Abstract
Patients affected by β-thalassemia major require lifelong transfusions because of insufficient or absent production of the β chain of hemoglobin (Hb). A minority of patients are cured by allogeneic bone marrow transplantation. In the most severe of the hitherto available mouse models of β-thalassemia, a model for human β-thalassemia intermedia, we previously demonstrated that globin gene transfer in bone marrow cells is curative, stably raising Hb levels from 8.0-8.5 to 11.0-12.0 g/dL in long-term chimeras. To fully assess the therapeutic potential of gene therapy in the context of a lethal anemia, we now have created an adult model of β0-thalassemia major. In this novel model, mice engrafted with β-globin–null (Hbbth3/th3) fetal liver cells succumb to ineffective erythropoiesis within 60 days. These mice rapidly develop severe anemia (2-4 g/dL), massive splenomegaly, extramedullary hematopoiesis (EMH), and hepatic iron overload. Remarkably, most mice (11 of 13) treated by lentivirus-mediated globin gene transfer were rescued. Long-term chimeras with an average 1.0-2.4 copies of the TNS9 vector in their hematopoietic and blood cells stably produced up to 12 g/dL chimeric Hb consisting of muα2:huβ2tetramers. Pathologic analyses indicated that erythroid maturation was restored, while EMH and iron overload dramatically decreased. Thus, we have established an adult animal model for the most severe of the hemoglobinopathies, Cooley anemia, which should prove useful to investigate both genetic and pharmacologic treatments. Our findings demonstrate the remarkable efficacy of lentivirus-mediated globin gene transfer in treating a fulminant blood disorder and strongly support the efficacy of gene therapy in the severe hemoglobinopathies.
Introduction
The β-thalassemias are caused by more than 200 mutations that lead to decreased or absent production of the β chain of hemoglobin (Hb).1-3 Most of the mutations are point mutations in the β-globin gene that either decrease the level of transcription or mRNA stability or lead to nonfunctional mRNA, as is the case in nonsense and frameshift mutations. Other mutations include deletions in the β-globin gene, as well as rare deletions located distally to the β-globin gene itself.1,3,4 There is a large spectrum in the severity of the disease found in homozygotes and compound heterozygotes. Patients affected by β-thalassemia intermedia do not require chronic transfusion therapy in the first years of life, but they often worsen over time, eventually developing hypersplenism, osteopenia, extramedullary hematopoiesis (EMH), and iron overload. Some patients eventually require splenectomy and blood transfusions. In patients with β-thalassemia major, or Cooley anemia, the absent or extremely reduced production of the β chain of Hb causes severe ineffective erythropoiesis, massive erythroid hyperplasia in the bone marrow and extramedullary sites, and hemolysis. The ensuing iron overload can lead to endocrine deficiencies, cirrhosis, and cardiac failure. In the absence of lifelong transfusions, the disease is lethal.5 Current disease management consists of prenatal diagnosis, transfusion therapy, or allogeneic bone marrow transplantation.1,3 4 Only the latter is curative, but this option is limited to a minority of patients for whom a histocompatible donor can be identified.
New approaches aimed at ameliorating the condition of patients with severe β-thalassemia are greatly needed. Genetic approaches based on the transfer of a regulated human β-globin gene in autologous hematopoietic stem cells (HSCs) represent an attractive potential treatment.6 Their implementation has been hampered by the difficulty of appropriately regulating expression of globin genes in the progeny of virally transduced HSCs. The therapeutic globin gene must indeed be expressed in a tissue-specific fashion and at a high level.7,8 We recently demonstrated that complex lentiviral vectors harboring the human β-globin gene and an optimized combination of proximal and distal transcriptional control elements direct erythroid-specific and sustained globin gene expression in murine bone marrow chimeras.9 In mice with β-thalassemia intermedia, the TNS9 lentiviral vector durably ameliorated anemia.9,10 A similar vector encoding a variant globin gene reduced sickle cell formation in 2 murine models of sickle cell disease (SCD).11 Obtained in hitherto available models of severe hemoglobinopathies, these encouraging findings do not address whether stem cell–based gene therapy is potentially curative for a deficiency as profound as that found in Cooley anemia.
Three mouse models of β-thalassemia have been generated to date. These mouse models are affected by different degrees of β-thalassemia intermedia. The th1 model results from the deletion of the βmajor gene; Hbbth1/th1homozygotes exhibit a moderate form of thalassemia.12 A second model (th2) was generated by insertional disruption of the βmajor gene, causing lethality in Hbbth2/th2 homozygotes but only a very mild phenotype in the heterozygotes.13 The third model,th3, was generated by deletion of both the βmajor and βminor genes.14Mice homozygous for this deletion die late in gestation, while heterozygotes are viable and thalassemic. Adult Hbbth1/th1 and Hbbth3/+mice exhibit anemia (slightly more severe in the latter model), abnormal red cell morphology, and splenomegaly, and develop spontaneous hepatic iron deposition similar to that found in humans with β-thalassemia intermedia. The lack of an animal model for Cooley anemia has limited the full investigation of the pathophysiology underlying this disease and hampered the evaluation of both pharmacologic and genetic treatments.
Here we describe a severe anemia model in which mice engrafted with β-globin–null (Hbbth3/th3) fetal liver cells succumb to ineffective erythropoiesis within 60 days. This novel model enabled us to investigate whether globin gene transfer could rescue a massive protein deficit. We show that treatment with the TNS9 vector durably rescues most treated chimeras. In hematopoietic chimeras with a mean vector copy number of 1.0 to 2.4 in hematopoietic and blood cells, stable production of chimeric Hb (Hbbhu, consisting of chimeric murine β2:human β2 tetramers) was achieved, reaching levels up to 12 g/dL and effectively treating the anemia. These findings strongly support the feasibility of a genetic treatment for β-thalassemia and have general implications for devising stem cell–based treatments for severe blood disorders.
Materials and methods
Hematopoietic chimeras
Donor fetal liver cells (FLCs) were harvested from embryonic day (ED) 14.5 embryos obtained by intercrossing Hbbth3/+ mice (Jackson Laboratories, Bar Harbor, ME). Embryo genotypes were screened by analyzing Hb by cellulose acetate electrophoresis (Figure 1A). FLCs were resuspended in X-VIVO-15 serum-free medium and supplemented with 10 ng/mL IL-1α, 100 U/mL IL-3, 150 U/mL IL-6, 10 ng/mL Kit ligand obtained from Genzyme (Cambridge, MA), 0.5 mM β-mercaptoethanol obtained from Sigma (St Louis, MO), 200 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. FLCs were then pelleted and resuspended in serum-free medium containing concentrated lentiviral supernatant and supplemented with 4 mg/mL polybrene obtained from Sigma, 200 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and cytokines as above, and cultured for 8 hours. Transduced FLCs (2 × 106) were then intravenously injected into each of the irradiated female recipients to establish bone marrow chimeras. Recipient mice (11- to 14-week-old C57/BL6 mice) were irradiated with 12 Gy (split dose 2 × 6 Gy) on the day of transplantation. Vector stocks were generated by triple transfection into 293T cells, as previously described.9 Concentrated viral stocks with titers of 1-2 × 108 infectious particles/mL were used to infect 2 × 106 FLCs in a final volume of 1 mL (multiplicity of infection = 50). The lentiviral vectors TNS9 and pHR'eGFP (enhanced green fluorescent protein), derived from pHR′LacZ,15 have been previously described.9
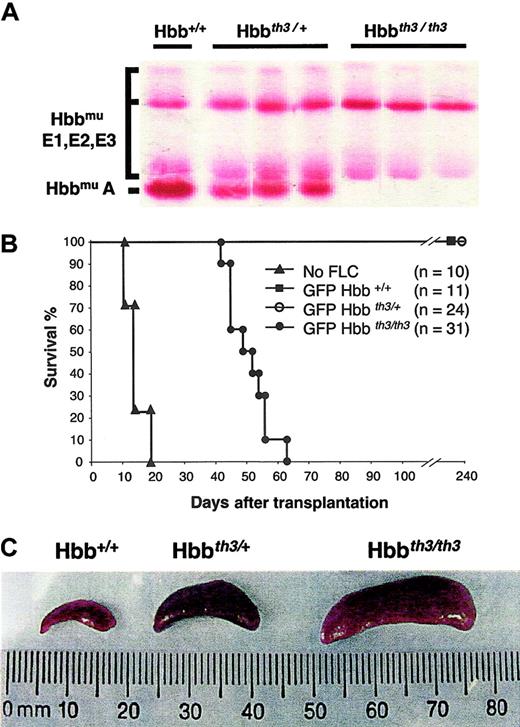
Establishment of an adult model of β0-thalassemia.
(A) Cellulose acetate analysis showing the hemoglobin patterns in ED 14.5 Hbb+/+, Hbbth3/+, and Hbbth3/th3 embryos. E1, E2, and E3 indicate the 3 embryonic hemoglobins. (B) Mice engrafted with Hbbth3/th3 hematopoietic FLCs die 6 to 9 weeks after receiving transplants. Donor FLCs harvested from ED 14.5 embryos were injected in irradiated C57BL/6 recipient mice. Mice engrafted with Hbbth3/th3 FLCs died or had to be killed 7 to 9 weeks after receiving transplants. Recipients of Hbb+/+ and Hbbth3/+ FLCs survived for at least 8 months (duration of follow-up). (C) Spleen sizes in mice engrafted with Hbb+/+, Hbbth3/+, and Hbbth3/th3 FLCs at 6 to 8 weeks after receiving transplants.
Establishment of an adult model of β0-thalassemia.
(A) Cellulose acetate analysis showing the hemoglobin patterns in ED 14.5 Hbb+/+, Hbbth3/+, and Hbbth3/th3 embryos. E1, E2, and E3 indicate the 3 embryonic hemoglobins. (B) Mice engrafted with Hbbth3/th3 hematopoietic FLCs die 6 to 9 weeks after receiving transplants. Donor FLCs harvested from ED 14.5 embryos were injected in irradiated C57BL/6 recipient mice. Mice engrafted with Hbbth3/th3 FLCs died or had to be killed 7 to 9 weeks after receiving transplants. Recipients of Hbb+/+ and Hbbth3/+ FLCs survived for at least 8 months (duration of follow-up). (C) Spleen sizes in mice engrafted with Hbb+/+, Hbbth3/+, and Hbbth3/th3 FLCs at 6 to 8 weeks after receiving transplants.
Hematologic studies
Blood samples were obtained by retro-orbital puncture under ether anesthesia. Total hemoglobin levels, red cell counts, hematocrit levels, neutrophil counts, platelet counts, and reticulocyte counts were measured on a blood cell count analyzer (H System, Bayer, Leverkusen, Germany). Plasma erythropoietin (EPO) concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) kit for human EPO (R&D Systems, Oxon, United Kingdom).
Protein analyses
Red cell lysates from freshly collected peripheral blood were analyzed by cellulose acetate electrophoresis obtained from Helena Laboratories (Beaumont, TX). Hemoglobin bands were visualized by Ponceau S staining and quantitated by densitometry, as previously described.9
Southern blot analysis
For quantification of vector copy number in HSC chimeras, genomic DNA from tissues was isolated, digested with BamHI, and studied by Southern blot analysis using a [32P]dCTP-labeled NcoI/BamHI fragment of the human β-globin gene as probe. TNS9-transduced single-copy murine erythroleukemia (MEL) clone controls also were digested with BamHI. Radioactive bands were quantitated by phosphorimager analysis (Molecular Dynamics).
Gene expression analysis
Total RNA was extracted from bone marrow (BM), spleen, and blood using TRIzol (Gibco-BRL). Quantitative primer extension assays were performed as previously described,9 using the Primer Extension System-AMV Reverse Transcriptase kit (Promega) with [32P]dATP end-labeled primers specific for lentiviral-encoded human β-globin (5′-CAGTAACGGCAGACTTCTCCTC-3′), mouse β-globin (5′-TGATGTCTGTTTCTGGGGTTGTG-3′), and mouse β-globin (5′-CCTTGATGTTGCTTTTGTCTTC-3′) with predicted extension products of 90 bp, 53 bp, and 69 bp, respectively. Radioactive bands were quantitated by phosphorimager analysis (Molecular Dynamics). All measurements were standardized to RNA samples obtained from YAC transgenic mice harboring one copy of the human β-globin gene (line A85.68,16) as previously described.9
Tissue pathology
Four-micron tissue sections were stained with hematoxylin and eosin or with a Gomori iron stain and examined under light microscopy, as previously described.10 Slides of control and treated mice were assessed in a blind manner.
Statistical analysis
For statistical analyses, we used the permutation rank sum statistic to determine whether hematologic parameters differed between treated and mock-treated groups. A low P value is evidence that the 2 populations are different. The Wilcoxon rank sum statistic was used to compare survival curves between groups.
Results
Establishment of an adult mouse model of β-thalassemia major
Homozygote mice lacking adult β-globin genes (Hbbth3/th3) die late in gestation.14 We therefore attempted to adoptively transfer the hematologic deficit to adult mice by transplanting FLCs harvested from screened ED 14.5 embryos (Figure 1A). Congenic C57BL/6 recipient mice were irradiated (12 Gy) before intravenous infusion of 2 × 106 FLCs. Mice engrafted with Hbb+/+ or Hbbth3/+ FLCs survived for at least 8 months, corresponding to the duration of this study (n = 11 and 24, respectively; Figure 1B). In contrast, recipients of Hbbth3/th3 cells showed a progressive decrease of body mass and activity, eventually requiring termination 7 to 9 weeks after transplantation (T50 = 50 days, n = 31). These mice died significantly later than radiation controls (T50 = 15 days, n = 10,P < .01), suggesting that the cause of death was neither failure to engraft nor pancytopenia.
Hbbth3/th3chimeras die of a selective erythroid defect
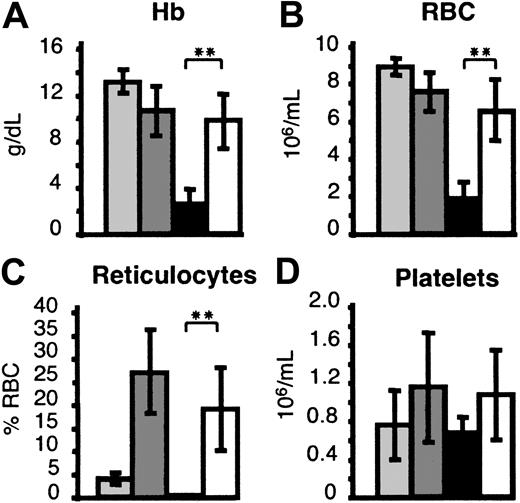
Hematologic analyses performed 6 to 8 weeks after transplantation in mice engrafted with Hbbth3/th3 FLCs revealed severe anemia (2.8 ± 0.8 g/dL of Hb levels, versus 13.2 ± 1.0 in 11 Hbb+/+ chimeras and 11.1 ± 2.1 in 24 Hbbth3/+ chimeras). Low red blood cell (RBC) counts, hematocrit values, and reticulocyte counts, together with high levels of serum erythropoietin (S.R., M.S., unpublished observations), further confirmed the development of a profound erythroid deficiency. Platelet counts (Figure2), as well as neutrophil counts (data not shown), were comparable in experimental and control groups, excluding aplasia or graft failure as the cause of death. Analyses of murine globin transcripts in BM, spleen, and blood by primer extension analysis showed that murine β-globin (muβ) expression was extremely low or undetectable in bone marrow, spleen, and blood of mice engrafted with Hbbth3/th3 FLCs (data not shown). The presence of the β-globin transcript further confirmed that these chimeras developed a selective erythroid defect caused by the lack of β-globin expression. The profound anemia settled in after 50 days, consistent with the clearance rate of the recipient's normal RBCs.17
Hematologic parameters in hematopoietic chimeras 6-8 weeks after transplantation.
(A) Hb: Hemoglobin levels. (B) RBC: Red blood cell counts. (C) Reticulocytes are represented as percentage of RBC. (D) Platelet counts. Donors: light gray indicates Hbb+/+ FLCs; dark gray, Hbbth3/+ FLCs; black, Hbbth3/th3 FLCs; and white, TNS9-transduced Hbbth3/th3.
Hematologic parameters in hematopoietic chimeras 6-8 weeks after transplantation.
(A) Hb: Hemoglobin levels. (B) RBC: Red blood cell counts. (C) Reticulocytes are represented as percentage of RBC. (D) Platelet counts. Donors: light gray indicates Hbb+/+ FLCs; dark gray, Hbbth3/+ FLCs; black, Hbbth3/th3 FLCs; and white, TNS9-transduced Hbbth3/th3.
Hbbth3/th3chimeras develop pathologic features that are characteristic of severe thalassemia
After 6 to 7 weeks, mice engrafted with Hbbth3/th3 FLCs exhibited massive splenomegaly, far exceeding that found in mice engrafted with Hbbth3/+ FLCs (Figure 1C). The massive splenomegaly in the Hbbth3/th3 chimeras was due to marked erythroid hyperplasia consisting mostly of immature nucleated erythroid cells that obliterated the normal microscopic architecture of the spleen. Mature RBCs, still present in the Hbbth3/+ chimera albeit in reduced numbers compared with Hbb+/+ chimera, were virtually absent in their bone marrow and spleen (Figure 3A-B). EMH was absent in the liver of Hbb+/+ chimeras, moderate in Hbbth3/+ chimeras, and extensive in Hbbth3/th3 chimeras (Figure 3C). We also examined hepatic iron accumulation, which is a major characteristic of the systemic disease found in patients with severe thalassemia, arising as a consequence of hemolysis, ineffective erythropoiesis, and increased gastrointestinal iron uptake. In Hbb+/+ and Hbbth3/+ chimeras, iron accumulation was, respectively, absent and moderate, predominantly within Kupffer cells (Figure 3D). In the Hbbth3/th3 chimeras, iron deposition in the liver was found as early as 6 weeks after transplantation, with preferential accumulation in the cytoplasm of the hepatocytes around the portal tracts (Figure 3D). Altogether, these pathologic findings confirmed the presence of severe ineffective erythropoiesis, extensive EMH, and rapid iron accumulation.
Histopathologic analysis of hematopoietic tissues in Hbb+/+, Hbbth3/+, and Hbbth3/th3 chimeras.
(A) Bone marrow (original magnification, × 60). Left: Hbb+/+ control. The marrow shows various stages of normal erythroid maturation. Megakaryocytes as well as a significant numbers of maturing myeloid cells are present. Center: Hbbth3/+ control. The marrow shows a relative increase in erythroid elements; some myeloid cells are still present. Right: Hbbth3/th3 chimera. Misshapen erythroid cells and red cell fragments are present in the sinuses. Some of the immature erythroid cells have dysmorphic nuclei. Mature erythroid cells are decreased. (B) Spleen (original magnification, × 60). Left: Hbb+/+ control. The red pulp contains a mixture of erythroid and myeloid cells. The sinuses are open. Center: Hbbth3/+ control. Significant expansion of red pulp compressing the sinuses. Right: Hbbth3/th3 chimera. The red pulp consists primarily of immature erythroid cells. Mitotic figures are easily identified. The immature erythroid cells often have abnormal nuclei. (C) Liver (original magnification, × 20). Left: Hbb+/+ control. EMH is absent. Center: Hbbth3/+ control. Moderate EMH is present. Right: Hbbth3/th3 chimera: Extensive EMH is present. (D) Liver (original magnification, × 20), Gomori stain). Left: Hbb+/+ control: undetectable iron. Center: Hbbth3/+ control. Moderate iron deposits predominantly in Kuppfer cells. Right: Hbbth3/th3 chimera: Diffuse iron accumulation, concentrated in the cytoplasm of the hepatocytes preferentially around the portal tracts. Control tissues from mice engrafted with eGFP-transduced Hbb+/+ or Hbbth3/+ FLCs were harvested 6 and 8 months after transplantation. Tissues from control Hbbth3/th3 chimeras were harvested 8 weeks after transplantation. Stained with hematoxylin and eosin (A-C).
Histopathologic analysis of hematopoietic tissues in Hbb+/+, Hbbth3/+, and Hbbth3/th3 chimeras.
(A) Bone marrow (original magnification, × 60). Left: Hbb+/+ control. The marrow shows various stages of normal erythroid maturation. Megakaryocytes as well as a significant numbers of maturing myeloid cells are present. Center: Hbbth3/+ control. The marrow shows a relative increase in erythroid elements; some myeloid cells are still present. Right: Hbbth3/th3 chimera. Misshapen erythroid cells and red cell fragments are present in the sinuses. Some of the immature erythroid cells have dysmorphic nuclei. Mature erythroid cells are decreased. (B) Spleen (original magnification, × 60). Left: Hbb+/+ control. The red pulp contains a mixture of erythroid and myeloid cells. The sinuses are open. Center: Hbbth3/+ control. Significant expansion of red pulp compressing the sinuses. Right: Hbbth3/th3 chimera. The red pulp consists primarily of immature erythroid cells. Mitotic figures are easily identified. The immature erythroid cells often have abnormal nuclei. (C) Liver (original magnification, × 20). Left: Hbb+/+ control. EMH is absent. Center: Hbbth3/+ control. Moderate EMH is present. Right: Hbbth3/th3 chimera: Extensive EMH is present. (D) Liver (original magnification, × 20), Gomori stain). Left: Hbb+/+ control: undetectable iron. Center: Hbbth3/+ control. Moderate iron deposits predominantly in Kuppfer cells. Right: Hbbth3/th3 chimera: Diffuse iron accumulation, concentrated in the cytoplasm of the hepatocytes preferentially around the portal tracts. Control tissues from mice engrafted with eGFP-transduced Hbb+/+ or Hbbth3/+ FLCs were harvested 6 and 8 months after transplantation. Tissues from control Hbbth3/th3 chimeras were harvested 8 weeks after transplantation. Stained with hematoxylin and eosin (A-C).
TNS9 rescues lethal β0-thalassemia
The establishment of a novel adult model of severe β-thalassemia allowed us to investigate the efficacy of lentivirus-mediated globin gene transfer in correcting the lethal phenotype and the anemia. Hbbth3/th3 as well as control Hbb+/th3 and Hbb+/+ FLCs were transduced with either the TNS9 vector or a control lentiviral vector encoding eGFP. All mice engrafted with eGFP-transduced Hbbth3/th3 FLCs (n = 20) died as expected within 60 days (Figure 4). In contrast, all mice engrafted with TNS9-transduced Hbbth3/th3 FLCs (n = 13) survived at least 4 months (P < .01, TNS9 versus eGFP-transduced Hbbth3/th3; Figure 4), thus confirming the deficit in β-globin synthesis to be the cause of death. These mice showed a remarkable improvement of their hematologic parameters (Figure 2) 8 weeks after transplantation, with Hbbhu accounting for 30%-65% of total Hb. Of the 13 mice TNS9-treated Hbbth3/th3 chimeras, 10 stably produced Hb consisting predominantly or exclusively of Hbbhu. Over 8 months (Figure 5A-B), 6 consistently showed levels of Hbbhu above 95% of total Hb levels, therefore surviving on the sole basis of sustained production of lentivirus-encoded human β-globin. Chimeras with more than 5% murine Hb were excluded from further analyses to rule out any contribution of residual host erythropoiesis to their pathology and survival. Secondary bone marrow chimeras derived 5 and 6 months after transplantation from 4 of the 6 healthy mice with more than 95% Hbbhu expressed high levels of the human β-globin levels in peripheral blood after 2-4 months (data not shown). These findings, consistent with our results obtained in normal mice,9 indicated that TNS9-treated chimeras were rescued by transduced fetal liver HSCs.
Lentivirus-mediated globin gene transfer rescues Hbb
th3/th3 hematopoietic chimeras from death. Donor Hbb+/+, Hbbth3/+, and Hbbth3/th3 FLCs harvested from ED 14.5 embryos were transduced with TNS9 or a control lentivirus encoding the eGFP protein. Of the 13 TNS9-treated chimeras, 6 stably and predominantly expressed Hbbhu (> 95% of total Hb). The asterisk indicates that healthy Hbb+/+, Hbbth3/+, and Hbbth3/th3 FLCs recipient mice were killed at 4 or 6 months after transplantation to perform pathologic and molecular analysis (see “Results” and Table 1). The remaining mice were killed 8 months after transplantation to complete the analysis. Two mice died 4 months after transplantation, showing profound anemia. Measurement of vector copy number in bone marrow, spleen, and peripheral blood showed, respectively, undetectable and very low vector copy number, thus establishing that these 2 chimeras had been temporally rescued by genetically modified, short-lived erythroid progenitor cells.
Lentivirus-mediated globin gene transfer rescues Hbb
th3/th3 hematopoietic chimeras from death. Donor Hbb+/+, Hbbth3/+, and Hbbth3/th3 FLCs harvested from ED 14.5 embryos were transduced with TNS9 or a control lentivirus encoding the eGFP protein. Of the 13 TNS9-treated chimeras, 6 stably and predominantly expressed Hbbhu (> 95% of total Hb). The asterisk indicates that healthy Hbb+/+, Hbbth3/+, and Hbbth3/th3 FLCs recipient mice were killed at 4 or 6 months after transplantation to perform pathologic and molecular analysis (see “Results” and Table 1). The remaining mice were killed 8 months after transplantation to complete the analysis. Two mice died 4 months after transplantation, showing profound anemia. Measurement of vector copy number in bone marrow, spleen, and peripheral blood showed, respectively, undetectable and very low vector copy number, thus establishing that these 2 chimeras had been temporally rescued by genetically modified, short-lived erythroid progenitor cells.
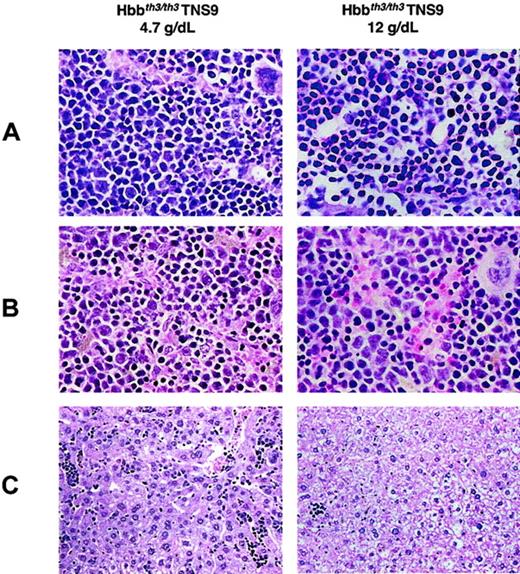
Correction of anemia and ineffective erythropoiesis in TNS9-treated Hbbth3/th3chimeras
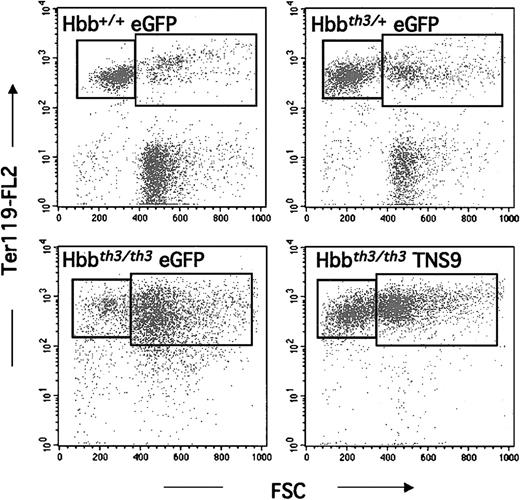
Our long-term studies focused on chimeras with less than 5% residual murine Hb levels in the 8 months following transplantation (n = 6). Over this period the mice showed stable and long-term expression of Hbbhu, averaging 6.5 ± 2.9 g/dL. This level approximates that of hemizygous Hb production (8.1 ± 0.3 g/dL in Hbbth3/+ chimeras, Table1). Pathologic examination performed between 5 and 8 months after transplantation showed variable degrees of ineffective erythropoiesis, commensurate to the degree of anemia. Tissue sections from 2 mice, one with a level of 4.7 g/dL Hb and the other with a level of 12 g/dL are shown in Figure6. The findings in the latter are similar to the eGFP-transduced Hbb+/+ control (Figure 3), except for small and rare foci of EMH in the liver (Figure 6 right column). Virtually no iron deposition was detected. The long-term chimera with 4.7 g/dL Hb levels showed features intermediate between control Hbbth3/th3 and Hbbth3/+ chimeras (Figure 6 left column). To further demonstrate the restoration of erythroid maturation, we analyzed splenic erythroid populations by fluorescence-activated cell-sorter scanner (FACS) analysis. In control eGFP-Hbb+/+ chimeras, the majority (72% ± 7%) of Ter-119+ cells corresponded to erythrocytes and reticulocytes,18 shown in the top left box of each quadrant in Figure 7. This fraction decreased to 52% ± 13% in eGFP-transduced Hbbth3/+ chimeras and was virtually absent in recipients of eGFP-Hbbth3/th3 FLCs. In TNS9-treated Hbbth3/th3 chimeras, this fraction was restored to 25% ± 8% (n = 4), thus directly demonstrating restoration of erythroblastic maturation.
Vector copy number (VCN) and Hb levels in long-term hematopoietic chimeras
| Group genotype . | n . | Months after transplantation . | VCN BM . | VCN blood . | Hb g/dL . | % Hbbhu . | Spleen mg . |
|---|---|---|---|---|---|---|---|
| GFP+/+ | 5 | 4-8 | NA | NA | 13.8 ± 1.0 | 0 | 108 ± 25 |
| GFP+/− | 5 | 4-8 | NA | NA | 8.1 ± 0.3 | 0 | 231 ± 58 |
| GFP−/− | 5 | 1.5-2 | NA | NA | 2.8 ± 0.8 | 0 | 800 ± 338 |
| TNS9−/− | 6 | 4-8 | 1.6 ± 0.6 | 1.2 ± 0.5 | 6.5 ± 2.9 | > 95 | 777 ± 382 |
| TNS9-1−/− | 1 | 4 | 1.7 | 1.6 | 5.7 | > 95 | 550 |
| TNS9-2−/− | 1 | 8 | 2.4 | 2.0 | 4.7 | > 97 | 1450 |
| TNS9-3−/− | 1 | 4 | 1.0 | 1.0 | 4.2 | > 97 | 829 |
| TNS9-4−/− | 1 | 5 | 0.9 | 1.0 | 5.0 | > 97 | 850 |
| TNS9-5−/− | 1 | 6 | 1.4 | 0.7 | 7.5 | > 97 | 650 |
| TNS9-6−/− | 1 | 6 | 2.2 | 1.1 | 12.0 | > 97 | 330 |
| Group genotype . | n . | Months after transplantation . | VCN BM . | VCN blood . | Hb g/dL . | % Hbbhu . | Spleen mg . |
|---|---|---|---|---|---|---|---|
| GFP+/+ | 5 | 4-8 | NA | NA | 13.8 ± 1.0 | 0 | 108 ± 25 |
| GFP+/− | 5 | 4-8 | NA | NA | 8.1 ± 0.3 | 0 | 231 ± 58 |
| GFP−/− | 5 | 1.5-2 | NA | NA | 2.8 ± 0.8 | 0 | 800 ± 338 |
| TNS9−/− | 6 | 4-8 | 1.6 ± 0.6 | 1.2 ± 0.5 | 6.5 ± 2.9 | > 95 | 777 ± 382 |
| TNS9-1−/− | 1 | 4 | 1.7 | 1.6 | 5.7 | > 95 | 550 |
| TNS9-2−/− | 1 | 8 | 2.4 | 2.0 | 4.7 | > 97 | 1450 |
| TNS9-3−/− | 1 | 4 | 1.0 | 1.0 | 4.2 | > 97 | 829 |
| TNS9-4−/− | 1 | 5 | 0.9 | 1.0 | 5.0 | > 97 | 850 |
| TNS9-5−/− | 1 | 6 | 1.4 | 0.7 | 7.5 | > 97 | 650 |
| TNS9-6−/− | 1 | 6 | 2.2 | 1.1 | 12.0 | > 97 | 330 |
NA indicates not applicable.
Hb analysis in TNS9-rescued mice.
Cellulose acetate electrophoresis of peripheral blood lysates was performed monthly. Hb levels (g/dL) are indicated at the bottom of each lane. Hbmu indicates mouse Hb tetramer; Hbhu, Hb tetramer composed of mouse α and human β chains; WT, wild-type mouse, showing Hb single; and TG, transgenic mouse blood,9,10 15 showing hemoglobin tetramers that incorporate human βA (Hbbhu, 14% of total Hb). TNS9: 4 TNS9-transduced Hbbth3/th3 FLCs mice (3 on one gel on the left and one on another gel to the right) 8 weeks (A) and 4 months (B) after transplantation.
Hb analysis in TNS9-rescued mice.
Cellulose acetate electrophoresis of peripheral blood lysates was performed monthly. Hb levels (g/dL) are indicated at the bottom of each lane. Hbmu indicates mouse Hb tetramer; Hbhu, Hb tetramer composed of mouse α and human β chains; WT, wild-type mouse, showing Hb single; and TG, transgenic mouse blood,9,10 15 showing hemoglobin tetramers that incorporate human βA (Hbbhu, 14% of total Hb). TNS9: 4 TNS9-transduced Hbbth3/th3 FLCs mice (3 on one gel on the left and one on another gel to the right) 8 weeks (A) and 4 months (B) after transplantation.
Histopathologic analysis of hematopoietic tissues in long-term TNS9-treated chimeras.
Left: 4.7 g/dL Hb, 8 months after transplantation. Right: 12.0 g/dL Hb, 6 months after transplantation. (A) Bone marrow (original magnification, × 60). Left: There is still a relative increase in the erythroid lineage but with more evidence of maturation than in untreated chimeras (Figure 3). Myeloid maturation is seen. Right: Normal erythroid maturation is present; the erythroid precursors are not as densely packed. Myeloid maturation, still decreased compared with normal, is more frequently seen than in the mouse with the lower Hb level (left). (B) Spleen (original magnification, × 60). Left: There is more maturation and less degenerative changes in the erythroid lineage than in the untreated mice (Figure 3B, right). Right: In comparison to the mouse with lower Hb level, the sinuses appear to be somewhat less compressed. The sinusoidal erythroid cells are more eosinophilic, suggesting better hemoglobinization. The nuclei are not as irregular as in either the mouse with lower Hb levels (left) or in the Hbbth3/th3 chimera. (C) Liver (original magnification, × 20). Left: Residual EMH, greater than in control Hbbth3/+ chimeras (Figure 3C, center) and approaching that of the Hbbth3/th3 chimeras (Figure 3C, left). Right: Minimal residual EMH, considerably less than in the Hbbth3/+ controls (Figure 3C, center). Stain: hematoxylin and eosin. Hepatic iron deposition, as detected by a Gomori iron stain: the 4.7 g/dL Hb chimera was similar to the Hbbth3/+ chimera (Figure 3D; center) and was minimal in the 12.0 g/dL Hb chimera (data not shown).
Histopathologic analysis of hematopoietic tissues in long-term TNS9-treated chimeras.
Left: 4.7 g/dL Hb, 8 months after transplantation. Right: 12.0 g/dL Hb, 6 months after transplantation. (A) Bone marrow (original magnification, × 60). Left: There is still a relative increase in the erythroid lineage but with more evidence of maturation than in untreated chimeras (Figure 3). Myeloid maturation is seen. Right: Normal erythroid maturation is present; the erythroid precursors are not as densely packed. Myeloid maturation, still decreased compared with normal, is more frequently seen than in the mouse with the lower Hb level (left). (B) Spleen (original magnification, × 60). Left: There is more maturation and less degenerative changes in the erythroid lineage than in the untreated mice (Figure 3B, right). Right: In comparison to the mouse with lower Hb level, the sinuses appear to be somewhat less compressed. The sinusoidal erythroid cells are more eosinophilic, suggesting better hemoglobinization. The nuclei are not as irregular as in either the mouse with lower Hb levels (left) or in the Hbbth3/th3 chimera. (C) Liver (original magnification, × 20). Left: Residual EMH, greater than in control Hbbth3/+ chimeras (Figure 3C, center) and approaching that of the Hbbth3/th3 chimeras (Figure 3C, left). Right: Minimal residual EMH, considerably less than in the Hbbth3/+ controls (Figure 3C, center). Stain: hematoxylin and eosin. Hepatic iron deposition, as detected by a Gomori iron stain: the 4.7 g/dL Hb chimera was similar to the Hbbth3/+ chimera (Figure 3D; center) and was minimal in the 12.0 g/dL Hb chimera (data not shown).
FACS analysis of splenic erythroid populations in long-term chimeras.
Y-axis: Ter-119 expression (BD PharMingen); X-axis: cell size (forward scatter). The left box corresponds to the smaller, more mature fraction of erythroid cells. Ter119+ cells represented, respectively, 47% ± 10% and 79% ± 18% of all spleen cells in eGFP-Hbb+/+ (n = 5) and eGFP-Hbbth3/+ (n = 5) chimeras. In recipients of eGFP-transduced Hbbth3/th3(n = 4), erythroid cells represented 93% ± 2% of splenocytes, and in TNS9-transduced Hbbth3/th3, 93% ± 5% (n = 4).
FACS analysis of splenic erythroid populations in long-term chimeras.
Y-axis: Ter-119 expression (BD PharMingen); X-axis: cell size (forward scatter). The left box corresponds to the smaller, more mature fraction of erythroid cells. Ter119+ cells represented, respectively, 47% ± 10% and 79% ± 18% of all spleen cells in eGFP-Hbb+/+ (n = 5) and eGFP-Hbbth3/+ (n = 5) chimeras. In recipients of eGFP-transduced Hbbth3/th3(n = 4), erythroid cells represented 93% ± 2% of splenocytes, and in TNS9-transduced Hbbth3/th3, 93% ± 5% (n = 4).
Discussion
The thorough evaluation of globin gene transfer as a potential treatment for severe hemoglobinopathies requires murine models of relevant severity in which the impact of gene transfer efficiency, transgene expression, chimerism, and the recipient's genetic background can be evaluated. Using the TNS9 vector, we previously demonstrated efficient gene transfer in bone marrow hematopoietic stem cells, tissue-specific transgene expression, and long-term correction in a mouse model of β-thalassemia intermedia.9 10 In order to fully evaluate the therapeutic potential of TNS9 and its potential clinical applicability to the most severe hemoglobinopathies, it is essential to investigate its efficacy in the context of a fatal anemia. Here we address this requirement by establishing a novel mouse model for the most severe form of β-thalassemia, Cooley anemia. We demonstrate that mice die as a consequence of extreme ineffective erythropoiesis and that they can be rescued and cured by lentivirus-mediated transfer of the human β-globin gene.
In this new model, a severe anemia gradually develops, reaching 2-3 g/dL of Hb levels within 6 to 9 weeks. These kinetics are consistent with the disappearance of host RBCs17 and the inability to generate functional RBCs from the graft. Moreover, these mice rapidly develop massive EMH and iron overload, features that are characteristic of severe β-thalassemia. This model should therefore prove useful to investigate novel treatments for congenital anemias, including pharmacologic and genetic approaches. Genetic approaches based on vectors encoding β-like globin genes, either β, γ, or mutants thereof, can be thoroughly tested in this extreme form of β-thalassemia. Lacking expression of any adult endogenous β-globin, this model provides a gold standard for quantifying globin transgene expression and comparing therapeutic vectors. By introducing selected genomic regions of the human β-like globin gene domain, in particular the γ-globin region, pharmacologic treatments for reactivating human fetal hemoglobin19-21 also could be investigated in this adult model of disease.
Our results establish lentivirus-mediated gene transfer as an effective, expeditious, and broadly applicable approach to study and rescue lethal hematopoietic phenotypes in animal models. In the absence of any selection, fetal liver stem cells were efficiently transduced with a lentiviral vector encoding a regulated, tissue-specific gene. Complex vectors like TNS9 provide a powerful tool for rescuing lethal phenotypes obtained by targeted gene disruption as well as a novel approach for investigating gene regulation. In contrast to the multicopy concatamers typically found in transgenic mice following pronuclear DNA injection,22 lentivirus-mediated gene transfer will be of great use in gene expression studies by enabling titration of gene delivery and thus facilitate the generation of single-copy mice. The combination of highly efficient gene transfer,23 the ability to package large genomic elements and thus enhance gene regulation,9 and the preferential integration into sites of active chromatin24 make lentiviral vectors a major tool for experimental gene transfer. The same vectors can be used to either generate hematopoietic chimeras or transgenic animals through infection of embryonic stem cells.25 26 As shown here, fetal liver cell transduction is highly efficient, enabling rapid and effective hematopoietic reconstitution and thus providing a significant practical advantage over transgenesis.
Using this model, we could determine for the first time the absolute level of human β chain production obtained in vivo in the absence of competing murine β chains. Unlike transgenic mice, hematopoietic chimeras harbor variable mixtures of transduced and nontransduced cells, with the former also varying in their vector copy number. Chimeras with endogenous repopulation in excess of 5% were not included in these analyses to rule out any contribution of residual host erythropoiesis to their pathology and survival. In the 6 long-term chimeras with negligible residual host erythropoiesis (< 5% Hbbmu), the mean Hb levels were 6.5 ± 2.9 g/dL, in conjunction with an average vector copy number of 1.6 ± 0.6. In one chimera showing stable production of 12 g/dL Hbbhu, the vector copy number was 2.2-2.4 in the different hematopoietic tissues. This mouse was virtually cured of anemia. The persistent, moderate EMH noted in this mouse, greater than expected for this Hb level,10 suggests that the chimeric Hbbhu may not be functionally equivalent to Hbbmu.
The TNS9 vector encodes the human β-globin gene, including an extended promoter and 2 proximal enhancers, 3 large genomic fragments derived from the human β-globin locus control region, and additional synthetic GATA-1 transcription factor binding sites. Globin transcriptional control elements have been extensively studied,27,28 without conclusively defining what minimal segments and what combination thereof are best suited to generate a miniature β-globin transcription unit. The relative roles of the locus control region and of other genetic elements spanning the human β-globin locus and its chromatin domain in regulating globin gene expression remain controversial.29-31 The combination adopted within the TNS9 vector results from a systematic analysis of numerous combinations (TNS 1 through 9; S.R., C.M., M.S., unpublished observations). As shown here, most Hbbth3/th3 chimeras were rescued by TNS9. This unequivocally demonstrates that a major therapeutic benefit can be achieved in a lethal hemoglobinopathy. Using a vector closely reproducing the structure of TNS9, termed β89, which differs in one amino acid in the β-globin gene, the promoter region (−615 from the cap site in TNS9 versus −266 in β89) and boundaries of the locus control region (LCR) elements (3.2 kb in TNS9 versus 2.7 kb in β89), Pawliuk et al achieved inhibition of red blood cell dehydration and sickling with correction of hematologic parameters and splenomegaly11 in 2 murine SCD models, Berkeley32 and SAD.33 This result was obtained with an average of 3 copies of the vector per cell.11 It is unclear whether such a high vector copy number is required to ameliorate anemia or increase lifespan in these models of SCD and whether this high vector copy number is also necessary to treat murine β-thalassemia intermedia or β-thalassemia major. It would be interesting to investigate β89 and other globin vectors in the present model of β0-thalassemia to determine what mean vector copy number is sufficient to rescue lethal anemia. Although seemingly minimal, the differences between TNS9 and β89 may well result in different levels of β-globin production and different susceptibility to position effects. In consideration of the potential risk of insertional oncogenesis associated with randomly integrated transgenes, it is essential in our view to optimize transgene expression so as to achieve therapeutic benefits with the lowest possible vector copy number.
Based on the results obtained in the model of Cooley anemia described here, we conclude that the TNS9 lentiviral vector expresses high enough levels of human β-globin to warrant its clinical evaluation. In addition to requiring elevated and regulated globin gene expression, the prospective clinical use of lentiviral vectors requires that other efficacy and safety features be ascertained. At present, the efficacy of lentiviral vectors in transducing human HSCs appears to be at least as good as that of oncoretroviral vectors. This matter is still under investigation,34-42 including, importantly, marking studies in nonhuman primates.43-45 The transduction efficiency of lentiviral vectors would have to prove to be vastly superior to that of oncoretroviral vectors to justify their clinical investigation for this reason alone. In the case of the severe hemoglobinopathies, the unparalleled efficacy of lentiviral vectors in permitting stable transfer of large genomic fragments and regulation of β-globin expression provides a powerful argument in favor of their use.46 The major safety concerns relate to the risk of generating a replication-competent lentivirus (RCL) and to that of insertional oncogenesis. The former depends on the establishment of safe packaging cell lines and dependable RCL detection assays. The hitherto positive experience with oncoretroviral vectors47provides an encouraging precedent for the clinical investigation of lentiviral vector systems48 49 and suggests that the generation of safe, stable, and high-titer lentiviral packaging cell lines should be feasible.
While insertional mutagenesis is unavoidable with retroviral vectors—as for any gene transfer method resulting in random integration—the risk of insertional oncogenesis is difficult to apprehend. Although insertional oncogenesis is deemed to be a rare event,50 2 recent reports51,52 raise the concern that its frequency may have been underestimated. In both instances, genes encoding molecules involved in the regulation of cell survival, ΔLNGFR51,53 and γc,52,54 were transduced in bone marrow cells under the nonspecific transcriptional control of the retroviral long terminal repeat (LTR). In both cases, integration was adjacent to oncogenes, respectively Evi-1 and LMO-2. The complete analysis of the transforming events associated with leukemogenesis in both instances is still pending and therefore preclude definitive conclusions. Importantly, lineage-restricted vectors with U3-deleted LTRs provide in principle a mean to reduce the risk of insertional oncogenesis.55 Globin vectors encompassing the β-globin promoter are transcribed only in late stages of proerythroblastic differentiation, prior to nuclear extrusion, except for possible low-level “leakiness” at some integration sites. While the risk of gene disruption is unchanged relative to LTR-driven vectors, the risk of oncogene activation would therefore be confined to a single lineage. The risk of promoter transactivation by vector-encoded enhancers could be further reduced by the incorporation into the vector of insulators or other elements with enhancer-blocking activity.56,57 A comprehensive definition of the risk of insertional oncogenesis will require extensive studies over time. Ultimately, like any other medical decision, therapy will be best guided by a rational risk-benefit assessment. Based on our prior reports9 10 and the present one, we conclude that lentiviral vectors are very promising drugs for the treatment of β-thalassemia intermedia and β-thalassemia major.
We thank Ping Zhu, Ellinor Peerschke, and Diane Magravati for technical assistance and Katerina Politi for reviewing the manuscript.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-10-3305.
Supported by National Institutes of Health grants HL-57612 (M.S.), HL-66952 (M.S., I.R.), CA-59350 (I.R., M.S.); a postdoctoral award from the Cooley's Anemia Foundation (S.R.); and the Fondazione Italiana L. Giambrone per la Guarigione dalla Thalassemia (M.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michel Sadelain, Box 182, MSKCC, 1275 York Ave, New York, NY 10021; e-mail:m-sadelain@ski.mskcc.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal