Antiphospholipid syndrome (APS) is an autoimmune disease characterized by the persistent presence of antiphospholipid antibodies (aPLs) and recurrent thrombosis or fetal loss. The thrombophilic state has been partially related to the induction of a proinflammatory and procoagulant endothelial cell (EC) phenotype induced by anti–β2-glycoprotein I (β2-GPI) antibodies that bind β2-GPI expressed on the EC surface. Anti–β2-GPI antibody binding has been shown to induce nuclear factor-κB (NF-κB) translocation leading to a proinflammatory EC phenotype similar to that elicited by interaction with microbial products (lipopolysaccharide [LPS]) and proinflammatory cytokines (interleukin 1β [IL-1β], tumor necrosis factor α [TNF-α]). However, the upstream signaling events are not characterized yet. To investigate the endothelial signaling cascade activated by anti–β2-GPI antibodies, we transiently cotransfected immortalized human microvascular endothelial cells (HMEC-1) with dominant-negative constructs of different components of the pathway (ΔTRAF2, ΔTRAF6, ΔMyD88) together with reporter genes (NF-κB luciferase and pCMV-β-galactosidase). Results showed that both human anti–β2-GPI IgM monoclonal antibodies as well as polyclonal affinity-purified anti–β2-GPI IgG display a signaling cascade comparable to that activated by LPS or IL-1. ΔTRAF6 and ΔMyD88 significantly abrogate antibody-induced as well as IL-1– or LPS-induced NF-κB activation, whereas ΔTRAF2 (involved in NF-κB activation by TNF) does not affect it. Moreover, anti– β2-GPI antibodies and LPS followed the same time kinetic of IL-1 receptor–activated kinase (IRAK) phosphorylation, suggesting an involvement of the toll-like receptor (TLR) family. Our findings demonstrate that anti–β2-GPI antibodies react with their antigen likely associated to a member of the TLR/IL-1 receptor family on the EC surface and directly induce activation.
Introduction
Antiphospholipid syndrome (APS) is characterized by recurrent arterial and venous thrombotic events or fetal loss (or both) in the presence of circulating antiphospholipid antibodies (aPLs).
The aPLs seem to play a pathogenic role rather than just being a simple diagnostic marker of the syndrome.1 They constitute a heterogeneous family of antibodies reacting with serum phospholipid (PL)–binding proteins; among them, β2-glycoprotein I (β2-GPI) represents the main target antigen.2
β2-GPI is a plasma PL-binding protein that can be expressed on the cell membrane of endothelial and trophoblast cells. Cell surface β2-GPI offers suitable epitopes targeting circulating anti–β2-GPI antibodies to these cells; once bound, anti–β2-GPI antibodies were reported to affect cell functions that have been related to the clinical manifestations of APS.1-7
Recently, anti–β2-GPI antibodies have been found to activate endothelial cells (ECs) by inducing a proinflammatory and procoagulant phenotype sustained by the up-regulation of adhesion molecule (E-selectin, intercellular adhesion molecule 1 [ICAM-1], and vascular cell adhesion molecule 1 [VCAM-1]) expression, synthesis and secretion of cytokines, chemokines, endothelin-1, and tissue factor.8 Such an effect was reported both in vitro and in vivo experimental models and strongly suggested to represent one of the pathogenic mechanisms for the thrombophilic diathesis in APS.7
In this regard, we have recently found that E-selectin up-regulation in ECs activated by anti–β2-GPI antibodies is dependent on nuclear factor κB (NF-κB) translocation in a manner quite comparable to that induced by proinflammatory cytokines (interleukin 1β [IL-1β], tumor necrosis factor α [TNF-α]) or by bacterial lipopolysaccharide (LPS).8
It is known that LPS and IL-1 interact with membrane proteins, namely, toll-like receptors (TLRs) and IL-1 receptor (IL-1R), respectively, which share a homologous cytoplasmic signaling domain, the toll/IL-1 receptor (TIR) domain,9 and use the same intracellular mediators in their activation pathway. Particularly, adapter molecule myeloid differentiation protein (MyD88) is the first protein that associates with both the TLRs and IL-1R through its TIR domain. Molecules successively involved in the signaling are IL-1 receptor–activated kinase (IRAK), IRAK2, and TNF receptor–associated factor 6 (TRAF6).10 The essential roles of MyD88 and TRAF6 in TLR and IL-1R activation pathway are confirmed by targeted deletion of their genes.11,12 On the other hand, TNF-α receptor (TNFR), recognized by TNF-α and not belonging to the IL-1R/TLR superfamily, uses different molecular frameworks among which TRAF2 plays a crucial role.13
Downstream signaling pathway of the 3 stimuli distal to TRAF6 and TRAF2 converges at the level of NF-κB–inducing kinase (NIK).13,14 Subsequently to NF-κB inhibitory protein (IκB) degradation, NF-κB translocates from cytoplasm to the nucleus and binds to the κB sites present in cis-acting regulatory regions of adhesion molecule, cytokine, and chemokine genes.15 16
TLRs have been initially identified in Drosophila melanogaster as receptors involved in embryonic development. They are a key component of the innate immune response and several of them appear to recognize specific microbial products, including LPS, bacterial lipoproteins, peptidoglycan, and bacterial DNA.10 To date, in mammals, 10 members of the TLR family have been found. All of them are expressed in lymphoid and nonlymphoid tissues, but the pattern of expression varies with the studied cell types and tissues.10 Human ECs, which actively take part in innate immune responses, have been reported to express a selected set of TLRs, one of which is TLR4.17 This receptor is now well established to be a crucial component of the LPS signaling receptor complex; in fact, mice in which the tlr4 gene has been deleted fail to respond to LPS.18
Here we report for the first time that anti–β2-GPI antibodies activate ECs through the MyD88-dependent pathway, therefore implicating members of the TLR family.
Study design
Patients
Table 1 reports the clinical and serologic characteristics of 3 patients from whom the IgG fractions were obtained. All of them had primary antiphospholipid syndrome (PAPS) diagnosed according to the Sapporo classification criteria.19
Clinical and serologic characteristics of the APS patients
| Patient . | Clinical manifestations . | aCL . | LA . | Anti-β2-GPI . | ANA‡ . | Anti-ENA‡ . | Anti-dsDNA‡ . | ||
|---|---|---|---|---|---|---|---|---|---|
| IgG . | IgM* . | IgG . | IgM† . | ||||||
| 1 | Stroke, recurrent fetal loss | 111 | 5 | Pos | 2.216 | 0.118 | Neg | Neg | Neg |
| 2 | Stroke | 87 | 1 | Neg | 2.145 | 0.017 | Neg | Neg | Neg |
| 3 | Fetal death | 98 | 31 | Pos | 2.119 | 1.666 | Neg | Neg | Neg |
| Patient . | Clinical manifestations . | aCL . | LA . | Anti-β2-GPI . | ANA‡ . | Anti-ENA‡ . | Anti-dsDNA‡ . | ||
|---|---|---|---|---|---|---|---|---|---|
| IgG . | IgM* . | IgG . | IgM† . | ||||||
| 1 | Stroke, recurrent fetal loss | 111 | 5 | Pos | 2.216 | 0.118 | Neg | Neg | Neg |
| 2 | Stroke | 87 | 1 | Neg | 2.145 | 0.017 | Neg | Neg | Neg |
| 3 | Fetal death | 98 | 31 | Pos | 2.119 | 1.666 | Neg | Neg | Neg |
aCL indicates anticardiolipin antibodies; LA, lupus anticoagulant; ANA, antinuclear antibodies; ENA, extractable nuclear antigens; dsDNA, double-stranded DNA.
Anticardiolipin antibodies are expressed as G antiphospholipid (GPL) and M antiphospholipid (MPL) units (normal values < 10 GPL or MPL units).20
Anti-β2-GPI antibodies are expressed as OD values (normal values < 0.150 for IgG and 0.280 for IgM).20Sera were considered positive if OD values were higher than the 95th percentile of 100 healthy controls.
Antinuclear antibodies were detected by indirect immunofluorescence on Hep2 cells, antiextractable nuclear antigens by counterimmunoelectrophoresis and immunoblot, and anti-dsDNA by indirect immunofluorescence on Crithidia luciliae as described.37-39
Antiphospholipid assays
EC culture
Human umbilical vein endothelial cells (HUVECs) were isolated from normal term umbilical cord vein by collagenase A (Roche Diagnostics, Mannheim, Germany) perfusion and cultured in E199 medium (ICN Biomedicals, Aurora, OH) supplemented with 20% heat-inactivated fetal calf serum (FCS; Euroclone, West York, United Kingdom) as previously reported.22
The immortalized human microvascular endothelial cells (HMEC-1),23 a generous gift from Dr N. Lindsey (Department of Biomedical Sciences, University of Bradford, Bradford, United Kingdom), were cultured in MCDB-131 medium (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated FCS and used between passages 10 and 14.
Human monoclonal anti–β2-GPI antibodies
Two human monoclonal IgM anti–β2-GPI antibodies (moAbs) derived from patients with APS were used. GR1D5 has been previously characterized as reacting with human β2-GPI, and TM1B9, which did not display any reactivity to β2-GPI, was used as a negative control. The characterization of these moAbs has been previously reported in detail.5 24
Human polyclonal anti–β2-GPI antibodies
Whole IgG from 3 patient sera were fractionated on protein G-Sepharose columns (HiTrap Protein G, Pharmacia Biotech Europe, Freiburg, Germany) and affinity purified on β2-GPI-N-hydroxysuccinamide-activated Sepharose as previously reported.21 The reactivity of affinity-purified IgG fractions toward human β2-GPI and CL is reported in Table 2.
Reactivity of affinity-purified anti-β2-GPI polyclonal IgG fractions
| IgG . | Anti-β2-GPI* . | Anti-CL† . |
|---|---|---|
| 1 | 2.098 ± 0.069 | 85 ± 8 |
| 2 | 2.275 ± 0.154 | 78 ± 3 |
| 3 | 1.938 ± 0.097 | 96 ± 7 |
| NHS | 0.015 ± 0.004 | 3 ± 0.2 |
| IgG . | Anti-β2-GPI* . | Anti-CL† . |
|---|---|---|
| 1 | 2.098 ± 0.069 | 85 ± 8 |
| 2 | 2.275 ± 0.154 | 78 ± 3 |
| 3 | 1.938 ± 0.097 | 96 ± 7 |
| NHS | 0.015 ± 0.004 | 3 ± 0.2 |
IgG anti-β2-GPI or aCL activity was evaluated by ELISA as described in “Study design”; the fractions were used at the protein concentration of 1 μg/mL.
Data are expressed as OD values (mean ± SD of triplicate experiments).
Values are expressed as GPL units (mean ± SD of triplicate experiments).
Expression vectors and transfection
Dominant-negative expression vectors of MyD88 (MyD88 152-296), TRAF2 (TRAF2 87-501), and TRAF6 (TRAF6 298-522) have been characterized.13
HMEC-1 or HUVECs were plated at a concentration of 50 000 cells/well in 24-well plates and the following day transiently cotransfected with FuGene 6 Transfection Reagent (Roche Molecular Biochemicals), according to manufacturer's instructions. Reporter genes pCMV-β-galactosidase (0.2 μg) and ELAM-NF-kB-luciferase (0.3 μg), pCMV empty vector (0.45 μg), or dominant-negative expression vectors (0.45 μg) were cotransfected overnight as previously described.15 Because the HMEC-1 cell line displayed a better transformation efficiency than HUVECs in our experimental conditions, we chose the cell line for further transfection experiments.
Cells were then stimulated for 3 hours and 30 minutes with: (1) standard agonists (10 ng/mL TNF-α, 50 U/mL IL-1β [both from R & D Systems, Minneapolis, MN], 20 ng/mL LPS [Sigma-Aldrich, St Louis, MO); (2) human monoclonal IgM (100 μg/mL) or affinity-purified IgG anti–β2-GPI antibodies (200 μg/mL); (3) irrelevant human monoclonal IgM (100 μg/mL) or normal human serum (NHS) IgG (200 μg/mL); or (4) medium alone. The cells were lysed in 100 μL reporter lysis buffer (Promega, Madison, WI) and luciferase activity was measured by a Promega kit and a luminometer. β-Galactosidase activity was determined by calorimetric method to normalize transfection efficiency as previously described.15
IRAK assay
HUVECs or HMEC-1 (5 × 106 cells/sample) were stimulated with IL-1β (1 U/mL), LPS (10 ng/mL), anti–β2-GPI IgG (200 μg/mL), NHS IgG (200 μg/mL), or medium alone for 10 or 45 minutes as indicated. Cells were then lysed in 1 mL lysis buffer (0.5% Nonidet-P40, 10% glycerol, 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9, 250 mM NaCl, 20 mM glycerophosphate, 5 mMp-nitrophenylphosphate, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM Na orthovanadate, 5 mM dithioerythrol, 1 × complete protease inhibitor [Roche]). IRAK was immunoprecipitated using 1 μg/sample anti-IRAK moAb, a kind gift from Tularik (San Francisco, CA). The in vitro kinase assay was performed as described.25-27 Briefly, immunoprecipitated IRAK was collected and washed twice in kinase buffer (20 mM HEPES, pH 6.5, 150 mM NaCl, 5 mM MgCl2, 5 mM MnCl2) and then incubated for 10 or 45 minutes in 30 μL kinase buffer supplemented with 1 μCi (0.037 MBq)γ-[32P]-adenosine triphosphate (ATP) per sample (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) at 37°C. The reaction was stopped by addition of 3 × Laemmli buffer followed by heating at 95°C for 10 minutes. Samples were resolved on a 7% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, after which the gel was dried and subjected to autoradiography at −80°C with intensifying screens. To evidence equal loading of the lanes, one tenth of each supernatant after IRAK immunoprecipitation was run on an SDS-PAGE gel and blotted against β-actin.
Endothelial E-selectin expression
E-selectin expression was evaluated by a cell ELISA as previously described.28 Briefly, HUVECs in 96-well plates were incubated for 5 hours with: (1) different agonists (1 U/mL IL-1β, 1 ng/mL TNF-α, and 10 ng/mL LPS); (2) 200 μg/mL affinity-purified or whole fraction anti–β2-GPI IgG; (3) 200 μg/mL NHS IgG or medium alone as controls, in a final volume of 100 μL. In some inhibition experiments, IL-1ra (1 μg/mL; Serotec, Oxford, United Kingdom) or blocking anti–IL-1RI (10 μg/mL; Immunex, Seattle, WA), anti–TNF-α (1 μg/mL; R & D Systems) antibodies28 were added 30 minutes before addition of IgG or agonists. The cells were then washed twice and incubated for 60 minutes with mouse antihuman E-selectin moAb (Serotec). The reaction was revealed by adding phosphatase-conjugated goat antimouse IgG (Sigma) and the appropriate substrate (Sigma). Optical density (OD) values at 405 nm were determined using an semiautomatic reader (Titertek Multiscan, Ayre, Scotland).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance for multiple comparisons. P < .05 was considered significant.
Results and discussion
Characterization of affinity-purified fractions
All the affinity-purified IgG fractions eluted from β2-GPI columns have been tested against a panel of antigens to check their antigen specificity. The affinity-purified preparations displayed a clear binding against type C-γ-irradiated plates coated with β2-GPI and with plates coated with CL and then incubated with the cofactor (Table 2). On the contrary they did not display any binding on plates coated with the following antigens: CL alone, ssDNA, bovine serum albumin (BSA), or tetanus toxoid (data not shown). All the 3 affinity-purified anti–β2-GPI IgG fractions were found to up-regulate E-selectin expression on HUVEC and HMEC-1 monolayers in a dose-dependent manner (from 200 to 25 μg/mL). These findings are in line with data previously reported.4 29
Anti–β2-GPI antibodies activate ECs by a MyD88-dependent pathway
To investigate the EC signaling cascade by anti–β2-GPI antibodies, we transiently cotransfected immortalized HMEC-1 with dominant-negative constructs of different components of the pathway (ΔTRAF2, ΔMyD88, and ΔTRAF6) and reporter genes (ELAM-NF-kB-luciferase and pCMV-β-galactosidase). Because HUVECs were found to be poor recipients for transfection in our experimental conditions, we used the immortalized HMEC-1 cell line.
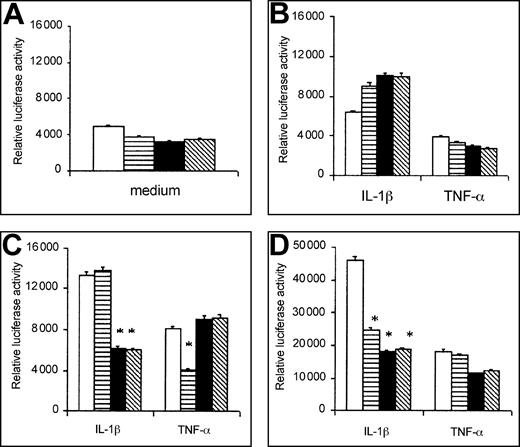
Preliminary time kinetic experiments have been carried out to discriminate the effect of ΔTRAF2, ΔMyD88, and ΔTRAF6 transfection on EC activation by IL-1β or TNF-α. As shown in Figure1, the effects of the transfection with the different negative mutants were negligible after 2 hours of incubation (Figure 1B), whereas 5-hour incubation times gave very high values of relative luciferase activity in all the experimental conditions (Figure 1D). After 3 hours and 30 minutes stimulation, the inhibitory effect of ΔTRAF6 and ΔMyD88 was higher than that of ΔTRAF2 in IL-1β–activated cells, whereas opposite results were observed in TNF-α–exposed cells (Figure 1C). On the basis of these observations and in agreement with previous results in the literature,15 we chose this time point for the experiments with both the classical agonists and the human polyclonal or monoclonal anti–β2-GPI antibodies.
Time kinetic experiments on IL-1β– or TNF-α–induced activation of cotransfected HMEC-1.
Relative luciferase activity in unstimulated or stimulated cells at 0 hour (A), 2 hours (B), 3 hours and 30 minutes (C), and 5 hours (D) of activation. HMEC-1 were cotransfected with p-CMV empty vector (■), ΔTRAF2 (▤), ΔTRAF6 (▪), ΔMyD88 (▧) and stimulated by IL-1β (50 U/mL) or TNF-α (10 ng/mL). Data are shown as cpm, mean ± SD of 3 different experiments. *P < .05 versus vector.
Time kinetic experiments on IL-1β– or TNF-α–induced activation of cotransfected HMEC-1.
Relative luciferase activity in unstimulated or stimulated cells at 0 hour (A), 2 hours (B), 3 hours and 30 minutes (C), and 5 hours (D) of activation. HMEC-1 were cotransfected with p-CMV empty vector (■), ΔTRAF2 (▤), ΔTRAF6 (▪), ΔMyD88 (▧) and stimulated by IL-1β (50 U/mL) or TNF-α (10 ng/mL). Data are shown as cpm, mean ± SD of 3 different experiments. *P < .05 versus vector.
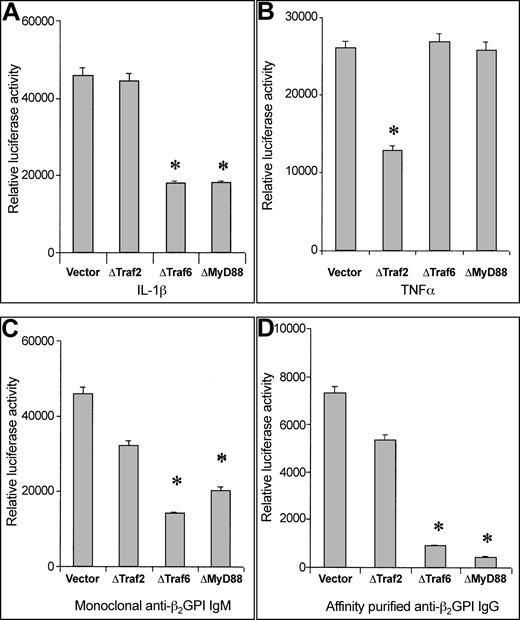
As shown in Figure 2, EC activation by human anti–β2-GPI IgM moAb as well as by polyclonal affinity-purified anti–β2-GPI IgG displays a signaling cascade comparable to that induced by IL-1β or LPS. In fact, ΔTRAF6 and ΔMyD88 significantly abrogate antibody-induced as well as IL-1–induced NF-κB activation, whereas ΔTRAF2 (involved in NF-κB activation by TNF-α) does not affect it. LPS gave results similar to IL-1β (data not shown).
Involvement of MyD88 pathway in EC activation induced by anti–β2-GPI antibodies.
Relative luciferase activity induced by IL-1β (50 U/mL), TNF-α (10 ng/mL), anti–β 2-GPI IgM moAb (100 μg/mL), or affinity-purified polyclonal anti–β2-GPI IgG (200 μg/mL) in cotransfected HMEC-1. Data are shown as mean ± SD of 3 different experiments. *P < .05 versus vector.
Involvement of MyD88 pathway in EC activation induced by anti–β2-GPI antibodies.
Relative luciferase activity induced by IL-1β (50 U/mL), TNF-α (10 ng/mL), anti–β 2-GPI IgM moAb (100 μg/mL), or affinity-purified polyclonal anti–β2-GPI IgG (200 μg/mL) in cotransfected HMEC-1. Data are shown as mean ± SD of 3 different experiments. *P < .05 versus vector.
Control experiments performed with irrelevant human anti–β2-GPI IgM (TM1B9) moAb and NHS IgG gave results comparable to medium alone in the different experimental conditions without inducing cell activation (Table3).
Relative luciferase activity by control antibodies or medium alone in transfected HMEC-1
| . | Vector . | ΔTRAF2 . | ΔTRAF6 . | ΔMyD88 . |
|---|---|---|---|---|
| Medium | 1250 ± 55 | 2015 ± 88 | 1803 ± 61 | 2315 ± 62 |
| TM1B9 (100 μg/mL) | 2223 ± 98 | 3241 ± 151 | 2958 ± 84 | 2561 ± 79 |
| NHS IgG (200 μg/mL) | 1354 ± 114 | 1985 ± 66 | 2240 ± 74 | 2351 ± 87 |
| . | Vector . | ΔTRAF2 . | ΔTRAF6 . | ΔMyD88 . |
|---|---|---|---|---|
| Medium | 1250 ± 55 | 2015 ± 88 | 1803 ± 61 | 2315 ± 62 |
| TM1B9 (100 μg/mL) | 2223 ± 98 | 3241 ± 151 | 2958 ± 84 | 2561 ± 79 |
| NHS IgG (200 μg/mL) | 1354 ± 114 | 1985 ± 66 | 2240 ± 74 | 2351 ± 87 |
Data are expressed as cpm, mean ± SD of 3 different experiments.
To rule out endotoxin contamination of the IgG or moAb preparations, we performed the same experiments in the presence of polymyxin B (5 μg/mL) and we did not observe any difference in the results (data not shown).
IRAK time kinetic phosphorylation
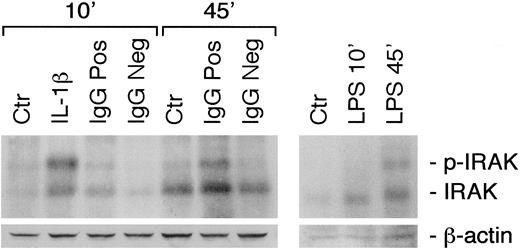
To further dissect the signaling pathway involved in EC activation on anti–β2-GPI antibody binding, we investigated IRAK phosphorylation. IRAK is the first kinase to be recruited by receptors of the IL-1/TLR superfamily, but not by other known receptors.30 Figure 3 shows that anti–β2-GPI but not NHS IgG induces IRAK activation. Although the shifted band is already barely detectable after 10 minutes of stimulation, it reaches a maximum at 45 minutes, in a way comparable to that described for LPS, at variance with IL-1β,25,30 which gets to the peak of phosphorylation at 10 minutes of incubation. The rapid IRAK activation by IL-1β (< 20 minutes) versus LPS (Figure 3) is consistent with previous results.25 30 Experiments carried out with human anti–β2-GPI IgM moAb (GR1D5) and the respective control (TM1B9) as agonists or with HMEC-1 monolayers gave comparable results (data not shown).
Time kinetics of IRAK phosphorylation induced by different agonists.
Medium alone (Ctr), IL-1β (1 U/mL), polyclonal anti–β2-GPI IgG (IgG Pos; 200 μg/mL), NHS IgG (IgG Neg; 200 μg/mL), and LPS (10 ng/mL) were used. To evidence equal loading of the lanes, in control experiments one tenth of each supernatant after IRAK immunoprecipitation was run on an SDS-PAGE gel and blotted against β-actin (lower panel).
Time kinetics of IRAK phosphorylation induced by different agonists.
Medium alone (Ctr), IL-1β (1 U/mL), polyclonal anti–β2-GPI IgG (IgG Pos; 200 μg/mL), NHS IgG (IgG Neg; 200 μg/mL), and LPS (10 ng/mL) were used. To evidence equal loading of the lanes, in control experiments one tenth of each supernatant after IRAK immunoprecipitation was run on an SDS-PAGE gel and blotted against β-actin (lower panel).
Effect of IL-1ra and anti–IL-1RI on E-selectin expression
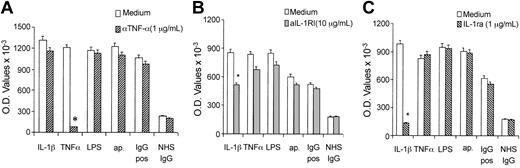
To further rule out the IL-1R involvement in anti–β2-GPI signaling pathway, we studied the endothelial activation by agonists or polyclonal anti–β2-GPI IgG (both affinity purified and whole IgG fraction) in the presence of IL-1ra and anti–IL-1RI antibodies. Figure4 shows that E-selectin expression on ECs preincubated with anti–IL-1RI or IL-1ra was significantly reduced when cells were incubated with IL-1β but not in any other experimental conditions. In addition, when blocking anti–TNF-α antibodies were added to the endothelial cultures, E-selectin expression was inhibited if stimulated by TNF-α, whereas no effect was observed in the presence of other agonists or anti–β2-GPI antibodies.
Effect of specific antagonists of IL-1R on E-selectin expression.
Anti–TNF-α (A) or anti–IL-1RI (B) blocking antibodies or IL-1ra (C) were used in cultures of HUVECs activated by polyclonal anti–β2-GPI (200 μg/mL) or NHS IgG fractions (200 μg/mL) or by TNF-α (1 ng/mL), IL-1β (1 U/mL), or LPS (10 ng/mL). Data are expressed as OD values, mean ± SD of 3 different experiments. *P < .05 versus medium.
Effect of specific antagonists of IL-1R on E-selectin expression.
Anti–TNF-α (A) or anti–IL-1RI (B) blocking antibodies or IL-1ra (C) were used in cultures of HUVECs activated by polyclonal anti–β2-GPI (200 μg/mL) or NHS IgG fractions (200 μg/mL) or by TNF-α (1 ng/mL), IL-1β (1 U/mL), or LPS (10 ng/mL). Data are expressed as OD values, mean ± SD of 3 different experiments. *P < .05 versus medium.
EC activation and anti–β2-GPI antibodies
Our findings demonstrate for the first time that anti–β2-GPI antibodies react with their antigen likely associated to TLRs on the surface and directly induce cell activation.
We and others previously reported that the binding between anti–β2-GPI antibodies and β2-GPI expressed on the endothelial membranes activates the cells, inducing a proadhesive and proinflammatory phenotype with up-regulation of adhesion molecule expression, cytokine, and chemokine secretion.6
In addition, we recently observed that this effect was consequent on NF-κB activation and translocation from cytoplasm to nucleus in a way similar to standard agonists, such as IL-1β, TNF-α, and LPS.8
To investigate the endothelial signaling cascade activated by anti–β2-GPI antibodies, we transiently cotransfected immortalized HMEC-1 with dominant-negative constructs of different components of the pathway (ΔTRAF2, ΔTRAF6, and ΔMyD88) together with reporter genes (NF-κB luciferase and pCMV-β-galactosidase).15 31
Results showed that human monoclonal IgM as well as polyclonal anti–β2-GPI IgG trigger an endothelial activation pathway using the same signaling molecules implicated in LPS or IL-1 cascade. In fact, dominant-negative versions of TRAF6 and MyD88 significantly abrogated antibody-induced as well as IL-1β– or LPS-induced NF-κB activation. On the other hand, ΔTRAF2 blocked NF-κB activation induced by TNF-α but not by anti–β2-GPI antibodies, IL-1β, or LPS.
To better characterize the cell signaling induced by anti–β2-GPI antibodies, we investigated IRAK phosphorylation. Actually, IRAK is the first kinase to be recruited by receptors of the IL-1/TLR superfamily, but not by other known receptors.30 The death domain of MyD88, interacting with the amino-terminal death domain of IRAK, takes IRAK to the receptor complex and, on recruitment, IRAK is autophosphorylated and associated with TRAF6.10
It is known that IRAK is autophosphorylated with different time kinetics depending on the agonist used: 45 or 10 minutes after exposure to LPS or IL-1, respectively.25 27 Our experiments indicated that anti–β2-GPI IgG but not NHS IgG induced IRAK activation. In addition, the shifted band was already barely detectable after 10 minutes of stimulation, but it reached a maximum at 45 minutes, in a way comparable to that described for LPS.
Taken together these findings suggest that anti–β2-GPI antibodies may activate ECs through direct or indirect involvement of one or more TLRs. Interestingly, ECs were found to express selected TLRs and to actively take part in innate immunity.17 The lack of any inhibition in the experiments carried out in the presence of IL-1ra or anti–IL-1RI further suggests the involvement of TLRs by anti–β2-GPI antibodies.
Signaling pathways other than NF-κB are known to be involved in EC activation.32 At the time points considered, however, anti–β2-GPI IgG did not induce c-Jun, p38, or erk1/2 phosphorylation (data not shown). A more accurate kinetic study is required to assess whether aPLs can activate different signaling pathways.
Recently, annexin II, an EC receptor for tissue plasminogen activator, was reported to behave as a receptor for β2-GPI.33 However, it is still unclear whether such a putative receptor is actually involved in cell activation; in fact, it does not span the cell membrane and the presence of an unknown “adaptor” protein was suggested to be necessary to induce activation.33 In addition, some authors suggested that the cell activation by aPL IgG can also result from the cross-linking of the surface receptors for the Fc fragment of the IgG molecules (FcγR).34 The fact that anti–β2-GPI moAbs of the IgM isotype were found able to activate ECs strongly suggests that the FcγR involvement does not represent a necessary requisite.6
The findings reported here indicate that anti–β2-GPI antibodies directly induce EC activation via the MyD88 pathway and suggest a possible association between β2-GPI and members of the TLR family. Of note, β2-GPI has been shown to share amino acid sequence in common with different microbial pathogens, supporting the hypothesis that β2-GPI might associate with TLRs.35
It has been shown that TRLs form functional signaling pairs (homodimers or heterodimers) on interaction with the proper ligand,9so we speculate that anti–β2-GPI antibodies might cross-link β2-GPI molecules likely together with TRLs, eventually favoring the receptor polymerization and the signaling cascade.
Interestingly, that a relationship between aPL-associated acute widespread thrombosis and infectious events does exist has recently been re-emphasized by the clinical association between the so-called catastrophic variant of APS and infections as triggering events.36
Hence, MyD88, using innate immunity receptors, may have a wider role in autoimmunity than recognized so far and may be a valuable therapeutic target.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/ blood-2002-08-2349.
Supported in part by Ricerca Corrente 2001 IRCCS Istituto Auxologico Italiano and by Ricerca Finalizzata 2001 IRCCS Istituto Auxologico Italiano (Interaction between endothelium and blood cells in the pathogenesis of brain stroke/inflammation; to P.L.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pier Luigi Meroni, Allergy and Clinical Immunology Unit, Department of Internal Medicine, University of Milan, IRCCS Istituto Auxologico Italiano, Via L. Ariosto, 13, 20145 Milan, Italy; e-mail: pierluigi.meroni@unimi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal