Interleukin-18 (IL-18) is a unique cytokine that modulates both TH1/TH2 responses, but its ability to modulate diseases through induction of TH2 cytokines is unclear. It has been shown to play an important role in allogeneic bone marrow transplantation (BMT). Because immune responses of allogeneic BM donors may affect acute graft-versus-host disease (GVHD), we investigated the effect of pretreating BM transplant donors with IL-18 on the severity of acute GVHD using a well-characterized experimental BMT model (BALB/c→B6). Pretreatment of allogeneic BM transplant donors with IL-18 significantly improved survival (80% vs 0%; P < .001), and reduced clinical, biochemical, and pathologic indices of acute GVHD in BM transplant recipients. IL-18 pretreatment was associated with reduced interferon γ (IFN-γ) and greater IL-4 secretion by donor T cells after BMT. Acute GVHD mortality was reduced when IL-18 was administered to donors deficient in IFN-γ and signal transducer and activator of transcription 4 (STAT4) but not STAT6 signaling molecules, suggesting a critical role for STAT6 signaling in IL-18's protective effect. IL-18 treatment did not alter donor CD8+ cytotoxic T-lymphocyte (CTL) activity and preserved graft-versus-leukemia (GVL) effects after allogeneic BMT (70% vs 10%; P < .01). Together these data illustrate that pretreatment of donors with IL-18 prior to allogeneic BMT attenuates acute GVHD in a STAT6-dependent mechanism while preserving GVL effects.
Introduction
Interleukin-18 (IL-18) was originally discovered as interferon γ (IFN-γ)–inducing factor and is produced by a wide variety of cells, such as macrophages, Kupffer cells, T cells, dendritic cells, keratinocytes, osteoblasts, and astrocytes.1,2 IL-18 is similar to IL-1 in its molecular structure, processing, and signaling, and exerts its biologic activities through a specific receptor consisting of 2 molecules that are members of IL-1R family: IL-1Rrp and AcP-like protein.3,4 The biologic activities of IL-18 and IL-1 are quite distinct; IL-18 shares functional similarity with IL-12, with which it synergizes to induce TH1 responses.2,5 IL-18 plays an important role by inducing TH1 polarization in various infectious and immunologic diseases, such as tuberculosis, Leishmaniasis, tuberculoid leprosy, Sjogren syndrome, rheumatoid arthritis, and Crohn disease.2 IL-18 also induces proinflammatory cytokines (eg, tumor necrosis factor α [TNF-α] and IL-1) and the secretion of several hematopoietic growth factors, such as granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6.2 6
In the absence of IL-12, however, IL-18 acts somewhat differently and can induce TH2 polarization.2,7-10 IL-18 transgenic mice produce increased amounts of both TH1 and TH2 cytokines.11 Patients with lepromatous leprosy, a TH2-like disease, exhibit high serum levels of IL-18.7 Thus it is unclear whether IL-18 can modulate certain disease processes in vivo through the induction of TH1 cytokines only or whether its effects on TH2 cytokines may also be important.
Acute graft-versus-host disease (GVHD) is the major toxicity of allogeneic bone marrow transplantation (BMT). The pathophysiology of acute GVHD is complex, and it involves donor T-cell responses to the host alloantigens and dysregulation of inflammatory cytokines cascades.12 The TH1 polarization of activated T-cell subsets plays an important role in the “cytokine storm” that characterizes several acute GVHD models, whereas a shift to TH2 polarization of donor cells can reduce acute GVHD.12-14 The TH1/TH2 balance as it relates to the pathophysiology of acute GVHD is complex and controversial. For example, administration of TH1-inducing cytokines early after BMT has been shown to paradoxically reduce the severity of acute GVHD.15-20 The Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathways control cytokine production by T cells: STAT4 regulates TH1 responses and STAT6 regulates TH2 responses. A recent study demonstrated that acute GVHD can occur in the absence of either STAT4 or STAT6 signaling in donor T cells.21 Furthermore, other studies have failed to demonstrate a reduction in acute GVHD after direct in vivo administration of TH2 cytokines to the recipients.21-26
IL-18, as well as its inhibitor IL-18–binding protein, is elevated in acute GVHD.27-30 We have previously demonstrated that administration of IL-18 to recipient mice early after BMT reduces the severity of acute GVHD in an IFN-γ–dependent fashion.20 In addition, IL-18 also prevents murine chronic GVHD.31 Because IL-18 is nontoxic7,8,32,33and can induce hematopoietic growth-factor secretion,2,6 and might therefore be suitable for administration to healthy donors, we investigated whether pretreatment of donors with IL-18 would modulate GVHD and GVL responses in a well-defined mouse experimental BMT system. Given that IL-18 can induce IFN-γ or induce TH2 polarization via STAT6, we also determined whether IFN-γ secretion and STAT signaling by donor cells are required for IL-18 to mediate its effect.2 7
Materials and methods
Mice
Female C57BL/6 (B6, H-2b, CD45.2+), B6.129S7-IFN-tm1Ts(GKO, H-2b, CD45.2+), and BALB/c (H-2d) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). B6 Ly5.2 (H-2b, CD45.1+) mice were purchased from Frederick Cancer Research Facility (Frederick, MD). BALB/c (H-2d STAT6−/−) and BALB/c (H-2dSTAT4−/−) were kindly provided by Dr Nick Lukacs, Department of Pathology, University of Michigan, who purchased them from Jackson Laboratories. Mice between 8- and 12-weeks of age were housed in sterilized microisolator cages and received filtered water and normal chow or autoclaved hyperchlorinated drinking water for the first 3 weeks after BMT.
BMT
Mice underwent transplantation according to a standard protocol described previously.34 Briefly, B6 recipients received 13 Gy total body irradiation (TBI) (137Cs source), split into 2 doses separated by 3 hours to minimize gastrointestinal (GI) toxicity. Recipient mice were injected with T-cell–depleted (TCD) bone marrow cells (5 × 106) plus 2 × 106splenic T cells from either syngeneic (B6) or allogeneic (BALB/c) donors. Donor T cells were negatively isolated by using major histocompatibility (MHC) class II+, and CD11b+MicroBeads and the autoMACS (Mittenyi Biotech, Gladbach, Germany) following nylon wool enrichment of T cells from splenocytes. Splenic T cells and TCD BM cells from respective allogeneic or syngeneic donors were resuspended in 0.25 mL Leibovitz L-15 media (GIBCO BRL, Gaithersburg, MD) and injected intravenously into recipients on day 0. For engraftment experiments, CD45.1+ (H-2b, B6 Ly5.2) animals were used as recipients. Survival was monitored daily and recipients' body weights and GVHD clinical scores were measured weekly. Donor cell numbers were determined by examining the percentage of CD45.1− cells in the recipient spleens at different time points.
Assessment of acute GVHD
The degree of systemic acute GVHD was assessed by a scoring system incorporating 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity, as published previously.35 At the time of analysis, mice from coded cages were evaluated and graded from 0 to 2 for each criterion. A clinical index was subsequently generated by summation of the 5 criteria scores (maximum index = 10). Mice that underwent transplantation were ear punched and individual scores were obtained and recorded on day 0, and weekly thereafter.
IL-18 treatment
Recombinant murine IL-18 was purchased from Research Diagnostic (Flanders, NJ) and reconstituted in phosphate-bufferend saline (PBS). Donor mice were injected intraperitoneally with IL-18 (1 μg/d/mouse) for 10 days from days −11 to −1 (10 injections total). Mice from the control groups received only the diluent in a similar schedule.
Leukemia induction
An EL4 leukemia model, as previously described, was used for the graft-versus-leukemia (GVL) experiments.16,36 EL4 is a subline of the B6 MHC class II−/− T-cell leukemia/lymphoma EL4 (H2b), and is thus syngeneic (H2b) to the B6 hosts and allogeneic (H2d) to the BALB/c donors. On day 0, 2000 EL4 cells were injected into each recipient along with syngeneic (B6) or allogeneic (BALB/c) BM and spleen T cells. Previous studies have demonstrated that as few as 500 cells/recipient can induce fatal leukemia in the syngeneic hosts.36 Injection of 2000 EL4 cells into animals that cannot reject this tumor is uniformly lethal and results in massive tumor infiltration and enlargement of various organs, particularly the liver, spleen, and kidneys.36 Survival was monitored daily and the cause of each death after BMT was determined by postmortem examination. Death from EL4 was defined by enlargement of the liver and spleen with macroscopic tumor nodules, whereas GVHD death was defined as the absence of tumor and the presence of GVHD as determined by the clinical scoring system described in “Assessment of acute GVHD.” Minimal residual disease was determined in surviving animals by fluorescence-activated cell sorting (FACS) analysis of the peripheral blood and spleen. The sensitivity of tumor detection by this method has been previously demonstrated by mixing experiments to be 0.2%.37
FACS analysis
Fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (MoAbs) to mouse CD3+-, Gr-1+-, CD4+-, and PE-conjugated MoAbs to B220+-, CD8+-, CD25+-, and APC-conjugated MoAbs CD45.1+ antigens were purchased from PharMingen (San Diego, CA). For determining the extent of donor T-cell number and engraftment (CD45.1+, anti-Ly5.2 MoAb) was used as host cell specific marker. The procedure was performed as described previously.34 Briefly, cells were first incubated with MoAb 2.4G2 for 15 minutes at 4°C and then with the relevant FITC-conjugated MoAb for 30 minutes at 4°C. Finally, cells were washed twice with PBS/0.2% bovine serum albumin and fixed with PBS/1% paraformaldehyde. Then 3-color flow cytometry was performed by using EPICS Elite ESP cell sorter (Beckman-Coulter, Miami, FL) and on FACS Vantage SE cell sorter (Becton Dickinson, San Jose, CA).
Mixed lymphocyte cultures
All culture media and incubation conditions have been previously described.38 39 Briefly, for secondary mixed lymphocyte reaction (MLR) cultures, T cells were isolated from IL-18 or diluent-treated BALB/c mice by negative selection (CD11b−, MHC II−) with magnetic beads and cultured with radiated B6 Ly5.2 splenocytes. After 96 hours, donor T cells were removed from cultures, layered over Ficoll, and adjusted for CD3+ cell number and then restimulated with irradiated B6 Ly5.2 peritoneal cells. Supernatants were collected after 48 hours proliferation was determined by incubation of cells with3H-thymidine for an additional 24 hours. For post-BMT cell culture analyses, splenocytes were removed from the animals 7 days after transplantation and 3 to 4 spleens were combined from each group. These cells were then layered over Ficoll-Paque (Pharmacia LKB Biotechnology, Piscataway, NJ) and centrifuged at 800g for 15 minutes. Cells were then collected from the interface and washed twice before suspension in supplemented 10% fetal calf serum (FCS)/Dulbecco modified Eagle medium. The cells were normalized for donor T cells (CD45.1− and CD3+) and were plated in 96-well flat-bottomed plates (Falcon Labware, Lincoln Park, NJ) at a concentration of 2 × 105 T cells (CD45.1−CD3+)/well with 2 × 105irradiated (2000 rad) splenocytes harvested from naive B6 (allogeneic) animals. At 48 hours, supernatants were collected for cytokine analysis and the cultures were pulsed with 3H-thymidine (1 μCi/well [0.037 MBq]) and proliferation was determined 20 hours later on a 1205 Betaplate reader (Wallac, Turku, Finland).
51Cr release assay
The cytotoxic function of T cells was analyzed by performing51Cr release assay as described previously.37 39 Briefly, 2 × 106allogeneic EL4 (H-2b) and syngeneic P815 (H2d) tumor targets were labeled with 100 μCi (3.7 MBq) of 51Cr sodium salt (NEN Life Sciences Products, Frederick, CO) for 2 hours. After washing 3 times, labeled targets were resuspended in 10% FCS RPMI and plated at 104 cells per well in U-bottom plates (Corning-Costar, Cambridge, MA). Recipient mice were killed on day 14 after BMT and the spleens were harvested. Splenocyte preparations were normalized for CD8+ cells and were added to quadruplicate wells at varying effector (CD8+)-to-target ratios and incubated for 5 hours. Maximal and background release was determined by the addition of Triton-X (Sigma Chemical, St Louis, MO) or media alone to targets, respectively. 51Cr activity in supernatants taken 5 hours later was determined in an autogamma counter (Packard Instrument, Meriden, CT), and lysis was expressed as a percentage of maximum: percentage of specific lysis = 100 × ([sample count − background count]/[maximum count − background count]).
Cytokine enzyme-linked immunosorbent assay (ELISA)
Antibodies were purchased from R&D Systems and PharMingen; assays were performed according to the manufacturer's protocol. Briefly, samples were diluted 1:5 to 1:10 and TNF-α, IFN-γ, or IL-4 was captured by the specific primary MoAb and detected by horseradish peroxidase (TNF-α) or biotin-labeled (IFN-γ or IL-4) secondary MoAbs. Plates were read at 450 nm using a microplate reader (Model 3550; Bio-Rad Labs, Hercules, CA). Recombinant murine TNF-α (rmTNF-α), mIFN-γ, and mIL-4 (PharMingen) were used as standards for ELISAs. All the samples and standards were run in duplicate.
Serum lipopolysacchride (LPS) estimation
The Limulus Amebocyte Lysate (LAL) assay (Bio Whittaker, Walkersville, MD) was performed according to the manufacturer's protocol to determine the endotoxin (LPS) concentration in serum. Briefly, serum samples were collected and analyzed using pyrogen-free materials, diluted 10% (vol/vol) in LAL reagent water, and heated to 70°C for 5 minutes to minimize nonspecific inhibition. Samples were then incubated with equal volumes of LAL for 10 minutes at 37°C and developed with equal volumes of substrate solution for 6 minutes. The absorbance of the assay plate was read at 405 nm using the same microplate reader used in cytokine assays. Samples and standards were run in duplicate and the lower limit of detection was 0.15 U/mL. All units expressed are relative to the US reference standard EC-6.
Histology
Formalin-preserved liver and small and large bowel were embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded fashion by a pathologist (C.L.). A semiquantitative scoring system, as described previously, was used to assess the following abnormalities known to be associated with GVHD.38 Specifically, 7 parameters each were scored for small bowel (villous blunting, crypt regeneration, crypt epithelial cell apoptosis, crypt loss, luminal sloughing of cellular debris, lamina propria inflammatory cell infiltrate, and mucosal ulceration) and large bowel (crypt regeneration, crypt epithelial cell apoptosis, crypt loss, surface colonocyte vacuolization, surface colonocyte attenuation, lamina propria inflammatory cell infiltrate, and mucosal ulceration), and 10 parameters for liver (portal tract expansion by an inflammatory cell infiltrate, lymphocytic infiltrate of bile ducts, bile duct epithelial cell apoptosis, bile duct epithelial cell sloughing, vascular endothelialitis, parenchymal apoptosis, parenchymal microabscesses, parenchymal mitotic figures, hepatocellular cholestasis, and hepatocellular steatosis).The scoring system denoted 0 as normal; 0.5 as focal and rare; 1.0 as focal and mild; 2.0 as diffuse and mild; 3.0 as diffuse and moderate; and 4.0 as diffuse and severe. Scores were added to provide a total score for each specimen. After scoring the codes were broken and data compiled.
Statistical analysis
The Mann-Whitney U test was used for the statistical analysis of cytokine data, LPS levels, clinical scores, weight loss, and histology, whereas the Wilcoxon rank test was used to analyze survival data. A P value less than .05 was considered statistically significant.
Results
IL-18 treatment of donors reduces GVHD mortality and morbidity after allogeneic BMT
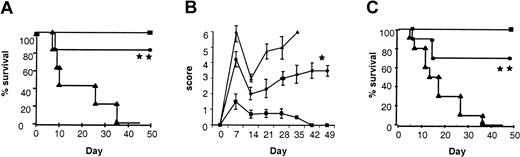
We first studied the effect of IL-18 administration to BM transplant donors in terms of morbidity and mortality from acute GVHD following allogeneic BMT. We used a well-characterized mouse model of acute GVHD directed against both MHC and minor histocompatibility antigens, BALB/c (H-2d)→B6 (H-2b). Donor BALB/c mice were pretreated intraperitoneally with either 1 μg/mouse of rmIL-18 or diluent for 10 days, a dose that has previously been shown to be nontoxic and to induce TH2 polarization.7 Recipient B6 mice were conditioned with 1300 cGy TBI and received transplants of TCD BM and splenic T cells from either IL-18 or diluent pretreated syngeneic (B6) or allogeneic (BALB/c) donors as described in “Materials and methods.” As shown in Figure 1A, animals that received allogeneic T cells from IL-18 pretreated donors showed a significantly better survival after BMT compared with controls (80% vs 0%; P < .001). Clinical GVHD was quantified with a clinical scoring system (“Materials and methods”), and was also less severe in animals receiving allogeneic BM transplants from IL-18–treated donors (Figure 1B; P < .05). As expected, the clinical scores of syngeneic animals gradually returned to baseline over this time. All BM transplant recipients displayed complete donor hematopoietic chimerism as determined by FACS analysis (data not shown). Longer follow-up of the BM transplant recipients from IL-18–treated donors demonstrated 40% survival with clinical signs of GVHD beyond day 100. Thus, pretreatment of donors with IL-18 reduced and delayed the morbidity and attenuated mortality from acute GVHD. Similar GVHD benefit was also observed in a different donor-recipient BMT model (B6 (H2b)→B6D2F1 (H2b/d)) ruling out strain-dependent artifact (Figure 1C; P < .001). Furthermore, donor treatment with a higher dose of IL-18 for a shorter duration (5 μg/mouse/d for 7 days) also demonstrated a similar reduction in acute GVHD mortality (50-day survival, 75% vs 0%;P < .01).
Pretreatment of donors with IL-18 attenuates acute GVHD mortality and morbidity.
BALB/c donor mice were injected intraperitoneally with 1 μg/mouse/d of IL-18 or the diluent (PBS) for 10 days. B6 mice were given 1300 cGy of TBI and received transplants of 5 × 106T-cell depleted BM cells and 2 × 106 T cells from either IL-18–treated (●, n = 10) or diluent-treated (▴, n = 10) BALB/c donors, as in “Materials and methods.” Syngeneic recipients (▪, n = 5) received similar transplants of cells from B6 donors. Data from 1 of 2 similar experiments is shown. (A) Percent survival after BMT (● vs ▴; **P < .001 by Wilcoxon rank test). (B) Animals were scored for clinical GVHD as described in “Materials and methods” (● vs ▴; *P < .05 by Mann-WhitneyU test from days 7 to 35). (C) B6 donors were injected with IL-18 or the diluent as above for 10 days. B6D2F1 mice were irradiated with 1300 cGy of TBI and received transplants, as described above, of cells from IL-18–treated (●, n = 10) or diluent-treated (▴, n = 10) B6 donors. Syngeneic recipients (▪, n = 4) received transplants of cells from B6D2F1 donors. Percent survival after BMT (● vs ▴; **P < .001 by Wilcoxon rank test). Data from 1 of 2 similar experiments is shown.
Pretreatment of donors with IL-18 attenuates acute GVHD mortality and morbidity.
BALB/c donor mice were injected intraperitoneally with 1 μg/mouse/d of IL-18 or the diluent (PBS) for 10 days. B6 mice were given 1300 cGy of TBI and received transplants of 5 × 106T-cell depleted BM cells and 2 × 106 T cells from either IL-18–treated (●, n = 10) or diluent-treated (▴, n = 10) BALB/c donors, as in “Materials and methods.” Syngeneic recipients (▪, n = 5) received similar transplants of cells from B6 donors. Data from 1 of 2 similar experiments is shown. (A) Percent survival after BMT (● vs ▴; **P < .001 by Wilcoxon rank test). (B) Animals were scored for clinical GVHD as described in “Materials and methods” (● vs ▴; *P < .05 by Mann-WhitneyU test from days 7 to 35). (C) B6 donors were injected with IL-18 or the diluent as above for 10 days. B6D2F1 mice were irradiated with 1300 cGy of TBI and received transplants, as described above, of cells from IL-18–treated (●, n = 10) or diluent-treated (▴, n = 10) B6 donors. Syngeneic recipients (▪, n = 4) received transplants of cells from B6D2F1 donors. Percent survival after BMT (● vs ▴; **P < .001 by Wilcoxon rank test). Data from 1 of 2 similar experiments is shown.
We next evaluated the effect of IL-18 administration on splenic-cell number and phenotype of donor mice. IL-18 increased the total number of splenocytes and the percent of Gr-1+ cells, but did not significantly alter the ratios of the CD4+, CD8+, and B220+ cells (Table1). IL-18 administration also did not change splenic T-cell function as measured by several responses, such as proliferation, IFN-γ, or IL-4 secretion to alloantigen in primary MLR (Table3). After restimulation in a secondary mixed leukocyte reaction (MLR), however, responder cells from IL-18–treated mice showed markedly elevated IL-4 production together with a significant reduction in IFN-γ secretion and less proliferation (Table 3). Increased levels of IL-4 were also apparent in the serum of treated mice (Table 2), as reported by Yoshimoto et al,7 confirming that IL-18 polarized T cells prior to BMT.
Effect of IL-18 on the percent of splenic cellular phenotype
| Group . | Total cells . | %CD4+ . | %CD8+ . | % B220+ . | %Gr-1+ . |
|---|---|---|---|---|---|
| Diluent | 110.4 ± 7.8 × 106 | 24% ± 5% | 13% ± 3% | 51% ± 9% | 2.5% ± 0.6% |
| IL-18 | 179.8 ± 12 × 106* | 19% ± 6% | 9% ± 3.8% | 46% ± 14% | 13.4% ± 1.27%* |
| Group . | Total cells . | %CD4+ . | %CD8+ . | % B220+ . | %Gr-1+ . |
|---|---|---|---|---|---|
| Diluent | 110.4 ± 7.8 × 106 | 24% ± 5% | 13% ± 3% | 51% ± 9% | 2.5% ± 0.6% |
| IL-18 | 179.8 ± 12 × 106* | 19% ± 6% | 9% ± 3.8% | 46% ± 14% | 13.4% ± 1.27%* |
BALB/c mice (n = 3/group) were injected with 1 μg/day of IL-18 or the diluent (control) for 10 days. Splenocytes were harvested on day 11 and analyzed for cellular phenotype by 2-colored flow cytometry. IL-18 treatment increased the total number of cells and the percent of Gr-1 cells (
P < .03) but did not significantly alter the ratios of CD4+, CD8+, or B220+ cells.
Effect of IL-18 administration on donor T-cell responses in primary and secondary MLR
| . | cpm . | IFN-γ, pg/mL . | IL-4, pg/mL . |
|---|---|---|---|
| Primary MLR | |||
| Syngeneic | 247 ± 39 | 38 ± 21 | UD |
| Allogeneic + control | 37 358 ± 1596 | 4473 ± 296 | UD |
| Allogeneic + IL-18 | 32 649 ± 4771 | 3362 ± 958 | UD |
| Seconday MLR | |||
| Syngeneic | 979 ± 254 | 675 ± 342 | 13 ± 6 |
| Allogeneic + control | 76 988 ± 9752 | 16 786 ± 1041 | 23 ± 7 |
| Allogeneic + IL-18 | 48 676 ± 6453* | 9863 ± 474* | 94 ± 12* |
| . | cpm . | IFN-γ, pg/mL . | IL-4, pg/mL . |
|---|---|---|---|
| Primary MLR | |||
| Syngeneic | 247 ± 39 | 38 ± 21 | UD |
| Allogeneic + control | 37 358 ± 1596 | 4473 ± 296 | UD |
| Allogeneic + IL-18 | 32 649 ± 4771 | 3362 ± 958 | UD |
| Seconday MLR | |||
| Syngeneic | 979 ± 254 | 675 ± 342 | 13 ± 6 |
| Allogeneic + control | 76 988 ± 9752 | 16 786 ± 1041 | 23 ± 7 |
| Allogeneic + IL-18 | 48 676 ± 6453* | 9863 ± 474* | 94 ± 12* |
BALB/c mice were injected with IL-18 or diluent (control) for 10 days, as in “Materials and methods.” Splenic T (CD3+) cells were harvested on day 11 and cultured in primary MLR (n = 3/group) with irradiated B6 splenocytes for 96 hours. Culture supernatants were collected for cytokine measurement and proliferation was determined by pulsing with 3H-thymidine (1 μCi/well [0.037 MBq/well]) for an additional 16 hours. Secondary MLR responder T cells from bulk primary MLRs were then removed and restimulated with irradiated B6 peritoneal cells. After 48 hours of culture, supernatants were collected for cytokine measurement, and proliferation was determined by pulsing with3H-thymidine (1 μCi/well [0.037 MBq/well]) for an additional 20 hours. *P < .05.
Effect of IL-18 administration on serum levels of IFN-γ and IL-4 in the donor mice
| Group . | IFN-γ, pg/mL . | IL-4, pg/mL . |
|---|---|---|
| WT + diluent | UD | UD |
| WT + IL-18 | UD | 44 ± 9.6* |
| STAT6−/− + diluent | 143 ± 41 | UD |
| STAT6−/− + IL-18 | 2338 ± 387** | UD |
| Group . | IFN-γ, pg/mL . | IL-4, pg/mL . |
|---|---|---|
| WT + diluent | UD | UD |
| WT + IL-18 | UD | 44 ± 9.6* |
| STAT6−/− + diluent | 143 ± 41 | UD |
| STAT6−/− + IL-18 | 2338 ± 387** | UD |
Wild-type (WT) (n = 3/group) and STAT6-deficient (n = 3/group) BALB/c mice were injected with IL-18 or diluent (control) for 10 days as in “Materials and methods.” Serum was collected on the eleventh day by retro-orbital venous puncture and analyzed for IFN-γ and IL-4 by ELISA. IL-18 administration significantly increased serum levels of IL-4 (*P < .05) but did not alter IFN-γ production in the WT mice; significantly increased serum IFN-γ (**P < .03), but not IL-4, was detected in the STAT6-deficient mice.
UD indicates undetectable.
Pretreatment of donors with IL-18 reduces GVHD target organ damage
We next evaluated the effect of IL-18 pretreatment of donors on 2 principal target organs of acute GVHD, the GI tract (small bowel and colon) and liver. Samples were taken from animals (n = 4/group) on day 7 after transplantation and scored in a coded fashion as described in “Materials and methods.” Recipients of allogeneic BM transplants from IL-18–treated donors demonstrated significantly less damage than controls in the liver (Figure 2A-C) and the GI tract (Figure 2D-F).
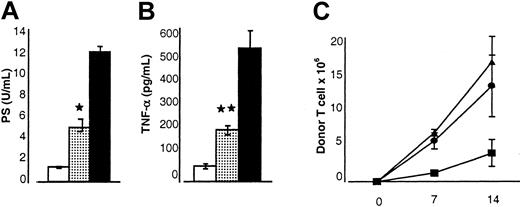
Serum levels of LPS and TNF-α correlate with the severity of acute GVHD and with damage to the GI tract.14,34 40 As shown in Figure 3, the serum levels of both LPS (5.3 ± 1.1 U/mL vs 12.4 ± 1.3 U/mL; Figure 3A) and TNF-α (212 ± 24 pg/mL vs 524 ± 69 pg/mL; Figure 3B) in animals receiving allogeneic BM transplants from IL-18–treated donors were significantly lower compared with allogeneic controls (P < .05). Thus, pretreatment of donors with IL-18 reduced the severity of acute GVHD as measured by clinical, pathologic, and serum biochemical markers.
IL-18 treatment of donor mice reduces the acute GVHD pathologic damage of liver and GI tract in the recipient.
Donor cells from IL-18–treated or diluent-treated (control) BALB/c mice were transplanted into B6 recipients as in Figure 1. Animals that underwent transplantation (n = 4/group) were killed; liver and gut were obtained for analysis on day 7 after BMT. Histology of the liver from the recipients of control-treated (A) and IL-18–treated (B) allogeneic donors. Coded slides were scored semiquantitatively for pathologic damage, as described in “Materials and methods.” BM transplant recipients of diluent-treated (▪, n = 4) or IL-18–treated (░, n = 4) allogeneic donors and of syngeneic donors (■, n = 4) are shown (C) (*P < .05; control [▪] vs IL-18 [░] allo group). Histology of the large bowel from the recipients of control-treated (D) and IL-18–treated (E) allogeneic donors is shown. Coded slides were scored semiquantitatively, as in “Materials and methods.” BMT recipients of diluent-treated (▪, n = 4) or IL-18–treated (░, n = 4) allogeneic donors and of syngeneic donors (■, n = 4) are shown (F). Total GVHD score: mean ±SE of the sum of scores for small bowel and colon from individual animals in each group (*P < .05 vs IL-18 allo group). Original magnification × 400 (A-B, D-E).
IL-18 treatment of donor mice reduces the acute GVHD pathologic damage of liver and GI tract in the recipient.
Donor cells from IL-18–treated or diluent-treated (control) BALB/c mice were transplanted into B6 recipients as in Figure 1. Animals that underwent transplantation (n = 4/group) were killed; liver and gut were obtained for analysis on day 7 after BMT. Histology of the liver from the recipients of control-treated (A) and IL-18–treated (B) allogeneic donors. Coded slides were scored semiquantitatively for pathologic damage, as described in “Materials and methods.” BM transplant recipients of diluent-treated (▪, n = 4) or IL-18–treated (░, n = 4) allogeneic donors and of syngeneic donors (■, n = 4) are shown (C) (*P < .05; control [▪] vs IL-18 [░] allo group). Histology of the large bowel from the recipients of control-treated (D) and IL-18–treated (E) allogeneic donors is shown. Coded slides were scored semiquantitatively, as in “Materials and methods.” BMT recipients of diluent-treated (▪, n = 4) or IL-18–treated (░, n = 4) allogeneic donors and of syngeneic donors (■, n = 4) are shown (F). Total GVHD score: mean ±SE of the sum of scores for small bowel and colon from individual animals in each group (*P < .05 vs IL-18 allo group). Original magnification × 400 (A-B, D-E).
Effect of IL-18 injection to donors before BMT on the biochemical indices of acute GVHD and donor T-cell proliferation.
B6 mice underwent transplantation as in Figure 1. Sera from the recipient animals (n = 3 mice/group) were obtained on day 7 after BMT and analyzed as described in “Materials and methods.” (A) Serum LPS levels are reduced in the recipients of IL-18–treated donor cells. Controls (▪) versus IL-18 allo recipients (░); *P < .05. Results from 1 of 2 similar experiments are shown. (B) Serum TNF-α levels are reduced. Controls (▪) versus IL-18–treated recipients (░); **P < .01. Data from 1 of 2 similar experiments are shown. (C) B6 Ly5.2 (CD45.1+) recipient mice underwent transplantation as in Figure 1. Splenocytes were harvested from the recipients at each time point as shown (n = 4/per group) and labeled with anti-CD3+ PE and anti-CD45.1+ FITC. The number of donor T cells (CD45.1−CD3+) was determined (▴, allo+control vs ●, allo+IL-18) at various time points, on days 7 and 14 after BMT. Recipients of syngeneic BM transplants (▪) demonstrated lower numbers of donor T cells at all time points. Data from 1 of 3 representative experiments are shown. Error bars represent standard error.
Effect of IL-18 injection to donors before BMT on the biochemical indices of acute GVHD and donor T-cell proliferation.
B6 mice underwent transplantation as in Figure 1. Sera from the recipient animals (n = 3 mice/group) were obtained on day 7 after BMT and analyzed as described in “Materials and methods.” (A) Serum LPS levels are reduced in the recipients of IL-18–treated donor cells. Controls (▪) versus IL-18 allo recipients (░); *P < .05. Results from 1 of 2 similar experiments are shown. (B) Serum TNF-α levels are reduced. Controls (▪) versus IL-18–treated recipients (░); **P < .01. Data from 1 of 2 similar experiments are shown. (C) B6 Ly5.2 (CD45.1+) recipient mice underwent transplantation as in Figure 1. Splenocytes were harvested from the recipients at each time point as shown (n = 4/per group) and labeled with anti-CD3+ PE and anti-CD45.1+ FITC. The number of donor T cells (CD45.1−CD3+) was determined (▴, allo+control vs ●, allo+IL-18) at various time points, on days 7 and 14 after BMT. Recipients of syngeneic BM transplants (▪) demonstrated lower numbers of donor T cells at all time points. Data from 1 of 3 representative experiments are shown. Error bars represent standard error.
Effects of IL-18 treatment of donors on T-cell function after BMT
Induction of GVHD fundamentally depends on the donor T-cell response to host alloantigens.41 We previously demonstrated that administration of IL-18 to recipient mice early in BMT reduced acute GVHD by attenuating donor T-cell expansion.20 We next evaluated the effects of IL-18 treatment of donors by measuring T-cell proliferation in the spleen after BMT. Pretreatment of donors with IL-18 did not significantly alter donor T-cell expansion in the recipient spleens in the first 2 weeks after BMT (Figure 3C).
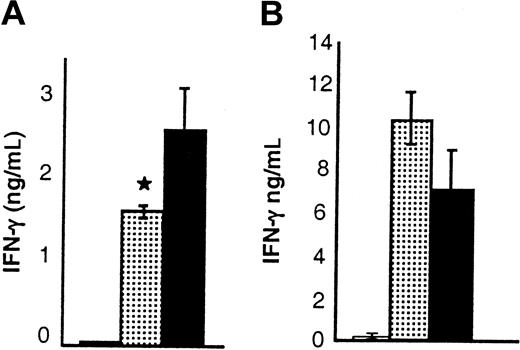
We further evaluated the effects of IL-18 administration on cytokine secretion of donor T cells after BMT. IL-18 can promote either TH1 or TH2 polarization depending on the context.2,7-10 Donor splenic T cells were harvested from BM transplant recipients 7 days after transplantation and used as responders in MLRs with host stimulator cells. IL-18–treated donor T cells secreted significantly less IFN-γ, but more IL-4, and demonstrated reduced proliferation (Figure 4A-C). The proliferation was not different between the groups in a primary MLR but was less in the IL-18 group both in a secondary MLR and in a post-BMT MLR (Figure 4C). These data are consistent with previous observations of reduced proliferation by TH2 cells after BMT.38 Consistent with these in vitro findings, recipients of allogeneic BM transplants from donors pretreated with IL-18 had significantly reduced serum IFN-γ levels on day 4 after transplantation (Figure 6A), but IL-4 was not detected. These results are consistent with previous reports that IL-4 was not measurable in the serum despite enhanced TH2 polarization of donor T cells in MLR cultures after BMT.42 43 Thus IL-18 administration to the donors produced a diminished TH1, and enhanced TH2 responses after BMT.
Donor T-cell cytokine and cytolytic functions after BMT.
B6 Ly5.2 (CD45.1+) animals were irradiated and received BM transplants from BALB/c donor mice that had been injected with control diluent (▪) or IL-18 (░) as in Figure 1. Splenocytes from the recipients (n = 3/group) were harvested and combined on day 7 after BMT. Splenocytes were normalized for donor T cells (CD45.1-and CD3+) and restimulated in quadruplicate with irradiated naive host (B6 Ly5.2) splenocytes in MLR cultures. Supernatants were collected after 48 hours of culture for cytokine measurement. Proliferation was determined by incubation of cells with3H-thymidine (1 μCi/well [0.037 MBq]) for an additional 24 hours. T cells from IL-18–treated donors produced significantly (A) less IFN-γ (▪ vs ░; *P < .05), (B) more IL-4 (▪ vs ░; **P < .01), and (C) less proliferation (▪ vs ░; *P < .05) in MLR cultures. (D) IL-18 treatment preserves CTL function after BMT. Splenocytes harvested from allogeneic animals on day 14 after BMT were pooled (n = 3/group), and normalized for donor CD8+ cells and used in a 51Cr release assay. CTL activity against allogeneic targets (EL4) in control (▴) and IL-18 (●) groups was similar, while there was no significant lysis of syngeneic targets (P815) by both the groups (○ and ▵). Error bars represent standard error.
Donor T-cell cytokine and cytolytic functions after BMT.
B6 Ly5.2 (CD45.1+) animals were irradiated and received BM transplants from BALB/c donor mice that had been injected with control diluent (▪) or IL-18 (░) as in Figure 1. Splenocytes from the recipients (n = 3/group) were harvested and combined on day 7 after BMT. Splenocytes were normalized for donor T cells (CD45.1-and CD3+) and restimulated in quadruplicate with irradiated naive host (B6 Ly5.2) splenocytes in MLR cultures. Supernatants were collected after 48 hours of culture for cytokine measurement. Proliferation was determined by incubation of cells with3H-thymidine (1 μCi/well [0.037 MBq]) for an additional 24 hours. T cells from IL-18–treated donors produced significantly (A) less IFN-γ (▪ vs ░; *P < .05), (B) more IL-4 (▪ vs ░; **P < .01), and (C) less proliferation (▪ vs ░; *P < .05) in MLR cultures. (D) IL-18 treatment preserves CTL function after BMT. Splenocytes harvested from allogeneic animals on day 14 after BMT were pooled (n = 3/group), and normalized for donor CD8+ cells and used in a 51Cr release assay. CTL activity against allogeneic targets (EL4) in control (▴) and IL-18 (●) groups was similar, while there was no significant lysis of syngeneic targets (P815) by both the groups (○ and ▵). Error bars represent standard error.
We next examined the effects of IL-18 pretreatment on cytolytic activity of donor cells to host antigens after BMT. Splenocytes harvested on day 14 after BMT from B6 recipients of both IL-18–treated and control donor cells (H-2d) showed strong lytic activity against host-type EL-4 (H-2b) targets (Figure 4D). Significant lysis of syngeneic, P815 (H-2d), targets was not observed, confirming allospecific cytotoxic activity. Thus IL-18 treatment of donors, in contrast to the posttransplantation MLR findings, did not alter the allospecific cytotoxic T-lymphocyte (CTL) response of donor cells after BMT.
STAT6 signaling in donor T cells is required for attenuation of GVHD by IL-18
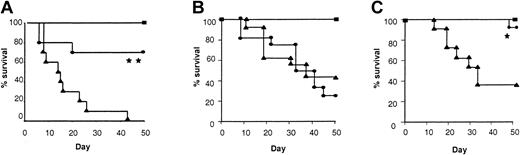
We hypothesized that reduction of acute GVHD observed after IL-18 treatment of donors was mediated through the T-cell component of the donor inoculum. To test this hypothesis we first performed in vivo mixing experiments. TCD BM from control-treated allogeneic donors was mixed with purified CD4+ (purity > 90%) cells from either IL-18– or control-treated mice. As shown in Figure5A, recipients of CD4+ cells from IL-18–treated donors demonstrated a significantly improved survival compared with the controls (70% vs 0%;P < .01).
Requirement of T-cell polarization and STAT6 but not STAT4 signaling in donor cells for IL-18–mediated GVHD protection.
(A) B6 mice were irradiated and received transplants of control-treated 5 × 106 TCD BM and with 2 × 106 purified CD4+ cells from either control-treated (▴, n = 10) or IL-18–treated (●, n = 10) allogeneic BALB/c donors. Syngeneic controls (▪, n = 5) received similar number of T and BM cells from B6 donors. BMT recipients of IL-18–treated CD4+ cells showed significantly improved survival (▴ vs ●; **P < .01). (B) B6 mice underwent transplantation as in Figure 1 with either syngeneic B6 (H2b) donors (▪, n = 6) or allogeneic STAT6-deficient (H2d) donor mice injected with control diluent (▴, n = 15) or IL-18 (●, n = 15) for 10 days. Data combined from 2 similar experiments are shown (▴ vs ●; P = NS). (C) B6 mice underwent transplantation as in Figure 1 with either syngeneic B6 (H2b) donors (▪, n = 6) or allogeneic STAT4-deficient (H2d) donor mice injected with control diluent (▴, n = 15) or IL-18 (●, n = 15) for 10 days (▴ vs ●; *P < .02). Data combined from 2 similar experiments are shown.
Requirement of T-cell polarization and STAT6 but not STAT4 signaling in donor cells for IL-18–mediated GVHD protection.
(A) B6 mice were irradiated and received transplants of control-treated 5 × 106 TCD BM and with 2 × 106 purified CD4+ cells from either control-treated (▴, n = 10) or IL-18–treated (●, n = 10) allogeneic BALB/c donors. Syngeneic controls (▪, n = 5) received similar number of T and BM cells from B6 donors. BMT recipients of IL-18–treated CD4+ cells showed significantly improved survival (▴ vs ●; **P < .01). (B) B6 mice underwent transplantation as in Figure 1 with either syngeneic B6 (H2b) donors (▪, n = 6) or allogeneic STAT6-deficient (H2d) donor mice injected with control diluent (▴, n = 15) or IL-18 (●, n = 15) for 10 days. Data combined from 2 similar experiments are shown (▴ vs ●; P = NS). (C) B6 mice underwent transplantation as in Figure 1 with either syngeneic B6 (H2b) donors (▪, n = 6) or allogeneic STAT4-deficient (H2d) donor mice injected with control diluent (▴, n = 15) or IL-18 (●, n = 15) for 10 days (▴ vs ●; *P < .02). Data combined from 2 similar experiments are shown.
Because pretreatment of donors with IL-18 resulted in a decrease in IFN-γ, and TH1 response of donor T cells is known to be critical to GVHD induction,12-14 we next tested the hypothesis that donor-derived IFN-γ might be critical for IL-18 to mediate its effect. We used IFN-γ–deficient (B6, H-2b) mice as donors and BALB/c (H-2d) mice as recipients. Preliminary experiments demonstrated that the severity of acute GVHD in this B6→BALB/c donor-recipient BMT system is comparable with the BALB/c→B6 system. Surprisingly, when IFN-γ–deficient mice were pretreated with rmIL-18 and used as donors, the protective effect of IL-18 was again observed in terms of GVHD morbidity (clinical scores, 3.75 ± 0.9 vs 6.25 ± 1.2; P < .05) and mortality (day 50 survival, 40% vs 0%; P = .034) The absence of IFN-γ secretion by donor T cells was therefore not critical for the IL-18–mediated GVHD protection.
In order to confirm these surprising results and to elucidate the molecular mechanism of the effect of IL-18 on donor T-cell polarization and GVHD protection, we used donor mice that lacked STAT4 and that had impaired TH1 responses.44 IL-18 treatment of BALB/c STAT4-deficient donor mice reduced GVHD in B6 recipients, confirming that STAT4 is not required for the protective effect of IL-18 (P < .02; Figure 5C). Taken together these 2 separate experimental models confirm each other and show that the alteration in TH1 response as determined by secretion of IFN-γ is not critical for the GVHD protection mediated by IL-18.
Pretreatment of wild-type donors with IL-18 also enhanced secretion of IL-4 by donor T cells after BMT (Figure 4), and Yoshimoto et al7 have recently demonstrated that STAT6 is critical for IL-18–mediated TH2 polarization. Therefore we next determined whether STAT6 signaling, which is critical to TH2 responses, is important for the protective effect of IL-18 on GVHD.44 We injected IL-18 or the diluent into BALB/c STAT6−/− (H2d) mice as described in “Materials and methods.” In contrast to its effects on wild-type donors, injection of IL-18 increased IFN-γ but not IL-4 in the sera of BALB/c STAT6-deficient mice (Table 2) and was not able to confer protection against acute GVHD mortality to B6 (H2b) recipients (Figure 5B). Furthermore recipients of allogeneic BM transplants from STAT6−/− donors pretreated with IL-18 had high levels of serum IFN-γ without any measurable IL-4 on day 4 after BMT (Figure 6B). These data therefore support the hypothesis that alteration of TH2 responses by donor T cells via STAT6 signaling is critical for the protective effect of IL-18.
Effect of IL-18 on serum levels of IFN-γ in the BM transplant recipients.
(A) B6 Ly5.2 (CD45.1+) animals were irradiated and received BM transplants from BALB/c donor mice that had been injected with control diluent (▪) or IL-18 (░) as in Figure 1. Serum was obtained (n = 3 mice/group) by retro-orbital venous puncture on day 4 after BMT; serum levels of IFN-γ were significantly reduced in recipients of IL-18–treated (░) versus diluent-treated (▪) donors; *P < .05. Results from 1 of 3 similar experiments are shown. (B) B6 animals were irradiated and received transplants of cells from either syngeneic B6 (H2b) donors (■) or allogeneic STAT6-deficient (H2d) donor mice injected with control diluent (▪) or IL-18 (░) for 10 days as in Figure 1. Serum was obtained (n = 4/group) on day 4 after BMT and analyzed for cytokines. IFN-γ was increased in the recipients of IL-18–treated (░) and control-treated (▪) allogeneic donor cells (▪ vs ░;P = NS). IL-4 was not detected. Error bars represent standard error.
Effect of IL-18 on serum levels of IFN-γ in the BM transplant recipients.
(A) B6 Ly5.2 (CD45.1+) animals were irradiated and received BM transplants from BALB/c donor mice that had been injected with control diluent (▪) or IL-18 (░) as in Figure 1. Serum was obtained (n = 3 mice/group) by retro-orbital venous puncture on day 4 after BMT; serum levels of IFN-γ were significantly reduced in recipients of IL-18–treated (░) versus diluent-treated (▪) donors; *P < .05. Results from 1 of 3 similar experiments are shown. (B) B6 animals were irradiated and received transplants of cells from either syngeneic B6 (H2b) donors (■) or allogeneic STAT6-deficient (H2d) donor mice injected with control diluent (▪) or IL-18 (░) for 10 days as in Figure 1. Serum was obtained (n = 4/group) on day 4 after BMT and analyzed for cytokines. IFN-γ was increased in the recipients of IL-18–treated (░) and control-treated (▪) allogeneic donor cells (▪ vs ░;P = NS). IL-4 was not detected. Error bars represent standard error.
Administration of IL-18 to donors preserves GVL activity after allogeneic BMT
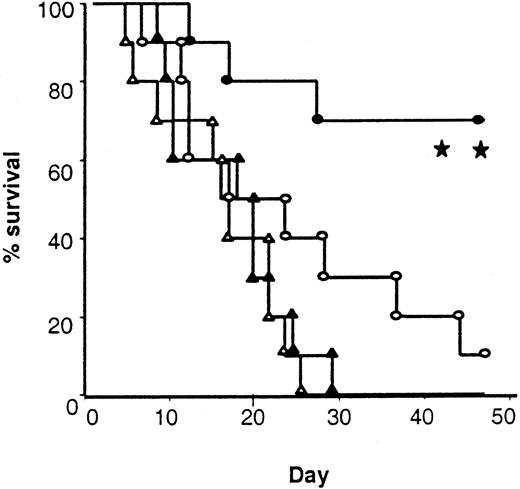
Maintenance of donor cytotoxic responses to host antigens (Figure4D) suggested that treatment of donors with IL-18 might preserve GVL activity even though it decreased acute GVHD. To test this hypothesis, we added 2000 EL-4 (T-cell leukemia/lymphoma) cells (H-2bCD45.2+) to the BM inoculum injected into each mouse on the day of BMT. In vivo EL4 behaves like leukemia, resulting in massive infiltration and enlargement of various organs, such as the liver, spleen, and kidneys, and an injection of as few as 500 cells is uniformly fatal.45 Injection of 2000 EL-4 cells with the BM transplant inoculum caused 100% mortality in syngeneic B6 hosts by day +28 with evidence of tumor infiltrating into liver and spleen (Figure 7 and data not shown). Identically treated recipients of IL-18–treated allogeneic BM transplant donors showed 70% survival compared with 10% in controls (P < .01; Figure 7). Necropsy performed on all animals (either on the day of death or at the end of the observation period) showed no residual tumor by macroscopic examination of liver, spleen, and kidneys or by FACS analysis of the spleens (data not shown). Taken together, these data confirm that allogeneic BM transplants from IL-18–treated donors reduce acute GVHD severity while preserving a GVL effect.
Survival after leukemia induction.
Total body irradiated B6 mice received 5 × 106 TCD BM and 2 × 106 splenic T cells from either allogeneic control (○, n = 10) and IL-18–pretreated (●, n = 10) BALB/c donors or syngeneic control (▵, n = 10) and IL-18–pretreated (▴, n = 10) B6 Ly5.2 donors. All the recipient mice were injected intravenously with 2000 EL4 tumor cells on day 0 (○ vs ●; **P < .01, and ▴ vs ●;P < .001).
Survival after leukemia induction.
Total body irradiated B6 mice received 5 × 106 TCD BM and 2 × 106 splenic T cells from either allogeneic control (○, n = 10) and IL-18–pretreated (●, n = 10) BALB/c donors or syngeneic control (▵, n = 10) and IL-18–pretreated (▴, n = 10) B6 Ly5.2 donors. All the recipient mice were injected intravenously with 2000 EL4 tumor cells on day 0 (○ vs ●; **P < .01, and ▴ vs ●;P < .001).
Discussion
Since its initial discovery in the sera of mice treated withPropionibacterium acnes and LPS, IL-18 has been shown to play a critical role in the immune modulation of several disease and biologic processes by inducing TH1 secretion.1,2 Yet mice deficient in IL-18 can eventually develop TH1 cells,46,47 and exogenous administration of IL-18 to naive mice can induce TH2 polarization.7,8 Recent studies have also revealed that although IL-18R expression is up-regulated in the presence of TH1 cytokines, naive T cells express low levels of IL-18R, and when cultured with IL-18 they produce TH2 cytokines.2,48 Furthermore IL-18 induces basophils and mast cells to produce IL-4 and IL-138 and also increases serum immunoglobulin E (IgE) levels in a dose-dependent manner.7,49 Although the reason for TH2 cytokine induction by IL-18 under some circumstances remains unexplained, this phenomenon is dependent on STAT6.7 More interestingly, IL-18 transgenic mice secrete higher than normal amounts of both TH1 and TH2 cytokines.11Thus IL-18 has the remarkable capacity to induce TH2 cytokine secretion in addition to TH1 cytokines, depending on the immunologic and stimulatory context.2
The ability of IL-18 to modulate various diseases by inducing TH1 cytokine production has been amply demonstrated,2 but its significance in modulating disease processes as a TH2-inducing cytokine is unclear. We made the surprising observation that pretreatment of allogeneic donors with IL-18 was associated with TH2 polarization of donor cells and attenuated acute GVHD severity and mortality. Given that GVHD in this system is mediated predominantly by CD4+ T cells,18 mixing experiments of control-treated TCD BM and purified CD4+ cells from IL-18–treated donor mice demonstrate that the effects of IL-18 on GVHD reduction are secondary to its immunomodulatory effects on donor T cells rather than accessory cells. Even though STAT6-independent TH2 induction has been reported,50,51 our data demonstrate that signaling via STAT6 is necessary for acute GVHD protection associated with TH2 polarization induced by IL-18. Allogeneic BM transplants from STAT4-deficient donors (which have increased propensity toward TH2 polarization) or IL-4–deficient donors (which have increased propensity toward TH1 polarization) can cause, albeit delayed, mortality from GVHD compared with STAT6-deficient or wild-type donors.21,25Administration of IL-18 retained its protective effect against acute GVHD mortality when given to the donors deficient in IFN-γ and STAT4, showing that alterations in TH1 cytokines play little or no role in mediating this effect. GVHD protection was lost, however, when STAT6-deficient mice were used as donors, suggesting that enhanced TH2 cytokine production via STAT6 is critical for the protection afforded by IL-18. It remains possible that the pathogenesis of GVHD in mice receiving STAT4−/− BM transplants differs from that in animals that received transplants from STAT6−/− donors, and IL-18 may affect one but not the other. Furthermore, although our data suggest a critical role for STAT6 signaling in the mechanism of action of IL-18, we cannot rule out the possibility that molecules upstream to STAT6, such as JAKs, might also be necessary. Experiments are currently in progress to explore whether secretion of IL-4 or other type-2 cytokines by STAT6-independent mechanisms might play a critical role in the beneficial effect mediated by IL-18.50 51
We have previously demonstrated that administration of IL-18 to BM transplant hosts early after transplantation attenuates acute GVHD by enhancing Fas-dependent apoptosis of donor T cells and that donor-derived IFN-γ is critical for this protection.20In this context the predominant effect of IL-18 is to induce TH1 cytokines. However when IFN-γ–deficient mice were pretreated with IL-18 and used as donors, the protective effect of IL-18 was observed. Clearly the data presented herein demonstrate that administration of IL-18 to BM transplant donors reduces acute GVHD by a different mechanism, one that is independent of donor-derived IFN-γ but requires STAT6 signaling in donor T cells. Thus IL-18 plays different roles in modulating cytokine responses and Th polarization depending on whether the donor T cells are resting or already activated by alloantigens and can reduce GVHD.
The role of TH1/TH2 polarization as it relates to acute GVHD is controversial. Although the “cytokine storm” amplified by the TH1 phenotype correlates with the development of acute GVHD,12-14 early TH1 polarization of donor T cells by administration of cytokines, such as IFN-γ, IL-2, and IL-12, to BM transplant recipients can attenuate acute GVHD.15-19 Furthermore the use of TH1 cytokine-deficient mice as BM transplant donors still results in GVHD21,25,52,53; and some studies failed to show a beneficial effect of TH2 polarization on acute GVHD.21-26However TH2 polarization of donor T cells by IL-4, both in vivo54-56 and ex-vivo,42,57 the use of G-CSF to mobilize donor cells (which induces TH2 polarization),43 administration of IL-11,38and the secretion of IL-4 by NK1.1+ T cells,58,59 all reduce acute GVHD, albeit the mechanisms of these effects are undetermined. Herein we demonstrate that pretreatment of donors is associated with TH2 polarization and attenuation of GVHD and go on to further determine that STAT6 is critical for this effect. Furthermore, a previous study suggested that TH1 and TH2 subsets cause injury of distinct target tissues21; TH2 cells were required for hepatic damage and TH1 cells for GI tract damage after allogeneic BMT. In contrast, our study shows that IL-18 administration to donors enhanced TH2 polarization and reduced GVHD damage in both the GI tract and liver. This difference may be due to time at which the organs were evaluated for damage. In our study, both target organs (liver and GI tract) were evaluated simultaneously on day 7, whereas in the previous study they were evaluated at various times between the groups, over an extended period.21 Taken together with our study all of these data demonstrate that the timing of administration and the systemic production of any given cytokine may be critical to the eventual outcome of acute GVHD.
The toxicity of GVHD is difficult to separate from the benefits of GVL effect, the physiology of which is complex and likely involves multiple antitumor mechanisms, including both cellular and inflammatory effectors.60-62 Donor cytotoxic functions are critical to the preservation of GVL effects after allogeneic BMT.63IL-18 administration to donors did not change antihost cytotoxic responses in vitro despite TH2 polarization and preserved GVL effects in vivo in this model as shown by improved leukemia-free survival after BMT. The induction of GVHD in this murine model is mediated predominantly by CD4+ T cells,18while GVL against EL-4, an MHC II−/− lymphoma, is mediated by CD8+ T cells.36 Thus, pretreatment of donors with IL-18 preserved CD8+ T-cell–mediated GVL effect while attenuating CD4+-dependent GVHD. It remains to be determined whether the IL-18 treatment will preserve CD4+-mediated GVL. The present study is consistent with previous reports that demonstrate that T-cell polarization does not affect CTL generation.13,38,64 Donor T cells mediate their cytolytic functions by several mechanisms, including granule exocytosis and the Fas pathway,63,65 and several recent studies have suggested that GVHD and GVL may be mediated by differential CTL lytic mechanisms.37,66 67 Studies are in progress to determine if donor T-cell polarization by IL-18 is associated with differential use of these cytotoxic mechanisms in mediating GVHD and GVL.
In summary, our data demonstrate a novel role for IL-18 in reducing the severity of acute GVHD via STAT6, independent of donor-derived IFN-γ, while preserving the beneficial GVL effects. Our findings emphasize the complex and diverse roles of IL-18 in modulating in vivo allo-immune reaction depending on the state of donor T-cell activation and the inflammatory milieu. Given that IL-18 is nontoxic7,8,32,33and can induce the secretion of various hematopoietic growth factors,6 these data suggest that IL-18 may serve as a novel adjunct to standard donor-cell mobilization that can attenuate acute GVHD without compromising CD8+-mediated GVL effect.
K.R.C. is a scholar of the National Marrow Donor Program (NMDP) Amy Strelzer-Manasevit Scholarship Program and a Fellow of the Robert Wood Johnson Minority Medical Faculty Development Program. J.L.M.F. is a Distinguished Clinical Scientist of the Doris Duke Foundation.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-08-2566.
Supported by National Institutes of Health (NIH) grant CA 49542 (J.L.M.F.), and a Young Investigator Award by the American Society of Clinical Oncology (P.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James L. M. Ferrara, 6410 CCGC, University of Michigan Cancer Center, 1500 E Medical Center Dr, Ann Arbor, MI 48109-0942; e-mail: ferrara@umich.edu.

![Fig. 2. IL-18 treatment of donor mice reduces the acute GVHD pathologic damage of liver and GI tract in the recipient. / Donor cells from IL-18–treated or diluent-treated (control) BALB/c mice were transplanted into B6 recipients as in Figure 1. Animals that underwent transplantation (n = 4/group) were killed; liver and gut were obtained for analysis on day 7 after BMT. Histology of the liver from the recipients of control-treated (A) and IL-18–treated (B) allogeneic donors. Coded slides were scored semiquantitatively for pathologic damage, as described in “Materials and methods.” BM transplant recipients of diluent-treated (▪, n = 4) or IL-18–treated (░, n = 4) allogeneic donors and of syngeneic donors (■, n = 4) are shown (C) (*P < .05; control [▪] vs IL-18 [░] allo group). Histology of the large bowel from the recipients of control-treated (D) and IL-18–treated (E) allogeneic donors is shown. Coded slides were scored semiquantitatively, as in “Materials and methods.” BMT recipients of diluent-treated (▪, n = 4) or IL-18–treated (░, n = 4) allogeneic donors and of syngeneic donors (■, n = 4) are shown (F). Total GVHD score: mean ±SE of the sum of scores for small bowel and colon from individual animals in each group (*P < .05 vs IL-18 allo group). Original magnification × 400 (A-B, D-E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-08-2566/3/m_h80734096002.jpeg?Expires=1769104295&Signature=CllXipvTIpmWJV~07jB0LHoikaYQms4-L4dAqShYa~8~VwzwkEj4nyRCRt9SursWVIwt~wrt5zv8Fj1iJ94C1wgFiKceOpPe-7wBu0ixMLeDPwmELs7Oa9vHr1omeMj4fnbBfWHwVbfPDtgzmvnObIl9QhPJcZm0QnDQCErvoebXtc22s4hlx-hvFXEbefd-7VNJgM9ZGbXsq0UkGDfQJg9F4yMP6SVQChltja24gH26PyaxTnsOP11D8oY~iobxn4P7UWE4s8okKSRmHQXrEyua9aHbGj~V69BsNLNwv02zpusCaiQIQvbb3iRJyQIzHZl2nWyg9j15JxstIEwPcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Donor T-cell cytokine and cytolytic functions after BMT. / B6 Ly5.2 (CD45.1+) animals were irradiated and received BM transplants from BALB/c donor mice that had been injected with control diluent (▪) or IL-18 (░) as in Figure 1. Splenocytes from the recipients (n = 3/group) were harvested and combined on day 7 after BMT. Splenocytes were normalized for donor T cells (CD45.1-and CD3+) and restimulated in quadruplicate with irradiated naive host (B6 Ly5.2) splenocytes in MLR cultures. Supernatants were collected after 48 hours of culture for cytokine measurement. Proliferation was determined by incubation of cells with3H-thymidine (1 μCi/well [0.037 MBq]) for an additional 24 hours. T cells from IL-18–treated donors produced significantly (A) less IFN-γ (▪ vs ░; *P < .05), (B) more IL-4 (▪ vs ░; **P < .01), and (C) less proliferation (▪ vs ░; *P < .05) in MLR cultures. (D) IL-18 treatment preserves CTL function after BMT. Splenocytes harvested from allogeneic animals on day 14 after BMT were pooled (n = 3/group), and normalized for donor CD8+ cells and used in a 51Cr release assay. CTL activity against allogeneic targets (EL4) in control (▴) and IL-18 (●) groups was similar, while there was no significant lysis of syngeneic targets (P815) by both the groups (○ and ▵). Error bars represent standard error.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-08-2566/3/m_h80734096004.jpeg?Expires=1769104295&Signature=iUzlilErVI5R8FUWACYLN4nfM3nRSSxglPw5GdCgMrD3w76Hm9sFQToOLBk8fX4GQSe1cbcsbJ1u8gkkunZQ96OmmCvH8KRngBPedte9gBvqrH56Mdx8FsPw91OKOKWBLvLevJE91bIAtKwPS8ghcoXoJSnDkTX2qLWIUVO035VIoDue-SxLo1Zhv2zDPfTCGkve7sUDFXiZKkH0nUr5QG7BgLrc9ceIKES~ebRuQn2zORToRJ8bZLEXLz0b6rfYoTQ7M6a1NhOlwswB8VSIpb9u-qAZpiMJjtmoWRLzYLVQTCtt9Jsf39v9Jqy003prOSas9lTs9zO7hnK0bi79tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal