Natural killer (NK) cells decrease in function during chronic myelogenous leukemia (CML) progression from chronic phase to blast crisis, and they can becomeBCR/ABL+ late in the disease course. To study this altered function, NK92 cells were transduced with the BCR/ABL oncogene. In contrast to the parental cells, which died when deprived of interleukin 2 (IL-2), p210+ NK92 cells proliferated and survived indefinitely in the absence of IL-2. BCR/ABL also decreased the natural cytotoxicity of NK92 cells against K562 targets, without affecting IL-2, interferon γ (IFN-γ), or tumor necrosis factor α (TNF-α) production. Although the ABL-specific tyrosine kinase inhibitor imatinib mesylate (STI-571) had no effect on parental NK92 cells, it markedly decreased the growth and survival of IL-2–independent p210+ NK92 cells. In contrast to the parental cell line, serial analysis of p210+ NK92 cells detected small populations that clonally expressed one or more killer immunoglobulin-like receptors (KIRs). Unlike the decreased natural cytotoxicity, the function of the activating CD158j receptor remained intact. Southern blotting and hybridization with an enhanced green fluorescence protein (eGFP) probe showed that KIR− and KIR+ NK92 cells were all derived from the same clone, suggesting that KIR acquisition remains dynamic at the maturational stage represented by the NK92 cell line. When tested in primary CD56+bright NK cells, p210 induced partial IL-2–independent growth and increased KIR expression similar to findings in NK92 cells. This is the first study to show thatBCR/ABL, well known for its effects on the myeloid lineage, can alter the function of lymphoid cells, which may be associated with the defect in innate immunity associated with CML progression.
Introduction
Natural killer (NK) cells play a role in immune surveillance against tumors and virus-infected cells. They are functionally defined by their ability to kill targets without prior immunization and secrete a broad spectrum of cytokines.1The mechanisms by which NK cells recognize and lyse their targets are complex. Aside from the involvement of adhesion receptors,2 activating and inhibitory receptors integrate a balance between positive and negative signals that influence the decision of whether a target will be killed. Of these, the killer immunoglobulin-like receptors (KIRs) and the CD94/NKG2 lectin-type receptors can signal activation or inhibition when class I major histocompatibility complex (MHC) is recognized.3-5The natural cytotoxicity receptors (NCRs) and 2B4 do not recognize MHC molecules and are mainly activating.6-9
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disorder characterized by the presence of the BCR/ABL gene that leads to the malignant phenotype and accumulation of myeloid precursors in peripheral blood.10 NK cells may be involved in the control of the malignant CML clone.11 CML NK cells gradually decrease in number during disease progression from chronic phase to blast crisis,12,13 and freshly isolated NK cells from patients with advanced phase CML have decreased cytotoxicity. All CML NK cells proliferate less in response to interleukin 2 (IL-2) stimulation.12 We have previously shown that IL-2–activated NK cells from patients with CML suppress autologous primitive CML progenitors in long-term culture.14 However, a clinical trial using autologous IL-2–stimulated NK cells failed to prevent relapse after autologous stem cell transplantation.15 This failure may be due, in part, to diminished NK cell function because of prior cytotoxic therapy or cellular dilution effects.13,16 An alternative hypothesis is that BCR/ABL diminishes NK cell function directly. Recently, BCR/ABL+ NK cells have been identified in the blood of patients with CML late in the disease course,17 18 but the function of these cells is unknown.
NK cell lines have been used to study the biology of NK cells.19 Among them, the NK92 cell line has been used extensively to analyze the mechanisms involved in NK cell cytotoxicity against tumor targets20 and virus-infected cells.21 Others have used NK92 cells to describe the molecular pathways important in cytotoxicity and cytokine production.22-24 The NK92 cell line phenotype matches that of normal NK cells (CD56+/CD3−), is dependent on IL-2 for growth and survival, and is highly cytotoxic against a variety of tumor targets.25 In this study, we use NK92 cells as a model to study the NK cell dysfunction observed in CML.
Materials and methods
Cells
NK92 cells (American Type Culture Collection [ATCC], Rockville, MD) were cultured in enriched alpha medium supplemented with fetal calf serum (FCS; 12.5%) and horse serum (12.5%) (HyClone Laboratories, Logan, UT), 100 U/mL penicillin, 100 U/mL streptomycin (Gibco Laboratories, Grand Island, NY), and in the presence of 500 U/mL recombinant human IL-2 (a gift from Amgen, Thousand Oaks, CA). Cells were maintained at 37°C in a humidified 5% CO2 environment. Primary NK cells were obtained from peripheral blood mononuclear cells (PBMNCs) from healthy donors and enriched by using a magnetically activated cell sorter (MACS) NK cell column (Miltenyi Biotech, Oberlin, CA) according to the manufacturer's instructions. CD56+bright/CD3−and CD56+dim/CD3− NK cells were obtained by flow cytometry. Primary NK cells were cultured in Dulbecco modified Eagle medium (DMEM) high glucose/Ham F12 (Gibco) supplemented with 24 μM 2-mercaptoethanol, 50 μM ethanolamine, 20 mg/L ascorbic acid, 50 μg/L sodium selenite (Na2SeO3), 100 U/mL penicillin, and 100 U/mL streptomycin (Gibco), 20% heat-inactivated human AB serum (North American Biologicals, Miami, FL) and 1000 U/mL IL-2 where indicated. The K562 and P815 mastocytoma cell lines (ATCC) were cultured using established conditions. The use of healthy donor blood was approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota.
Retroviral vectors and transduction of NK cells
The full-length b3a2 BCR/ABL cDNA26 (a gift from Dr J. Y. Wang, University of California, San Diego) was cloned upstream from the internal ribosomal entry site of the murine stem cell virus (MSCV)–enhanced green fluorescence protein (eGFP) vector27 (a gift from Dr W. Pear, University of Pennsylvania, Philadelphia) as described.17 TheBCR/ABL-containing vector was termed p210-eGFP (ie, p210). Supernatants obtained from the 293kj cell line28 (a kind gift from Dr M. Haas, University of California, San Diego) were screened for potency by transducing HEL cells (ATCC).
NK92 or primary sorted NK cells (after 72 hours of prestimulation with 1000 U/mL IL-2 alone) were placed (3 × 105) in tissue culture–treated Transwells of 6-well plates that had previously been incubated with 50 μg/mL of the recombinant CH-296 fibronectin fragment (Takara Shuzo, Otsu, Japan). p210-eGFP or eGFP-containing retroviral supernatant (2 mL) was filtered through the Transwell, and this process was repeated twice during 48 hours. Forty-eight hours after the initial exposure to retroviral supernatant the cells were harvested and maintained in culture for 72 hours. CD56+eGFP+ NK cells were selected by fluorescence-activated cell sorter (FACS; FACStarPlus with FACStation G3; Becton Dickinson, Franklin Lakes, NJ). Parental or sorted eGFP+ and p210-eGFP+ NK92 cells were cultured in media with or without IL-2. After sorting, primary NK cells were cultured in the presence of IL-2, phytohemagglutinin (PHA; 2.2 μg/mL; Remel, Lenexa, KS) and PBMNC/EBV (Epstein-Barr virus) feeders as described29 (EBV feeders, a kind gift from Dr P. Leibson, Mayo Clinic, Rochester, MN). Feeders were added again at culture expansion 2 weeks later, 1 week prior to IL-2 withdrawal.
Flow cytometry and detection of cytokines
Fluorescein (FITC)– and phycoerythrin (PE)–coupled mouse isotype-matched immunoglobulins were used to control for nonspecific labeling. The phenotype of eGFP+ and p210-eGFP+clones was determined using APC/PE/FITC-coupled monoclonal antibodies directed at CD56 (BD PharMingen, San Diego, CA), CD158a (clone EB6; Beckman Coulter, Miami, FL), CD158b/j (clone GL183; Beckman Coulter), CD158e (clone DX9; BD Bioscience, San Jose, CA), and CD158i (clone FES172; Immunotech, Marseille, France). Specific KIRs are referred to according to the recently published nomenclature.30
Cytokine production was determined for NK92 cells grown under basal conditions (unstimulated) and after stimulation with phorbol 12-myristate 13-acetate (10−9 M)/Ca2+ionophore (0.1 μg/mL) (P/I) (Sigma, St Louis, MO) or rhIL-12 (10 U/mL) (Genetics Institute, Cambridge, MA). Parental and transduced NK92 cells (1 × 106/mL) were stimulated with P/I or rhIL-12, in the presence of GolgiStop (4 μL/culture condition) for 6 hours at 37°C and were subsequently washed and stained for interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and IL-2 (BD PharMingen) according to the manufacturer's recommendations. All analyses were performed with a FACSCalibur and CELLQuest software (Becton Dickinson).
Cell growth and survival
NK cells were cultured with or without IL-2, and the viable cells were counted using the trypan blue exclusion assay. Alternatively, cells were analyzed for proliferation using a3H-thymidine incorporation assay. NK92 cells (1 × 104 cells/100 μL) were plated in 96-well U-bottomed microtiter plates (Falcon Microtest III, Becton Dickinson) and incubated at 37°C and 5% CO2 for 72 hours in the presence or absence of 500 U/mL IL-2 and with or without the addition of 0, 1, or 10 μM imatinib mesylate (STI-571; a gift from Novartis, Basel, Switzerland). 3H-thymidine (0.0111 MBq [0.3 μCi]) (Amersham, Oakville, ON, Canada) was added to each well, and the samples were collected 16 hours later using a 96 Mach II harvester. The H3 release was measured by a Betaplate scintillation counter.
The apoptotic effect of imatinib mesylate on p210-eGFP+NK92 cells was analyzed using the annexin-V–PE/7-amino-actinomycin (7-AAD) binding assay (BD PharMingen). Parental, eGFP, and p210-eGFP–transduced NK92 cells (1 × 105 cells/mL) were treated with 0, 1, and 10 μM imatinib mesylate for 48, 72, 96 hours, and 7 days, and apoptosis was assessed with a FACSCalibur (Becton Dickinson) based on annexin-V binding, according to the manufacturer's protocol. K562 cells were used as a positive control.
Cytotoxicity
Parental and transduced NK cells were tested in 4-hour51Cr release cytotoxicity assays against the K562 cell line (ATCC) as previously described.13 To determine imatinib mesylate's effect on the lytic function of NK cells, p210+ NK92 cells were cultured in the presence or absence of IL-2 (500 U/mL), and imatinib mesylate (10 μM) was added for 48 hours, 7 days, and 14 days prior to cytotoxic testing. All cytotoxicity assays used effector/target ratios from 20:1 to 0.08:1 in triplicate as previously described.13 Fc receptor–positive P815 cells (ATCC) were used in a reverse ADCC (antibody-dependent cell-mediated cytotoxicity) assay to test receptor function as previously described,31 either in the absence or presence of purified monoclonal antibodies (mAbs) (3 μL 0.5 mg/mL initial concentration) (anti-CD56 clone MY31 [BD Pharmingen], anti-CD158b/j clone GL183 [Immunotech], and anti-CD158e clone DX9 [BD PharMingen]).
Western and Southern blotting
Parental and transduced NK92 cells (5 × 104) were lysed in 20 μL lysis buffer (50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl [pH 7.4], 250 mM NaCl, 2mM EDTA (ethylenediaminetetraacetic acid), 1% NP-40, 50 mM NaF, 2 mM NaVO4, 1 mM NaPO4, and complete protease inhibitor cocktail [Roche, Palo Alto, CA]), to which 20 μL sample buffer (2% sodium dodecyl sulfate [SDS], 10% glycerol, 0.96 M 2-mercaptoethanol, 0.3 M Tris [pH 6.8], and 0.02% bromophenol blue) was added prior to boiling for 5 minutes. The samples were then separated on an 8% SDS-PAGE (polyacrylamide gel electrophoresis) gel and then transferred to Immunoblot polyvinylidene fluoride (PVDF) membrane (BioRad, Hercules, CA). The membrane was blocked using either 5% bovine serum albumin (Sigma) or 4% nonfat dry milk (BioRad) in phosphate-buffered saline–Tween (PBS-T; pH 7.6, 0.05% Tween-20) and incubated for 90 minutes with either the 4G10 antiphosphotyrosine antibody (Upstate, Lake Placid, NY) or monoclonal Abl-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive bands were visualized using secondary horseradish peroxidase (HRP)–conjugated antibody (1:5000 dilution) (Santa Cruz Biotechnology) and chemiluminescence (Amersham Life Science, Arlington Heights, IL).
Genomic DNA (10 μg) extracted from eGFP+ and p210-eGFP+ NK92 clones was digested with 1.5 μLEcoRI restriction enzyme (Gibco) for 4 hours at 37°C. The digested DNA was then electrophoresed on a 0.7% agarose gel with ethidium bromide, transferred to positively charged nylon membrane, and probed with a 32P-labeled eGFP probe (106cpm/mL) to determine clonality.
Statistical analysis
Results of experimental points obtained from multiple experiments were reported as the mean ± SEM. Significance levels were determined by 2-sided Student t test analysis.
Results
p210+ NK92 cells are IL-2 independent and exhibit diminished natural cytotoxicity
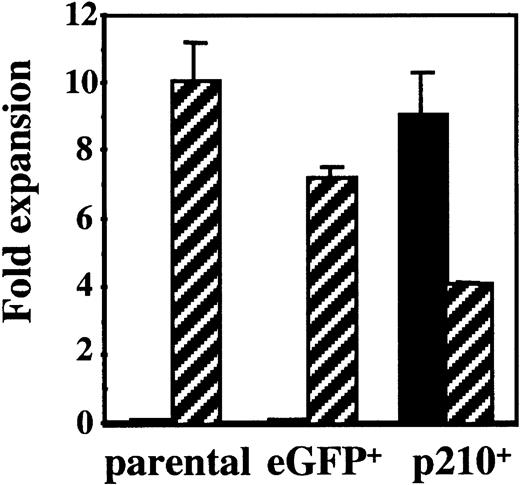
NK92 cells are IL-2 dependent and start dying within 72 hours after removal of IL-2. To explore the role of the BCR/ABLgene on NK cell function in CML, NK92 cells were transduced with an MSCV retrovirus containing p210BCR/ABL-eGFP (p210) or eGFP alone (control). After sorting for eGFP+cells, NK92 cells were cultured in media with and without IL-2. In the presence of IL-2 all cells survive and grow like the parental NK92 cell line. When IL-2 is removed, both the parental and eGFP-transduced control cells die within 7 days. However, p210-transduced cells survive and become IL-2 independent (Figure 1). To determine whether p210-transduced cells had a competitive advantage versus untransduced cells, eGFP and p210-transduced NK92 cells were cultured without prior sorting, in the presence or absence of IL-2. Fourteen days later, phenotypic analysis for eGFP showed no survival of control (eGFP+) cells in the absence of IL-2, whereas the p210+ cells survived and grew indefinitely, resulting in outgrowth of eGFP+ cells (data not shown). These data prove that the IL-2 withdrawal selects for p210+ NK92 cells, and that p210 transduction induces the IL-2 independence of the NK92 cells.
NK92 cells transduced with p210 become IL-2 independent.
Untransduced (parental) NK92 cells were transduced with a control vector (eGFP) and a vector containing BCR/ABL (p210). Parental and sorted eGFP+ (termed eGFP) and p210-eGFP+ NK92 cells (termed p210+) (3 × 105 cells/mL) were cultured in the presence (▨) or absence (▪) of 500 U/mL IL-2, and the number of viable cells was determined after 14 days of culture. Parental and eGFP+control NK92 cells died in the absence of IL-2, whereas p210+ cells without IL-2 expanded at similar rates as parental NK92 cells in the presence of IL-2.
NK92 cells transduced with p210 become IL-2 independent.
Untransduced (parental) NK92 cells were transduced with a control vector (eGFP) and a vector containing BCR/ABL (p210). Parental and sorted eGFP+ (termed eGFP) and p210-eGFP+ NK92 cells (termed p210+) (3 × 105 cells/mL) were cultured in the presence (▨) or absence (▪) of 500 U/mL IL-2, and the number of viable cells was determined after 14 days of culture. Parental and eGFP+control NK92 cells died in the absence of IL-2, whereas p210+ cells without IL-2 expanded at similar rates as parental NK92 cells in the presence of IL-2.
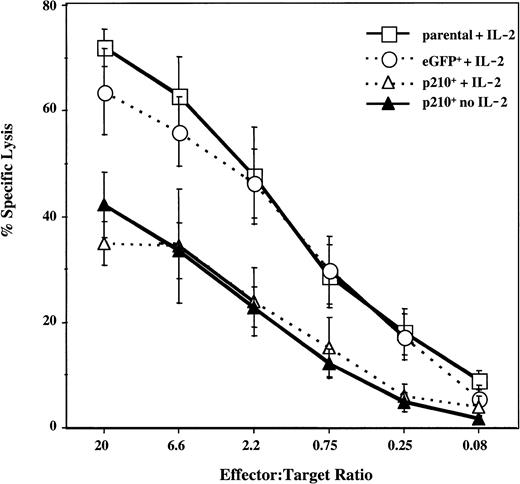
To test for the effect of BCR/ABL on NK cell function, the cytotoxicity of the parental and transduced NK92 cells was measured against K562 targets. Both parental and control eGFP+ NK92 cells exhibited potent cytotoxic activity. In contrast, p210+ NK92 cells killed K562 cells significantly less well, regardless of whether IL-2 was present (P < .01) (Figure2).
Natural cytotoxicity of p210+ NK92 cells is decreased.
Untransduced (parental), eGFP+, and p210-eGFP+(p210+) NK92 cells were tested against the K562 targets, at effector-to-target ratios ranging from 20:1 to 0.08:1. eGFP+ and p210+ cells were cultured in the presence or absence of 500 U/mL IL-2 between 14 days and up to 12 months before cytotoxicity testing, and results at all time points were similar. The difference between p210+cells (with or without IL-2) and eGFP+ cells with IL-2 is highly significant at all but the lowest E/T ratio (P < .01). Data shown as the mean ± SEM of 5 different experiments performed in triplicate.
Natural cytotoxicity of p210+ NK92 cells is decreased.
Untransduced (parental), eGFP+, and p210-eGFP+(p210+) NK92 cells were tested against the K562 targets, at effector-to-target ratios ranging from 20:1 to 0.08:1. eGFP+ and p210+ cells were cultured in the presence or absence of 500 U/mL IL-2 between 14 days and up to 12 months before cytotoxicity testing, and results at all time points were similar. The difference between p210+cells (with or without IL-2) and eGFP+ cells with IL-2 is highly significant at all but the lowest E/T ratio (P < .01). Data shown as the mean ± SEM of 5 different experiments performed in triplicate.
NK cells function not only as direct cytotoxic cells but they also produce cytokines important in the immune response.32 33NK92 cells were therefore analyzed for production of IL-2, IFN-γ, and TNF-α by intracellular cytokine (IC) staining. There was no significant difference between the cytokine profiles of the parental, eGFP+ control, and the p210+ NK92 cells (data not shown), demonstrating that their IL-2 independence is not due to autocrine production of IL-2.
Imatinib mesylate inhibits the growth and survival of the p210+ NK92 cells
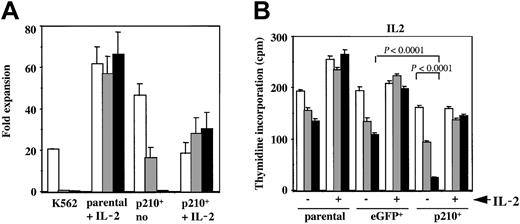
The selective inhibitor of the BCR/ABL tyrosine kinase, imatinib mesylate, leads to growth inhibition ofBCR/ABL-expressing myeloid cells34 and induces a variable degree of apoptosis in human CML cell lines.35To assess the specific effect onBCR/ABL+ NK cells, untransduced and eGFP or p210-eGFP–transduced NK92 cells were cultured in the presence or absence of imatinib mesylate, with or without IL-2. Parental NK92 cells expanded 60-fold regardless of whether imatinib mesylate was present. In contrast, imatinib mesylate inhibited the growth of the IL-2–independent p210+ cells in a dose-dependent manner (Figure 3A). Furthermore, in the presence of imatinib mesylate, the addition of IL-2 rescued p210+ NK92 cells from death and allowed continuous expansion. This finding demonstrates that imatinib mesylate's effects are reversible.
Imatinib mesylate markedly inhibits the growth of IL-2–independent p210+ NK92 cells, which is reversible by IL-2.
(A) Untransduced (parental) and p210-eGFP+(p210+) NK92 cells (3 × 105 cells/mL) were cultured for 14 days with or without IL-2 and with or without the addition of 0 (■), 1 (░), or 10 (▪) μM imatinib mesylate. K562 cells were completely inhibited by imatinib mesylate after 7 days of culture. p210+ NK92 cells exhibited a dose-dependent decline in growth, and all cells died from 10 μM imatinib mesylate. In contrast, p210+ NK92 cells continued to grow despite imatinib mesylate when IL-2 was re-added to cultures, showing that imatinib mesylate's effects are reversible. Imatinib mesylate had no effect on the growth of the parental NK92 cells (negative control). (B) Parental, eGFP+, and p210-eGFP–transduced (p210+) NK92 cells (1 × 104 cells/100 μL) were treated with imatinib mesylate for 72 hours in the presence or absence of IL-2, and then tested for proliferation using3H-thymidine incorporation. Concentrations of imatinib mesylate are as follows: ■, no imatinib mesylate; ░, 1 μM imatinib mesylate; ▪ 10 μM imatinib mesylate. The IL-2–independent p210+ NK92 cells show marked decrease in proliferation from imatinib mesylate when compared with control eGFP+ cells (P < .0001), and the addition of IL-2 reversed this effect. Both parental and eGFP+ NK92 cells proliferate less in the absence of IL-2, and imatinib mesylate further accelerates this decline, whereas in the presence of IL-2, imatinib mesylate has no effect (P = NS). Each bar shows the mean ± SEM of 12 replicates for each condition.
Imatinib mesylate markedly inhibits the growth of IL-2–independent p210+ NK92 cells, which is reversible by IL-2.
(A) Untransduced (parental) and p210-eGFP+(p210+) NK92 cells (3 × 105 cells/mL) were cultured for 14 days with or without IL-2 and with or without the addition of 0 (■), 1 (░), or 10 (▪) μM imatinib mesylate. K562 cells were completely inhibited by imatinib mesylate after 7 days of culture. p210+ NK92 cells exhibited a dose-dependent decline in growth, and all cells died from 10 μM imatinib mesylate. In contrast, p210+ NK92 cells continued to grow despite imatinib mesylate when IL-2 was re-added to cultures, showing that imatinib mesylate's effects are reversible. Imatinib mesylate had no effect on the growth of the parental NK92 cells (negative control). (B) Parental, eGFP+, and p210-eGFP–transduced (p210+) NK92 cells (1 × 104 cells/100 μL) were treated with imatinib mesylate for 72 hours in the presence or absence of IL-2, and then tested for proliferation using3H-thymidine incorporation. Concentrations of imatinib mesylate are as follows: ■, no imatinib mesylate; ░, 1 μM imatinib mesylate; ▪ 10 μM imatinib mesylate. The IL-2–independent p210+ NK92 cells show marked decrease in proliferation from imatinib mesylate when compared with control eGFP+ cells (P < .0001), and the addition of IL-2 reversed this effect. Both parental and eGFP+ NK92 cells proliferate less in the absence of IL-2, and imatinib mesylate further accelerates this decline, whereas in the presence of IL-2, imatinib mesylate has no effect (P = NS). Each bar shows the mean ± SEM of 12 replicates for each condition.
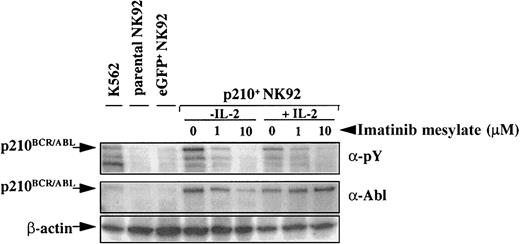
To determine whether imatinib mesylate inhibited proliferation or promoted apoptosis of the p210+ NK92 cells, we tested short-term proliferation using thymidine incorporation and monitored apoptosis using annexin-V. As expected, parental and eGFP+control NK92 cells proliferated significantly less in the absence of IL-2, and imatinib mesylate further contributed to this effect. When IL-2 was present, imatinib mesylate had no effect on proliferation of the parental or control eGFP+ NK92 cells (Figure 3B). In marked contrast, the IL-2–independent p210+ NK92 cells demonstrated a significant dose-dependent decrease in proliferation when imatinib mesylate was present (P < .0001), and the addition of IL-2 restored their proliferation close to baseline (Figure3B). Imatinib mesylate potently inhibited BCR/ABL-kinase activity both in the presence or absence of IL-2, as demonstrated by the reduced phosphorylation of cellular proteins, including p210BCR/ABL itself (Figure4).
p210-transduced cells express
BCR/ABL, and imatinib mesylate inhibits p210BCR/ABL phosphorylation. Parental (untransduced), eGFP+, and p210-eGFP-transduced (p210+) NK92 cells (5 × 104 cells) were incubated with or without imatinib mesylate for 48 hours in the presence or absence of IL-2 and analyzed by Western blot with antiphosphotyrosine (α-pY) or anti-Abl antibody. Cells treated with imatinib mesylate demonstrate a dose-dependent inhibition of p210BCR/ABL phosphorylation, both in the presence and absence of IL-2. p210BCR/ABLexpression is present in all p210-transduced NK92 and K562 (positive control) cells.
p210-transduced cells express
BCR/ABL, and imatinib mesylate inhibits p210BCR/ABL phosphorylation. Parental (untransduced), eGFP+, and p210-eGFP-transduced (p210+) NK92 cells (5 × 104 cells) were incubated with or without imatinib mesylate for 48 hours in the presence or absence of IL-2 and analyzed by Western blot with antiphosphotyrosine (α-pY) or anti-Abl antibody. Cells treated with imatinib mesylate demonstrate a dose-dependent inhibition of p210BCR/ABL phosphorylation, both in the presence and absence of IL-2. p210BCR/ABLexpression is present in all p210-transduced NK92 and K562 (positive control) cells.
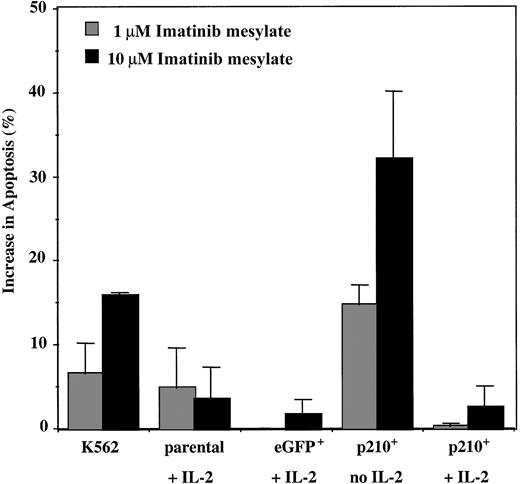
Imatinib mesylate has been shown to induce apoptosis ofBCR/ABL+ myeloid cell lines,34,35 partly by blocking the antiapoptotic signaling pathways and also by stimulating BCR/ABL nuclear entry.36 K562 cells underwent significant apoptosis when imatinib mesylate was present (15% increase from baseline), whereas little effect was observed on parental and eGFP+ control NK92 cells maintained in IL-2. In contrast, p210+ NK92 cells in the absence of IL-2 showed a major increase in apoptosis in the presence of imatinib mesylate (P < .001), which was reversed by the addition of IL-2 (Figure5). These findings suggest that the growth inhibitory effect of imatinib mesylate on the IL-2–independent p210+ NK92 cells is due both to reduced proliferation (90% reduction from 72 hours of treatment with 10 μM imatinib mesylate; Figure 3B) and increase in apoptosis (30% after 96 hours of treatment; Figure 5). Using this assay, no significant apoptosis was detected for the p210+ NK92 cells with less than 96-hour exposure to imatinib mesylate, suggesting that the imatinib mesylate influence in3H-thymidine assays may be due to an antiproliferative effect.
Imatinib mesylate induces apoptosis of the IL-2–independent p210+ NK92 cells.
Parental, eGFP+, and p210-eGFP+(p210+) NK92 cells (1 × 105cells/mL) were incubated with 1 and 10 μM imatinib mesylate for 96 hours, and then apoptosis was analyzed using the annexin-V binding assay. Data shown are the increase in apoptosis over the baseline (without imatinib mesylate) for each condition. p210+ NK92 cells without IL-2 demonstrate significantly increased apoptosis from imatinib mesylate compared with control eGFP+ NK92 cells (P < .01), and adding IL-2 reverses this effect (P = NS). Each bar represents the mean ± SEM of 3 experiments for each condition.
Imatinib mesylate induces apoptosis of the IL-2–independent p210+ NK92 cells.
Parental, eGFP+, and p210-eGFP+(p210+) NK92 cells (1 × 105cells/mL) were incubated with 1 and 10 μM imatinib mesylate for 96 hours, and then apoptosis was analyzed using the annexin-V binding assay. Data shown are the increase in apoptosis over the baseline (without imatinib mesylate) for each condition. p210+ NK92 cells without IL-2 demonstrate significantly increased apoptosis from imatinib mesylate compared with control eGFP+ NK92 cells (P < .01), and adding IL-2 reverses this effect (P = NS). Each bar represents the mean ± SEM of 3 experiments for each condition.
Because p210BCR/ABL decreases NK92-mediated natural cytotoxicity against K562 targets, we thought that imatinib mesylate might improve their reduced function back to that of control eGFP+ NK92 cells. However, after 48 hours of incubation (before apoptosis can be detected), imatinib mesylate decreased the lytic function of IL-2–independent p210+ NK92 cells (6.5% lysis at E/T ratio 20:1) (n = 3), and the addition of IL-2 restored their killing rates back to baseline (36% lysis) (P = .01), but not to the level of control cells without p210.
p210+ NK92 cells acquire KIR receptors
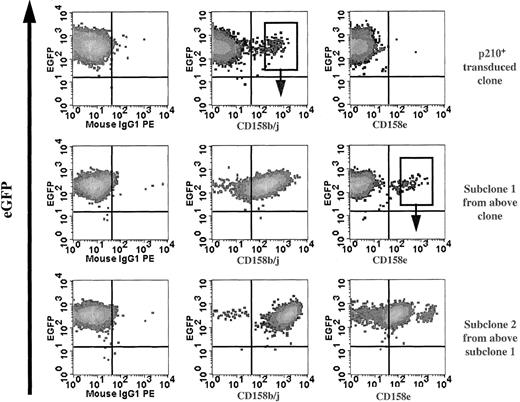
NK cell effector function is regulated by the balance between inhibitory and activating receptors, which endow NK cells with cytokine secretion and cytotoxicity against various target cells. It is currently unknown what determines the NK cell receptor repertoire, but data suggest that the receptor acquisition occurs after NK-cell lineage commitment.37 The NK92 parental cell line lacks the expression of most KIR (except CD158d [KIR2DL4]), but expresses NCR and lectin-type (CD94, NKG2, etc) receptors.38 Serial phenotypic analysis of p210+ NK92 clones for KIR expression detected small subpopulations that preferentially expressed CD158b/j (KIR2DL2/L3/S2 recognized by the monoclonal antibody GL183). Subclones of CD158b/j+ cells maintained receptor expression indefinitely. CD158e- (KIR3DL1, recognized by the monoclonal antibody DX9) or CD158i- (KIR2DS4, recognized by the monoclonal antibody FES172) expressing cells were not found from the original KIR-negative p210-transduced cells. However, all CD158b/j+ clones also expressed CD158i (data not shown). In addition, the CD158b/j+ p210+ clones continued to acquire new KIRs, and subclones expressing CD158e have been established (Figure6).
Serial clones of p210+ NK92 cells acquire KIR.
Serial phenotype analysis of p210-eGFP+(p210+) NK92 clones for KIR expression detected small subpopulations (top row) that expressed CD158b/j allowing selection of subclones. Subclone 1 (CD158b/j+; top row, middle panel) continued to acquire KIR (middle row), and subclone 2 expressing CD158e was established (bottom row). The sorting gates shown are representative of those used to establish subsequent subclones.
Serial clones of p210+ NK92 cells acquire KIR.
Serial phenotype analysis of p210-eGFP+(p210+) NK92 clones for KIR expression detected small subpopulations (top row) that expressed CD158b/j allowing selection of subclones. Subclone 1 (CD158b/j+; top row, middle panel) continued to acquire KIR (middle row), and subclone 2 expressing CD158e was established (bottom row). The sorting gates shown are representative of those used to establish subsequent subclones.
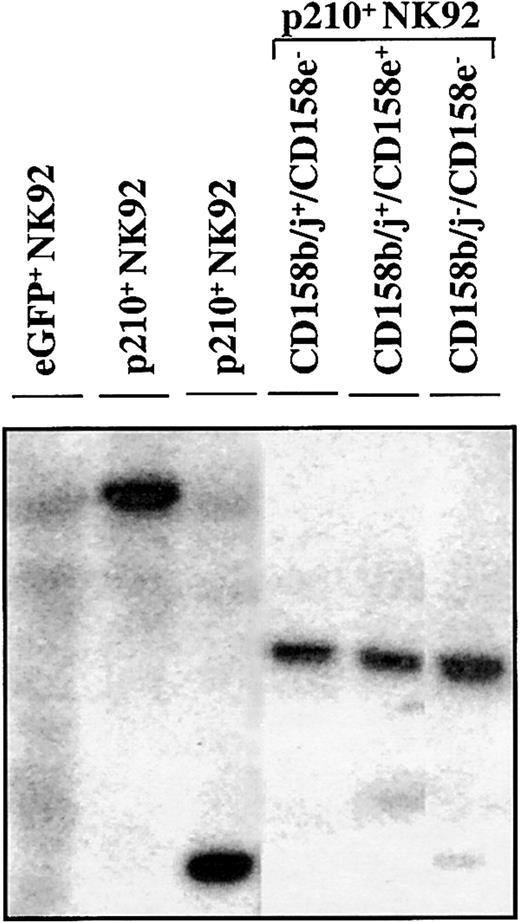
The CD158b/j+/CD158e−, CD158b/j+/CD158e+, and CD158b/j−/CD158e− p210+ cell populations were derived from the same clone, as determined by Southern blot analysis, showing a distinct dominant band after probing for the presence of eGFP in the genomic DNA (Figure7). This finding shows that KIR acquisition occurred in the progeny of a single cell marked by a retrovirus with a unique random integration site. KIR+ NK92 cells from the parental or control eGFP+ NK92 cells were difficult to find. However, after a year of subcloning from the parental clones, a CD158b/j+/CD158e− subclone has been established, suggesting that BCR/ABL is not specifically required for KIR acquisition in the NK92 cell line.
KIR expressing p210+ NK92 cells derive from the same clone.
In a Southern blot analysis, genomic DNA from parental, eGFP+, and p210-eGFP+(p210+) NK92 cells was digested withEcoRI, electrophoresed on a 0.7% agarose gel, and probed with a 32P-eGFP probe. Representative lanes show that CD158b/j+/CD158e− (lane 4), CD158b/j+/CD158e+ (lane 5), and CD158b/j−/CD158e− (lane 6) p210+NK92 cell populations express identical dominant bands, indicating that they derive from the same clone. KIR-negative eGFP+ NK92 cells show a smear (lane 1), and 2 different p210+ clones show a different unique band (lanes 2-3).
KIR expressing p210+ NK92 cells derive from the same clone.
In a Southern blot analysis, genomic DNA from parental, eGFP+, and p210-eGFP+(p210+) NK92 cells was digested withEcoRI, electrophoresed on a 0.7% agarose gel, and probed with a 32P-eGFP probe. Representative lanes show that CD158b/j+/CD158e− (lane 4), CD158b/j+/CD158e+ (lane 5), and CD158b/j−/CD158e− (lane 6) p210+NK92 cell populations express identical dominant bands, indicating that they derive from the same clone. KIR-negative eGFP+ NK92 cells show a smear (lane 1), and 2 different p210+ clones show a different unique band (lanes 2-3).
The CD158b/j receptor acquired by the p210+ clones can be activating (CD158j, KIR2DS2) or inhibitory (CD158b, KIR2DL2/L3). To determine the function of the CD158b/j surface expression, we performed reverse ADCC assays against the 51Cr-labeled P815 targets. The GL183 mAb mediated a specific increase in lysis of the P815 cells (60%-80% lysis) by CD158b/j+ p210+ NK92 cells, whereas no significant effect (< 10% lysis) was seen without antibody, with control anti-CD56 mAb, or with NK92 cells lacking CD158b/j surface expression. The GL183 mAb induced significant lysis (58%) by the CD158b/j+/CD158e+p210+ effector cells, and this was suppressed by the concomitant addition of the DX9 mAb (38% lysis). Therefore, reverse ADCC identifies the CD158b/j+ cells as expressing the activating receptor CD158j (KIR2DS2) and suggests that CD158e (KIR3DL1) has at least partial, but not complete, overriding inhibitory function. Therefore, in contrast to natural cytotoxicity, BCR/ABL does not block KIR function.
p210+ primary NK cells
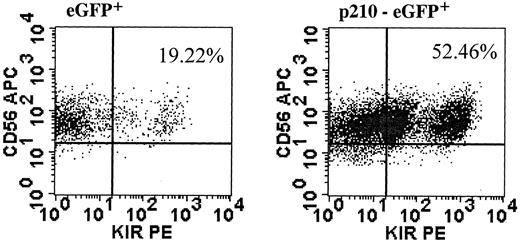
Findings in NK92 cells may be limited by the immortalized nature of cell lines. Therefore, we designed experiments to test the effects of BCR/ABL in primary NK cells. CD56+bright and CD56+dim NK cells from healthy donors were transduced with eGFP (control) or p210-eGFP. We were unable to transduce CD56+dim NK cells at a frequency sufficient for further testing. In contrast, CD56+bright NK cells were transduced, albeit at low frequency, after IL-2 prestimulation, and CD56+/eGFP+ cells were sorted and expanded using conditions with feeders described for NK cell cloning.29 Unlike NK92 cells, primary p210+ NK cells killed K562 targets similar to control cells (data not shown). However, primary p210+ NK cells gave similar results to NK92 cells with an altered response to IL-2 and increased KIR acquisition. When IL-2 was removed from 3-week expanded transduced cells, eGFP+ control cells had no growth after an additional 14 days in culture, whereas the p210+ NK cells expanded up to 6-fold (similar results from 2 separate experiments).BCR/ABL-transduced cells did not proliferate further after 14 days of IL-2 withdrawal, showing that BCR/ABL alone does not lead to indefinite growth like the transformed NK92 cells. Finally, fresh sorted CD56+bright NK cells, which express a paucity of KIR,39 acquire at least one KIR on a higher percentage of cultured cells when transduced with p210-eGFP compared with eGFP alone (Figure 8).
p210+ primary NK cells acquire KIR.
CD56+bright/CD3− NK cells were prestimulated with IL-2 alone and transduced with p210-eGFP+ and eGFP+ (control). CD56+/eGFP+ NK cells were sorted and expanded for 3 weeks, then NK cells were analyzed for expression of at least one KIR, using a cocktail of KIR antibodies against CD158a, CD158b/j, and CD158e. The fold increase in KIR-expressing NK cells was similar from another donor (data not shown).
p210+ primary NK cells acquire KIR.
CD56+bright/CD3− NK cells were prestimulated with IL-2 alone and transduced with p210-eGFP+ and eGFP+ (control). CD56+/eGFP+ NK cells were sorted and expanded for 3 weeks, then NK cells were analyzed for expression of at least one KIR, using a cocktail of KIR antibodies against CD158a, CD158b/j, and CD158e. The fold increase in KIR-expressing NK cells was similar from another donor (data not shown).
Discussion
Although the role of BCR/ABL has been extensively studied in the myeloid lineage in vitro40-45 and in mice,46-48 its effect on lymphocytes is unknown. It has been previously thought that the NK cell lineage is not part of theBCR/ABL+ clone in CML. However, Nakajima et al17 have recently demonstrated that small numbers of BCR/ABL+ NK cells can be found in advanced stage CML.17 Although it was hypothesized that BCR/ABL may intrinsically block the differentiation of NK cells from primitive CD34+progenitors, accounting for decreased circulating NK cells, this was not the case. Instead, decreased NK differentiation observed in CML is due to a competitive suppression by the extrinsic interaction withBCR/ABL+ myeloid cells. To explore whether BCR/ABL affects NK cell effector function, we used the NK92 cell line and primary cells sharing similar characteristics (CD56+bright NK cells) transduced with a vector containing p210BCR/ABL.
p210BCR/ABL promotes the malignant myeloid phenotype in CML mostly by increasing cell proliferation and survival.49 Our data, both in NK92 cells and primary NK cells, show that BCR/ABL induces an altered response to IL-2 without stimulating its autocrine production. This finding is similar to the IL-3–independent growth seen with myeloid cells. In vitro studies to explain this finding invoked constitutive activation of mitogenic signaling proteins, such as JAK/STAT,50Raf-1,51 and p42 mitogen–activated protein (MAP) kinase.52 Alternatively, an acquired autocrine production of IL-3 and granulocyte colony-stimulating factor (G-CSF),53 54 leading to growth factor independence, has also been described. It is not yet known whether these same signaling pathways are stimulated in p210BCR/ABL+ NK cells.
Primary NK cells from patients with CML exhibit diminished natural cytotoxicity, with decreased killing of K562 targets.13,55 56 Similar to this finding, p210+ NK92 cells have a significant decrease in natural cytotoxicity against the K562 targets, which is not restored by IL-2. This cytotoxicity defect is not due to global cell dysfunction, because IFN-γ and TNF-α production are not affected, and the IL-12–induced stimulation of the IFN-γ pathway is intact. Although the use of primary cell transductions validate the NK92 data for several results, the finding that p210-transduced CD56+bright NK cells did not exhibit a decrease in natural cytotoxicity suggests several possibilities. First, the small unique subset of CD56+bright NK cells were testable only in this model, and they may not be at a maturation stage equivalent to NK cells previously studied by us and others in patients with CML. Alternatively, this finding is consistent with the possibility that other physiologically relevant factors, in addition to p210, have not yet been identified. Both of these possibilities warrant further study.
Because the tyrosine kinase inhibitor imatinib mesylate manifests significant activity againstBCR/ABL+ myeloid cells,34 one would expect a similar inhibitory effect on the growth and survival of p210BCR/ABL+ NK cells. Indeed, imatinib mesylate causes a major decrease in the proliferation and survival of the p210+ NK92 cells but only in the absence of IL-2. IL-2 can directly activate mitogenic and antiapoptotic substrates, such as JAK-STAT (Janus kinase/signal transducer and activator of transcription), Ras-MAP kinase, and phosphatidyl inositol 3-kinase.57 Thus, the complete reversal of imatinib mesylate's effects after the addition of IL-2 is likely due to its direct stimulation of substrates downstream from the p210 tyrosine kinase. These data are similar to reports by Druker et al,34 Carroll et al,58 and Okuda et al59 regarding IL-3–independentBCR/ABL+ 32D cells, and TEL/ABL-, TEL/platelet-derived growth factor β receptor (PDGFβR)–, and TEL/ABL-related gene (ARG)–transformed BaF3 myeloid cells, in which the inhibitory effects of imatinib mesylate on cell growth and survival could be reversed with the addition of IL-3.
NK cell function is dependent on the balance between activating and inhibitory signals, mediated by activating or inhibitory receptors.60 CD94 and KIR receptor acquisition during NK cell development from single hematopoietic stem cells occurs after lineage commitment and late in the maturation process.37In vitro experiments have shown that KIR receptor expression depends on the presence of lymphocyte-stimulating cytokines (eg, IL-2 or IL-15).37 Although the parental NK92 cell line does not express most KIRs, p210+ cells acquired the receptors CD158i and CD158j, and subsequently CD158e. The increase in KIRs was also supported by findings using primary cells. In both NK92 cells and primary cells, KIR expression could occur in the absence of p210 but at a lower frequency. Thus, KIR acquisition cannot be causally related to p210BCR/ABL, but p210 may be a contributing factor by facilitating receptor acquisition, or alternatively, by merely enhancing the survival of KIR+ subclones. In marked contrast to the inhibition seen in natural cytotoxicity, functional assays showed that p210 does not impair CD158j function in NK92 cells. To our knowledge, this is the first IL-2–independent NK cell line with functional NK cell receptors, which may provide a unique tool to dissect mechanisms of NK cell function in CML, without confounding effects of IL-2.
In summary, NK cells show altered IL-2 dependence and increased KIR expression after transformation with the BCR/ABL oncogene. Although only true in NK92 cells, natural cytotoxicity was decreased as seen in patients with CML. This study is unique because the role of p210 on lymphocytes has not been previously studied. The altered function of p210+ NK cells may allow malignant cells to escape from immune control. Further understanding of the basic mechanisms involved in the NK cell dysfunction in CML may facilitate therapeutic strategies to effectively target and correct specific immune defects, aid in the malignant clone recognition, and prevent disease progression.
We thank Brad Anderson for his expertise in flow cytometry and Valarie McCullar and Karen Brungard for their excellent technical assistance.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-04-1172.
Supported by National Institutes of Health grants R01-HL-55417 and PO1-CA-65493 and supported in part by grant M01-RR00400 from the National Cancer Institute for Research Resources.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jeffrey S. Miller, University of Minnesota Cancer Center, MMC 806, Division of Hematology, Oncology and Transplantation, Harvard St at East River Rd, Minneapolis, MN 55455; e-mail: mille011@tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal