PMNs (○) or PMNs prestimulated with PMA (10 ng/mL) for 10 minutes at 37°C (●) were incubated with the indicated concentrations of gp96-FITC (A) or LPS-FITC (B) and analyzed by FACS. The increase in mean fluorescence was statistically significant for both Gp96-FITC (50 μg/mL) or LPS-FITC (30 μg/mL) binding in 5 independent experiments (n = 5; P < .05 orP < .001, respectively, by Student ttest).
Enhanced binding of Gp96-FITC or LPS-FITC by activated PMNs.
Unstimulated PMNs (○) or PMNs prestimulated with PMA (10 ng/mL) for 10 minutes at 37°C (●) were incubated with the indicated concentrations of gp96-FITC (A) or LPS-FITC (B) and analyzed by FACS. The increase in mean fluorescence was statistically significant for both Gp96-FITC (50 μg/mL) or LPS-FITC (30 μg/mL) binding in 5 independent experiments (n = 5; P < .05 orP < .001, respectively, by Student ttest).
Gp96 increases phagocytic activity of PMNs
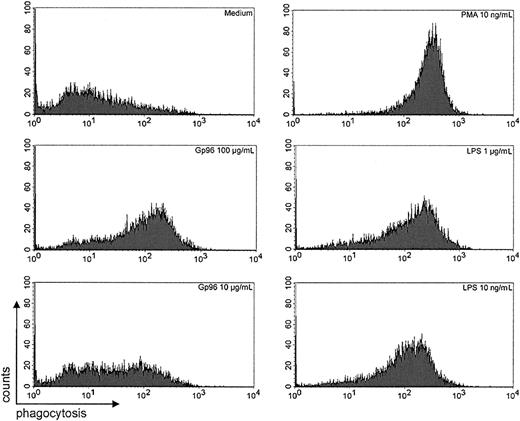
The innate defense mechanisms of PMNs depend on their ability to ingest and subsequently eliminate pathogens by oxidative and nonoxidative processes. Antibody and complement receptors, as well as bacterial products such as LPS, mediate activation of human PMNs.22 To assess the influence of Gp96 on phagocytic activity of PMNs, we used a model of unopsonized fluorochrome-labeled polystyrene beads as targets, and phagocytosis was quantitated by FACS analysis.25 PMNs showed a low spontaneous phagocytosis of polystyrene beads in the absence of exogenous stimuli, but in the presence of Gp96 as well as LPS or phorbolester there was a marked enhancement of phagocytic activity (Figure3). Even at low concentrations of Gp96 (10 μg/mL), there was an increase of phagocytosis detectable (n = 4; P < .05 by Student t test), suggesting that Gp96 is able to activate PMNs.
Phagocytosis of fluorochrome-labeled polystyrene beads by PMNs.
Purified PMNs (2 × 105) were incubated with the indicated stimuli in the presence of 5 × 106fluorochrome-labeled microspheres for 45 minutes at 37°C. Cells were washed and fixed in 1% paraformaldehyde. Fluorescence intensity was evaluated by FACS. The results shown are representative of 4 independent experiments with different donors. Statistical analysis of all experiments showed a significant increase in mean fluorescence with PMA, LPS, or Gp96 compared with the medium control (n = 4;P < .05 by Student t test).
Phagocytosis of fluorochrome-labeled polystyrene beads by PMNs.
Purified PMNs (2 × 105) were incubated with the indicated stimuli in the presence of 5 × 106fluorochrome-labeled microspheres for 45 minutes at 37°C. Cells were washed and fixed in 1% paraformaldehyde. Fluorescence intensity was evaluated by FACS. The results shown are representative of 4 independent experiments with different donors. Statistical analysis of all experiments showed a significant increase in mean fluorescence with PMA, LPS, or Gp96 compared with the medium control (n = 4;P < .05 by Student t test).
Production of IL-8 by PMNs and monocytes
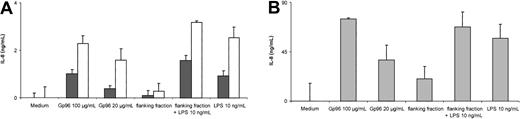
IL-8 is an important PMN-attracting chemokine released by a variety of cells, including PMNs and monocytes upon activation.21 22 In order to assess the production of IL-8 in PMNs and monocytes, supernatants of purified PMNs or mononuclear cells were analyzed by ELISA. We observed that high amounts of IL-8 are present after incubating these cells with Gp96 or LPS as control (Figure 4). To confirm that the cells were activated by Gp96 and not by potential endotoxin contaminations, flanking fractions of the same Gp96 purification were analyzed in parallel. No significant increase in IL-8 release was detected. To exclude an inhibitory effect of the flanking fractions themselves due to differences in the salt concentration, the fractions were spiked with LPS (10 ng/mL). PMNs and monocytes were activated to levels comparable with LPS in normal medium. These controls demonstrate that Gp96, and not potential endotoxin contaminations, is responsible for the observed release of IL-8.
Analysis of IL-8 in supernatant of PMNs or MNCs by ELISA.
Cells (4 × 105) were incubated in the presence of LPS, Gp96, or flanking fractions of the Gp96 purification scheme. Culture supernatants were harvested after 6 hours at 37°C with 7.5% CO2 and assayed for IL-8 by ELISA. To evaluate the contribution of cells other than PMNs to the release of IL-8, supernatants from PMNs (▪), PMNs with 5% MNCs added (■) (A) or MNCs only (B) were analyzed. All samples were taken in duplicates. The results shown are representative of 3 independent experiments. Statistical analysis of all 3 experiments indicated a significant increase in the release of IL-8 in the presence of Gp96 (at 100 μg/mL or 20 μg/mL for PMNs, P < .001; for PMNs+MNC or MNCs alone, P < .05) or LPS (at 10 ng/mL for PMNs,P = .0017; for PMNs+MNCs or MNCs, P < .05; flanking fraction+LPS, 10 ng/mL for PMNs, P < .001; for MNCs, P < .05), but not for flanking fraction alone (by Student t test).
Analysis of IL-8 in supernatant of PMNs or MNCs by ELISA.
Cells (4 × 105) were incubated in the presence of LPS, Gp96, or flanking fractions of the Gp96 purification scheme. Culture supernatants were harvested after 6 hours at 37°C with 7.5% CO2 and assayed for IL-8 by ELISA. To evaluate the contribution of cells other than PMNs to the release of IL-8, supernatants from PMNs (▪), PMNs with 5% MNCs added (■) (A) or MNCs only (B) were analyzed. All samples were taken in duplicates. The results shown are representative of 3 independent experiments. Statistical analysis of all 3 experiments indicated a significant increase in the release of IL-8 in the presence of Gp96 (at 100 μg/mL or 20 μg/mL for PMNs, P < .001; for PMNs+MNC or MNCs alone, P < .05) or LPS (at 10 ng/mL for PMNs,P = .0017; for PMNs+MNCs or MNCs, P < .05; flanking fraction+LPS, 10 ng/mL for PMNs, P < .001; for MNCs, P < .05), but not for flanking fraction alone (by Student t test).
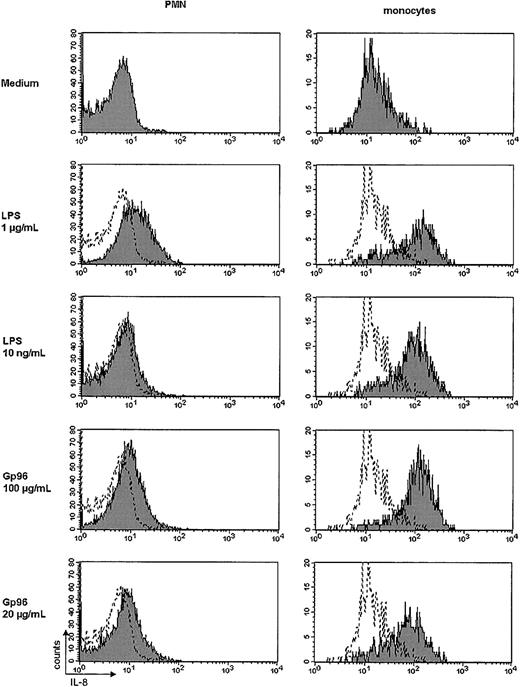
Because the preparation of PMNs usually renders a purity of approximately 95% to 98%, it could not be excluded that the production of IL-8 was exclusively due to the presence of monocytes. Therefore, we added 5% MNCs to the PMN preparation, resulting in an increase of monocytes (CD14+ cells) from 0.9% to 1.2% by FACS analysis (for comparison, the MNC preparation contained 23% CD14+ cells; not shown). Increasing the number of monocytes resulted in a 2- to 3-fold increase of the released amount of IL-8. To demonstrate that both PMNs and monocytes contribute to the release of IL-8, we investigated the production of IL-8 by intracellular staining and FACS anaylsis. Here we found a remarkable increase of intracellular IL-8 in monocytes after stimulation with Gp96 (Figure5, right panel), but not in T or B cells using CD3 and CD19 as markers, respectively (not shown). PMNs also showed a slight increase in IL-8 (Figure 5, left panel), but to a lesser extent. These experiments demonstrate that monocytes as well as PMNs contribute to the production of IL-8 after stimulation with Gp96.
Intracellular IL-8 staining in PMNs and monocytes.
Purified PMNs or MNCs (2 × 105) were incubated for 4 hours at 37°C with 7.5% CO2 with the indicated stimuli in the presence of brefeldin A (1 μg/mL) or monensin (GolgiStop, 2 μM). Cells were washed and stained as described in “Materials and methods.” FITC-labeled mAbs against CD66b or CD14 were used to identify PMNs or monocytes, respectively, and a PE-labeled mAb against IL-8 was used to evaluate the amount of IL-8 produced by the cells. The shaded histograms represent the fluorescence of stimulated cells; the white histograms, the respective medium control. The results shown are representative of 3 independent experiments. The increase in mean fluorescence of Gp96 or LPS-stimulated monocytes was statistically significant compared with the medium control (P < .05 by Student ttest).
Intracellular IL-8 staining in PMNs and monocytes.
Purified PMNs or MNCs (2 × 105) were incubated for 4 hours at 37°C with 7.5% CO2 with the indicated stimuli in the presence of brefeldin A (1 μg/mL) or monensin (GolgiStop, 2 μM). Cells were washed and stained as described in “Materials and methods.” FITC-labeled mAbs against CD66b or CD14 were used to identify PMNs or monocytes, respectively, and a PE-labeled mAb against IL-8 was used to evaluate the amount of IL-8 produced by the cells. The shaded histograms represent the fluorescence of stimulated cells; the white histograms, the respective medium control. The results shown are representative of 3 independent experiments. The increase in mean fluorescence of Gp96 or LPS-stimulated monocytes was statistically significant compared with the medium control (P < .05 by Student ttest).
Discussion
The HSP Gp96 is normally localized in the ER of cells and released into the extracellular space during necrotic cell death,5(ie, after cell damage due to viral infection6 or excessive trauma [S. Herter et al, manuscript in preparation]). Extracellular Gp96 activates dendritic cells and macrophages by releasing inflammatory cytokines and inducing DC maturation.5,13 This feature, combined with the ability to transfer antigenic peptides for their MHC class I–restricted presentation,2 3 allows Gp96, together with other HSPs such as HSP70 and HSP90, to function as an efficient messaging system alerting the organism to bacterial or viral infection and possibly injury.
Given the fact that during the early phase of inflammation, in particular, the majority of cells recruited to an inflammatory focus are mainly PMNs and monocytes,20 it can be assumed that these cells will encounter contact with Gp96 early on in inflammation. Therefore, we investigated whether PMNs and monocytes, in addition to macrophages and DCs, are able to interact with Gp96. We found that both cell types bind FITC-labeled Gp96. The binding of labeled Gp96 could be competed with excess of unlabeled Gp96, but not in the presence of the same amount of an irrelevant protein (Figure 1), suggesting that Gp96 is able to specifically interact with monocytes as well as PMNs.
The α2-macroglobulin receptor (CD91) and the class B scavenger receptor (CD36) have been described as receptors for Gp96 on DCs, macrophages, and also on platelets.14-16,19 Both of these receptors are expressed by monocytes, suggesting that interaction with Gp96 on monocytes is also mediated by these receptors. Interestingly, PMNs do not express CD3628 or CD91 (data not shown). Considering these differences and the comparable binding capacity for Gp96 on PMNs and monocytes, it seems likely that a major receptor for Gp96 binding by PMNs has not yet been identified. The increased binding of Gp96-FITC on activated PMNs (Figure 2A) suggests that the receptor responsible is up-regulated upon stimulation. Moreover, our results showing an increased binding of LPS (Figure 2B), and the ability of unlabeled Gp96 to interfere with the binding of FITC-labeled LPS and vice versa (Figure 1E), indicate that LPS and Gp96 may share a binding receptor on PMNs. This notion is also supported by our results showing that fucoidan, a homopolymer of sulfated fucose and a known ligand for scavenger receptors or selectins,26,27 was able to compete with the binding of LPS-FITC or Gp96-FITC. Because the expression of CD14 is not altered on PMA-activated PMNs and L-selectin is completely lost (not shown), these 2 known receptors for LPS are not likely to be the main receptor for Gp96 on PMNs. In addition, the expression of TLR2 as well as the TLR2-mediated secretion of IL-8 in PMNs are enhanced by granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas this is not the case for TLR4.29 This proves that signaling via TLR2 is relevant for PMNs and can be increased upon stimulation. Furthermore, Haziot et al show an enhanced responsiveness of PMNs to LPS in CD14 knock-out mice or TLR4 knock-out mice, suggesting that other receptors are important in PMNs.30In inflammatory responses induced by necrotic cells, TLR2 signaling is essential for the induction of MIP-2 and KC, a mouse homologue of GRO-α.31 The release of Gp96 after necrotic cell death5 and our results pointing to a common receptor for Gp96 and LPS indicate that TLR2 might be a binding receptor for Gp96. However, additional studies are necessary to investigate this hypothesis.
In addition to the binding of Gp96 to PMNs and monocytes, we also examined whether Gp96 can mediate activation of these cells. PMNs play a crucial part in innate host defense with their ability to accumulate rapidly and to clear an inflammatory site by using their potent oxidative and nonoxidative effector mechanisms. One important function is the ingestion of particles (ie, bacteria and fungi) by phagocytosis.20 Therefore, we assessed the phagocytic activity of PMNs and found that the presence of Gp96 greatly enhances the phagocytic activity of these cells, even at low concentrations of Gp96 (Figure 3). Thus, Gp96 may represent an additional signal for PMNs to clear an inflammatory site.
IL-8 is one of the first chemokines to be described; it was originally identified as a strong chemoattracting agent for PMNs produced by LPS-stimulated monocytes.32 In addition to its chemotactic effects, IL-8 also mediates activation for PMNs directly by enhancing degranulation.22 There is broad evidence to demonstrate the importance of IL-8 in leukocyte trafficking homeostasis and also in disease.33 We were able to show that both PMNs and monocytes produce IL-8 upon activation by Gp96 (Figure 4). Despite similar binding capacity for Gp96 of both cell types (Figure 1) and the ability to produce IL-8 after stimulation with Gp96 (Figure 5), there is a marked difference in the amount of IL-8 produced by PMNs or monocytes (Figure 4). We propose that Gp96 induces monocytes to attract PMNs to an inflammatory site via IL-8 secretion and that PMNs clear the site by enhanced phagocytic activity. Whether the phagocytic activity is mediated by Gp96 directly via receptor-ligand interactions on PMNs or indirectly by activation via IL-8 is not yet clear and will be the subject of further studies.
In conclusion, Gp96 binds specifically to both PMNs and monocytes and mediates the release of large amounts of IL-8 by both cell types. Furthermore, we show that in addition to the production of IL-8, Gp96 also stimulates the phagocytic activity of PMNs, possibly to clear a site of cellular debris or microbial particles. Altogether, this work supports the notion that Gp96 acts as a danger signal to the innate immune system, now including also monocytes and PMNs as cell types responsive to Gp96. However, there are probably differences in the receptors involved in Gp96-mediated activation of monocytes and PMNs, the latter expressing neither CD36 nor CD91. Further studies will be necessary to elucidate the receptors and signaling pathways for Gp96-mediated interactions.
We thank Dr Birgitt Schönfisch from the Department of Biomathematics, University of Tübingen (Germany) for the support concerning the statistical analyses of the data.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-07-2261.
Supported by grants from the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 510, C1 to H.S.), the European Union (EC Project QLK3-CT-1999-00064), and the University of Tübingen (AKF- and IZKF-Programm).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hansjoerg Schild, Institute for Cell Biology, Department Immunology, Auf der Morgenstelle 15, D-72076 Tübingen, Germany; e-mail:hansjoerg.schild@uni-tuebingen.de.

![Fig. 1. Binding of FITC-labeled Gp96 to PMNs and monocytes. / PBLs (2 × 105) were incubated with Gp96-FITC (50 μg/mL) and analyzed by FACS gating on PMNs (CD66b+) or monocytes (CD14+). Gp96-FITC binding was competed in the presence of 5 times excess of unlabeled Gp96 on PMNs (A) or monocytes (C), but not by IgG2b as irrelevant protein (PMNs [B] or monocytes [D]). (E) The summarized results of normalized LPS-FITC or Gp96-FITC binding to PMNs from 4 independent experiments are presented with standard deviation. LPS-FITC or Gp96-FITC binding was competed using fucoidan (1 mg/mL), unlabeled LPS (5-fold), or Gp96 (5-fold) (*P < .004; **P < .05, by Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-07-2261/3/m_h80734063001.jpeg?Expires=1765935711&Signature=zxRaa1p27GXh6R~2vRGg0qogUOrvUMTF42wsjMNwx5XEhzBVEJfd-4adqRWpWXTTYQ~HyhNwAC2x7gLsQ~yUrvLqV-wAFCAN6ECEsWF6L41KlXCAmBwe-VjFwXyZTkPAWlnSIl~pbPKfrjVru3sitF9o1EHyrIbTsawgFEDjwo-1YBZ8Oxc8bHgFnDqh2ajVNUyJFS~8TIsLmIF9wIR-0vF1gNuKJMJWE4l6s4zgBvsqewQYoT~oMcxeHTxZmg2G3CHVeFTS-tkY30F28WtA8OCmdqz94VErA0Mf-rBxvOl~xkUFkpSWyZ5g9vxEKKQaHLW0UY-TQc4u4eoWUigDwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal