Abstract
Selectin-dependent leukocyte rolling is one of the earliest steps of an acute inflammatory response and, as such, contributes to many inflammatory diseases. Although inhibiting leukocyte rolling with selectin antagonists is a strategy that promises far-reaching clinical benefit, the perceived value of this strategy has been limited by studies using inactive, weak, or poorly characterized antagonists. Recombinant P-selectin glycoprotein ligand–1–immunoglobulin (rPSGL-Ig) is a recombinant form of the best-characterized selectin ligand (PSGL-1) fused to IgG, and is one of the best prospects in the search for effective selectin antagonists. We have used intravital microscopy to investigate the ability of rPSGL-Ig to influence leukocyte rolling in living blood vessels and find that it can reduce rolling dependent on each of the selectins in vivo. Interestingly, doses of rPSGL-Ig required to reverse pre-existing leukocyte rolling are 30-fold higher than those required to limit inflammation, suggesting additional properties of this molecule. In support of this, we find that rPSGL-Ig can bind the murine chemokine KC and inhibit neutrophil migration toward this chemoattractant in vitro.
Introduction
Recruitment of leukocytes, although essential for host defense, can also be deleterious, for example in ischemia-reperfusion (I/R) injury,1arthritis,2 and sepsis.3 As an early and necessary step in the leukocyte recruitment process, selectin-dependent rolling, therefore, presents an attractive drug target.
Although the selectins bind with moderate affinity to sialylated, fucosylated carbohydrate ligands, typified by the tetrasaccharide sialyl Lewisx (sLex),4 preferred natural ligands are more complex glycoconjugates.5 The best-characterized selectin ligand is P-selectin glycoprotein ligand–1 (PSGL-1),6,7 a homodimeric sialomucin found on leukocytes8,9 and platelets.10 PSGL-1, in its correct glycoform, binds to P-, E- and L-selectins in vitro5 and represents an important functional ligand for all of these molecules.11-15
A recombinant fusion protein, recombinant PSGL-1–immunoglobulin (rPSGL-Ig), consisting of 47 amino acids from the NH2 terminal of human PSGL-1 linked to the Fc portion of human IgG1 has been developed.16 To preserve the function of the natural ligand, rPSGL-Ig is expressed in cell lines containing enzymatic machinery for posttranslational sulfation and glycosylation.17 Many investigators believe that rPSGL-Ig will compete with cell surface selectin ligands to reduce leukocyte rolling and thus limit inflammation.
Anti-inflammatory effects of rPSGL-Ig have been described. Treatment with rPSGL-Ig accelerates thrombolysis and prevents reocclusion of injured porcine arteries18; protects against traumatic shock,19 hemorrhage reinfusion injury,20 and I/R injury in rats21; reduces myocardial I/R injury in cats22; and limits acid-induced lung inflammation in mice.23 Leukotriene C4(LTC4)–induced rolling is also reduced by rPSGL-Ig.17 In all of these studies, rPSGL-Ig was administered prior to inflammatory stimulation, and direct effects on individual selectins were not investigated. We have used well-characterized models of selectin-dependent leukocyte rolling to demonstrate, for the first time, that rPSGL-Ig can directly inhibit leukocyte rolling on all 3 selectins in vivo. We also report that rPSGL-Ig inhibits inflammation at doses that only moderately alter established leukocyte rolling and has supplementary activity (inhibition of chemokine binding and function) not related to inhibition of selectin-dependent rolling.
Materials and methods
Animals
Male wild-type C57BL/6 (Harlan, Bicester, United Kingdom), P-selectin−/− (in-house colony derived from C57BL/6J-Selptm1Bay; The Jackson Laboratory, Bar Harbor, ME) and E-selectin−/− mice (in-house colony derived from breeding pairs supplied by Dr B. A. Wolitzky)24 weighing between 23 and 26 g were used in these experiments. Mice were anesthetized with an intraperitoneal injection (12.5 μL/g) of a mixture consisting of 10 mg/mL ketamine hydrochloride (Ketaset; Willows Francis Veterinary, Crawley, United Kingdom), 1 mg/mL xylazine hydrochloride (Bayer, Suffolk, United Kingdom), and 0.02 mg/mL atropine sulfate (Phoenix Pharmaceuticals, Gloucester, United Kingdom). Mice were given additional anesthesia (pentobarbital 100 μL, 0.5 mg bolus) every 30 minutes (Sagatal, Rhone Merieux, Maidenhead, United Kingdom).
All procedures performed were approved by the University of Sheffield ethics committee and by the Home Office Animals (Scientific Procedures) Act 1986 of the United Kingdom.
rPSGL-Ig and controls
Active and low-affinity rPSGL-Ig were kind gifts from Wyeth/Genetics Institute (Andover, MA). Low-affinity rPSGL-Ig has a sequence identical to rPSGL-Ig, but was produced in a cell line that lacks the enzymes required for expression of fully active PSGL-1.19 Either saline (vehicle) or CD4-Ig were selected as controls for in vivo experiments. CD4-Ig was a kind gift from Dr S. Watson (Genentech, South San Francisco, CA). Previous investigations19 and in vitro studies presented herein found that the effects of low-affinity rPSGL-Ig (unavailable in sufficient quantities for in vivo experiments) could not be distinguished from those of saline or CD4-Ig.
Cytokines and antibodies
Murine recombinant tumor necrosis factor–α (TNFα), purchased from R&D Systems (Abingdon, Oxon, United Kingdom), was dissolved at 0.1 mg/mL in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA), sterile filtered, and stored in 500-ng aliquots at −20°C. Murine KC, purchased from Peprotech (Rocky Hill, NJ), was reconstituted in PBS containing 0.1% BSA at 10−4 M and stored in 10-μL aliquots at −20°C. Rat antimouse antibodies against P-selectin (RB40.34, IgG1), L-selectin (Mel-14, IgG2a), PSGL-1 (2PH1, IgG1), Ly-6G (RB6-8C5, IgG1), CD2 (rm2-5, IgG2b), CD5 (53-7.3, IgG2a), and CD45R (RA3-6B2, IgG2a) were purchased from Pharmingen (Oxford, United Kingdom). Rat antimouse intercellular adhesion molecule–1 (ICAM-1 [YN1/1]) was a gift from Dr C. Wegner (Abbott Laboratories, Abbott Park, IL). Rat antimouse F4/80 antigen (CI:A3-1), fluorescein isothiocyanate (FITC)–labeled rat antimouse CD18 (C71/16), and phycoerythrin (PE)–labeled rat IgG2a isotype negative control were purchased from Serotec (Kidlington, United Kingdom). Goat antirat IgG microbeads were obtained from Miltenyi Biotech (Bisley, United Kingdom). The isotype control for RB40.34 (8B9, rat IgG1) was a kind gift from Dr B. A. Wolitzky (Hoffman-La Roche, Nutley, NJ). Rat antimouse E-selectin (10E6, rat IgG2b) used in these experiments was also a kind gift from Dr B. A. Wolitzky. All of these antibodies have been studied extensively by us and others.11,14 25-30 Antibodies were stored at 1 mg/mL in 10-μg aliquots at −20°C. Before injection into mice, cytokine and antibody aliquot volumes were expanded to 200 μL by addition of sterile saline.
Intravital microscopy
Leukocyte rolling was observed in postcapillary venules of the murine cremaster muscle. For P-selectin–dependent leukocyte rolling, wild-type mice were used, and cremasters were stimulated merely by the surgery required for microscopic observation. For E-selectin–dependent/L-selectin–dependent leukocyte rolling, P-selectin−/− mice were used, and cremasters were stimulated with TNFα (500 ng, intrascrotal) 2.5 hours prior to observation. Leukocyte rolling with a strong requirement for L-selectin has been described in E-selectin/P-selectin double-null mice stimulated for 6 hours with TNFα and treated with heparin to prevent coagulation.31 Since these mice were not available in our laboratory, E-selectin−/− mice were pretreated with P-selectin blocking antibody (30 μg), TNFα (500 ng), and heparin (50 U) 6 hours prior to observation. Immediately prior to exteriorization of the cremaster for intravital microscopy, the following cannulations were performed: the trachea to facilitate respiration; the jugular vein to allow intravenous injection of additional anesthesia and injection of rPSGL-Ig, vehicle, and antibodies; and the carotid artery to permit blood sampling. Body temperature was maintained at 37°C by means of a thermocontrolled heat pad (PDtronics, Sheffield, United Kingdom).
The cremaster muscle was prepared for microscopic observation as described previously.27 Briefly, the testis, epididymis, and cremaster were extracted through a small incision in the scrotum. The tip of the cremaster was pinned so that the testis and cremaster were positioned across a 10-mm cover glass composing part of a specialized microscopy stage. Fat and connective tissue were carefully removed, and an excision was made through the cremaster allowing the muscle to be pinned out across the glass coverslip. Throughout this procedure and during intravital microscopy, the cremaster muscle was superfused with thermocontrolled (36°C) bicarbonate buffer solution (131.9 mM NaCl, 18 mM NaHCO3, 4.7 mM KCl, 2.0 mM CaCl2, and 2 mM MgSO4) through which a gas mixture of 5% CO2 in N2 was bubbled.
Microscopic observations were made by means of a light microscope (Nikon Eclipse E600-FN; Nikon United Kingdom, Kingston, Surrey, United Kingdom) equipped with a water immersion objective (40 ×/0.80 W). Venules between 16- and 62-μm diameter were selected for observation. Images of rolling leukocytes were recorded by means of a charge-coupled device (CCD) camera (DC-330E; DAGE MTI, Michigan City, MI) onto S-VHS videocassettes.
To investigate the instantaneous effects of rPSGL-Ig, venules were recorded continuously beginning 1 minute before treatment and continuing until 5 minutes after injection. In some experiments, attention was focused on areas immediately downstream of points where capillaries converged with small venules, allowing formation of new attachments to be visualized and quantified. In these experiments, attachment rates before and after treatments were determined for 10 minutes. In other experiments, leukocytes were tracked from the point of initial attachment through the network of vessels to points that could no longer be observed. Blood samples (10 μL) were drawn from the carotid artery at 10-minute intervals and at 2 minutes before and after treatments. These were analyzed for total systemic leukocyte concentrations.
Venular blood flow velocity was measured as previously described,32 by means of a dual photodiode and digital online cross-correlation program (Microvessel Velocity OD-RT System; Circusoft Instrumentation LLC, Hockessin, DE).
Leukocyte rolling induced by lower-body ischemia-reperfusion injury
Wild-type C57BL/6 mice were anesthetized, and neck surgery was performed as described. The abdominal cavity was opened, and the intestines were moved carefully to one side. The aorta was uncovered in the abdominal cavity, gently detached from the vena cava, and occluded at a point immediately below the renal vasculature by means of a vessel clamp. Lower-body ischemia was maintained for 60 minutes, at which time the clamp was carefully removed.
After clamp removal, the intestines were replaced, and the abdominal cavity was sutured. Vessels were reperfused for 30 minutes, after which time the cremaster muscle was exteriorized and leukocyte rolling observed by intravital microscopy. In some experiments, mice were given rPSGL-Ig (1 mg/kg) or vehicle as a pretreatment 55 minutes into the ischemic period (ie, just before reperfusion). Additional groups of animals were given rPSGL-Ig (1 mg/kg) or anti–P-selectin antibody (10 μg) 30 minutes after observation of rolling induced by I/R. Sham-treated mice received identical surgical manipulation followed by 60 minutes' sham ischemia and 30 minutes' sham reperfusion.
Intravital microscopy data analysis
After collection of intravital microscopy images, sequences of interest were digitized (Miromotion DC30; Pinnacle Systems, Mountain View, CA) and stored on a Macintosh (Seattle, WA) G3/400 computer prior to analysis. Rolling flux percentage was calculated as previously described.25 To allow easy comparison of rolling before and after application of inhibitors, some data are presented as the percentage of baseline rolling. Vessel geometry and leukocyte rolling velocities were measured by means of the public domain National Institutes of Health (NIH)–Image program (available at: http://rsb.info.nih.gov/nih-image) as described.33 Leukocyte tethering was quantified by counting the number of new attachments forming in a defined area of observation per unit of time. Tethering rate after inhibitor treatment is expressed as a percentage of the rate measured immediately prior to treatment. Some leukocytes were tracked through the network of cremasteric venules until they either detached, adhered, or rolled beyond the range of our imaging setup. These data are presented as percentages.
Leukocyte accumulation in thioglycollate-induced peritonitis
Negative controls (200 μL saline and 1 mg/kg CD4-Ig), rPSGL-Ig (1 mg/kg), anti–P-selectin antibody (10 μg RB40.34 per mouse), or anti–E-selectin antibody (10 μg 10E6 per mouse) were given as a pretreatment to wild-type mice. In these experiments, rPSGL-Ig was injected subcutaneously rather than intravenously. This allowed treatment of a large number of mice in close succession since preliminary experiments identified temporal variation as a major determinant of experimental error. At 15 minutes after pretreatment, 1-mL injections of 3% thioglycollate broth or 0.9% saline were administered intraperitoneally. Mice were killed 4 hours after thioglycollate by cervical dislocation. Heparinized saline (5 mL, 10 U/mL) was injected into the peritoneum; the abdomen was gently massaged; and lavage fluid was recovered via a small incision into the abdominal cavity. Samples of lavage fluid were taken for total leukocyte counts, which were performed by means of a modified Neubauer hemocytometer, and differential counts, which were performed on Giemsa-stained cytospin slides. Thioglycollate-induced peritonitis data are presented as the total number of neutrophils per peritoneum.
Preparation and investigation of rPSGL-Ig–coated beads
Nonfluorescent, protein A–coated 1-μm latex beads were purchased from Bangs Laboratories (Fishers, IN). Stock (1% wt/vol) beads were diluted 1/10 for labeling. Then, 200 μL of 0.1% (wt/vol) beads were incubated overnight at 4°C with 50 μg rPSGL-Ig or low-affinity rPSGL-Ig. Residual protein A sites on the beads were then blocked by incubation with an excess of Blockaid (Molecular Probes, Eugene, OR) for 15 minutes at room temperature. Beads were washed by centrifugation and resuspended at 0.1% wt/vol. Murine KC was fluorescently labeled by means of a kit according to the manufacturer's (Molecular Probes) instructions. Then, 10 μg fluorescent KC was incubated with 5 μL rPSGL-Ig– or low-affinity rPSGL-Ig–coated beads for 30 minutes at 4°C in a final volume of 100 μL. In some experiments, fluorescent KC was incubated with free rPSGL-Ig (10 μg/mL) or low-affinity rPSGL-Ig (10 μg/mL) prior to incubation with beads. Binding of fluorescent KC to nonfluorescent beads was assessed by means of flow cytometry.
Purification of murine neutrophils
Neutrophils were isolated from mouse blood as described.26 Briefly, blood was collected from the carotid artery into heparin (100 U/mL) and mixed with dextran (1.25% wt/vol in saline) to a final volume of 10 mL for each 1 mL blood collected. After erythrocyte sedimentation, leukocyte-rich plasma was collected and washed in PBS containing BSA (0.5% wt/vol). Antibodies against CD2, CD5, CD45R, F4/80, and ICAM-1 were added to target nonneutrophil cell types. Anti–ICAM-1 also recognizes neutrophils, but the dose of this antibody was titrated to preferentially target monocyes.26Secondary antibody–coated magnetic microspheres were then added, and labeled cells removed by passing the cell suspension over a BS cell-separation column that was attached to a VarioMACS magnet (Miltenyi Biotech). After removal of residual erythrocytes by hypotonic lysis, neutrophils were washed once more in PBS plus BSA and resuspended at 2 × 106/mL.
In vitro transmigration of murine neutrophils
We used an in vitro model to investigate the influence of rPSGL-Ig on chemoattractant-induced migration of murine neutrophils. Neutrophils were treated with either CD4-Ig, low-affinity rPSGL-Ig, or rPSGL-Ig at different concentrations and then immediately dispensed (25 μL of 2 × 106/mL suspension) onto the filter membrane of a ChemoTx microplate (Neuro Probe, Gaithersburg, MD). The framed filter array was then positioned over a microplate loaded with different concentrations (10−8 to 10−6 M, 25 μL per well) of the murine chemoattractant KC or controls. Microplates were incubated for 3 hours at 37°C in 5% CO2. After incubation, nonmigrated neutrophils were removed from the top of the filter by wiping and washing 3 times with RPMI. Migrated neutrophils were pelleted to the bottom of microplate wells by centrifugation (300g, 10 minutes) and then resuspended in 25 μL RPMI. Numbers of migrated neutrophils were determined by means of a hemocytometer, and results expressed as the percentage of neutrophils migrated or the percentage of control migration.
Statistics
Differences between data sets were analyzed by means of GraphPad Prism software (San Diego, CA). Data were analyzed for deviation from a normal distribution, and tests for parametric or nonparametric data were used appropriately. Posttests (Dunn, Dunnet, or Tukey Kramer) for multiple comparisons were used when required.
Online supplemental material
Four QuickTime movies are included on the Bloodwebsite with this manuscript; see the Supplemental Videos link at the top of the online article. Movies were digitized from video archives and prepared for online viewing by means of Adobe Premiere version 5.1 (www.adobe.com/premiere) and QuickTime Pro version 5 (www.apple.com/quicktime) as described.33
Results
rPSGL-Ig inhibits leukocyte rolling on all 3 selectins in vivo
We used intravital microscopy to study the ability of rPSGL-Ig to prevent binding of natural selectin ligands and thereby disrupt leukocyte rolling in vivo.
Surgical preparation of the mouse cremaster muscle for intravital microscopy induces leukocyte rolling that is almost exclusively dependent on P-selectin.25 Baseline rolling was observed 30 minutes after such surgery and recorded for 1 minute. Indicated doses of rPSGL-Ig were injected intravenously, and the effects on existing leukocyte rolling were observed. Low doses (1 mg/kg, 10 mg/kg) of rPSGL-Ig did not alter the proportion of leukocytes rolling through observed venules, whereas higher doses (30 mg/kg, 100 mg/kg) did (Figure 1A). Interestingly, the incomplete inhibition given by 30 mg/kg rPSGL-Ig was not exceeded by a higher dose. Quicktime movies showing P-selectin–dependent leukocyte rolling before (movie 1) and after (movie 2) 100 mg/kg rPSGL-Ig are included with the online version of this manuscript. A PSGL-1 blocking antibody, 2PH1, inhibited leukocyte rolling to a level similar to that of rPSGL-Ig, whereas P-selectin blocking antibody all but abolished surgically induced rolling. A Quicktime movie comparing the effect of P-selectin blocking antibody with that of rPSGL-Ig (movie 3) is included with the online version. As well as reducing the proportion of leukocytes rolling through observed venules, rPSGL-Ig also increased the velocity of leukocytes continuing to roll (Figure 1B). Increased rolling velocity was measurable with doses of rPSGL-Ig that did not reduce the proportion of leukocytes rolling (ie, 1 mg/kg).
Effect of rPSGL-Ig on pre-existing leukocyte rolling.
(A-B) Effect on P-selectin–dependent rolling. Leukocyte rolling was allowed to develop for 30 minutes after surgical stimulation of wild-type (WT) mice. Control rolling was recorded for 1 minute followed by injection of rPSGL-Ig at the indicated dose. Effects on proportion of leukocytes rolling (A) and rolling velocity (B) were determined and compared with controls. Anti–P-selectin or PSGL-1 antibodies were injected at the end of experiments. (C-D) Effect on E-selectin–dependent rolling. P-selectin−/− mice were stimulated for 2 hours with TNFα, after which time cremasters were prepared for intravital microscopy. Control rolling was recorded for 1 minute followed by injection of rPSGL-Ig at the indicated dose. Effects on the proportion of leukocytes rolling (C) and leukocyte rolling velocity (D) were determined and compared with controls. Anti–E-selectin antibody was injected at the end of experiments. (E) Effect on L-selectin–dependent rolling. E-selectin−/− mice were stimulated for 6 hours with TNFα. Mice also received P-selectin blocking antibody plus 50 U heparin at the time of TNFα injection. Cremasters were prepared for intravital microscopy, and rolling was allowed to develop for a further 30 minutes. Indicated doses of rPSGL-Ig were injected at 6 hours 31 minutes, and the effects on the proportion of leukocytes rolling were determined. Anti–L-selectin antibody was injected at the end of all experiments. All experiments were repeated in at least 4 mice. Velocities of 6 leukocytes were measured for each of at least 10 observed venules. The dashed line in panels A, C, and E indicates baseline rolling. Data in panels A, C, and E are presented as mean ± standard errors of the mean (SEMs). Significant difference from baseline rolling is indicated by *P < .05 or **P < .01.
Effect of rPSGL-Ig on pre-existing leukocyte rolling.
(A-B) Effect on P-selectin–dependent rolling. Leukocyte rolling was allowed to develop for 30 minutes after surgical stimulation of wild-type (WT) mice. Control rolling was recorded for 1 minute followed by injection of rPSGL-Ig at the indicated dose. Effects on proportion of leukocytes rolling (A) and rolling velocity (B) were determined and compared with controls. Anti–P-selectin or PSGL-1 antibodies were injected at the end of experiments. (C-D) Effect on E-selectin–dependent rolling. P-selectin−/− mice were stimulated for 2 hours with TNFα, after which time cremasters were prepared for intravital microscopy. Control rolling was recorded for 1 minute followed by injection of rPSGL-Ig at the indicated dose. Effects on the proportion of leukocytes rolling (C) and leukocyte rolling velocity (D) were determined and compared with controls. Anti–E-selectin antibody was injected at the end of experiments. (E) Effect on L-selectin–dependent rolling. E-selectin−/− mice were stimulated for 6 hours with TNFα. Mice also received P-selectin blocking antibody plus 50 U heparin at the time of TNFα injection. Cremasters were prepared for intravital microscopy, and rolling was allowed to develop for a further 30 minutes. Indicated doses of rPSGL-Ig were injected at 6 hours 31 minutes, and the effects on the proportion of leukocytes rolling were determined. Anti–L-selectin antibody was injected at the end of all experiments. All experiments were repeated in at least 4 mice. Velocities of 6 leukocytes were measured for each of at least 10 observed venules. The dashed line in panels A, C, and E indicates baseline rolling. Data in panels A, C, and E are presented as mean ± standard errors of the mean (SEMs). Significant difference from baseline rolling is indicated by *P < .05 or **P < .01.
We have previously used TNFα-stimulated P-selectin−/−mice to study inhibition of E-selectin–dependent leukocyte rolling.27 Although this model is also partially sensitive to L-selectin blockade, E-selectin inhibitors produce a more pronounced effect and a characteristically increased leukocyte rolling velocity.34 Low-dose (1 mg/kg) rPSGL-Ig did not alter the proportion of leukocytes rolling (Figure 1C), but did elevate their velocity. Higher doses (30 to 100 mg/kg) of rPSGL-Ig convincingly reduced TNFα-induced rolling in P-selectin−/− mice (Figure 1C). That rolling velocity was also elevated (Figure 1D) strongly indicates inhibition of E-selectin. E-selectin antibody all but abolished leukocyte rolling in these mice.
To investigate L-selectin–dependent leukocyte rolling, we treated E-selectin−/− mice with P-selectin blocking antibody plus TNFα for 6 hours. Mice also received 50 U heparin to prevent coagulation in response to TNFα. Cremasters were exteriorized at 6 hours, and baseline rolling was observed at 6 hours 30 minutes. Application of rPSGL-Ig at 30 or 100 mg/kg significantly inhibited leukocyte rolling in these animals, indicating direct inhibition of L-selectin–dependent leukocyte rolling (Figure 1E). The lower dose (1 mg/kg) of rPSGL-1 did not alter the proportion of leukocytes rolling in these mice. L-selectin blocking monoclonal antibody (mAb) also reduced leukocyte rolling substantially, confirming the L-selectin dependence of the model. Reapplication of P-selectin blocking antibody did not alter leukocyte rolling (data not shown), confirming that the antibody approach used had reproduced the previously described double-knockout experiment. The number of rolling leukocytes at 6 hours 30 minutes after TNFα in mice lacking E- and P-selectin function was too low to permit accurate determination of leukocyte rolling velocities.
By making these observations in the same vessels before and after treatments, we controlled for vessel diameter as a possible influence on leukocyte rolling. Although vessels selected for observation in TNFα-stimulated P-selectin–knockout and E-selectin–knockout mice were larger than those selected in WT mice (Table 1), no comparisons were made between these groups. Treatment with rPSGL-Ig tended to slightly increase circulating leukocyte counts and blood flow velocities, although these effects were not statistically significant (Table 1; effect of highest dose rPSGL-Ig shown).
Effect of rPSGL-Ig treatment on hemodynamic parameters
| Mouse genotype . | Treatment . | Circulating leukocytes, cells per μL blood . | Centerline blood flow velocity, μm/s . | Vessel diameter, μm . | ||
|---|---|---|---|---|---|---|
| Before treatment* . | After treatment† . | Before treatment* . | After treatment† . | |||
| WT | Surgical stimulation | 8200 ± 907 | 10 167 ± 2161 | 2157 ± 159 | 2664 ± 329 | 51.0 ± 4.1 |
| P-selectin−/− | TNFα stimulation for 2 hours 30 minutes | 4566 ± 753 | 5 200 ± 1159 | 2470 ± 413 | 2891 ± 278 | 39.8 ± 2.7 |
| E-selectin−/− | P-selectin blocking antibody + heparin + 6 hours of TNFα | 3400 ± 184 | 3 500 ± 217 | 2564 ± 357 | 2782 ± 427 | 39.4 ± 5.2 |
| Mouse genotype . | Treatment . | Circulating leukocytes, cells per μL blood . | Centerline blood flow velocity, μm/s . | Vessel diameter, μm . | ||
|---|---|---|---|---|---|---|
| Before treatment* . | After treatment† . | Before treatment* . | After treatment† . | |||
| WT | Surgical stimulation | 8200 ± 907 | 10 167 ± 2161 | 2157 ± 159 | 2664 ± 329 | 51.0 ± 4.1 |
| P-selectin−/− | TNFα stimulation for 2 hours 30 minutes | 4566 ± 753 | 5 200 ± 1159 | 2470 ± 413 | 2891 ± 278 | 39.8 ± 2.7 |
| E-selectin−/− | P-selectin blocking antibody + heparin + 6 hours of TNFα | 3400 ± 184 | 3 500 ± 217 | 2564 ± 357 | 2782 ± 427 | 39.4 ± 5.2 |
Inhibitor treatment was 100 mg/kg throughout.
At 2 minutes before treatment.
At 2 minutes after treatment
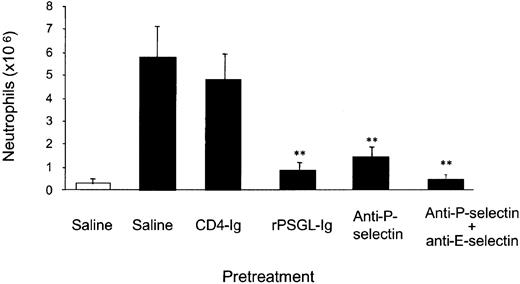
Low-dose rPSGL-Ig inhibits thioglycollate-induced peritonitis
Low-dose (1 mg/kg) rPSGL-Ig failed to reduce rolling in any of these studies. We found this result surprising since others have described pronounced anti-inflammatory effects of this molecule at doses as low as 0.25 mg/kg. We therefore tested the anti-inflammatory activity of rPSGL-Ig in a peritonitis model.
Few neutrophils were detected in peritoneal lavage samples of unstimulated mice. Thioglycollate treatment caused approximately 5 million neutrophils to be recruited; a response that was not modified by CD4-Ig (Figure 2). In contrast, rPSGL-Ig (1 mg/kg) pretreatment inhibited thioglycollate-induced recruitment of neutrophils to the peritoneum by 87%. The P-selectin blocking antibody RB40.34 inhibited neutrophil recruitment by approximately 77%, and a combination of RB40.34 and the E-selectin blocking antibody 10E6 reduced recruitment by more than 90%. Thus, in agreement with previous investigators, we find pronounced anti-inflammatory activity of rPSGL-Ig at a relatively low dose.
Effect of rPSGL-Ig pretreatment on thioglycollate-induced peritonitis.
Mice were pretreated (15 minutes) with either saline, CD4-Ig (1 mg/kg, subcutaneously), rPSGL-Ig (1 mg/kg, subcutaneously), RB40.34 (10 μg, subcutaneously), or 10E6 (10 μg, subcutaneously), followed 15 minutes later by 1 mL intraperitoneal injection of thioglycollate (▪) or saline (■). Peritoneal lavage was performed 4 hours after stimulation, and neutrophil numbers were determined. The data represent the means ± SEMs of 7 mice in vehicle, CD4-Ig–, and rPSGL-Ig–pretreated groups, and 8 mice in all other groups. Significant difference from vehicle control is indicated by **P < .01.
Effect of rPSGL-Ig pretreatment on thioglycollate-induced peritonitis.
Mice were pretreated (15 minutes) with either saline, CD4-Ig (1 mg/kg, subcutaneously), rPSGL-Ig (1 mg/kg, subcutaneously), RB40.34 (10 μg, subcutaneously), or 10E6 (10 μg, subcutaneously), followed 15 minutes later by 1 mL intraperitoneal injection of thioglycollate (▪) or saline (■). Peritoneal lavage was performed 4 hours after stimulation, and neutrophil numbers were determined. The data represent the means ± SEMs of 7 mice in vehicle, CD4-Ig–, and rPSGL-Ig–pretreated groups, and 8 mice in all other groups. Significant difference from vehicle control is indicated by **P < .01.
Low-dose rPSGL-Ig inhibits I/R-injury–induced leukocyte rolling
We also tested the effects of low-dose rPSGL-Ig in a model of I/R injury. I/R injury was induced by clamping the aorta for 1 hour followed by 30 minutes of reperfusion. Leukocyte rolling was observed by means of intravital microscopy of cremaster muscles prepared at the end of the reperfusion period. Mice subjected to sham I/R injury followed by preparation of the cremaster muscle had levels of leukocyte rolling similar to those of mice receiving cremaster surgery alone, whereas I/R-injured mice had elevated levels of leukocyte rolling (Figure 3A). Treatment with rPSGL-Ig (1 mg/kg, intravenously) 5 minutes before reperfusion, inhibited the increased leukocyte rolling caused by I/R, although apparently not that caused by surgical exposure of the cremaster muscle 30 minutes later (Figure 3A). Systemic leukocyte concentrations, blood flow velocities, and diameters of vessels examined in I/R experiments are shown in Table 2. Mice subjected to the I/R procedure had significantly higher cremasteric blood flow but leukocyte concentrations similar to those of sham-treated mice. Pretreatment with rPSGL-Ig did not significantly influence any of the hemodynamic parameters studied in I/R experiments, and similarly sized vessels were selected.
Effect of rPSGL-Ig on ischemia/reperfusion–induced leukocyte rolling.
(A) Effect of rPSGL-Ig pretreatment. Mice were subjected to 1 hour lower-body ischemia by application of an infrarenal cross-clamp. Surgery control mice received a laparotomy but no clamp. Some mice received rPSGL-Ig (1 mg/kg, intravenously) 5 minutes prior to release of the aortic clamp. Mice were prepared for intravital microscopy 30 minutes after release of the aortic clamp, and leukocyte rolling in venules was recorded. (B) Effect of rPSGL-Ig treatment after development of I/R-induced rolling. Mice were subjected to 1-hour lower-body ischemia and prepared for intravital microscopy after aortic clamp removal. Low-dose (1 mg/kg) rPSGL-Ig was injected 30 minutes after cremaster surgery, and P-selectin blocking antibody was injected after a further 10 minutes. Data are presented as mean ± SEMs of n = 4 to 6 experiments. Significant difference between rPSGL-Ig– (1 mg/kg) and vehicle-pretreated I/R groups are indicated by *P < .05 or **P < .01.
Effect of rPSGL-Ig on ischemia/reperfusion–induced leukocyte rolling.
(A) Effect of rPSGL-Ig pretreatment. Mice were subjected to 1 hour lower-body ischemia by application of an infrarenal cross-clamp. Surgery control mice received a laparotomy but no clamp. Some mice received rPSGL-Ig (1 mg/kg, intravenously) 5 minutes prior to release of the aortic clamp. Mice were prepared for intravital microscopy 30 minutes after release of the aortic clamp, and leukocyte rolling in venules was recorded. (B) Effect of rPSGL-Ig treatment after development of I/R-induced rolling. Mice were subjected to 1-hour lower-body ischemia and prepared for intravital microscopy after aortic clamp removal. Low-dose (1 mg/kg) rPSGL-Ig was injected 30 minutes after cremaster surgery, and P-selectin blocking antibody was injected after a further 10 minutes. Data are presented as mean ± SEMs of n = 4 to 6 experiments. Significant difference between rPSGL-Ig– (1 mg/kg) and vehicle-pretreated I/R groups are indicated by *P < .05 or **P < .01.
Comparison of hemodynamic parameters in control and rPSGL-Ig–pretreated mice
| Treatments prior to IVM . | Circulating leukocytes, cells per μL blood . | Centerline blood flow velocity, μm/s . | Vessel diameter, μm . |
|---|---|---|---|
| Surgery control, sham I/R | 6706 ± 511 | 1109 ± 182 | 31.2 ± 2.2 |
| Vehicle pretreatment + I/R | 4214 ± 326 | 2007 ± 457* | 34.6 ± 1.2 |
| rPSGL-Ig pretreatment at 1 mg/kg + I/R | 5471 ± 428 | 2203 ± 210* | 31.2 ± 1.0 |
| Treatments prior to IVM . | Circulating leukocytes, cells per μL blood . | Centerline blood flow velocity, μm/s . | Vessel diameter, μm . |
|---|---|---|---|
| Surgery control, sham I/R | 6706 ± 511 | 1109 ± 182 | 31.2 ± 2.2 |
| Vehicle pretreatment + I/R | 4214 ± 326 | 2007 ± 457* | 34.6 ± 1.2 |
| rPSGL-Ig pretreatment at 1 mg/kg + I/R | 5471 ± 428 | 2203 ± 210* | 31.2 ± 1.0 |
IVM indicates intravital microscopy.
Indicates significant difference from mice subjected to sham I/R.
Rolling in the cremaster muscle induced by I/R injury is known to be exclusively P-selectin dependent at early time points.35Given that 1 mg/kg rPSGL-Ig failed to reduce surgically induced (P-selectin–dependent) rolling in earlier experiments (Figure 1A), we were surprised by its potent actions on I/R-induced rolling. To investigate the possibility that pretreatment with rPSGL-Ig confers some advantage to its activity, we tested the effects of 1 mg/kg rPSGL-Ig on pre-existing I/R-induced leukocyte rolling. Data in Figure3B clearly demonstrate that 1 mg/kg rPSGL-Ig does not alter I/R-induced rolling if it is given after rolling has been established. This same rolling is all but abolished by a P-selectin blocking antibody, supporting earlier reports that such rolling is P-selectin dependent.35
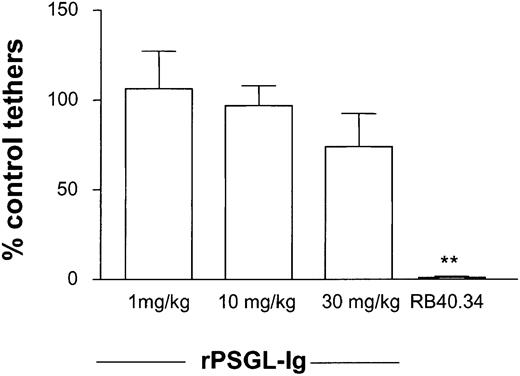
Effect of rPSGL-Ig on leukocyte tethering
Data in Figure 3 suggest considerable advantage to giving rPSGL-Ig as a pretreatment. Before leukocytes can begin rolling along vascular endothelium, they must first tether to it. Formation of the first molecular bond between a free-flowing leukocyte and endothelium may present more of a physical challenge than formation of subsequent bonds (which are facilitated by the existence of the first) and may therefore be more sensitive to inhibition. We designed experiments to test whether low-dose rPSGL-Ig could inhibit leukocyte tethering. Attention was focused on points where tethering was noted to preferentially occur and could be clearly demarked. This was most easily achieved by observing points where capillaries fed into venules (movie 4 in the online version of this article). Control tether formation was counted 30 minutes after surgical preparation of the cremaster. The tethering rate was not significantly altered by application of rPSGL-Ig at any of the doses studied. This suggests that the effect of 30 mg/kg rPSGL-Ig on rolling flux was not due to a reduction in the number of cells forming attachments to postcapillary venular endothelium. Tethering was abolished by P-selectin blocking antibody (Figure4).
Effect of rPSGL-Ig treatment on leukocyte-tethering rate.
WT mice were prepared for intravital microscopy, and surgically induced leukocyte rolling was allowed to develop for 10 minutes. Attention was focused on regions where new tethers could be clearly distinguished, and control tethering rate was determined for 5 to 10 minutes. Low-dose (1 mg/kg) rPSGL-Ig was then injected intravenously, and tethering determined for a further 5 to 10 minutes. Anti–P-selectin antibody was injected at the end of experiments as a positive control. Tethering rates were determined in 6 mice for each dose of rPSGL-Ig studied. Significant difference from control is indicated by **P < .01. Data are presented as mean ± SEM.
Effect of rPSGL-Ig treatment on leukocyte-tethering rate.
WT mice were prepared for intravital microscopy, and surgically induced leukocyte rolling was allowed to develop for 10 minutes. Attention was focused on regions where new tethers could be clearly distinguished, and control tethering rate was determined for 5 to 10 minutes. Low-dose (1 mg/kg) rPSGL-Ig was then injected intravenously, and tethering determined for a further 5 to 10 minutes. Anti–P-selectin antibody was injected at the end of experiments as a positive control. Tethering rates were determined in 6 mice for each dose of rPSGL-Ig studied. Significant difference from control is indicated by **P < .01. Data are presented as mean ± SEM.
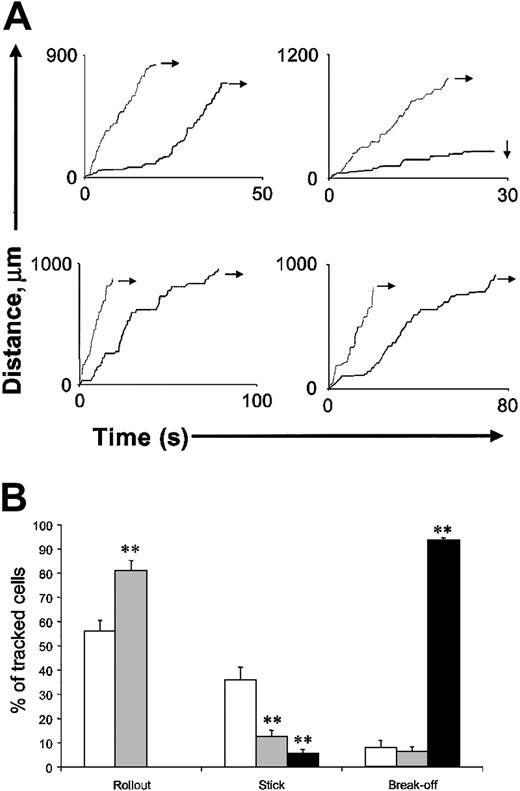
Effect of rPSGL-I on the fate of rolling leukocytes
We hypothesized that rPSGL-Ig might destabilize rolling, allowing leukocytes to roll only short distances before detaching. Such an effect might explain the advantage of pretreatment and the potent anti-inflammatory activity of this molecule. To test this, we tracked leukocytes from their point of attachment in the smallest postcapillary venules of the cremaster to points where they could no longer be visualized (ie, as they entered very large vessels or left the cremaster). Velocities of leukocytes tracked through 4 different routes are shown in Figure 5A. Of the 4 leukocytes tracked prior to application of rPSGL-Ig (denoted by the black lines), 1 rolled for some distance and then firmly adhered (denoted by downward-pointing arrow), whereas the others rolled continuously from their point of attachment to points beyond observation (indicated by rightward-facing arrows). Mice were subsequently treated with 1 mg/kg rPSGL-Ig, and leukocyte velocities tracked through the same vessel networks (denoted by the gray lines). Although leukocytes rolled more quickly following 1 mg/kg rPSGL-Ig treatment, they were not more prone to detachment. The fate of a larger number of cells is shown in Figure 5B. Low-dose (1 mg/kg) rPSGL-Ig increased the percentage of cells rolling all the way out of the tissue by reducing the proportion that firmly adhered. Detachment of leukocytes from the endothelium was an infrequent event that was not appreciably altered by rPSGL-Ig at 1 mg/kg. In contrast, detachment was the most likely fate of leukocytes rolling in venules of mice treated with rPSGL-Ig at 30 mg/kg. Firm adhesion was rare in these mice, and no leukocytes rolled all the way out of the cremaster.
Effect of low-dose (1 mg/kg) rPSGL-Ig treatment on the fate of rolling leukocytes.
WT mice were prepared for intravital microscopy, and surgically induced leukocyte rolling was allowed to develop for 10 minutes. After this period, leukocytes were tracked from a point of obvious attachment (as in Figure 3) through postcapillary venules to points where confident tracking could no longer proceed (ie, if vessels were too large or passed out of the viewable area. (A) Cumulative distance/time plots for individual leukocytes. Each plot shows tracks of 2 leukocytes traveling through the same vessel network before (black line) and after (gray line) low-dose (1 mg/kg intravenously) rPSGL-Ig. Rightward-facing arrows indicate leukocytes rolling out of the observable region. Downward-facing arrows indicate firm adhesion. (B) Combined fate of leukocytes traveling through multiple networks. After surgical preparation of the cremaster and a 10-minute stabilization period, mice received either rPSGL-Ig or vehicle. Multiple leukocytes were tracked through different vessel networks of n = 4 mice per treatment (control, 46 cells, ■; 1 mg/kg rPSGL-Ig, 48 cells, ░; 30 mg/kg rPSGL-Ig, 54 cells, ▪), and their fate was determined as either rollout (leaving the observable area without detachment), stick (remaining stationary for longer than 30 seconds), or break-off (detaching from the endothelium, rejoining the free circulation, and disappearing from view). **Indicates a statistically significant difference from control, P < .01. Data are presented as mean ± SEM.
Effect of low-dose (1 mg/kg) rPSGL-Ig treatment on the fate of rolling leukocytes.
WT mice were prepared for intravital microscopy, and surgically induced leukocyte rolling was allowed to develop for 10 minutes. After this period, leukocytes were tracked from a point of obvious attachment (as in Figure 3) through postcapillary venules to points where confident tracking could no longer proceed (ie, if vessels were too large or passed out of the viewable area. (A) Cumulative distance/time plots for individual leukocytes. Each plot shows tracks of 2 leukocytes traveling through the same vessel network before (black line) and after (gray line) low-dose (1 mg/kg intravenously) rPSGL-Ig. Rightward-facing arrows indicate leukocytes rolling out of the observable region. Downward-facing arrows indicate firm adhesion. (B) Combined fate of leukocytes traveling through multiple networks. After surgical preparation of the cremaster and a 10-minute stabilization period, mice received either rPSGL-Ig or vehicle. Multiple leukocytes were tracked through different vessel networks of n = 4 mice per treatment (control, 46 cells, ■; 1 mg/kg rPSGL-Ig, 48 cells, ░; 30 mg/kg rPSGL-Ig, 54 cells, ▪), and their fate was determined as either rollout (leaving the observable area without detachment), stick (remaining stationary for longer than 30 seconds), or break-off (detaching from the endothelium, rejoining the free circulation, and disappearing from view). **Indicates a statistically significant difference from control, P < .01. Data are presented as mean ± SEM.
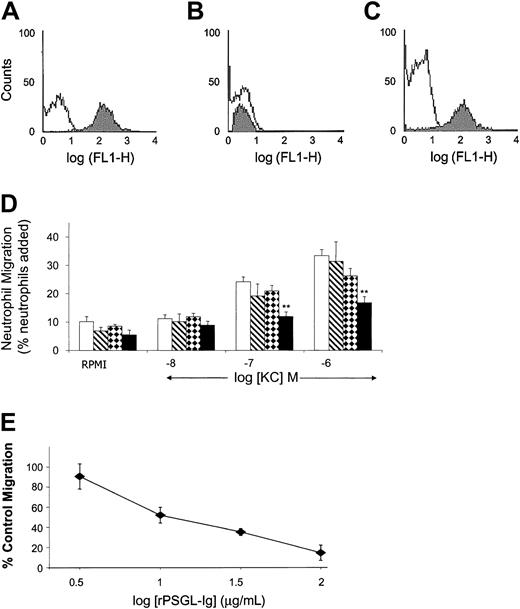
rPSGL-Ig binds the chemokine KC and inhibits its function
PSGL-1 has certain functional requirements in common with chemokine receptors, including posttranslational sulfation and glycosylation.36 To investigate the possibility of chemokine binding to rPSGL-Ig, we coupled rPSGL-Ig to 1-μm beads and measured binding of fluorescent KC (a murine CXC chemokine) using flow cytometry. Fluorescent KC bound to beads coated with rPSGL-Ig (Figure6A), but not to beads coated with a low-affinity form of rPSGL-Ig (Figure 6B). Preincubating fluorescent KC with soluble rPSGL-Ig (10 μg/mL) prevented subsequent binding to rPSGL-Ig–coated beads, whereas preincubation with low-affinity rPSGL-Ig (10 μg/mL) did not (Figure 6C).
Effect of rPSGL-Ig on the murine chemokine KC.
(A-C) Fluorescent KC binds to rPSGL-Ig–coated beads. Differently coated nonfluorescent beads were incubated with FITC-KC, and association was determined by means of flow cytometry. In panel A, open histogram indicates nonfluorescent rPSGL-Ig–coated beads; filled histogram indicates rPSGL-Ig–coated beads incubated with FITC-KC. In panel B, open histogram indicates nonfluorescent low-affinity rPSGL-Ig–coated beads; filled histogram indicates low-affinity rPSGL-Ig–coated beads incubated with FITC-KC. In panel C, open histogram indicates FITC-KC incubated with rPSGL-Ig prior to incubation with rPSGL-Ig–coated beads; filled histogram indicates FITC-KC incubated with low-affinity rPSGL-Ig prior to incubation with rPSGL-Ig–coated beads. (D) Migration of murine neutrophils toward KC is inhibited by rPSGL-Ig. Purified mouse neutrophils were incubated with vehicle (open bars), CD4-Ig (hatched bars), low-affinity rPSGL-Ig (checked bars), or rPSGL-Ig (filled bars) (all at 10 μg/mL) and allowed to migrate over ChemoTx chambers in response to the chemokine KC. The percentage of migration was calculated by expressing the number of migrated neutrophils as a percentage of the total number added to the chamber. Results are presented as means ± SEMs of 5 or 6 experiments. **Indicates a statistically significant difference from vehicle, P < .01. (E) Concentration-dependent inhibition of murine neutrophil migration by rPSGL-Ig. Purified mouse neutrophils were incubated with different concentrations of rPSGL-Ig prior to migration in response to 10−6 M KC. Results are presented as the percentage of control migration, where 100% is the percentage induced by 10−6 M KC with nonspecific migration toward vehicle (RPMI) subtracted. Means ± SEMs of n = 4 experiments are shown.
Effect of rPSGL-Ig on the murine chemokine KC.
(A-C) Fluorescent KC binds to rPSGL-Ig–coated beads. Differently coated nonfluorescent beads were incubated with FITC-KC, and association was determined by means of flow cytometry. In panel A, open histogram indicates nonfluorescent rPSGL-Ig–coated beads; filled histogram indicates rPSGL-Ig–coated beads incubated with FITC-KC. In panel B, open histogram indicates nonfluorescent low-affinity rPSGL-Ig–coated beads; filled histogram indicates low-affinity rPSGL-Ig–coated beads incubated with FITC-KC. In panel C, open histogram indicates FITC-KC incubated with rPSGL-Ig prior to incubation with rPSGL-Ig–coated beads; filled histogram indicates FITC-KC incubated with low-affinity rPSGL-Ig prior to incubation with rPSGL-Ig–coated beads. (D) Migration of murine neutrophils toward KC is inhibited by rPSGL-Ig. Purified mouse neutrophils were incubated with vehicle (open bars), CD4-Ig (hatched bars), low-affinity rPSGL-Ig (checked bars), or rPSGL-Ig (filled bars) (all at 10 μg/mL) and allowed to migrate over ChemoTx chambers in response to the chemokine KC. The percentage of migration was calculated by expressing the number of migrated neutrophils as a percentage of the total number added to the chamber. Results are presented as means ± SEMs of 5 or 6 experiments. **Indicates a statistically significant difference from vehicle, P < .01. (E) Concentration-dependent inhibition of murine neutrophil migration by rPSGL-Ig. Purified mouse neutrophils were incubated with different concentrations of rPSGL-Ig prior to migration in response to 10−6 M KC. Results are presented as the percentage of control migration, where 100% is the percentage induced by 10−6 M KC with nonspecific migration toward vehicle (RPMI) subtracted. Means ± SEMs of n = 4 experiments are shown.
We also used an in vitro neutrophil transmigration assay to investigate whether rPSGL-Ig could inhibit KC-induced responses. Mouse neutrophils (5 × 104/25 μL) placed onto filters of ChemoTx chambers migrated in a dose-dependent fashion toward the chemokine KC located in the wells of the microplate (Figure 6D). Interestingly, 10 μg/mL rPSGL-Ig inhibited migration toward KC. This concentration of rPSGL-Ig is roughly equivalent to the blood concentration given by the 1 mg/kg dose. Migration was not significantly affected by either CD4-Ig or low-affinity rPSGL-Ig. Inhibition of KC-induced neutrophil migration by rPSGL-Ig was dose dependent (Figure 6E).
Discussion
It is proposed that selectin antagonists such as rPSGL-Ig function by competing with cell-bound selectin ligands to prevent leukocyte–endothelial cell interaction. Since leukocyte rolling depends on constant formation and rupture of selectin-ligand bonds, such competitive action should allow selectin antagonists to reverse established leukocyte rolling. In spite of extensive investigation of rPSGL-Ig, its selectin-binding properties16 and anti-inflammatory actions,17-22 37 ours is the first evidence that rPSGL-Ig does precisely this in vivo. We have shown that while high doses of rPSGL-Ig reduce pre-existing leukocyte rolling on all 3 selectins, lower doses (previously shown to be anti-inflammatory) have modest effects, unless given as a pretreatment. These observations support, but do not prove, the assumption that rPSGL-Ig is a competitive selectin inhibitor.
Low-dose (1 mg/kg) rPSGL-Ig did not reduce established P-selectin–dependent leukocyte rolling in venules of the mouse cremaster, although we did detect moderately increased rolling velocities following treatment at 1 mg/kg. We could consider leukocyte rolling in simple terms, such as athletes on a running circuit and our point of observation as the start/finish line. According to such a simple model, doubling velocity should also double the events at our point of observation (providing the same number of runners show up, none of them stop, and no one runs off the track). Studies with other selectin inhibitors (particularly E-selectin inhibitors in TNFα-stimulated mice) have noted that rolling flux and velocity can increase concomitantly,14,34,38 although this is not always the case.27 There are many potential influences on how many cells roll past a fixed point, including rate of attachment, rate of detachment, and rate of firm adhesion. Of these factors, we find that only firm adhesion is influenced by rPSGL-Ig at 1 mg/kg and that firm adhesion is reduced (an effect that would add to the rolling flux). The running circuit model is clearly an oversimplification of the mouse microcirculation, and we are currently unable to explain why rolling flux remains stable as velocity increases in surgically stimulated WT mice following rPSGL-Ig at 1 mg/kg. Increasing the dose of rPSGL-Ig increases rolling velocity but reduces rolling flux. In this instance, we can attribute reduced rolling flux to reduced ability of rolling leukocytes to maintain contact with the endothelium.
Leukocyte rolling is not just a braking mechanism, allowing cells to decelerate as they near their target; it also enables them to sample their environment. Thus, rolling leukocytes are exposed to chemoattractants presented by vascular endothelial cells,39 and slow rolling enhances this exposure and promotes recruitment.38 40 Agents, such as rPSGL-Ig, that increase leukocyte rolling velocity may have profound anti-inflammatory effects by limiting the duration of contact between leukocytes and endothelium. The significance of such an effect is supported by the observation that 1 mg/kg rPSGL-Ig reduced the proportion of tracked leukocytes firmly adhering in venules of surgically stimulated mice without altering the number of leukocytes rolling through those venules.
Although it is likely that the effect of rPSGL-Ig on leukocyte rolling velocity contributes to its impressive anti-inflammatory activity, we do not believe this to be the full explanation. There seems to be considerable advantage in giving this molecule as a pretreatment, which is not readily explained by this mechanism. Low-dose rPSGL-Ig (1 mg/kg or lower) given as a pretreatment is able to reduce leukocyte rolling in response to various stimuli (this and other17,19 20 studies), whereas the same dose given after rolling is established merely alters rolling velocity.
An appealing explanation for the advantage of pretreatment is that low-dose rPSGL-Ig, rather than inhibiting leukocyte rolling per se, prevents initial attachment of leukocytes to the endothelium. Established rolling may be more resistant to inhibition, as one molecular bond between leukocyte and endothelial cell will promote formation of subsequent bonds between molecules that are held in proximity to one another. This explanation can be ruled out, however, by the observation that rPSGL-Ig does not alter tethering rate when given after stimulation of the cremaster.
Since rPSGL-Ig is a large, complex molecule posttranslationally modified to express sialylated fucosylated glycans and sulfate, it may have the potential to interfere with multiple stages of the inflammatory response. Others have shown effects of rPSGL-Ig that are not readily related to competitive inhibition of selectin–selectin ligand binding, including changes in de novo synthesis of endogenous PSGL-119 and E-selectin mRNA.37 Our transmigration experiments suggest inhibition of chemoattractant-driven responses as an alternative or additional mechanism of action. A rationale for this observation may be that chemokine (and other chemoattractant) receptors require sulfation and posttranslational glycosylation similar to that of PSGL-1 in order to function36 and that various sulfated glycans (eg, heparan sulfate, chondroitin sulfate) are known to affect both chemokine41 and selectin42 binding. Large sulfated, glycosylated molecules such as rPSGL-Ig, and perhaps natural soluble PSGL-1, may present attractive surfaces for binding of chemokines. This binding may have proinflammatory or anti-inflammatory activities depending on the location of the molecule.
Although the anti-inflammatory activity of low-dose rPSGL-Ig cannot be attributed entirely to direct inhibition of selectin–selectin ligand interaction (at least in mice), higher doses (30 to 100 mg/kg) of this molecule are able to reduce pre-existing rolling. Furthermore, this activity is apparent in models that favor either E-, L-, or P-selectin–dependent rolling. Although rPSGL-Ig can inhibit rolling by all 3 selectins in vivo, its activity appears to be incomplete, in that a significant proportion of rolling remains after rPSGL-Ig treatment in all experimental systems studied. In our model of surgically induced (P-selectin–dependent) rolling, for example, we see approximately 50% to 60% inhibition at 30 mg/kg and no further inhibition at 100 mg/kg. Treatment with P-selectin antibody, in contrast, abolishes leukocyte rolling in this model. Interestingly, antibodies against PSGL-1 are similarly unable to abolish P-selectin–dependent rolling,11,43,44 whereas gene-targeted mice lacking PSGL-1 have virtually no surgically induced rolling.45 We believe these data can be reconciled if we consider the partnership between the correctly modified N-terminus of PSGL-1 and P-selectin as a dominant, but not exclusive, interaction that can be inhibited by either PSGL-1 blocking antibodies or rPSGL-Ig. Data from PSGL-1 knockout mice suggest that residual rolling is still PSGL-1 dependent but may involve other parts of the molecule.
There are other possible explanations for the observation that direct inhibition of leukocyte rolling by rPSGL-Ig is incomplete and requires such high doses. Firstly, rPSGL-Ig is based on the structure of human and not murine PSGL-1. Although human and murine selectins and selectin ligands have some sequence homology,5,7 we cannot rule out the possibility that species differences underlie the requirement for high doses of rPSGL-Ig and the incomplete effect. This argument does not explain the incomplete inhibition given by 2PH1, which is specifically directed against murine PSGL-1. Another antibody (4RA10) against murine PSGL-1 blocks more rolling than 2PH1,44 but is still not as effective as P-selectin blocking antibody.
In summary, we have investigated the ability of rPSGL-Ig to influence leukocyte rolling. Although we find that rPSGL-Ig can inhibit leukocyte rolling via all 3 selectins in vivo if given in sufficient doses, the mechanism of its anti-inflammatory activity remains somewhat mysterious. Low-dose rPSGL-Ig has pronounced anti-inflammatory effects and can reduce leukocyte rolling if given as a pretreatment. The chemoattractant-driven responses that follow leukocyte rolling are also inhibited by rPSGL-Ig. The mechanism of this effect requires further investigation.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-07-2329.
Supported by grants FS/98051, FS/2001007, and FS/2002004 from the British Heart Foundation and grants 061305 and 057108 from the Wellcome Trust.
A.E.R.H. and S.L.N. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Keith Norman, Cardiovascular Research Group, University of Sheffield, Clinical Sciences Centre, Northern General Hospital, Herries Rd, Sheffield S5 7AU, United Kingdom; e-mail:k.norman@sheffield.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal