We recently developed a murine protocol for the induction of allogeneic mixed chimerism and tolerance employing nonmyeloablative total body irradiation (TBI), standard-dose bone marrow transplantation (BMT), and costimulation blockade (cobl) with an anti-CD154 monoclonal antibody (mAb) plus CTLA4Ig. We now evaluated whether a short course (1 month) of immunosuppressive drugs, which would be ethically required in the clinical setting of organ transplantation to prevent graft loss in case tolerance is not achieved, interferes with tolerance induced with this regimen. Our results show that calcineurin inhibitors (cyclosporin A [CyA] or tacrolimus [FK]) inhibit development of long-term chimerism and abrogate tolerance induction in this model. Rapamycin (rapa), methylprednisolone (MP), FTY720, and mycophenolate mofetil (MMF), in contrast, have no negative effect on chimerism or tolerance development. Peripheral deletion of donor-reactive T cells, which usually occurs in the weeks following BMT in this model, is blocked by CyA and FK, but not by the other drugs tested. Furthermore, we found that the additional use of compatible immunosuppressive drugs (rapa plus MMF plus MP) allows the dose of TBI to be reduced, so that mixed chimerism and donor skin-graft acceptance can be achieved with 1 Gy using clinically feasible cell numbers. Thus, this protocol of BMT with costimulation blockade can be safely combined with a clinically tested immunosuppressive regimen to permit success with a lower dose of irradiation. These results should facilitate clinical application of this tolerance strategy.
Introduction
Despite substantial progress in the field of organ transplantation, long-term outcome is currently limited by several factors, such as chronic graft rejection and the substantial side effects of lifelong immunosuppression. A possible solution to these problems is the induction of donor-specific tolerance, which can be reliably achieved through the establishment of mixed chimerism.1 The toxicity of previous bone marrow transplantation (BMT) protocols for the induction of mixed chimerism is a major factor hampering clinical application of this approach.2-7 Recently developed murine protocols using costimulation blockade (cobl) as part of BMT protocols are much milder and thus hold promise to become applicable as tolerance strategies in clinical organ transplantation.8-17 To be acceptable in the clinical setting, however, a tolerance regimen should be compatible with the transient use of standard immunosuppressive therapy, which would serve as a “safety net” preventing loss of the organ graft in case tolerance is not achieved in a particular patient.18The immunosuppressive drugs could be withdrawn several weeks after transplantation, once evidence for successful tolerance induction is obtained. Therefore, we tested whether the short-term application of commonly used immunosuppressive agents (cyclosporin A [CyA], tacrolimus [FK], rapamycin [rapa], methylprednisolone [MP], FTY720, and mycophenolate mofetil [MMF]) interferes with our recently developed murine tolerance protocol using nonmyeloablative total body irradiation (TBI; 3 Gy), fully allogeneic BMT, and cobl with anti-CD154 monoclonal antibody (mAb) plus CTLA4Ig.8
Various immunosuppressive drugs have been shown to interfere with tolerance development in models using costimulation blocking antibodies but not involving BMT and mixed chimerism.19 CyA abrogated tolerance induction in murine skin and heart graft models,20,21 whereas rapa was found to act synergistically with cobl.21,22 In a nonhuman primate renal allograft model employing anti-CD154 mAb, the administration of FK or the chronic use of steroids plus MMF was found to be detrimental, whereas a short-term course of steroids followed by long-term MMF treatment had no demonstrable negative effect.23 In another monkey model using anti-B7 mAbs, renal allograft survival was not inhibited by chronic steroid treatment and was even promoted by CyA administration.24 In a rat model, FTY720 given at high doses prolonged cardiac allograft survival in recipients that were transfected with CTLA4Ig.25
Tolerance induction through BMT with cobl relies at least in part on mechanisms other than those that lead to graft prolongation through cobl without BMT.19 Results from models without BMT are therefore not readily transferable to models involving BMT. Little is presently known, however, about the effects of immunosuppressive drugs on tolerance induction after BMT with cobl.
Immunosuppression can also influence allogeneic bone marrow (BM) engraftment, irrespective of possible effects on the action of cobl. In a murine BMT model not including cobl, treatment of recipients with CyA before BMT promoted chimerism and tolerance development, but this effect was abolished when a steroid bolus was given in addition to CyA.26 Short-term treatment with CyA after BMT is critical for the success of a monkey protocol inducing (transient) mixed chimerism and durable tolerance toward renal allografts.27CyA is routinely used in the prophylaxis of clinical graft-versus-host disease (GVHD), and MMF and FK are also increasingly used in this setting. In a murine haplotype-mismatched BMT model, FTY720 markedly inhibited the development of GVHD (Y.-M. Kim, T. Sachs, W. Asavaroengchai, R. Bronson, M.S., manuscript submitted, 2002). Of note, FTY720 shortened allograft survival in an intestinal transplant but not in a heart transplant model when used in combination with donor-specific blood transfusion.28 Storb et al have shown that posttransplantation immunosuppression using CyA and MMF permits reduction of the dose of TBI to 2 Gy in a major histocompatibility complex (MHC)–matched canine BMT model29 and that the addition of CTLA4Ig to this protocol allowed a further reduction to 1 Gy.30 When FTY720 was given instead of CTLA4Ig before transplantation in this dog model, it did not promote engraftment.31
In an attempt to increase the clinical applicability of our regimen, we therefore also examined whether the addition of a combination of compatible immunosuppressive drugs to our protocol would allow a further reduction of the dose of irradiation.
Materials and methods
Animals
Mice were purchased from the Institute for Laboratory Animals of the University of Vienna (Himberg, Austria) and were kept under specific pathogen-free conditions. All experiments were approved by the local review board of the University of Vienna and were performed in accordance with national and international guidelines of laboratory animal care.
BMT protocol
Hosts received a nonmyeloablative dose (1-3 Gy, as indicated; 0.9 Gy/min) of TBI 20 to 24 hours before BMT (day −1). Age-matched (6- to 12-week-old) female C57BL/6 (B6: H-2b) mice were injected intravenously with a dose of 15 to 20 × 106 unseparated bone marrow cells (BMCs) harvested from the tibiae, femurs, and humeri of fully MHC- and minor transplantation antigen–mismatched Balb/c (H-2d) donors (6 to 12 weeks old). Cells were diluted in cold BM media (500 mL Medium 199 [Sigma, Vienna, Austria], supplemented with 5 mL HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] buffer [ICN, Biomedica, Vienna, Austria], 5 mg DNAse [Sigma], and 2 mg gentamycin [Sigma]) and were injected in a volume of 1 mL into the tail vein of recipient mice (day 0). In addition, recipients were treated with a hamster anti-mouse CD154 mAb (MR1; 1-2 mg injected intraperitoneally on day 0) and with murine CTLA4Ig or human CTLA4Ig (0.5 mg injected intraperitoneally on day 2). MR1 was purchased from Bioexpress (West Lebanon, NH) or produced from an MR1 hybridoma kindly provided by Dr Randolph J. Noelle (Dartmouth Medical School, Lebanon, NH). Human CTLA4Ig was generously provided by Bristol-Myers, Squibb Pharmaceuticals (Princeton, NJ), and murine CTLA4Ig was produced from a transfected cell line kindly provided by Dr Terry Strom (Beth Israel/Deaconess Hospital, Boston, MA).
Flowcytometric analysis (FCM)
Two-color FCM was used to distinguish donor and host cells of particular lineages by staining with fluorescein isothiocyanate–conjugated antibodies against CD4, CD8, B220, MAC1, and biotinylated H-2Dd (34-2-12, developed with phycoerythrin streptavidin) and isotype controls. To analyze the expression of Vβ subunits, staining was performed with fluorescein isothiocyanate–conjugated antibodies against Vβ8.1/2, Vβ11, and Vβ5.1/2 (or isotype control) and phycoerythrin-conjugated antibodies against CD4 and CD8 (all antibodies from Pharmingen, San Diego, CA). Propidium iodide staining was used to exclude dead cells. Mice were considered chimeric if they showed at least 2% donor cells within the myeloid lineage and at least one lymphoid lineage. An Epics XL-MCL flow cytometer (Beckman Coulter, Fullerton, CA) was used for acquisition and EXPO32 ADC Software (Applied Cytometry Systems, Sheffield, United Kingdom) was used for analysis of flow-cytometric data.
Skin grafting
Full-thickness tail skin from Balb/c mice (donor specific) and fully mismatched C3H/HeHanHim mice (C3H: H-2k; third-party) was grafted 4 to 10 weeks after BMT. Grafts were considered rejected when less than 10% of the graft remained viable.
Immunosuppression
In the indicated groups, mice were injected with immunosuppressive drugs from day 0 to day 28. Drugs were used at the following doses: CyA, 20 mg/kg/d; FK, 2 mg/kg/d; rapa, 0.2 mg/kg/d (or 3 mg/kg/d in one experiment, as indicated); MP, 20 mg/kg/d for the first 10 days, 10 mg/kg/d for the remaining 19 days; FTY720, 0.5 mg/kg/d; MMF, 20 mg/kg/d. Rapa was diluted in sterilized medium consisting of 500 mL deionized water containing 1 g sodiumcarboxymethylcellulose (Sigma-Aldrich, Vienna, Austria) and 1.25 g Tween 80 (Sigma), using an ultrasound bath. CyA (100 mg) was diluted in a medium consisting of 1.3 g polyethoxylated castor oil (Cremophor EL; Fluka, Vienna, Austria) made to a volume of 2 mL with ethanol 94%. Before subcutaneous injection, it was diluted with 0.9% saline every other day. MP was dissolved in 1 mL distilled water and diluted with 0.9% saline every third day. FTY720 was diluted with distilled water. MMF was dissolved in 5% dextrose every day. FK was diluted in 0.9% saline. Drugs were injected in a volume of 0.25 mL. All drugs were injected intraperitoneally except CyA, which was administered subcutaneously. FTY720 and CyA were kindly provided by Novartis Pharma (Basel, Switzerland), FK by Fujisawa (Munich, Germany), rapa by Wyeth-Ayerst (Saint Davids, PA), and MMF by Roche (Vienna, Austria).
Mixed lymphocyte reaction (MLR)
Spleen cells were washed with phosphate-buffered saline (PBS) and resuspended in MLR medium (42.5 mL RPMI 1640 [Bio-Whittaker, Verviers, Belgium], 7.5 mL CPSR-2 [Sigma], 0.5 mL HEPES buffer [ICN, Biomedica], 1.55 mL nutrient mixture [10 mL Aqua destillata, 5 mL nonessential amino acids, 0.055 g sodium pyruvate, 0.146 g l-glutamine, 5000 U penicillin, 5000 μg streptomycin], and 100 μL 2-mercaptoethanol [dilution 1:3000]). Responder cells (4 × 105) were incubated at 37°C in 5% CO2 with 4 × 105 irradiated (30 Gy) stimulator cells of Balb/c, C3H, or B6 mice or with medium. After 3 or 4 days, cells were pulsed with 3H-thymidine and incubated for 12 to 18 hours. After being harvested on filter plates, they were analyzed with a Microbeta 1450 beta counter (PerkinElmer, Wellesley, MA). Stimulation indices (SIs) were calculated by dividing the mean counts per minute (cpm) from responses against host (B6), donor (Balb/c), or third-party (C3H) stimulator cells by mean background cpm (ie, cpm with no stimulator population).
Histology
At the time the mice were killed, tissue samples from spleen, colon, liver, and skin were fixed in formalin and embedded in paraffin. Sections (4 μm) were stained with hematoxylin/eosin and reviewed as coded samples for signs of GVHD by a transplant pathologist.
GVHD observations
Mice were weighed at least every other week and were screened frequently for weight loss, diarrhea, hair loss, skin changes, and hunched posture.
Statistical analysis
A 2-tailed Student t test for comparison of means with unequal variances was used for comparing Vβ deletion between groups; the χ2 test was used for comparing rates of chimerism and skin-graft acceptance between groups.P < .05 was considered statistically significant. Skin-graft survival was calculated according to the Kaplan-Meier product limit method.
Results
Effects of immunosuppressive drugs on the development of long-term chimerism
B6 mice underwent 3 Gy TBI, were injected with 15 to 20 × 106 fully mismatched, unseparated Balb/c BMCs approximately 24 hours later, and were then treated with costimulation blocking reagents (anti-CD154 [CD40L] mAb immediately after BM injection and CTLA4Ig 2 days later). Six groups received, in addition, one of the tested immunosuppressive drugs for 4 weeks following BMT, to enable us to study whether these drugs affect chimerism, deletion, or tolerance induction.
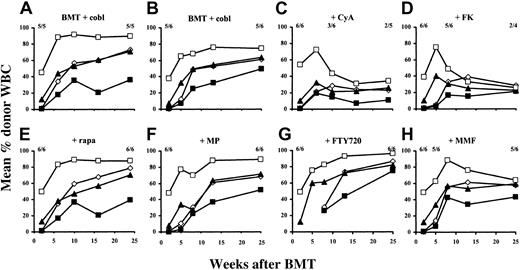
Two groups received our previously published regimen8consisting of TBI, BMC, and cobl without immunosuppressive drugs (termed “standard protocol” in this paper). In these 2 groups, 5 of 5 mice and 5 of 6 mice developed high levels of multilineage chimerism, similar to our previous experience8 (mean 67% donor CD4+ cells, 43% donor CD8+ cells, 67% donor B cells, and 82% donor myeloid cells approximately 6 months after BMT [combined results from 2 experiments]). Chimerism levels were stable for the duration of the observation period (Figure 1A-B), suggesting successful engraftment of donor stem cells.
Initial chimerism was established in all mice treated additionally with CyA (n = 6), but levels began to decline after week 6, and half of the mice had lost chimerism by week 10. At the end of follow-up, only 2 of the remaining 5 mice still alive were chimeric (Figure 1C; for the purpose of this study we defined multilineage chimerism as at least 2% donor reconstitution in the myeloid lineage and at least one lymphoid lineage). Similarly, early donor chimerism was induced in all recipients treated with FK (n = 6), but chimerism declined sharply in 4 of 6 mice from week 5 on. Two of 4 remaining mice still alive were still chimeric at the end of follow-up, but one of them had only low levels of donor reconstitution (12% in CD4+ cells, 3% in CD8+cells, 5% in B cells, and 2% in myeloid cells). One other mouse in this group showed chimerism only in the lymphoid lineages, but no myeloid chimerism (Figure 1D).
In contrast to rapa, MP, FTY720, or MMF, CyA and FK inhibit development of long-term chimerism after BMT with cobl.
Results from 2 separate experiments are shown. All B6 mice (n = 5 or 6 per group) received 3 Gy TBI (day −1), 15 to 20 × 106Balb/c BMCs (day 0), anti-CD154 mAb (day 0), and CTLA4Ig (day 2). The percentages of donor-derived CD4+ cells (⋄), CD8+ cells (▪), B cells (▴), and monocytes/granulocytes (■) were evaluated by FCM. The fractions on each panel indicate the fraction of analyzed mice showing chimerism at the time point below. Standard-protocol groups (A, n = 5; B, n = 6) did not receive any additional treatment. Other groups received additionally CyA (C), FK (D), rapa (E), MP (F), FTY720 (G), or MMF (H). Each immunosuppressive drug was given for the first 4 weeks after BMT.
In contrast to rapa, MP, FTY720, or MMF, CyA and FK inhibit development of long-term chimerism after BMT with cobl.
Results from 2 separate experiments are shown. All B6 mice (n = 5 or 6 per group) received 3 Gy TBI (day −1), 15 to 20 × 106Balb/c BMCs (day 0), anti-CD154 mAb (day 0), and CTLA4Ig (day 2). The percentages of donor-derived CD4+ cells (⋄), CD8+ cells (▪), B cells (▴), and monocytes/granulocytes (■) were evaluated by FCM. The fractions on each panel indicate the fraction of analyzed mice showing chimerism at the time point below. Standard-protocol groups (A, n = 5; B, n = 6) did not receive any additional treatment. Other groups received additionally CyA (C), FK (D), rapa (E), MP (F), FTY720 (G), or MMF (H). Each immunosuppressive drug was given for the first 4 weeks after BMT.
In contrast, all mice that received additional rapa (n = 6) developed and maintained levels of multilineage chimerism comparable to those in the standard-protocol groups (Figure 1E). Likewise, when MP or FTY720 was given (n = 6 in each group), donor repopulation was also not compromised, as evidenced by the development and maintenance of substantial chimerism in all mice (Figure 1F-G). One of 6 mice treated with MMF lost its chimerism very early, but in the other 5 animals donor reconstitution persisted (Figure 1H).
Rapa, MP, FTY720, MMF, and FK were also examined in small control groups (n = 3 in each group) of mice injected only with BM, but without cobl, for their potential to substitute for cobl. Except for FK-treated mice, which showed transient mixed chimerism (in 2 of 3 cases) that vanished by week 8, no chimerism was detectable in any group (data not shown).
These results were confirmed in separate repeat experiments (n = 5-8 per group), in which chimerism in rapa-, MP-, FTY720- or MMF-treated mice again followed a course comparable to that of standard-protocol groups, whereas CyA and FK again inhibited the development of long-term chimerism (data not shown). Taken together, these results show that calcineurin inhibitors led to a significantly lower rate of long-term chimerism (P < .05 for the calcineurin inhibitor groups vs the standard-protocol groups), whereas rapa, MP, FTY720, and MMF did not significantly influence long-term chimerism development (P > .05 for rapa, MP, FTY720, or MMF vs the standard-protocol groups).
At the end of follow-up, chimerism was also evaluated in BM, spleen, and thymus. Chimerism levels in BM and spleen correlated with levels measured in peripheral blood. In groups treated with rapa, MP, FTY720, or MMF, 6% to 14% of thymocytes were donor MHC class I–positive (overall donor representation among thymocytes is probably higher, as only relatively mature thymocytes express MHC class I and are thus detected by the antibody used; data not shown).
These data indicate that CyA and FK allowed chimerism establishment early after BMT with cobl, but inhibited the development of long-term chimerism, whereas rapa, MP, FTY720, and MMF did not interfere negatively with chimerism induction in this protocol. As expected, immunosuppressive drugs could not be substituted for the use of cobl.
Effects of immunosuppressive drugs on tolerance induction
Skin-graft survival.
To evaluate whether the immunosuppressive drugs had an effect on tolerance induction, donor and third-party (C3H) skin was transplanted 4 to 10 weeks after BMT. Three of 5 mice from the standard-protocol group in the first experiment and all mice treated with rapa (6 of 6) accepted their donor grafts for more than 130 days. In contrast, only 1 of the 6 mice treated with CyA accepted its graft permanently (> 130 days; Figure 2A). In the second experiment (Figure 2B), the majority of mice (5 of 6) receiving the standard protocol accepted their donor grafts for more than 160 days. Four of 6 mice in the group treated with MP were tolerant, with 1 very late graft loss (day 158) among the 2 that rejected the grafts. Four of 6 mice treated with FTY720 and 4 of 6 mice receiving MMF accepted the donor skin for more than 160 days. Strikingly, all FK-treated mice (n = 6) rejected the donor grafts within 18 days. All mice in all groups rejected third-party grafts within 17 days, indicating that tolerance was donor specific and suggesting immunocompetence.
In contrast to rapa, MP, FTY720, or MMF, CyA and FK inhibit donor skin-graft survival after BMT with cobl.
Results from 2 experiments are shown. Each group consisted of 5 or 6 mice. (A) Skin-graft survival is depicted for third-party grafts (●, all groups), donor skin grafts for standard-protocol group (♦), additionally given rapa (▴) and CyA (⋄). (B) Skin-graft survival is shown for third-party grafts (●, all groups), for donor skin grafts for the standard-protocol group (♦) and for groups that were treated additionally with MP (▵), FTY720 (▪), MMF (■), or FK (⋄).
In contrast to rapa, MP, FTY720, or MMF, CyA and FK inhibit donor skin-graft survival after BMT with cobl.
Results from 2 experiments are shown. Each group consisted of 5 or 6 mice. (A) Skin-graft survival is depicted for third-party grafts (●, all groups), donor skin grafts for standard-protocol group (♦), additionally given rapa (▴) and CyA (⋄). (B) Skin-graft survival is shown for third-party grafts (●, all groups), for donor skin grafts for the standard-protocol group (♦) and for groups that were treated additionally with MP (▵), FTY720 (▪), MMF (■), or FK (⋄).
Skin grafting produced similar results in the separate repeat experiments (data not shown). Seven of 8 CyA-treated and 4 of 4 FK-treated mice rejected their donor grafts. Four of 5 mice receiving the standard protocol, 7 of 7 treated with rapa, 4 of 6 treated with MP, 5 of 6 treated with FTY720, and 5 of 6 treated with MMF accepted their donor grafts long-term. Taken together, the results of these experiments demonstrate that the calcineurin inhibitors significantly inhibited the development of skin-graft tolerance (P < .001 for the calcineurin inhibitor groups vs the standard-protocol groups). Rapa led to an improved donor skin-graft survival rate (P < .05 for the rapa groups vs the standard-protocol groups), whereas MP, FTY720, and MMF did not significantly alter skin-graft survival (P > .05 for MP, FTY720, or MMF vs the standard-protocol groups).
Because calcineurin inhibitors are still the mainstay of most clinical immunosuppressive regimens, we evaluated whether delaying the initiation of CyA administration (CyA administration from day 7 to day 35) would allow its safe use in our protocol. Such delayed therapy with CyA is currently common clinical practice in some centers aiming to ensure sufficient kidney function before CyA therapy is begun after renal transplantation.32 Seven of 8 mice receiving delayed CyA demonstrated multilineage chimerism 6 weeks after BMT. However, 18 weeks after BMT, 3 of 7 mice remained chimeric, whereas 4 of 6 from the standard-protocol group demonstrated chimerism at this time point. All CyA-treated mice rejected donor skin (at 8, 12, 14, 16, 93, 143, 143, and 156 days after skin grafting; in the standard-protocol group in this experiment, 4 of 7 rejected; P < .05), suggesting that starting CyA treatment one week after BMT with cobl still negatively affected chimerism and tolerance induction.
In vitro tolerance.
To evaluate tolerance further, MLR assays were performed at the time the mice were killed, 28 to 40 weeks after BMT. As shown in Table1, in groups receiving the standard protocol, as well as in groups treated with additional rapa, MP, FTY720, or MMF, responsiveness toward donor antigens was lower than responsiveness toward third-party antigens. In contrast, mice treated with additional CyA or FK showed a higher response toward donor stimulators than toward third-party stimulators, similar to naive B6 mice. The reduced responsiveness against the donor is reflected in a ratio of donor SI vs third-party SI lower than 1 for the standard-protocol and the rapa, MP, FTY720, and MMF groups, but a ratio higher than 1 for naive B6 mice and the CyA and FK groups.
In vitro evaluation of tolerance through mixed lymphocyte reaction assays
| Group . | Stimulation index . | D:3rd . | ||
|---|---|---|---|---|
| Antihost . | Antidonor . | Anti–third party . | ||
| Normal B6 | 1.5 ± 0.3 | 5.8 ± 7.0 | 2.4 ± 0.9 | 2.1 ± 1.3 |
| Normal Balb/c | 15.5 ± 9.8 | 1.9 ± 0.9 | 4.3 ± 2.1 | 0.5 ± 0.2 |
| BMT + cobl* | 1.5 ± 0.4 | 1.4 ± 0.6 | 2.3 ± 1.3 | 0.7 ± 0.4 |
| + CyA | 1.5 ± 0.5 | 7.3 ± 6.0 | 2.4 ± 0.4 | 3.1 ± 2.6 |
| + FK | 1.4 ± 0.2 | 2.6 ± 2.3 | 1.9 ± 0.9 | 1.2 ± 0.5 |
| + Rapa | 2.5 ± 0.9 | 1.7 ± 0.6 | 3.6 ± 0.6 | 0.5 ± 0.1 |
| + MP | 1.6 ± 0.6 | 1.1 ± 0.2 | 2.3 ± 1.4 | 0.6 ± 0.3 |
| + FTY720 | 1.6 ± 0.4 | 1.3 ± 0.3 | 2.4 ± 1.1 | 0.6 ± 0.2 |
| + MMF | 1.6 ± 0.9 | 1.6 ± 1.2 | 2.6 ± 1.8 | 0.8 ± 0.7 |
| Group . | Stimulation index . | D:3rd . | ||
|---|---|---|---|---|
| Antihost . | Antidonor . | Anti–third party . | ||
| Normal B6 | 1.5 ± 0.3 | 5.8 ± 7.0 | 2.4 ± 0.9 | 2.1 ± 1.3 |
| Normal Balb/c | 15.5 ± 9.8 | 1.9 ± 0.9 | 4.3 ± 2.1 | 0.5 ± 0.2 |
| BMT + cobl* | 1.5 ± 0.4 | 1.4 ± 0.6 | 2.3 ± 1.3 | 0.7 ± 0.4 |
| + CyA | 1.5 ± 0.5 | 7.3 ± 6.0 | 2.4 ± 0.4 | 3.1 ± 2.6 |
| + FK | 1.4 ± 0.2 | 2.6 ± 2.3 | 1.9 ± 0.9 | 1.2 ± 0.5 |
| + Rapa | 2.5 ± 0.9 | 1.7 ± 0.6 | 3.6 ± 0.6 | 0.5 ± 0.1 |
| + MP | 1.6 ± 0.6 | 1.1 ± 0.2 | 2.3 ± 1.4 | 0.6 ± 0.3 |
| + FTY720 | 1.6 ± 0.4 | 1.3 ± 0.3 | 2.4 ± 1.1 | 0.6 ± 0.2 |
| + MMF | 1.6 ± 0.9 | 1.6 ± 1.2 | 2.6 ± 1.8 | 0.8 ± 0.7 |
CyA- and FK-treated mice demonstrate increased donor reactivity compared with mice receiving the standard protocol or additional rapa, MP, FTY720, or MMF. Combined results from 2 experiments are shown as mean stimulation index (SI) ± SD. Three to 6 mice per group were analyzed. SIs were calculated by dividing the mean cpm from responses against host (B6), donor (Balb/c), or third-party (C3H) stimulator cells by mean background cpm (ie, cpm with no stimulator population). D:3rd denotes the ratio of antidonor to anti–third-party SI.
Standard-protocol group.
The skin-graft and MLR results taken together show that CyA and FK impaired tolerance induction, whereas rapa, MP, FTY720, and MMF had no detectable negative effect.
Effects of immunosuppressive drugs on the peripheral deletion of donor-reactive T cells
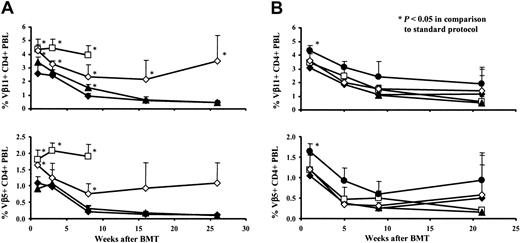
As peripheral deletion of donor-reactive T cells was shown to be an important mechanism for the induction of tolerance in this model,8,33,34 we evaluated deletion of donor-reactive CD4+ peripheral blood lymphocytes (PBLs) and splenocytes by following Vβ11+ and Vβ5+ CD4+cells that recognize endogenous superantigens presented by donor but not recipient MHC.35-37
The standard-protocol groups showed significant deletion of Vβ11+ and Vβ5+ CD4+ PBLs at every time point analyzed after BMT (P < .05 compared with naive B6 mice; P not shown in Figure3). Deletion of these populations at 1 week after BMT was completely blocked in CyA-treated mice (P < .05 compared with the standard-protocol group), and although deletion was seen in this group at later time points, it was substantially diminished throughout the follow-up period (P < .05 compared with the standard-protocol group for Vβ11+ at all analyzed time points; P < .05 for Vβ5+ CD4+ PBLs at week 1 and week 8; Figure 3A). Similarly, FK blocked deletion completely at week 1 after BMT (P < .05 compared with the standard-protocol group), and deletion was less pronounced than in the standard-protocol group at later time points (though this trend was not statistically significant; Figure 3B). In contrast, the mice treated additionally with rapa (0.2 mg/kg/d) demonstrated significant deletion of Vβ11+ and Vβ5+ CD4+ PBLs (P < .05 compared with naive B6 mice; P not shown in Figure 3), to a similar degree as that seen with the standard protocol (P > .05 for comparison with the standard-protocol group; Figure 3A). The group that had received only BMT and rapa, but no cobl, did not demonstrate deletion of either Vβ11+ or Vβ5+ CD4+ cells. Because rapa was used at higher doses in a model (not involving mixed chimerism) in which it acted synergistically with cobl in inducing permanent skin-graft acceptance,21 we also tested this higher dosage (3 mg/kg/d) in our model. However, again rapa did not enhance deletion (data not shown). MP, FTY720, and MMF allowed significant deletion (P < .05 compared with naive B6 mice; P not shown in Figure 3), similar to the standard protocol (Figure 3B). The percentage of Vβ8+ CD4+ cells, which was followed in all experiments to test whether deletion was specific, was not significantly reduced in any group (data not shown). Deletion in FTY720-treated groups could not be evaluated at the first 2 time points of analysis, because FTY720 leads to the transient disappearance of CD4+ and CD8+ T cells from peripheral blood.38
CyA and FK block deletion of donor-reactive CD4+ PBLs following BMT with cobl.
The percentage of Vβ11+ and Vβ5+CD4+ PBLs was examined by 2-color FCM at multiple time points. Results are shown as mean + SD. Vβ8+ CD4+ cells were not deleted in any group (data not shown), indicating that deletion occurring with this protocol was specific. All mice were treated with 3-Gy TBI, BMT, and cobl. Standard-protocol groups (♦; n's = 5 and 6) did not receive any further treatment. (A) ■ denotes a control group, which received only BMT and rapa (deletion did not occur in this group). Other groups were treated additionally with CyA (⋄; n = 6) or rapa (▴; n = 6). (B) Groups received additionally MP (■; n = 6), FTY720 (▴; n = 6), MMF (⋄; n = 6), or FK (●; n = 6). P values were calculated for comparison with deletion from the standard-protocol group of the respective experiment.
CyA and FK block deletion of donor-reactive CD4+ PBLs following BMT with cobl.
The percentage of Vβ11+ and Vβ5+CD4+ PBLs was examined by 2-color FCM at multiple time points. Results are shown as mean + SD. Vβ8+ CD4+ cells were not deleted in any group (data not shown), indicating that deletion occurring with this protocol was specific. All mice were treated with 3-Gy TBI, BMT, and cobl. Standard-protocol groups (♦; n's = 5 and 6) did not receive any further treatment. (A) ■ denotes a control group, which received only BMT and rapa (deletion did not occur in this group). Other groups were treated additionally with CyA (⋄; n = 6) or rapa (▴; n = 6). (B) Groups received additionally MP (■; n = 6), FTY720 (▴; n = 6), MMF (⋄; n = 6), or FK (●; n = 6). P values were calculated for comparison with deletion from the standard-protocol group of the respective experiment.
At the time the mice were killed, deletion of CD4+ and CD8+ splenocytes was also examined. Rapa, MP, FTY720, and MMF did not compromise deletion. CyA and FK also allowed deletion, but as in PBLs, deletion was diminished compared with the standard-protocol groups. Mature CD8+ T cells do not react efficiently with MHC class II; thus, Vβ11+ and Vβ5+CD8+ PBLs cannot be deleted in the periphery but can be deleted only intrathymically at the double-positive stage of T-cell development.8 39 We observed a reduction of Vβ11+ and Vβ5+ CD8+ splenocytes in the standard-protocol groups and the rapa-, MP-, FTY720-, and MMF-treated mice that is evidence for central deletion of donor-reactive thymocytes in these animals (data not shown).
Chimerism, tolerance, and deletion with reduced TBI
To evaluate whether this protocol could still be successful with a lower dose of TBI, we examined the effect of 1-, 1.5-, and 2-Gy TBI on chimerism induction in comparison with our usual dosage of 3 Gy.8,33 40 In the group receiving 1 Gy (n = 7), cobl, and no immunosuppressive drugs, only one mouse developed multilineage chimerism, which was low (this mouse died 8 weeks after BMT). All 5 mice that were irradiated with 1.5 Gy, 5 of 6 mice that received 2 Gy, and 3 of 4 mice from the standard-protocol group (3 Gy) developed lasting (> 5 months) multilineage chimerism (data not shown). Hence, 1.5 Gy seems to be the lowest dose of TBI leading to stable multilineage chimerism after BMT and cobl.
Correlating with chimerism development, the percentages of Vβ11+ and Vβ5+ CD4+ PBLs were significantly reduced at week 20 in the groups receiving 1.5-, 2-, or 3-Gy TBI (P < .05 compared with mice that experienced only 1-Gy TBI or naive mice; Table 2).
Deletion of donor-reactive CD4+ PBLs at week 20 in groups receiving different doses of irradiation
| Group . | CD4+ PBLs . | ||
|---|---|---|---|
| Vβ8+, % . | Vβ11+,% . | Vβ5+,% . | |
| Normal B6 | 16.9 ± 0.2 | 4.9 ± 0.3 | 1.9 ± 0.3 |
| Normal Balb/c | 17.4 ± 0.8 | 0.4 ± 0.2 | 0.0 ± 0.0 |
| 1 Gy | 17.2 ± 1.3* | 4.4 ± 1.1* | 1.3 ± 0.5* |
| 1.5 Gy | 21.5 ± 1.5 | 0.8 ± 0.2 | 0.1 ± 0.1 |
| 2 Gy | 21.8 ± 1.8 | 0.8 ± 0.3 | 0.2 ± 0.3 |
| 3 Gy | 20.4 ± 2.5 | 1.4 ± 1.5 | 0.4 ± 0.6 |
| Group . | CD4+ PBLs . | ||
|---|---|---|---|
| Vβ8+, % . | Vβ11+,% . | Vβ5+,% . | |
| Normal B6 | 16.9 ± 0.2 | 4.9 ± 0.3 | 1.9 ± 0.3 |
| Normal Balb/c | 17.4 ± 0.8 | 0.4 ± 0.2 | 0.0 ± 0.0 |
| 1 Gy | 17.2 ± 1.3* | 4.4 ± 1.1* | 1.3 ± 0.5* |
| 1.5 Gy | 21.5 ± 1.5 | 0.8 ± 0.2 | 0.1 ± 0.1 |
| 2 Gy | 21.8 ± 1.8 | 0.8 ± 0.3 | 0.2 ± 0.3 |
| 3 Gy | 20.4 ± 2.5 | 1.4 ± 1.5 | 0.4 ± 0.6 |
All mice were injected with BM and cobl. Different groups received TBI of 1, 1.5, 2, or 3 Gy on the day before BMT. Percentages shown are mean ± SD.
P < .05 for comparison of irradiated groups with the 3 Gy irradiated (standard-protocol) group.
Mice that received BMT with 1-Gy TBI rejected their donor skin grafts within 16 days, except for one animal with low transient myeloid chimerism, which rejected the graft one month after skin grafting. Two of 5 mice treated with 1.5 Gy, 4 of 7 mice treated with 2 Gy, and 4 of 6 mice from the 3-Gy group accepted their donor grafts long-term (P < .05 compared with mice treated with 1 Gy), with one animal experiencing very late graft loss at day 128 (data not shown). All groups promptly rejected third-party grafts.
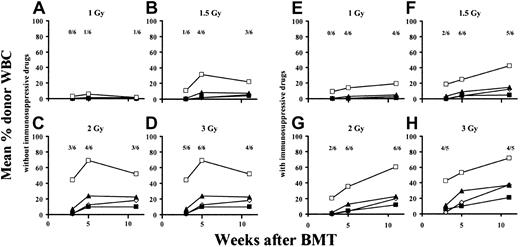
The effect of immunosuppressive drugs on the minimal required dose of TBI
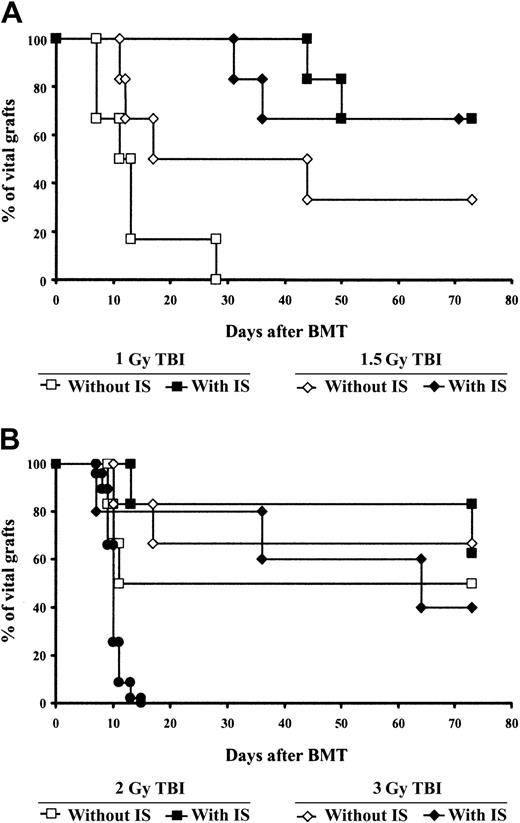
We next examined whether immunosuppressive drugs would allow a further reduction in the irradiation dose in our model. Again, mice received 1-, 1.5-, 2-, or 3-Gy TBI (n = 6 per group) and were injected with donor-derived BMCs and costimulation-blocking antibodies, as described above. In the same experiment, 4 groups (also irradiated with 1, 1.5, 2, or 3 Gy; n = 6 per group) were injected for 4 weeks after BMT with a combination of rapa plus MP plus MMF, which, as shown in “Effects of immunosuppressive drugs on tolerance induction,” can be combined safely with this protocol as single agents; these groups were compared with groups that did not receive any immunosuppressive treatment. The immunosuppressive regimen consisting of rapa, MP, and MMF (without FTY720) was chosen because this calcineurin inhibitor–free regimen has been used successfully in clinical trials after renal transplantation.41 Eleven weeks after BMT, low B-cell and myeloid chimerism was seen in 1 of 6 mice from the 1-Gy group not receiving immunosuppressive drugs (Figure4A). No chimerism was found among CD4+ or CD8+ cells in this group. In the 1-Gy group receiving additional drug treatment, in contrast, 4 of 6 mice developed multilineage chimerism (in the chimeric animals the percentage of donor-derived cells ranged from 2% to 7% for CD4 cells, from 5% to 10% for B cells, and from 15% to 43% for myeloid cells; Figure 4E). Following 1.5-Gy irradiation, 3 of 6 mice not receiving immunosuppressive treatment (Figure 4B) and 5 of 6 mice receiving immunosuppressive treatment (Figure 4F) became chimeric. Similarly, in the groups irradiated with 2 Gy, 3 of 6 mice treated without immunosuppressive drugs (Figure 4C) and 6 of 6 mice receiving immunosuppressive drugs (Figure 4G) developed multilineage chimerism. Irradiation with 3 Gy allowed lasting donor repopulation in 4 of 6 mice not receiving the immunosuppressive combination (Figure 4D) and in 4 of 5 mice receiving the combination (Figure 4H). Thus, there was a clear trend toward a higher rate of chimerism development in the groups receiving immunosuppressive drugs (though only the difference among the 2-Gy groups achieved statistical significance; P < .05). Similarly, skin-graft survival was better in the 1-, 1.5-, and 2-Gy groups receiving immunosuppressive treatment (Figure 5A-B). Most strikingly, no animal from the 1-Gy group that did not receive immunosuppressive drugs demonstrated long-term skin-graft survival (Figure 5A), whereas 4 of 6 mice irradiated with the same dose of TBI that did receive immunosuppressive drugs accepted donor skin for more than 70 days (P < .05 for comparison of the 2 1-Gy groups; 2 nonchimeric mice from this group showed prolonged graft survival).
The addition of a combination of compatible immunosuppressive drugs (rapa + MP + MMF) allows a further reduction of the minimal required dose of TBI.
To evaluate whether immunosuppressive drugs could partially replace TBI in this model, groups of mice were irradiated with different doses of TBI and some were additionally treated with a combination of immunosuppressive drugs. Mice received only TBI at doses of 1 Gy (A), 1.5 Gy (B), 2 Gy (C), or 3 Gy (D; standard-protocol group) or received a combination of rapa, MP, and MMF for 4 weeks in addition to TBI with 1 Gy (E), 1.5 Gy (F), 2 Gy (G), or 3 Gy (H). Fractions shown in each panel indicate the fraction of analyzed mice showing chimerism at the time point indicated below. In graphs, ⋄ indicates CD4 cells, ▪ indicates CD8 cells, ▴ indicates B cells, and ■ indicates monocytes/granulocytes.
The addition of a combination of compatible immunosuppressive drugs (rapa + MP + MMF) allows a further reduction of the minimal required dose of TBI.
To evaluate whether immunosuppressive drugs could partially replace TBI in this model, groups of mice were irradiated with different doses of TBI and some were additionally treated with a combination of immunosuppressive drugs. Mice received only TBI at doses of 1 Gy (A), 1.5 Gy (B), 2 Gy (C), or 3 Gy (D; standard-protocol group) or received a combination of rapa, MP, and MMF for 4 weeks in addition to TBI with 1 Gy (E), 1.5 Gy (F), 2 Gy (G), or 3 Gy (H). Fractions shown in each panel indicate the fraction of analyzed mice showing chimerism at the time point indicated below. In graphs, ⋄ indicates CD4 cells, ▪ indicates CD8 cells, ▴ indicates B cells, and ■ indicates monocytes/granulocytes.
The addition of immunosuppressive drugs (rapa + MP + MMF) improves donor skin-graft survival in mice receiving reduced doses of TBI.
All mice were injected with BM and cobl and irradiated with 1, 1.5, 2, or 3 (standard protocol) Gy. Some received a combination of rapa, MP, and MMF for 4 weeks after BMT and some received no further treatment in the same experiment. Third-party skin grafts (●) were promptly rejected in all groups. Results are shown for 1 and 1.5 Gy (A) and 2 and 3 Gy (B) with and without the immunosuppressive (IS) combination.
The addition of immunosuppressive drugs (rapa + MP + MMF) improves donor skin-graft survival in mice receiving reduced doses of TBI.
All mice were injected with BM and cobl and irradiated with 1, 1.5, 2, or 3 (standard protocol) Gy. Some received a combination of rapa, MP, and MMF for 4 weeks after BMT and some received no further treatment in the same experiment. Third-party skin grafts (●) were promptly rejected in all groups. Results are shown for 1 and 1.5 Gy (A) and 2 and 3 Gy (B) with and without the immunosuppressive (IS) combination.
These data indicate that in our protocol of BMT with cobl, multilineage chimerism can be achieved with doses of TBI as low as 1.5 Gy. Furthermore, immunosuppressive drugs improve chimerism and skin-graft survival and thus allow a further reduction of the dose of TBI.
GVHD
Mice were followed closely for the development of clinical signs of GVHD. Neither diarrhea, hair loss, nor hunched posture were observed. Tissue samples from skin, liver, spleen, and colon were histologically reviewed as coded samples for signs of GVHD by a pathologist in one of the experiments. Although some animals had to be killed because of skin ulcers, no histological evidence for GVHD was found in any analyzed animal (5 standard-protocol mice, 6 of 6 rapa-treated mice, 6 of 6 CyA-treated mice, and 2 of 6 FK-treated mice were examined).
Discussion
Experimental protocols for inducing tolerance through BMT with cobl have attracted much attention in recent years. It is hoped that these new strategies will bring the mixed-chimerism approach to clinical application in organ transplant recipients, thereby improving long-term graft survival while at the same time avoiding drug-associated morbidity. It would be important for ethical reasons to minimize the potential risk to a patient agreeing to undergo such experimental tolerance regimens.18 Therefore, an immunosuppressive “umbrella,” protecting against loss of the transplanted organ if tolerance is not successfully induced, should be applicable without blocking the effect of the tolerance protocol. We therefore aimed to find a clinically relevant immunosuppressive regimen that is compatible with a tolerance protocol using BMT with cobl.
The studies presented here show that long-term chimerism and tolerance induction were impeded by CyA and FK. In the calcineurin inhibitor groups, early engraftment levels were unaffected, but chimerism began to decline in the second month after BMT and the majority of mice had lost multilineage chimerism by 6 months. Importantly, more than 85% of mice treated with CyA and all mice treated with FK rejected their donor skin grafts, indicating that the use of calcineurin inhibitors substantially inhibits tolerance induction, the primary goal of this regimen. The negative effect on tolerance was confirmed in repeat experiments, and this trend was also evident when the start of CyA treatment was delayed until 1 week after BMT. We therefore conclude that CyA or FK should not be used with this tolerance protocol.
Calcineurin inhibitors demonstrated detrimental effects in other models using cobl without mixed chimerism,20,21,23 and this has been attributed to their inhibitory effect on the apoptosis of alloreactive T cells.21 Consistent with this hypothesis, CyA and FK blocked early deletion of donor-reactive T cells in our experiments. Deletion did occur at later time points, but to a significantly lesser degree than in drug-free recipients, and we think that the reduced, delayed deletion impairs tolerance induction. We think that the impairment of deletion several weeks after BMT, but not the blockade very early, is more likely to be responsible for the tolerance-abrogating effect of the calcineurin inhibitors, because in a model of BMT with anti-CD154 in which recipient CD8 cells are eliminated, tolerance occurred even though CyA inhibited early but not late deletion).42 This is compatible with our previous finding that both activation-induced cell death and passive cell death occur after BMT with cobl,33 with calcineurin inhibitors presumably impeding activation-induced cell death.43 Thus, we think that calcineurin inhibitors exert at least part of their detrimental effect on chimerism and tolerance after BMT with cobl by abrogating the deletion of mature donor-reactive T cells, thereby obstructing the lasting tolerization of the pre-existing T-cell repertoire. The decline in chimerism after CyA or FK treatment roughly coincides with the end of the drug treatment period (1 month after BMT), leading us to speculate that CyA and FK delayed the rejection of the donor BM by virtue of their immunosuppressive action. Because the tolerizing properties of cobl were diminished by the calcineurin inhibitors, however, the BM was gradually rejected in most mice once the effect of drug treatment wore off.
Our results regarding CyA and FK are partly at odds with the recent findings of Taylor et al.44 They observed that CyA did not decrease engraftment and that FK diminished chimerism levels only moderately in a mixed-chimerism model also using anti-CD154 (but no CTLA4Ig). Both drugs lowered donor T-cell reconstitution, something we did not observe in our experiments. Of note, donor skin grafts were accepted by fewer than half the recipients treated with the regimen of Taylor et al, and CyA did not have any obvious effect on this outcome (skin grafts were not investigated in detail after FK treatment). Differences between the model used by Taylor et al and our model, potentially explaining some of the observed discrepancy in outcome, include the drug doses given, the period of drug treatment (which started 1 day before BMT and lasted only 2 weeks in the study of Taylor et al), the dose of TBI (2 or 1 Gy), the dose rate of irradiation, the amount of BM (which was approximately twice as high in the study of Taylor et al), the dosing of anti-CD154, and the lack of CTLA4Ig treatment in Taylor and colleagues' study.
It is noteworthy that CyA also did not impair long-term chimerism or tolerance in a model using anti-CD154 (without CTLA4Ig) in which recipient CD8 cells were depleted before BMT.11,42 The isolated elimination of CD4 cells at the time of BMT with cobl12,17 (also Y. Takeuchi and M.S., unpublished data, May 2001), but not the combined elimination of CD4 and CD8 cells,13 has been reported to prevent tolerance induction after BMT with cobl. It has also been established in various models that CD4 cells are readily inhibited by anti-CD154 treatment, whereas CD8 cells are more resistant to tolerance induction through cobl.11 45-47 Thus, because CyA is detrimental only when CD8 cells are present in the host at the time of BMT and cobl, it may interfere with the tolerizing effect that CD4 cells exert on CD8 cells.
Rapa, MP, MMF, and FTY720 were found to be compatible with our tolerance protocol. These drugs did not significantly diminish the development of stable chimerism and did not abrogate donor skin-graft survival. Rapa even significantly improved the acceptance rate of donor grafts. These data are in line with several studies reporting rapa's compatibility with cobl in other models.21,43,48,49Surprisingly, we did not observe any effect of rapa on the degree of peripheral deletion, which we expected given that rapa increased apoptosis of alloreactive cells in another model.22 Even when we used a very high dose of rapa (3 mg/kg/d), deletion followed similar kinetics after BMT as in drug-free mice. Potential explanations for this lack of a detectable effect include the possibility that BMT plus cobl already leads to such a high degree of apoptosis that it cannot be increased further, or that rapa affects only certain types of apoptosis, which do not play a prominent role in our model. Encouragingly, neither MP, FTY720, nor MMF inhibited chimerism or tolerance, even though their use has been associated with negative effects in various tolerance models.23,26,28,49 Because to date no true monotherapy (without any induction therapy or short-term steroid use) has been shown to be effective in clinical organ transplantation, we considered it important to find a regimen of compatible drugs that has been proven effective in clinical trials. With the combination of rapa plus steroids plus MMF, this goal has been reached.41 FTY720, which is currently not yet approved for clinical use, could serve as an alternative or additive component to this regimen.
Another finding that might increase clinical acceptance of our protocol is the possibility of safely reducing the dose of TBI. TBI at a dose of 2 Gy is commonly used in the clinical setting of “minitransplant” BMT protocols for hematological indications with only moderate associated morbidity,50 and such a low dose of irradiation is linked only with a very low risk of long-term tumor induction.51 When TBI was used without immunosuppressive drugs, 1.5 Gy was the lowest dose to lead to stable multilineage chimerism and tolerance. The addition of rapa plus steroids plus MMF showed a clear beneficial effect in that it resulted in a higher rate of chimerism and skin-graft acceptance at all tested doses of irradiation. Strikingly, it permitted the development of chimerism and skin-graft prolongation with 1 Gy TBI. Thus, not only can an immunosuppressive regimen be combined safely with this tolerance protocol, it can even improve its outcome.
We want to thank Franz Winkler for helpful technical assistance and the staff of the Institute of Biomedical Research for expert animal care.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-10-3014.
Supported by research grant no. 8795 from the Jubilee Fund of the Austrian National Bank, T.W. by grant CI 110578928 from the Roche Organ Transplantation Research Foundation, and by National Institutes of Health grant RO1 HL44-9915 (M.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Wekerle, Department of Surgery—21A, Vienna General Hospital, Waehringer Guertel 18, A 1090 Vienna, Austria; e-mail: thomas.wekerle@akh-wien.ac.at.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal