Abstract
The vasoocclusive crisis is the major clinical feature of sickle cell anemia, which is believed to be initiated or sustained by sickle (SS) red blood cell (RBC) adhesion to the vascular wall. SS RBCs, but not unaffected (AA) RBCs, adhere avidly to multiple components of the vascular wall, including laminin. Here we report a novel role for epinephrine and cyclic adenosine monophosphate (cAMP) in the regulation of human SS RBC adhesiveness via the laminin receptor, basal cell adhesion molecule/Lutheran (BCAM/Lu). Our data demonstrate that peripheral SS RBCs contain greater than 4-fold more cAMP than AA RBCs under basal conditions. Forskolin or the stress mediator epinephrine further elevates cAMP in SS RBCs and increases adhesion of SS RBCs to laminin in a protein kinase A (PKA)–dependent manner, with the low-density population being the most responsive. Epinephrine-stimulated adhesion to laminin, mediated primarily via the β2-adrenergic receptor, occurred in SS RBC samples from 46% of patients and was blocked by recombinant, soluble BCAM/Lu, implicating this receptor as a target of cAMP signaling. Thus, these studies demonstrate a novel, rapid regulation of SS RBC adhesion by a cAMP-dependent pathway and suggest that components of this pathway, particularly PKA, the β2-adrenergic receptor, and BCAM/Lu, should be further explored as potential therapeutic targets to inhibit SS RBC adhesion.
Introduction
Sickle (SS) red blood cells (RBCs), unlike unaffected (AA) RBCs, adhere avidly to components of the vascular wall, and this abnormal adhesion is believed to contribute to the painful vasoocclusive crises that occur in patients with sickle cell anemia. Laminin is an extracellular matrix (ECM) protein distributed throughout most vascular beds,1 with isoforms 10 and 11 specifically supporting more SS RBC adhesion than other ECM proteins tested under physiologic shear and flow conditions.2Patients with sickle cell anemia have extensive endothelial damage,3 likely bringing the underlying matrix laminin into direct contact with flowing blood. In addition, plasma levels of laminin are elevated in sickle cell disease,4 suggesting that laminin may be deposited on the surface of the endothelium where it could also serve as an adhesive substrate. Udani et al5have demonstrated that the basal cell adhesion molecule and its isoform Lutheran (BCAM/Lu) are the major RBC receptors for laminin under basal conditions. Furthermore, recent studies demonstrating that the adhesive state of SS RBCs can be modulated by signal transduction6set a precedent to explore the regulation of SS RBC adhesion to laminin.
RBCs have traditionally been viewed as simple conduits for oxygen transport; however, these cells contain a broad range of signaling molecules.7 Some of the most well-studied signaling pathways in RBCs are mediated by the second messenger cyclic adenosine monophosphate (cAMP),8,9 generated by the conversion of adenosine triphosphate (ATP) to cAMP via membrane-associated adenylyl cyclase. The downstream effects of cAMP are primarily attributed to cAMP-dependent protein kinase A (PKA), the most well-studied downstream effector of cAMP.10 This pathway is important in down-regulating the adhesion of other hematopoietic cells, such as the rapid cAMP- and PKA-dependent reduction in platelet adhesiveness,11-16 and a similar, albeit more gradual, reduction in leukocyte adhesiveness mediated by this pathway.17,18 In contrast, more limited reports describe a gradual increase in the adhesion of leukocytes to various substrates,19 as well as the adhesion of T lymphocytes to laminin,20 in response to cAMP- and PKA-mediated signaling pathways. However, the adhesive effects of this pathway on RBCs, whether normal or diseased, have never been explored.
Here, we describe an unusually rapid cAMP- and PKA-dependent activation of SS RBC adhesion to laminin. The physiologic stress mediator epinephrine, acting largely through the β2-adrenergic receptor, increased the adhesion of SS RBCs from 46% of patients tested. Finally, we demonstrate that the cell surface adhesion receptor BCAM/Lu mediates the stimulated adhesion of SS RBCs to laminin. These results describe a novel effect of the cAMP-signaling pathway on SS RBC adhesion.
Materials and methods
Reagents
Human laminin (isoforms 10 and 11) was obtained from Gibco BRL (Grand Island, NY), and forskolin (Coleus forskohlii) (reconstituted in dimethyl sulfoxide [DMSO]), 14-22 amide protein kinase A inhibitor (PKAI), adenosine 3′,5′-cyclic monophosphate, N6O2′-dibutyryl-, sodium salt (db cAMP), and 3-isobutyl-1-methylxanthine (IBMX) (reconstituted in DMSO) from Calbiochem (La Jolla, CA). Epinephrine, propranolol, and butoxamine were from Sigma-Aldrich (St Louis, MO). Unless otherwise indicated, reagents were reconstituted and diluted in phosphate-buffered saline (PBS).
Red blood cell preparation
SS RBCs were obtained from patients with sickle cell anemia during clinic visits to The University of North Carolina (UNC) Comprehensive Sickle Cell Center following the guidelines of the Human Subjects Committee at The University of North Carolina at Chapel Hill. AA RBCs were obtained from laboratory personnel. Blood was drawn by venipuncture into 0.13 M sodium citrate and centrifuged at 150g for 15 minutes at 25°C to isolate RBCs. The buffy coat and the top layer of RBCs were aspirated to ensure maximal white blood cell (WBC) elimination. All buffers were prewarmed to 37°C prior to use. RBCs were washed 3 times in a CGS buffer (1.29 mM sodium citrate, 3.33 mM glucose, 124 mMsodium chloride, pH = 7.2) and centrifuged at 400g for 10 minutes in PBS. For flow adhesion assays, a 1% hematocrit was then prepared by diluting 30 μL packed RBCs per 1.5 mL perfusion media (Hanks balanced salt solution [HBSS] supplemented with 0.3% bovine serum albumin, phenol red, and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH = 7.4). For cAMP assays, packed RBCs were diluted in HBSS without phenol red and bovine serum albumen (BSA) to prevent interference with the colorimetric reading.
Arabinogalactan density gradient RBC fractionation
Washed RBCs were diluted 1:1 in an isotonic universal diluent (Larex, St Paul, MN) for 5 minutes. Next, 2 mL undiluted arabinogalactan cell separation medium (Larex) was layered at the bottom of an ultracentrifuge tube, followed by 3 mL of 1 part universal diluent to 2 parts cell separation medium solution. Finally, 3 mL of 1 part packed RBCs to 1 part diluent solution was layered on top of the gradient. The RBCs were centrifuged at 74 000g for 45 minutes at 20°C. The top 15% of RBCs (low-density fraction) was carefully aspirated, followed by the bottom 15% of RBCs (high-density fraction). These fractions were packed in PBS at 400g for 10 minutes and resuspended in HBSS.
Cyclic AMP enzyme-immunoassay
Total cAMP was measured in 1 × 108 RBCs as described in the Amersham Pharmacia Biotechnology (Piscataway, NJ) cAMP enzyme-immunoassay system protocol 4 (total cellular cAMP measurement). Briefly, 160 μL of 1 × 108 RBCs was added per well in a 96-well tissue culture plate. Next, RBCs were incubated at 37°C, and 20 μL desired cAMP-modulating agent was added. RBCs were lysed in 20 μL lysis reagent (2.5% solution of dodecyltrimethylammonium bromide). The 96-well plate was agitated for 30 minutes to ensure complete lysis, and total cAMP levels were measured according to the manufacturer's instructions.
Construction, expression, and purification of soluble BCAM/Lu recombinant protein
A cDNA construct of the BCAM/Lu extracellular domain was generated from the full-length Lutheran cDNA by polymerase chain reaction (PCR), using the forward oligonucleotide primer 5′-AACATGGAGCCCCCGGACGCA-3′ and the reverse primer 5′-CTGGGGGCTCACGGCGC-3′. The resulting cDNA encoding the extracellular domain fragment Met1 to Gln543 was subcloned into the pcDNA3.1/V5-His-TOPO expression vector (Invitrogen, Carlsbad, CA), which provides both a V5 epitope and a polyhistidine C-terminal sequence. This construct was stably transfected into 293 cells with Lipofectin (Invitrogen, Carlsbad, CA) and subsequently adapted to 293 serum-free medium (SFM) II containing 0.5 mg/mL Geneticin (Invitrogen). The secreted recombinant Lutheran protein (BCAM/Lu) produced by transfected 293 cells was purified using the Xpress protein purification system (Invitrogen) under native conditions.
Flow adhesion assay
RBC adhesion to laminin was measured under physiologic flow conditions using a parallel plate flow chamber as previously described.21 22 Briefly, 0.75 μg purified human laminin in PBS was immobilized on identical wells formed by a silicon gasket seated into a 35-mm polystyrene tissue culture dish (Becton Dickinson, Franklin Lakes, NJ) by incubating for 2 hours at 37°C. A 1% hematocrit of RBCs (1.5 mL) was flowed across the immobilized laminin at a constant flow rate of 1.0 mL/minute and shear stress of 1 dyne/cm2. After a 6-minute wash, adherent RBCs were quantified in 4 representative areas by microscopy (×200). Occasional contaminating WBCs were excluded from all counts.
Staining of adherent RBCs following the flow assay
Following the flow adhesion assay, the vacuum seal was gently removed followed by separation of the chamber from the underlying tissue culture dish. Each well was gently washed twice with HBSS and twice with PBS. Immediately afterward, adherent RBCs were simultaneously fixed and stained with methylene blue and washed with deionized, distilled (dd) H2O. Adherent RBCs were counted microscopically (40× oil immersion).
Data analysis
Figures represent averages across patients, shown with standard deviation bars, or selected representative patients. A 2-tailed pairedt test was used to assess the statistical significance between cAMP levels or adhesion of treated versus untreated samples where indicated. All other data analyses relied on descriptive and graphical methods.
Results
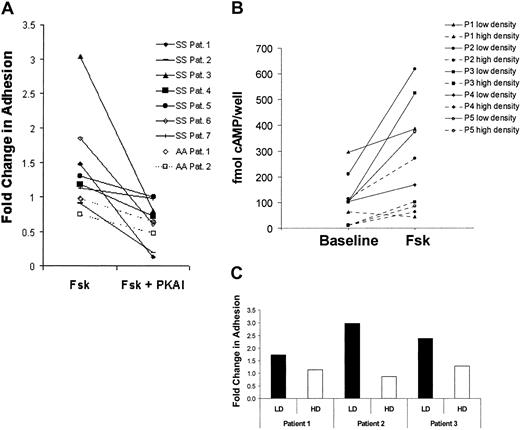
To compare the basal levels of cAMP in SS versus AA RBCs, total cellular cAMP levels were measured under unstimulated conditions. We found that SS RBCs exhibited a broad range of basal cAMP levels, 4.4-fold higher on average than the consistently low levels measured in AA RBCs (Figure 1A). We then asked if cAMP production could be stimulated beyond the elevated basal levels in SS RBCs via forskolin-mediated activation of adenylyl cyclase. A time course of forskolin treatment of SS RBCs from 3 separate patients demonstrated that cAMP levels increased approximately 3-fold on average above baseline (Figure 1B). Although the absolute cAMP levels induced by forskolin varied from patient to patient, maximum stimulation consistently occurred within 15 minutes. In contrast, minimal cAMP production occurred in forskolin-treated AA RBCs (Figure 1B). To our knowledge, these data represent the first evidence of differential basal and stimulated cAMP levels between intact peripheral SS and AA RBCs.
SS RBCs have higher basal and stimulated cAMP levels compared with AA RBCs.
Cyclic AMP levels in AA or SS RBCs were measured by enzyme-immunoassay (“Materials and methods”). (A) cAMP levels were measured under basal/unstimulated conditions in AA RBCs (n = 4) and SS RBCs (n = 6). Each dot represents the measured cAMP value in 1 × 108 RBCs from one patient. The horizontal bars represent the average values of the individual patient data points. (B) SS (n = 3) and AA (n = 2) RBCs samples (1 × 108 cells per condition) were treated with 80 μM forskolin (Fsk) for 0, 1, 5, 15, and 30 minutes as indicated. After each treatment condition, the RBCs were lysed, and total cellular cAMP levels were measured.
SS RBCs have higher basal and stimulated cAMP levels compared with AA RBCs.
Cyclic AMP levels in AA or SS RBCs were measured by enzyme-immunoassay (“Materials and methods”). (A) cAMP levels were measured under basal/unstimulated conditions in AA RBCs (n = 4) and SS RBCs (n = 6). Each dot represents the measured cAMP value in 1 × 108 RBCs from one patient. The horizontal bars represent the average values of the individual patient data points. (B) SS (n = 3) and AA (n = 2) RBCs samples (1 × 108 cells per condition) were treated with 80 μM forskolin (Fsk) for 0, 1, 5, 15, and 30 minutes as indicated. After each treatment condition, the RBCs were lysed, and total cellular cAMP levels were measured.
To determine how the stimulated cAMP levels affect adhesion of SS versus AA RBCs, we again treated SS RBCs with forskolin and found that SS RBC adhesion to laminin was stimulated to various extents (ie, greater than 1.5 fold) in 3 patients, a modest stimulation in 1 patient, and no stimulation in 3 other patients (Figure2A). However, preincubation of SS RBCs with a protein kinase A inhibitor (PKAI) prior to forskolin stimulation inhibited forskolin-stimulated adhesion to below basal levels in most patients (P < .05). These data suggest that cAMP-mediated PKA activation not only stimulates a more adhesive state in SS RBCs from some patients but also contributes to basal SS RBC adhesion to laminin.
Forskolin stimulates SS but not AA RBC adhesion to laminin.
(A) SS RBCs (n = 7, solid lines) and AA RBCs (n = 2, dashed lines) were pretreated with 200 μM IBMX for 2 hours to inhibit phosphodiesterase activity. The RBCs were subsequently treated with 36 nM 14-22 amide PKA inhibitor (PKAI) for 1 hour, 80 μM forskolin (Fsk) for 15 minutes, or a combination of Fsk and PKAI as indicated. Adhesion to laminin was measured in the flow adhesion assay (“Materials and methods”). Basal adhesion was normalized to 1. (B-C), SS RBCs patient samples were separated into a low-density (reticulocyte-enriched) and a high-density (reticulocyte-depleted) population using an arabinogalactan density gradient (“Materials and methods”). (B) cAMP levels were measured in 1 × 108low-density (solid lines) or high-density (dotted lines) SS RBCs/condition from 5 separate patients during baseline and forskolin-treated conditions (80 μM for 15 minutes) as indicated. (C) Fold change in adhesion of low-density (LD, ▪) and high-density (HD, ■) SS RBCs to laminin was measured in the flow adhesion assay (“Materials and methods”) in response to forskolin (80 μM for 15 minutes) in SS RBCs from 3 separate patients. Basal adhesion is normalized to 1.
Forskolin stimulates SS but not AA RBC adhesion to laminin.
(A) SS RBCs (n = 7, solid lines) and AA RBCs (n = 2, dashed lines) were pretreated with 200 μM IBMX for 2 hours to inhibit phosphodiesterase activity. The RBCs were subsequently treated with 36 nM 14-22 amide PKA inhibitor (PKAI) for 1 hour, 80 μM forskolin (Fsk) for 15 minutes, or a combination of Fsk and PKAI as indicated. Adhesion to laminin was measured in the flow adhesion assay (“Materials and methods”). Basal adhesion was normalized to 1. (B-C), SS RBCs patient samples were separated into a low-density (reticulocyte-enriched) and a high-density (reticulocyte-depleted) population using an arabinogalactan density gradient (“Materials and methods”). (B) cAMP levels were measured in 1 × 108low-density (solid lines) or high-density (dotted lines) SS RBCs/condition from 5 separate patients during baseline and forskolin-treated conditions (80 μM for 15 minutes) as indicated. (C) Fold change in adhesion of low-density (LD, ▪) and high-density (HD, ■) SS RBCs to laminin was measured in the flow adhesion assay (“Materials and methods”) in response to forskolin (80 μM for 15 minutes) in SS RBCs from 3 separate patients. Basal adhesion is normalized to 1.
We also examined the effects of forskolin stimulation and PKA inhibition on the adhesive state of AA RBCs. It should be noted that absolute basal AA RBC adhesion to laminin was approximately 10-fold lower than basal SS RBC adhesion, consistent with previous observations.2 23 Furthermore, AA RBC adhesion to laminin was unaffected by forskolin treatment (Figure 2A). These results are consistent with the inability of forskolin to stimulate cAMP production in peripheral AA RBCs versus SS RBCs (Figure 1B) and may reflect a reduced capacity of circulating AA RBCs to produce cAMP, potentially because of lower levels and/or activity of adenylyl cyclase.
Patients with sickle cell anemia have a much higher percentage of circulating reticulocytes than RBCs from unaffected control subjects, and the average age of their “mature” RBCs is markedly less.24 RBCs lose various signaling components as they age7; thus, their capacity to produce and respond to cAMP may be age dependent. To determine if a particular population of SS RBCs was more responsive to cAMP-mediated activation, SS RBCs were separated into low- (reticulocyte-enriched) and high-density (reticulocyte-depleted) fractions by centrifugation over an arabinogalactan density gradient. When forskolin-stimulated cAMP production and adhesion in each fraction were compared, the low-density SS RBC fraction exhibited a substantially greater increase in both cAMP production (Figure 2B) and adhesion (Figure 2C) versus the high-density fraction. Control experiments confirmed that WBC contamination did not account for the increased cAMP production in the low-density fraction (data not shown). These results suggest that the younger, low-density SS RBCs are most responsive to forskolin-induced increases in cAMP production and adhesion.
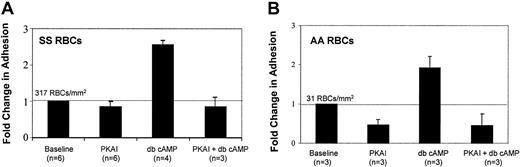
Next, we asked if RBC adhesion to laminin could be stimulated by directly introducing cAMP, thus bypassing adenylyl cyclase activation. Both SS and AA RBCs were preincubated with dibutyryl cAMP (db cAMP), a stable, membrane-soluble cAMP analog that activates PKA. Like forskolin, db cAMP treatment elevated SS RBC adhesion to laminin almost 3-fold (Figure 3A), further supporting the notion that elevated intracellular cAMP increases SS RBC adhesiveness. Interestingly, db cAMP induced a similar fold elevation in adhesion in AA RBCs (Figure 3B), suggesting that AA RBCs retain the signaling machinery downstream of adenylyl cyclase required to increase adhesion to laminin; however, the absolute number of adherent AA RBCs was 10-fold less than that of SS RBCs.
Cyclic AMP stimulates SS and AA RBC adhesion to laminin in a PKA-dependent manner.
SS RBCs (A) or AA RBCs (B) were treated with 36 nM PKA inhibitor (PKAI), for 1 hour, 180 μM of the stable cAMP analog dibutyryl cAMP (db cAMP) for at least 1 hour, or a combination of both as indicated. Adhesion to laminin was measured in the flow adhesion assay (“Materials and methods”). Baseline adhesion is set at 1, and fold change from baseline is indicated. The absolute number of RBCs adhering under basal conditions is shown above the baseline bar of each graph.
Cyclic AMP stimulates SS and AA RBC adhesion to laminin in a PKA-dependent manner.
SS RBCs (A) or AA RBCs (B) were treated with 36 nM PKA inhibitor (PKAI), for 1 hour, 180 μM of the stable cAMP analog dibutyryl cAMP (db cAMP) for at least 1 hour, or a combination of both as indicated. Adhesion to laminin was measured in the flow adhesion assay (“Materials and methods”). Baseline adhesion is set at 1, and fold change from baseline is indicated. The absolute number of RBCs adhering under basal conditions is shown above the baseline bar of each graph.
Next, the potential physiologic implications of cAMP-stimulated adhesion of SS RBCs were explored. Epinephrine, the major mediator of the physiologic stress response, elevates cAMP levels in RBCs25; however, the effects of epinephrine on cAMP levels or adhesion of SS RBCs have never been explored. Treatment of SS RBCs with epinephrine induced a maximal stimulation of total cellular cAMP in SS RBCs within 1 minute (Figure 4A) and a corresponding peak in SS RBC adhesion to laminin within 1 to 5 minutes of treatment (Figure 4B), suggesting a correlation between these 2 events. Consistent with our previous observations with forskolin (Figure 2B-C), epinephrine-stimulated cAMP levels were more pronounced in the low-density population of SS RBCs (data not shown).
Epinephrine-stimulated cAMP production and adhesion to laminin exhibit similar time courses.
(A) SS RBCs from 2 different patients were treated with 1 × 10−2 μM epinephrine and lysed after 1, 5, 15, or 30 minutes as indicated. Total cAMP levels were measured in 1 × 108 SS RBCs/condition by enzyme-immunoassay (“Materials and methods”). (B) Adhesion of SS RBCs from 2 different patients to immobilized laminin was measured using the flow adhesion assay after treatment for 1, 5, 15, and 30 minutes with 1 × 10−2 μM epinephrine. Baseline adhesion is set at 1, and fold change from baseline adhesion at each time point is shown (lines connecting the individual time points do not represent the best fit line, but serve as a visual aid).
Epinephrine-stimulated cAMP production and adhesion to laminin exhibit similar time courses.
(A) SS RBCs from 2 different patients were treated with 1 × 10−2 μM epinephrine and lysed after 1, 5, 15, or 30 minutes as indicated. Total cAMP levels were measured in 1 × 108 SS RBCs/condition by enzyme-immunoassay (“Materials and methods”). (B) Adhesion of SS RBCs from 2 different patients to immobilized laminin was measured using the flow adhesion assay after treatment for 1, 5, 15, and 30 minutes with 1 × 10−2 μM epinephrine. Baseline adhesion is set at 1, and fold change from baseline adhesion at each time point is shown (lines connecting the individual time points do not represent the best fit line, but serve as a visual aid).
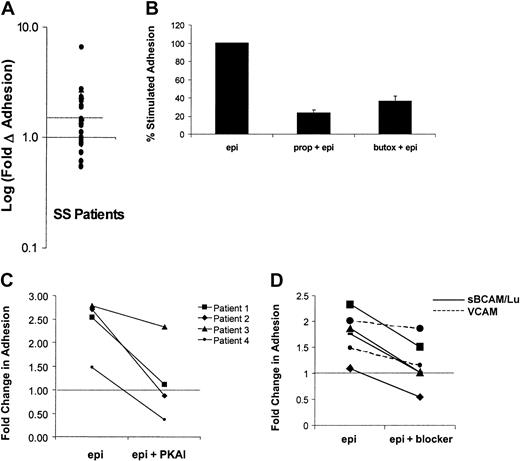
Because previous data indicated that the degree of adhesive response to cAMP stimulation varied from patient to patient, the effects of epinephrine on SS RBC adhesion to laminin was measured in samples obtained from a larger group of patients (n = 28). A statistically significant stimulation of SS RBC adhesion above basal levels was observed (P < .01). SS RBCs from a subset of patients (46%) exhibited more than 1.5-fold (50%) elevation in adhesion to laminin and were classified as responders (Figure5A), whereas adhesion was slightly decreased in a smaller subset of patients (11%). These data suggest that almost half of patients with sickle cell anemia may be susceptible to epinephrine-stimulated adhesion of SS RBCs.
Mechanisms of epinephrine-stimulated adhesion in SS RBCs from the responsive patient subpopulation.
Adhesion of SS RBCs to immobilized laminin was measured in the flow adhesion assay (“Materials and methods”) under basal conditions, following epinephrine stimulation (1 × 10−2 μM epinephrine for 1 minute), or in the presence of one of the inhibitors described in B, C, and D. (A) Baseline adhesion is set at 1 (solid line), and the log of the fold change from basal adhesion following epinephrine stimulation is indicated on the y-axis. Patients with more than 1.5-fold (50%) elevation in adhesion (on or above dotted line) were classified as responders, and patients below the dotted line were classified as nonresponders. Each dot represents a separate patient (n = 28). Inhibition of epinephrine-stimulated adhesion was measured in the presence of (B) the nonspecific β-AR antagonist propranolol (170 μM for 30 minutes) or the β2-specific AR antagonist butoxamine (160 μM for 30 minutes (P < .05, n = 5), (C) a PKA inhibitor (PKAI) (36 nM for 1 hour) (P < .05, n = 4), or (D) 100 μg/mL soluble BCAM/Lu (solid line) or 100 μg/mL soluble VCAM (control) (dotted line). The portion of adhesion stimulated by epinephrine was inhibited by an average of 96% (n = 4,P < .05). (The dotted line in C and D indicates basal adhesion normalized to 1).
Mechanisms of epinephrine-stimulated adhesion in SS RBCs from the responsive patient subpopulation.
Adhesion of SS RBCs to immobilized laminin was measured in the flow adhesion assay (“Materials and methods”) under basal conditions, following epinephrine stimulation (1 × 10−2 μM epinephrine for 1 minute), or in the presence of one of the inhibitors described in B, C, and D. (A) Baseline adhesion is set at 1 (solid line), and the log of the fold change from basal adhesion following epinephrine stimulation is indicated on the y-axis. Patients with more than 1.5-fold (50%) elevation in adhesion (on or above dotted line) were classified as responders, and patients below the dotted line were classified as nonresponders. Each dot represents a separate patient (n = 28). Inhibition of epinephrine-stimulated adhesion was measured in the presence of (B) the nonspecific β-AR antagonist propranolol (170 μM for 30 minutes) or the β2-specific AR antagonist butoxamine (160 μM for 30 minutes (P < .05, n = 5), (C) a PKA inhibitor (PKAI) (36 nM for 1 hour) (P < .05, n = 4), or (D) 100 μg/mL soluble BCAM/Lu (solid line) or 100 μg/mL soluble VCAM (control) (dotted line). The portion of adhesion stimulated by epinephrine was inhibited by an average of 96% (n = 4,P < .05). (The dotted line in C and D indicates basal adhesion normalized to 1).
Next, the mechanism of this epinephrine-stimulated adhesion was further explored in the responsive patient population. Previous studies suggested that the β-adrenergic receptor (β-AR) is the primary mediator of the physiologic effects of epinephrine on RBCs.9,26,27 As a result, we asked if β-AR inhibition could block the epinephrine-stimulated adhesion. The nonselective β-AR antagonist propranolol inhibited epinephrine-stimulated SS RBC adhesion by 77% (P < .01), whereas the β2-specific antagonist butoxamine inhibited adhesion by 62% (P < .001) (Figure 5B). These data are consistent with previous studies demonstrating that epinephrine acts on normal RBCs primarily via the β2-AR.28 However, the incomplete inhibition of stimulated SS RBC adhesion suggests that other ARs may also contribute.
Because the most well-described downstream effector of cAMP is PKA, we explored the role of PKA in epinephrine-mediated SS RBC adhesion. As shown in Figure 5C, epinephrine-stimulated adhesion was completely blocked by PKAI in 3 responders (patients 1, 2, and 4) and partially blocked in one responder (patient 3). These data suggest that PKA activation is required for epinephrine-stimulated adhesion to laminin in the responsive population of patients with sickle cell anemia.
Studies by Udani et al5 and Zen et al29 have shown that the primary laminin receptor on unstimulated SS RBCs is basal cell adhesion molecule and its isoform Lutheran (BCAM/Lu); we, therefore, asked if BCAM/Lu also mediated the epinephrine-stimulated adhesion. Immobilized laminin was incubated with recombinant, soluble BCAM/Lu, which significantly inhibited the epinephrine-stimulated portion of SS RBC adhesion by an average of 96% (n = 4, P < .05), whereas a control protein, soluble vascular cell adhesion molecule (VCAM), had no statistically significant effect on stimulated adhesion (n = 2) (Figure 5D). These data indicate a role for BCAM/Lu in epinephrine-stimulated SS RBC adhesion to laminin. Because BCAM/Lu contains several serine residues that represent potential PKA phosphorylation sites, we asked if PKA directly affects BCAM/Lu function via serine phosphorylation. However, we were unable to detect serine phosphorylation of BCAM/Lu when immunoprecipitated from epinephrine- or forskolin-stimulated SS RBCs and Western blotted with an antiphosphoserine antibody (data not shown), suggesting that PKA activation is an intermediate step in a pathway that modulates BCAM/Lu function.
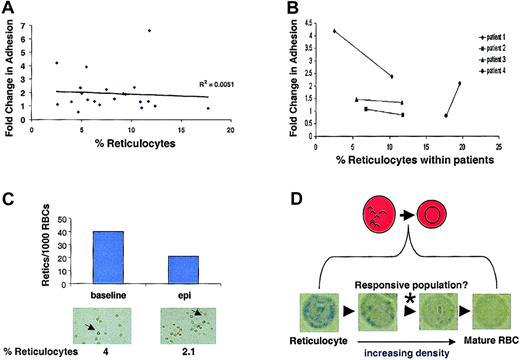
Reticulocytes, which are RBC precursors, retain more of their signaling machinery than other more mature circulating RBCs and exist at higher levels in the blood of patients with sickle cell anemia. To determine if the observed variability in epinephrine-stimulated SS RBC adhesion (Figure 5A) was directly related to patient reticulocyte counts per se, the relationship between fold change in adhesion versus percentage of reticulocyte count was examined. The percentage of reticulocyte values did not independently predict SS RBC responsiveness to epinephrine between patients (Figure 6A). Moreover, when individual patients were followed over time, there was no consistent correlation between reticulocyte counts within a given patient and stimulated adhesion (Figure 6B). Further, when adherent SS RBCs were fixed and stained with methylene blue following the flow adhesion assay, the percentage of adherent reticulocytes actually decreased following epinephrine stimulation (Figure 6C). Thus, the major epinephrine-responsive population of SS RBCs is not the RNA-containing reticulocytes detected with our staining procedure, but likely a subpopulation between the methylene blue–detectable reticulocyte–senescent SS RBC continuum (Figure 6D).
Reticulocyte counts do not predict epinephrine responsiveness.
SS RBC adhesion was measured in the flow adhesion assay (“Materials and methods”). SS RBCs were treated with 1 × 10−8 M epinephrine for 1 minute or with buffer control. (A) Fold change in adhesion was plotted against percentage of reticulocyte counts (n = 21). (B) Percentage of reticulocyte counts was measured in 4 different SS patients at 2 separate time points. The 2 time points for each patient are connected by a solid line to show the relationship between epinephrine-stimulated adhesion and temporal reticulocyte counts. (C) Adherent SS RBCs were stained immediately following a flow adhesion assay (“Materials and methods”). The percentage of reticulocytes/1000 SS RBCs was counted microscopically for basal and epinephrine-stimulated conditions. Arrows indicate cells classified as reticulocytes. (Reticulocytes in A, C, and D were detected by methylene blue staining. In A and B, basal adhesion is normalized to 1.) (D) Between the most immature reticulocytes and the senescent RBCs exist as a continuum of RBCs at different stages of maturation. Methylene blue staining detects only the reticulocytes containing sufficient remnant RNA to allow visualization. Panels A, B, and C all suggest that these detectable reticulocytes are not responsible for the epinephrine-stimulated adhesion, whereas Figure 3B shows that the responsive population is likely not the senescent, dense RBCs. Therefore, the responsive population likely exists between these extremes, as indicated by the (*).
Reticulocyte counts do not predict epinephrine responsiveness.
SS RBC adhesion was measured in the flow adhesion assay (“Materials and methods”). SS RBCs were treated with 1 × 10−8 M epinephrine for 1 minute or with buffer control. (A) Fold change in adhesion was plotted against percentage of reticulocyte counts (n = 21). (B) Percentage of reticulocyte counts was measured in 4 different SS patients at 2 separate time points. The 2 time points for each patient are connected by a solid line to show the relationship between epinephrine-stimulated adhesion and temporal reticulocyte counts. (C) Adherent SS RBCs were stained immediately following a flow adhesion assay (“Materials and methods”). The percentage of reticulocytes/1000 SS RBCs was counted microscopically for basal and epinephrine-stimulated conditions. Arrows indicate cells classified as reticulocytes. (Reticulocytes in A, C, and D were detected by methylene blue staining. In A and B, basal adhesion is normalized to 1.) (D) Between the most immature reticulocytes and the senescent RBCs exist as a continuum of RBCs at different stages of maturation. Methylene blue staining detects only the reticulocytes containing sufficient remnant RNA to allow visualization. Panels A, B, and C all suggest that these detectable reticulocytes are not responsible for the epinephrine-stimulated adhesion, whereas Figure 3B shows that the responsive population is likely not the senescent, dense RBCs. Therefore, the responsive population likely exists between these extremes, as indicated by the (*).
Next, we explored the possibility that epinephrine-stimulated adhesion may be a generalized feature of clinical conditions displaying reticulocytosis (eg, acute blood loss, nonsickle cell hemolytic anemias). To test this possibility, we obtained RBCs from 4 patients with elevated reticulocyte counts but without sickle cell anemia and observed their adhesive response following epinephrine treatment. Although one patient (reticulocyte count = 6.5%) exhibited an elevated adhesion to laminin in response to epinephrine, the other 3 (reticulocyte counts ranging from 2.8% to 9.4%) demonstrated no significant increase (data not shown). Thus, epinephrine stimulation did not cause a consistent change from basal adhesion. However, it would be premature to suggest that epinephrine-stimulated adhesion is a property limited mainly to SS RBCs but may be a function of the mean cell age in any given peripheral RBC population. Our results are summarized in a model that illustrates the role of the β-AR, adenylyl cyclase, PKA, and BCAM/Lu in epinephrine-stimulated SS RBC adhesion to laminin (Figure 7).
Proposed model for epinephrine-stimulated SS RBC adhesion to laminin.
During periods of stress, increased circulating levels of epinephrine act on the RBC β2-AR, thus activating Gαs, which stimulates adenylyl cyclase (AC). This enzyme catalyzes the conversion of ATP to cAMP, leading to PKA activation, an intermediate step in the up-regulation of BCAM/Lu-mediated adhesion. (Phosphodiesterase [PDE] breaks down cAMP into AMP.) Events between PKA activation and enhancement of BCAM/Lu-mediated adhesion are currently unknown. Although this pathway may not be limited to RBCs from patients with sickle cell anemia, the vasculature of patients with sickle cell anemia probably exists in a much more pro-adhesive state. These factors acting in concert suggest that epinephrine-stimulated adhesion may play a clinically significant role in the context of sickle cell anemia.
Proposed model for epinephrine-stimulated SS RBC adhesion to laminin.
During periods of stress, increased circulating levels of epinephrine act on the RBC β2-AR, thus activating Gαs, which stimulates adenylyl cyclase (AC). This enzyme catalyzes the conversion of ATP to cAMP, leading to PKA activation, an intermediate step in the up-regulation of BCAM/Lu-mediated adhesion. (Phosphodiesterase [PDE] breaks down cAMP into AMP.) Events between PKA activation and enhancement of BCAM/Lu-mediated adhesion are currently unknown. Although this pathway may not be limited to RBCs from patients with sickle cell anemia, the vasculature of patients with sickle cell anemia probably exists in a much more pro-adhesive state. These factors acting in concert suggest that epinephrine-stimulated adhesion may play a clinically significant role in the context of sickle cell anemia.
Discussion
In this study, we provide the first evidence that cAMP/PKA-mediated signaling pathways can promote RBC adhesion to a substrate—in this case, laminin, which may be particularly important in the context of sickle cell anemia. The stimulation of adhesion by cAMP and PKA was somewhat unexpected, because PKA activation is widely described as a negative regulator of cell adhesion in various cell types.17,18,30-32 Although there is limited evidence demonstrating a role for PKA in promoting adhesion of human neutrophils19 and human fibrosarcoma cells,33our data now provide a role for cAMP and PKA in regulating RBC adhesion.
Unlike AA RBCs, SS RBCs exhibit significantly greater basal and forskolin-stimulated levels of cAMP. Because AA RBC adhesion (albeit to much lower levels) could be stimulated with an exogenously added cAMP analog, we speculate that reduced activity or levels of adenylyl cyclase may be the molecular point of divergence between peripheral AA and SS RBCs. SS RBCs have an expanded population of young RBCs, and our data are consistent with reports demonstrating that mature RBC membranes contain only 10% to 20% of the adenylyl cyclase activity of younger RBC membranes.34
The ability of epinephrine to stimulate SS RBC adhesion to laminin is of particular clinical interest. Epinephrine is elevated during periods of stress, and previous studies have shown a direct relationship between stress and sickle cell-associated pain.35 36 These data, therefore, suggest a biologic link between stress and the pathophysiology of sickle cell anemia. Also of interest is that epinephrine mediates this stimulated adhesion in a specific subset of patients (46%), suggesting that there may be an identifiable population of patients predisposed to epinephrine-mediated increases in SS RBC adhesion. Although our study focused primarily on patients with sickle cell anemia, these results illustrate the potential importance of understanding the genetic background on which the sickle hemoglobin mutation is expressed to better predict an individual patient's physiologic response. Comparison of the genetic and protein expression profiles from each subset of patients may yield insight into the factors responsible for the variable responses to epinephrine. One factor that may contribute to the patient-to-patient variability observed in SS RBC adhesive responses to epinephrine is a difference in adenylyl cyclase copy number or enzymatic activity in RBCs from different patients, because we can bypass adenylyl cyclase in AA RBCs and observe a response.
Additionally, we demonstrate that epinephrine acts primarily on the β2-AR to stimulate adhesion to laminin (Figure 5B). Although previous studies have demonstrated a role for PKA in promoting adhesion in response to various cell surface stimuli, this is the first evidence, to our knowledge, of PKA-stimulated adhesion via an adrenergic receptor in any cell type. These data suggest that β-AR antagonists, which are commonly used in cardiovascular disease therapy, may have utility in inhibiting epinephrine-stimulated SS RBC adhesion in vivo.
Our data also demonstrate that immature SS RBC populations are more responsive to cAMP-mediated adhesion to laminin, compared with more mature SS RBC populations (Figure 2C), suggesting that patients with a younger mean RBC age (hemolytic anemia, blood loss, etc) may be more susceptible to cAMP-stimulated adhesion. In fact, we also observed epinephrine-stimulated adhesion in a patient with a hemolytic anemia independent of sickle cell disease (data not shown). Interestingly, stimulated SS RBC adhesion is not directly related to reticulocyte counts per se, as increased reticulocyte counts did not correlate with an increase in stimulated adhesion (Figure 6A-B), and reticulocyte adhesion did not increase in response to epinephrine (Figure 6C). Because reticulocytes retain more signaling capacity, this may appear counterintuitive. However, the increased signaling capacity may also be manifested as more active inhibitory pathways that prevent BCAM/Lu activation. As is the case with many other signaling pathways, potential inhibitory pathways may become lost as the reticulocytes mature into the intermediate RBC population illustrated in Figure 6D, explaining how this intermediate population could be the most responsive to stimulated adhesion. Taken together, these data suggest that the responsiveness of young circulating RBCs to agonists resulting in increased adhesiveness might not be specific to sickle cell anemia. However, why patients with sickle cell anemia are predisposed to vasoocclusive crises and other patients are not, is likely due to other factors that are more common features of this disease. For example, there is a large inflammatory component that contributes to an activated and denuded endothelium.3 The highly adhesive RBCs in sickle cell anemia are much more likely to mediate vasoocclusion in this pro-adhesive environment than reactive RBCs in other disease settings in which these contributing factors are generally absent.
In this report, we also implicate BCAM/Lu as the receptor mediating stimulated adhesion (Figure 5D). Although cAMP-mediated PKA activation has been reported to induce integrin-mediated neutrophil adhesion,19 our results with BCAM/Lu suggest that PKA may elevate cell adhesion via a broader range of adhesion receptors than previously realized. Further studies are needed to understand the role of the BCAM/Lu-laminin interaction in vivo. Elucidation of the specific pathways between PKA activation and stimulated BCAM/Lu adhesion may uncover additional potential therapeutic targets.
In conclusion, cAMP signaling may play a significant role in the pathophysiology of sickle cell anemia. In support of this, we provide the first evidence that the adhesive state of RBCs can be regulated by cAMP and demonstrate a role for BCAM/Lu in mediating this activated adhesion. Further, these studies show that epinephrine, acting primarily via the β2-adrenergic receptor, stimulates adhesion in a PKA-dependent manner (Figure 7). Thus, this report provides the basis to further study the role of epinephrine and other cAMP-modifying agonists in the pathophysiology of sickle cell disease in vivo. Specific antagonists of the β-adrenergic receptor, cAMP signaling pathway, and the BCAM/Lu receptor may ultimately prove to be valuable therapeutics. Finally, further studies to identify genetic markers predisposing patients to epinephrine-stimulated adhesion may bring us a step closer to providing more individualized therapy for patients with sickle cell anemia.
We thank Sheritha Lee, Julia Brittain, and Chris Anderson for their invaluable scientific and technical assistance; Dell Strayhorn, Sandra Santucci, and the UNC Comprehensive Sickle Cell Center for their assistance in patient recruitment and clinical perspectives; and Paul Stewart (Department of Biostatistics, UNC-CH) for statistical consultation.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2001-12-0289.
Supported by the United Negro College Fund (UNCF)–Merck Graduate Science Research Dissertation Fellowship (P.C.H.), a grant from the March of Dimes (M.J.T.), and grants HL58939 (M.J.T. and L.V.P.), HL067440 (L.V.P.), HL63409 (M.J.T.), and RR0046 (E.P.O.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leslie V. Parise, Department of Pharmacology, University of North Carolina at Chapel Hill, CB#7365, Chapel Hill, NC 27599-7365; e-mail:parise@med.unc.edu.



![Fig. 7. Proposed model for epinephrine-stimulated SS RBC adhesion to laminin. / During periods of stress, increased circulating levels of epinephrine act on the RBC β2-AR, thus activating Gαs, which stimulates adenylyl cyclase (AC). This enzyme catalyzes the conversion of ATP to cAMP, leading to PKA activation, an intermediate step in the up-regulation of BCAM/Lu-mediated adhesion. (Phosphodiesterase [PDE] breaks down cAMP into AMP.) Events between PKA activation and enhancement of BCAM/Lu-mediated adhesion are currently unknown. Although this pathway may not be limited to RBCs from patients with sickle cell anemia, the vasculature of patients with sickle cell anemia probably exists in a much more pro-adhesive state. These factors acting in concert suggest that epinephrine-stimulated adhesion may play a clinically significant role in the context of sickle cell anemia.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/8/10.1182_blood-2001-12-0289/4/m_h80834158007.jpeg?Expires=1767755921&Signature=P0~Ux4BWAJnNE8s3savkn3VIo6apJnyy3e6KOifduSyvhoPDJ0rOLfdGBKwOpVw~kJZyg4u0UmchwczC-Zcf8QT9pHcW-LUVQZqOfIT0ceL8Tr1XAkas8x1YPLvi9lclKJZ6Dl6aSyQDXMreqEs86GJYFac7otoiYvRBq-0ss4T8QTQ3xd7mfe59Ikc94QF2DKj-PhhUMGD0zXmIOXLc5NTR27CDfJHoftxy57Ht5tIZIV4TQqRmvLdHnBlPp8PyxBY6huBLqPdOxDoA-shyQr9Cv9-ThJE-G2DRSy5-ALYZghrWZm4UBaflC7kaoFvP9XDBXsflsg5gtpBDhhwOLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal