Rosmarinic acid (RosA) is a hydroxylated compound frequently found in herbal plants and is mostly responsible for anti-inflammatory and antioxidative activity. Previously, we observed that RosA inhibited T-cell antigen receptor (TCR)– induced interleukin 2 (IL-2) expression and subsequent T-cell proliferation in vitro. In this study, we investigated in detail inhibitory mechanism of RosA on TCR signaling, which ultimately activates IL-2 promoter by activating transcription factors, such as nuclear factor of activated T cells (NF-AT) and activating protein-1 (AP-1). Interestingly, RosA inhibited NF-AT activation but not AP-1, suggesting that RosA inhibits Ca2+- dependent signaling pathways only. Signaling events upstream of NF-AT activation, such as the generation of inositol 1,4,5-triphosphate and Ca2+ mobilization, and tyrosine phosphorylation of phospholipase C-γ1 (PLC-γ1) were strongly inhibited by RosA. Tyrosine phosphorylation of PLC-γ1 is largely dependent on 3 kinds of protein tyrosine kinases (PTKs), ie, Lck, ZAP-70, and Itk. We found that RosA efficiently inhibited TCR-induced tyrosine phosphorylation and subsequent activation of Itk but did not inhibit Lck or ZAP-70. ZAP-70–dependent signaling pathways such as the tyrosine phosphorylation of LAT and SLP-76 and serine/threonine phosphorylation of mitogen-activated protein kinases (MAPKs) were intact in the presence of RosA, confirming that RosA suppresses TCR signaling in a ZAP-70–independent manner. Therefore, we conclude that RosA inhibits TCR signaling leading to Ca2+ mobilization and NF-AT activation by blocking membrane-proximal events, specifically, the tyrosine phosphorylation of inducible T cells kinase (Itk) and PLC-γ1.
Introduction
Rosmarinic acid (α-o-caffeoyl-3, 4-dihydroxyphenyl lactic acid; RosA) is a naturally occurring hydroxylated compound widely distributed in Labiatae herbs, such as rosemary, sweet basil, and perilla. RosA appears to be a substance of considerable interest because it has a broad range of applications, from food preservatives to cosmetics, and has medicinal use because of its antimicrobial and antioxidant activity.1-3 Several studies also indicated that RosA has an anti-inflammatory activity and have demonstrated that this characteristic can be used to treat various inflammatory disorders. The manner in which RosA exerts its anti-inflammatory effects is not well understood, although it has been ascribed to its ability to block complement activation4 and to inhibit lipoxygenase5 and cyclooxygenase6 activity. In vivo studies have shown that RosA inhibits several complement-dependent inflammatory processes, including cobra venom factor–induced paw edema and ovalbumin/antiovalbumin–mediated passive cutaneous anaphylaxis.7
Recently, the inhibition of T-cell antigen receptor (TCR)–mediated signaling was identified as one of the working mechanisms of many anti-inflammatory drugs. Antirheumatic drugs, such as hydroxychloroquine,8 nonsteroidal anti-inflammatory drugs,9 and a traditional Chinese antirheumatic medicine TWHf10 were found to inhibit TCR-induced signaling events, such as Ca2+ mobilization, the activation of p38 mitogen–activated protein kinase (MAPK), and the expression of CD40 ligand, despite the fact that their points of action are quite different. Moreover, leflunomide represses the activity of 2 major Src family protein tyrosine kinases, Fyn and lymphocyte-specific cytoplasmic protein tyrosine kinase (Lck), which are implicated in the signal transduction of T cells. Leflunomide also blocks TCR-induced tyrosine phosphorylation of the phospholipase C-γ1 (PLC-γ1) and ζ-chain and consequently inhibits Ca2+mobilization, interleukin 2 (IL-2) secretion, and IL-2 receptor expression.11
The T-cell response is initiated by the interaction between the antigen presented in antigen-presenting cells and the TCR complex. Stimulation of the TCR leads to the rapid activation of tyrosine kinases, which phosphorylate a variety of signal-transducing proteins. A considerable weight of evidence supports the view that the phosphorylation of immunoreceptor tyrosine–based activation motifs within the TCR complex is required to initiate TCR signaling.12-14 The phosphorylation of immunoreceptor tyrosine–based activation motifs by Lck recruits and activates ζ-associated protein-70 (ZAP-70), the Syk family protein tyrosine kinase (PTK).15-17 ZAP-70 phosphorylates 2 major adaptor molecules, linker for activation of T cells (LAT) and SH2-containing leukocyte protein (SLP-76).18-21 This event results in the assembly of LAT with downstream signaling molecules such as PLC-γ1, growth factor receptor–bound protein 2 (Grb2), and Gad–SLP-76 complex, and so forth.22-25 This event is thought to be required for PLC-γ1 tyrosine phosphorylation and activation, as well as the activation of extracellular signal–regulated kinase (Erk). It has been proposed that the LAT-associated complex colocalizes PLC-γ1 with the activated PTK (possibly inducible T cells kinase [Itk]), which in turn phosphorylates and activates PLC-γ1.26-30 Tyrosine phosphorylation of PLC-γ1 converts this enzyme to an active state, which then hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to yield inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG).31,32 The former product regulates the levels of intracellular Ca2+ levels, whereas the latter activates the protein kinase C and Ras–guanyl nucleotide-releasing protein (GRP) and leads to Ras activation.33 The binding of IP3to the IP3 receptor on the endoplasmic reticulum leads to the opening of Ca2+ channel and to a subsequent increase in cytosolic Ca2+ level.34 The resulting cytoplasmic Ca2+ increase activates calcineurin, which helps the translocation of the nuclear factor for activation of T cells (NF-AT) into the nucleus by dephosphorylating NF-AT.35 Erk activation proceeds primarily through the sequential activation of Ras, Raf-1, and MAPK/Erk kinase (MEK). One mechanism involves the activation of protein kinase C (PKC),36 which can activate Raf-1 directly,37-40 and another involves Ras-GRP, which is expressed at high levels in lymphocytes, and was recently identified as a phorbol ester–activated (and presumably DAG-activated) guanine nucleotide exchange factor for Ras.41-43 However, other mechanisms of Ras activation have also been found in T cells. It has been suggested that Ras is activated in TCR-stimulated T cells via the recruitment of Grb2-associated son of sevenless (SOS), a guanine nucleotide exchange factor for Ras, to the plasma membrane by virtue of the ability of the Src homology domain 2 (SH2) domains of Grb2 to bind to membrane-resident, tyrosine-phosphorylated proteins such as LAT.28-30,44 This is analogous to what has been reported for Ras activation after the engagement of growth factor receptors.45 Therefore, many signaling pathways are likely to connect TCR engagement to Ras and subsequent Erk activation.
Recent reports regarding the suppressive effects of various anti-inflammatory agents on TCR signaling and our previous findings on the antiproliferative effects of RosA on T cells led us to study the role of RosA in TCR signaling. We found that RosA inhibited TCR-induced NF-AT activation but not activating protein-1 (AP-1) activation, which suggests that RosA specifically inhibits Ca2+-dependent signaling pathways. This finding was further confirmed by the fact that RosA effectively suppressed TCR-induced tyrosine phosphorylation of PLC-γ1 and its associated downstream signaling events such as IP3 increase and Ca2+ mobilization. RosA inhibited neither the kinase activities nor the TCR-induced tyrosine phosphorylation of Lck and ZAP-70. However, RosA effectively inhibited the TCR-induced tyrosine phosphorylation and subsequent activation of Itk, which was implicated as a direct kinase of PLC-γ1. We conclude that RosA inhibits TCR signaling by blocking upstream TCR-signaling events, specifically the tyrosine phosphorylation of Itk and PLC-γ1. Accordingly, this study shows that RosA inhibits the Ca2+-dependent pathways of TCR signaling and demonstrates that this occurs in a ZAP-70–independent manner.
Materials and methods
Reagents
RosA was obtained from Indofine Chemical Company (Somerville, NJ) and solubilized in ethanol. Phorbol 12-myrisate 13-acetate (PMA) and ionomycin were purchased from Sigma (St Louis, MO) and Calbiochem (San Diego, CA), respectively. Protease inhibitor tablets were purchased from Boehringer Mannheim (Indianapolis, IN).
Antibodies
OKT3 and OKT4 were harvested as ascites fluid from Balb/c mice 14 days after inoculating 2 × 106 cells of OKT3 or OKT4 (ATCC, Manassas, VA). UCHT1, purified mouse antihuman CD3 monoclonal antibodies (mAbs) were obtained from Pharmingen (San Diego, CA). Itk mouse ascites was purchased from Upstate Biotechnology (Lake Placid, NY); antiphosphotyrosine mAb (4G10), anti–PLC-γ1 mAb, anti–ZAP-70 mAbs, and rabbit polyclonal anti-Itk and anti-LAT antibodies were from Upstate Biotechnology; anti-TCR ζ chain mAb and anti-Lck mAb and rabbit polyclonal anti–PLC-γ1 and anti–SLP-76 were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-Grb2 mAb was from Transduction Laboratories (Lexington, KY); mAb specific for dual-phosphorylated Erk1/2 and rabbit polyclonal anti-Erk1/2 were from New England Biolabs (Beverly, MA); and goat antimouse/rabbit immunoglobulin G (IgG) antibodies were purchased from Sigma.
Cell cultures
Jurkat T cells (ATCC) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), antibiotics (Gibco BRL, Grand Island, NY), and 50 μM β-mercaptoethanol (Sigma). Human peripheral blood mononuclear cells were isolated from adult healthy donors by density-gradient centrifugation on Ficoll-Paque PLUS (Amersham Pharmacia Biotech, Uppsala, Sweden). Following isolation, cells were plated at a density of 2 × 106/mL and left in tissue culture flasks at 37°C in 5% CO2 incubator for 2 hours to remove adherent cells. The resulting human peripheral blood lymphocytes (hPBLs) were resuspended in complete RPMI medium and used within 24 hours of isolation. The purified hPBLs consisted of 58.9% of T cells and 11.3% of B cells according to the fluorescence-activated cell sorter (FACS) analysis using anti-CD3 and anti-CD19 antibodies, respectively.
Luciferase assay
A total of 2 × 106 Jurkat T cells was transfected with 2 μg NF-AT– or AP-1–Luciferase reporter plasmids (gifts from Gerald Crabtree, Stanford University, Stanford, CA) using Lipofectamine according to the manufacturer's protocol (Gibco BRL). After incubating with DNA-Lipofectamine mixtures for 24 hours, cells were preincubated in the presence or in the absence of RosA (30 μΜ) for 2 hours before being stimulated. Cells were activated either with anti-CD3 mAb (5 μg/mL, UCHT1, IgG2a isotype) coated on a plate or with PMA (5 ng/mL) and ionomycin (0.5 μg/mL) for 16 hours. In the RosA-treated group, RosA was present throughout the 16-hour incubation process. After stimulation, the cells were washed, lysed, and assayed for luciferase activity, according to the manufacturer's instructions (Luciferase Assay System kit; Promega, Madison, WI) with a Microplate luminometer LB96V (Perkin-Elmer, Foster City, CA).
Ca2+ mobilization assay
Jurkat T cells were washed twice with Hanks balanced salt solution (HBSS) containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.4), 1 mM MgCl2, 2 mM CaCl2, and 20 mM dextrose, and then incubated with 2 μM fura-2/am (Calbiochem) for 45 minutes at 37°C. Cells were washed extensively and resuspended at 5 × 106/3 mL in HBSS.46 For calcium studies in primary human T cells, hPBLs were loaded in calcium buffer (25 mM HEPES, 125 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 0.1% glucose, 500 μM MgCl2, 10 mM CaCl2, 0.1% bovine serum albumin [pH 7.4]) with fura-2/am (10 μM) for 30 minutes at 37°C.8 Loaded cells were washed once, resuspended at 5 × 106/3 mL in calcium buffer, and immediately placed on ice. Fura-2–loaded cells were placed in a water-jacked cuvette and preincubated in the absence or presence of various concentrations of RosA for 15 minutes at 37°C. Following 100 to 150 seconds of data acquisition, cells were then incubated with anti-CD3 mAb (3 μg/mL, UCHT1, IgG1 isotype) or OKT3 ascites (1:1000), and fluorescence levels were measured using a Luminescence spectrometer LB50B (Perkin-Elmer) before and after stimulating with antimouse IgG antibody (10 μg/mL) to crosslink the anti-CD3 mAb. Data are presented in arbitrary units as a function of fluorescence (relative intracellular calcium) versus time.
Measurement of IP3
Jurkat T cells (2 × 106/reaction) were incubated in the presence or absence of RosA for 4 hours at 37°C before harvesting. The cells were activated by incubating with OKT3 ascites (1:200), and cross-linking OKT3 with goat antimouse IgG antibody (40 μg/mL) for 3 minutes. The TCR-mediated stimulation was terminated by adding 10 μL ice-cold 100% trichloroacetic acid and then incubating on ice for 15 minutes. IP3 was quantified in duplicate with the use of an IP3 [3H] Radioreceptor Assay Kit (NEN Life Science Products, Boston, MA) and by liquid scintillation counting according to the manufacturer's instructions.
Cell stimulation, lysis, immunoprecipitation, and Western blot analysis
Jurkat T cells or hPBLs were stimulated by incubating with OKT3 or OKT4 ascites (1:200), and this was followed by cross-linking OKT3/4 with goat antimouse IgG antibody (40 μg/mL) for the indicated time at 37°C. For immunoprecipitation or Western blot, Jurkat T cells (5 × 107) or hPBLs (4 × 107) were lysed with ice-cold lysis buffer (50 mM Tris (tris(hydroxymethyl)aminomethane) [pH 8.0], 150 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), 0.5% deoxycholate, 1% Nonidet P-40 or Brij97, 1 mM Na3VO4, 5 mM NaF, and one tablet proteinase inhibitor per 50 mL lysis buffer) for 1 hour on ice and then centrifuged at 12 000g at 4°C for 30 minutes. The supernatant was saved, precleared for 1 hour at 4°C by incubating with 25 μL mouse or rabbit serum-conjugated agarose (Sigma), and then incubated with the appropriate antibodies overnight at 4°C. This was followed by incubation with 25 μL protein A–conjugated agarose (Sigma) for 3 hours at 4°C. Immunoprecipitates were then either subjected to an in vitro kinase assay or immunoblotting. For immunoblot, the immunoprecipitated complexes were washed 3 times in ice-cold lysis buffer, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and blotted onto nitrocellulose membranes (Bio-rad, Hercules, CA). Blots were blocked overnight at 4°C and probed with the indicated antibodies in Tris-buffered saline (2 mM Tris [pH 7.5], 30 mM NaCl), which included 0.05% Tween 20 and 5% skim milk. Signals were detected using horseradish peroxidase–labeled secondary antibodies (antimouse and antirabbit, 1 μg/mL; Transduction Laboratories). Where indicated, blots were reprobed after stripping blots at 60°C for 30 minutes in 62.5 mM Tris (pH 6.7), 2% SDS, and 100 mM β-mercaptoethanol. Blots were finally detected by the enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL).
In vitro kinase assay
Jurkat cells (5 × 107) were immunoprecipitated with anti-Lck or Itk mAb (5 μg/mL), and kinase reactions were carried out with these immunoprecipitates (1/10 of the total immunoprecipitated complexes) at 30°C for 10 minutes in 15 μL kinase buffer (0.1 M Pipes-NaOH, pH 6.8, and 20 mM MnCl2) containing 0.37 MBq (10 μCi) 32P-γ-ATP (Amersham, Buckinghamshire, United Kingdom) and 2 μL enolase (5 mg/mL; Sigma) as an exogenous substrate. Reactions were stopped by adding 3 × reducing SDS sample loading buffer (New England Biolabs) and analyzed by electrophoresis on a 12% SDS-PAGE. 32P incorporation into enolase (41 kDa) or Itk (72 kDa) was measured by exposure to Kodak (Rochester, NY) XAR film at −70°C, or to a BAS 2040 cassette and then quantified on a Bio-Imager analyzer (BAS 1000, FUJIX). The remainder of the Lck immunoprecipitates was used to detect the autophosphorylating activity of Lck by running them on 10% SDS-PAGE and performing Western blot analysis.
Results
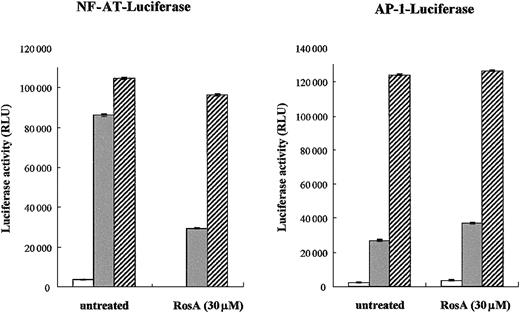
RosA inhibits NF-AT activation but not AP-1 in Jurkat T cells
Our previous study indicated that RosA inhibits TCR-induced IL-2 promoter activation and subsequent T-cell proliferation.47Moreover, this process seemed to occur at the membrane proximal point of TCR signaling, because RosA-mediated inhibition was limited to TCR-induced but not to PMA/ionomycin-induced IL-2 promoter activation.47 Because PMA and ionomycin can directly activate PKC and Ca2+ release, thus bypassing the requirement for the early activation of protein tyrosine kinases, our previous results strongly support the notion that RosA works upstream of PKC and Ca2+ flux. The IL-2 promoter contains several regulatory elements that can bind different transcription factors, such as NF-AT, AP-1, Oct-1, and nuclear factor κB (NF-κB).35 Initially, we determined the effect of RosA on 2 major transcription factors induced by TCR signaling, namely, NF-AT and AP-1. Jurkat T cells were transiently transfected with either NF-AT or AP-1 luciferase reporter plasmid, and luciferase activities were determined after TCR or PMA/ionomycin stimulation in the presence or in the absence of RosA. As shown in Figure1, neither of these promoter activities was inhibited in response to PMA/ionomycin stimulation, which was in agreement with our previous data. RosA treatment strongly repressed TCR-induced NF-AT promoter activity but not AP-1. In several cases, RosA-treated Jurkat cells showed a slight increase in AP-1 activity (Figure 1). These results indicate that RosA inhibits TCR-induced IL-2 promoter activation by suppressing NF-AT activation, and RosA acts in the membrane-proximal point(s) of the TCR signaling upstream of PMA/ionomycin working sites.
NF-AT reporter activity but not AP-1 is inhibited by RosA.
(A-B) Jurkat cells (2 × 106) were transfected with NF-AT (A) or AP-1 (B) reporter. Twenty-four hours after transfection, Jurkat cells were preincubated in the presence or absence of RosA (30 μM) for 2 hours and then stimulated with immobilized anti-CD3 mAb (UCHT1, 5 μg/mL) (░) or PMA (5 ng/mL) and ionomycin (0.5 μg/mL) (▨) for 16 hours. In the RosA-treated group, RosA was present throughout the whole 16 hours of incubation. Jurkat cells without any stimulation are marked as open bars (■). Shown are the relative light units (RLU), indicated as the average RLU and standard error of 2 independent experiments.
NF-AT reporter activity but not AP-1 is inhibited by RosA.
(A-B) Jurkat cells (2 × 106) were transfected with NF-AT (A) or AP-1 (B) reporter. Twenty-four hours after transfection, Jurkat cells were preincubated in the presence or absence of RosA (30 μM) for 2 hours and then stimulated with immobilized anti-CD3 mAb (UCHT1, 5 μg/mL) (░) or PMA (5 ng/mL) and ionomycin (0.5 μg/mL) (▨) for 16 hours. In the RosA-treated group, RosA was present throughout the whole 16 hours of incubation. Jurkat cells without any stimulation are marked as open bars (■). Shown are the relative light units (RLU), indicated as the average RLU and standard error of 2 independent experiments.
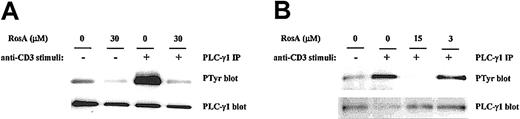
RosA blocks TCR-induced tyrosine phosphorylation of PLC-γ1 and its downstream signaling events such as IP3 generation and intracellular Ca2+ mobilization
The selective inhibitory effect of RosA on NF-AT activity strongly suggests that RosA affects Ca2+-dependent signaling. To investigate the effects of RosA on TCR-induced Ca2+mobilization, Jurkat T cells were loaded with the indicator dye fura-2/am and treated with RosA for 15 minutes at 37°C before CD3 stimulation. CD3 stimulation by anti-CD3 mAb (UCHT1), followed by cross-linking anti-CD3 mAb with antimouse IgG antibody transiently increased Ca2+ levels within 40 to 80 seconds. The incubation of Jurkat T cells with RosA (30 μM) gave either undetectable or much lower Ca2+ fluxes than untreated cells (Figure 2A). The observation that RosA had no effect on PMA/ionomycin-induced NF-AT activity indicates that inhibition by RosA occurs upstream of Ca2+ mobilization. There are many ways of blocking such Ca2+ mobilization, for example, by inhibiting IP3/IP3 receptor interaction or by inhibiting generation of IP3.34 In this study, we observed that RosA-treated Jurkat T cells released much less IP3 in response to CD3 stimulation (Figure 2B), suggesting that this is the likely explanation for the defective Ca2+ release. IP3 is generated by PLC-γ1–mediated hydrolysis of PIP232; therefore, we tested the activation status of PLC-γ1. To examine the effect of RosA on the TCR-induced tyrosine phosphorylation of PLC-γ1, Jurkat cells were preincubated with various concentrations of RosA for 4 hours and then stimulated with OKT3. Because PLC-γ1 is tyrosine phosphorylated within 2 minutes and rapidly dephosphorylated right after, we stimulated Jurkat cells for 2 minutes.26 PLC-γ1 immunoprecipitates were resolved on 12% SDS-PAGE, and tyrosine phosphorylated PLC-γ1 was detected by antiphosphotyrosine mAb (Figure 3, top panels). The identity of PLC-γ1 was confirmed by reblotting with anti–PLC-γ1 mAb (Figure 3, bottom panels). RosA completely abrogated TCR-induced tyrosine phosphorylation of PLC-γ1 at the concentration between 15 and 30 μM, however, and failed to inhibit at 3 μM (Figure 3B, top panel). In conclusion, these results suggest that reduced Ca2+ mobilization and IP3 induction in RosA-treated cells (Figure 2) are due to improper PLC-γ1 activation. Notably, RosA exerted no appreciable effect on viability of Jurkat cells during the course of these experiments and up to 16 hours at 30 μM (data not shown).
RosA suppresses TCR-induced elevation of intracellular Ca2+ and IP3 production in Jurkat T cells.
(A) Reduced TCR-induced elevation of intracellular Ca2+release by RosA. Jurkat cells were loaded with the indicator dye fura-2/am for 45 minutes, as described in “Materials and methods.” Fura-2–loaded Jurkat cells (5 × 106 cells/3 mL/sample) were preincubated in the absence or presence of various concentrations of RosA for 15 minutes and stimulated with anti-CD3 mAb (UCHT1) followed by cross-linking with antimouse IgG antibody. The arrows indicate time points for the addition of anti-CD3 mAb and antimouse IgG antibody. The Rmax/Rmin ratios of Ca2+ are representative of 4 independent experiments. (B) Inhibition of TCR-induced IP3 production by RosA. Jurkat cells were preincubated with or without the indicated concentrations of RosA. RosA-treated Jurkat T cells were either unstimulated or stimulated as indicated earlier for 3 minutes. The amount of IP3 was quantified using a competitive [3H] IP3 binding assay kit. The graph shows the average and the standard error of at least 3 independent experiments.
RosA suppresses TCR-induced elevation of intracellular Ca2+ and IP3 production in Jurkat T cells.
(A) Reduced TCR-induced elevation of intracellular Ca2+release by RosA. Jurkat cells were loaded with the indicator dye fura-2/am for 45 minutes, as described in “Materials and methods.” Fura-2–loaded Jurkat cells (5 × 106 cells/3 mL/sample) were preincubated in the absence or presence of various concentrations of RosA for 15 minutes and stimulated with anti-CD3 mAb (UCHT1) followed by cross-linking with antimouse IgG antibody. The arrows indicate time points for the addition of anti-CD3 mAb and antimouse IgG antibody. The Rmax/Rmin ratios of Ca2+ are representative of 4 independent experiments. (B) Inhibition of TCR-induced IP3 production by RosA. Jurkat cells were preincubated with or without the indicated concentrations of RosA. RosA-treated Jurkat T cells were either unstimulated or stimulated as indicated earlier for 3 minutes. The amount of IP3 was quantified using a competitive [3H] IP3 binding assay kit. The graph shows the average and the standard error of at least 3 independent experiments.
RosA inhibits TCR-induced tyrosine phosphorylation of PLC-γ1.
Jurkat cells (5 × 107) were preincubated with 30 μM (A) or 3 to 15 μM RosA (B) for 4 hours and then either unstimulated or stimulated with OKT3 ascites (1:200) and OKT3–cross-linking antimouse IgG antibody (40 μg/mL) at 37°C for 2 minutes. PLC-γ1 was immunoprecipitated with anti–PLC-γ1 mAb (5 μg/mL), and tyrosine phosphorylation (PTyr) status was determined by blotting with antiphosphotyrosine mAb (0.5 μg/mL). After stripping, the nitrocellulose membrane was reblotted with rabbit polyclonal anti–PLC-γ1 antibody (1 μg/mL) to confirm equal precipitation of PLC-γ1. Results represent 1 of 4 independent experiments.
RosA inhibits TCR-induced tyrosine phosphorylation of PLC-γ1.
Jurkat cells (5 × 107) were preincubated with 30 μM (A) or 3 to 15 μM RosA (B) for 4 hours and then either unstimulated or stimulated with OKT3 ascites (1:200) and OKT3–cross-linking antimouse IgG antibody (40 μg/mL) at 37°C for 2 minutes. PLC-γ1 was immunoprecipitated with anti–PLC-γ1 mAb (5 μg/mL), and tyrosine phosphorylation (PTyr) status was determined by blotting with antiphosphotyrosine mAb (0.5 μg/mL). After stripping, the nitrocellulose membrane was reblotted with rabbit polyclonal anti–PLC-γ1 antibody (1 μg/mL) to confirm equal precipitation of PLC-γ1. Results represent 1 of 4 independent experiments.
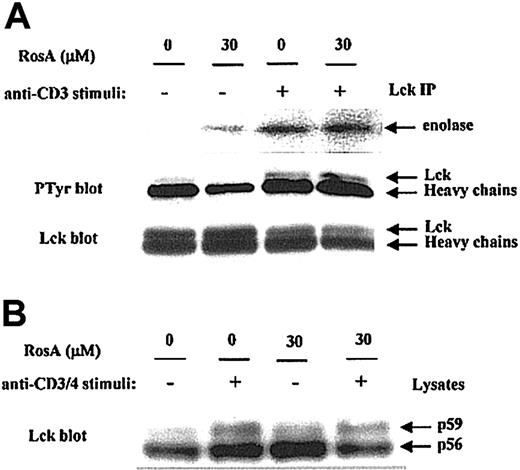
RosA does not modulate Lck kinase activity
There are 3 well-known PTKs that are important for the full activation of PLC-γ1, namely, Lck, ZAP-70, and Itk. Their cooperative actions are essentially required to assemble signaling molecules and to finally phosphorylate PLC-γ1.48 A significant amount of debate has taken place over the identity of the kinase(s) that directly phosphorylates PLC-γ1, although it is generally accepted that Itk is responsible. Itk needs to be phosphorylated for activation and, at the same time, requires adaptors to approach PLC-γ1. Lck activates Itk by phosphorylation and indirectly provides adaptors by phosphorylating ZAP-70, which in turn phosphorylates LAT and SLP-76.18,20,48 49 Finally, Lck-activated Itk may phosphorylate PLC-γ1 in association with SLP-76 and LAT.
First, we determined the effect of RosA on Lck kinase activity by immunoprecipitation kinase assay. Jurkat T cells preincubated in the presence or absence of RosA were either untreated or activated by OKT3. The catalytic activity of Lck in immunoprecipitates was measured by determining its ability to phosphorylate an exogenous substrate, enolase. The results obtained indicate that RosA treatment did not suppress Lck-mediated phosphorylation of enolase (Figure 4A, top panel), thus indicating that RosA does not inhibit Lck kinase activity. Autophosphorylation of Tyr394 in the activation loop of Lck is necessary for its kinase activity, probably by inducing steric changes that allow the catalytic region to fold into an active structure.50 Consistent with this data (Figure 4A, top panel), the present study showed that RosA did not inhibit the autophosphorylation of Lck (Figure 4A, middle panel).
Lck kinase activity is not inhibited by RosA.
(A) The effect of RosA on TCR-induced activation of Lck kinase activity. Lck was immunoprecipitated by anti-Lck mAb (5 μg/mL) from Jurkat cell lysates and analyzed for its ability to phosphorylate enolase (top panel). Lck kinase assay was performed as described in “Materials and methods.” Autophosphorylation status (middle panel) and equivalent immunoprecipitation of Lck (bottom panel) was confirmed by Western blotting using antiphosphotyrosine antibody and anti-Lck mAb (1 μg/mL), respectively. (B) The effect of RosA on OKT3/4-induced Lck activation. After preincubation with or without RosA, Jurkat cells were harvested and activated with OKT3/4 for 2 minutes. Jurkat cell lysates (2 × 106 cells per lane) were subjected to 12% SDS-PAGE, transferred to the nitrocellulose membrane, and blotted with anti-Lck mAb (1 μg/mL). Lck isoforms p56 and p59 are indicated by arrows. Results are representative of 2 separate experiments.
Lck kinase activity is not inhibited by RosA.
(A) The effect of RosA on TCR-induced activation of Lck kinase activity. Lck was immunoprecipitated by anti-Lck mAb (5 μg/mL) from Jurkat cell lysates and analyzed for its ability to phosphorylate enolase (top panel). Lck kinase assay was performed as described in “Materials and methods.” Autophosphorylation status (middle panel) and equivalent immunoprecipitation of Lck (bottom panel) was confirmed by Western blotting using antiphosphotyrosine antibody and anti-Lck mAb (1 μg/mL), respectively. (B) The effect of RosA on OKT3/4-induced Lck activation. After preincubation with or without RosA, Jurkat cells were harvested and activated with OKT3/4 for 2 minutes. Jurkat cell lysates (2 × 106 cells per lane) were subjected to 12% SDS-PAGE, transferred to the nitrocellulose membrane, and blotted with anti-Lck mAb (1 μg/mL). Lck isoforms p56 and p59 are indicated by arrows. Results are representative of 2 separate experiments.
Anti-CD3 activation alone minimally activates Lck, and maximal Lck activation is best induced by CD3/4 co–cross-linking. To determine the RosA effect on fully activated Lck, Jurkat cells were stimulated with OKT3/4 co–cross-linking in the presence or absence of RosA (Figure4B). In agreement with other reports,26 51 OKT3/4 co–cross-linking induced Lck shift from p56 to p59, indicating serine and threonine phosphorylation on SDS-PAGE. RosA did not inhibit this Lck shift, an indicator of Lck activation.
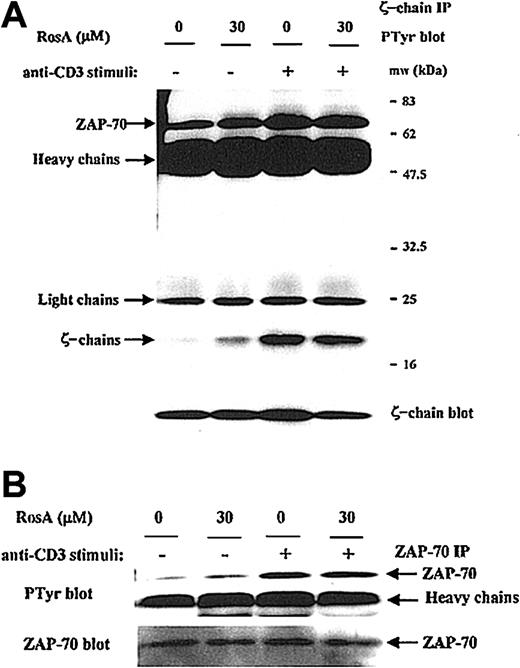
The inability of RosA to inhibit Lck kinase activity was further confirmed by determining the phosphorylation status of several in vivo Lck substrates. In response to TCR stimulus, Lck phosphorylates TCR ζ-chain and ZAP-70.15-17 The ζ-chain immunoprecipitates were blotted with antiphosphotyrosine antibody, and Figure 5A shows that RosA does not inhibit TCR-induced tyrosine phosphorylation of the TCR ζ-chain as well as ζ-chain–associated ZAP-70 (Figure 5A, top panel). The tyrosine phosphorylation status of ZAP-70 was further confirmed by ZAP-70 immunoprecipitation and Western blot analysis. RosA did not inhibit tyrosine phosphorylation of ZAP-70 in response to TCR stimulus of from 30 seconds to 10 minutes (data not shown, Figure 5B). These results suggest that RosA does not inhibit Lck kinase activity.
RosA does not interfere with the TCR-induced tyrosine phosphorylation of the TCR ζ-chain and ζ-chain–associated ZAP-70.
(A) The effect of RosA on phosphorylation status of ζ-chain and ζ-chain–associated ZAP-70. Jurkat cells were preincubated with or without RosA (30 μM) for 4 hours and stimulated for 3 minutes. Jurkat cells (5 × 107) were then lysed, immunoprecipitated with anti-TCR ζ-chain mAb (5 μg/mL), and separated by 12% SDS-PAGE. The tyrosine phosphorylation status of the ζ-chain and ζ-chain–associated ZAP-70 was verified by Western blot using antiphosphotyrosine mAb (top panel). The same membrane was reprobed with anti-TCR ζ-chain mAb (1 μg/mL) (bottom panel) to confirm the equal immunoprecipitation of the ζ-chain. (B) The effect of RosA on the tyrosine phosphorylation of ZAP-70. ZAP-70 was immunoprecipitated using anti–ZAP-70 mAb (5 μg/mL) and probed with antiphosphotyrosine mAb (top panel). The same blot was stripped and reprobed with anti–ZAP-70 mAb (bottom panel) (1 μg/mL). Results represent one of 3 independent experiments.
RosA does not interfere with the TCR-induced tyrosine phosphorylation of the TCR ζ-chain and ζ-chain–associated ZAP-70.
(A) The effect of RosA on phosphorylation status of ζ-chain and ζ-chain–associated ZAP-70. Jurkat cells were preincubated with or without RosA (30 μM) for 4 hours and stimulated for 3 minutes. Jurkat cells (5 × 107) were then lysed, immunoprecipitated with anti-TCR ζ-chain mAb (5 μg/mL), and separated by 12% SDS-PAGE. The tyrosine phosphorylation status of the ζ-chain and ζ-chain–associated ZAP-70 was verified by Western blot using antiphosphotyrosine mAb (top panel). The same membrane was reprobed with anti-TCR ζ-chain mAb (1 μg/mL) (bottom panel) to confirm the equal immunoprecipitation of the ζ-chain. (B) The effect of RosA on the tyrosine phosphorylation of ZAP-70. ZAP-70 was immunoprecipitated using anti–ZAP-70 mAb (5 μg/mL) and probed with antiphosphotyrosine mAb (top panel). The same blot was stripped and reprobed with anti–ZAP-70 mAb (bottom panel) (1 μg/mL). Results represent one of 3 independent experiments.
RosA does not inhibit the ZAP-70–mediated tyrosine phosphorylation of SLP-76 and LAT, subsequent association of LAT with SLP-76 or Grb2, and Erk1/2 activation
According to Figure 5B, the Lck-mediated tyrosine phosphorylation of ZAP-70 was intact in the presence of RosA; therefore, it is likely that ZAP-70 should be fully active. To confirm this, we investigated the phosphorylation status and association of 2 ZAP-70 substrates, eg, LAT and SLP-76.18 20 SLP-76 was precipitated from Jurkat T cells in the absence or presence of a TCR stimulus. SLP-76 was phosphorylated within 2 minutes and decreased gradually during 30 minutes (Figure 6A). Maximal phosphorylation of LAT was seen between 30 seconds and 2 minutes of stimulation, and LAT was quickly dephosphorylated. There was no noticeable effect of RosA on the kinetics of SLP-76 phosphorylation and its association with LAT (Figure 6A). Kinetics of SLP76-associated LAT phosphorylation was also not affected by RosA. The tyrosine phosphorylation of LAT was further confirmed by LAT immunoprecipitation and Western blot analysis (Figure 6B). Tyrosine phosphorylation of LAT was readily detectable within 2 minutes of stimulation in RosA-treated Jurkat T cells. As was the case for ZAP-70, the phosphorylation of SLP-76 and LAT and their association were not inhibited by RosA treatment, suggesting that abolished tyrosine phosphorylation of PLC-γ1 in RosA-treated Jurkat T cells does not arise from a blockade of ZAP-70 activation and the subsequent failure of LAT, SLP-76, and PLC-γ1 to assemble.
TCR-induced tyrosine phosphorylation of SLP-76 and LAT, and the association of LAT with SLP76 and Grb2 are not inhibited by RosA treatment.
Jurkat cells were preincubated with 30 μM RosA for 4 hours and stimulated for the indicated periods of time (A) or 2 minutes (B). Cells were then lysed, precleared with rabbit serum agarose, and immunoprecipitated with rabbit polyclonal anti–SLP-76 (5 μg/mL) (A) or rabbit polyclonal anti-LAT antibodies (5 μg/mL) (B), respectively. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine mAb (0.5 μg/mL) (top panels) or specific antibodies (0.5 μg/mL) (middle and bottom panels) as indicated. Representative data from more than 3 independent experiments are shown.
TCR-induced tyrosine phosphorylation of SLP-76 and LAT, and the association of LAT with SLP76 and Grb2 are not inhibited by RosA treatment.
Jurkat cells were preincubated with 30 μM RosA for 4 hours and stimulated for the indicated periods of time (A) or 2 minutes (B). Cells were then lysed, precleared with rabbit serum agarose, and immunoprecipitated with rabbit polyclonal anti–SLP-76 (5 μg/mL) (A) or rabbit polyclonal anti-LAT antibodies (5 μg/mL) (B), respectively. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine mAb (0.5 μg/mL) (top panels) or specific antibodies (0.5 μg/mL) (middle and bottom panels) as indicated. Representative data from more than 3 independent experiments are shown.
In Jurkat T cells, TCR-stimulated, ZAP-70–mediated tyrosine phosphorylation of the adapter proteins SLP-76 and LAT has been correlated with the efficient activation of Ras and Erk.19,52,53 Tyrosine phosphorylation of LAT results in the recruitment and SH2-dependent binding of the adapter molecule Grb2, and thus the binding of SOS, an upstream activator of Ras.25 28 Ras activates the Raf-MEK-Erk protein kinase cascade, which controls the level and the activity of the Jun/Fos dimeric transcription factor known as AP-1. The blot of LAT immunoprecipitates used in Figure 6B was reprobed with anti-Grb2 mAb to confirm RosA's effect on the association of LAT with Grb2 in each fraction (Figure 6B, middle panel). Amounts of Grb2 coprecipitated with tyrosine phosphorylated LAT following OKT3 stimulation were not changed significantly by RosA treatment. Furthermore, immunoblotting for the dually phosphorylated, activated forms of Erk1/2, a downstream effector of the Ras pathway, revealed that Erk1/2 activation occurs normally in the presence of RosA (Figure 7). Because Erk activation ultimately controls the activity of AP-1, this result agrees well with the data, which showed that RosA does not inhibit TCR-induced AP-1 activation (Figure 1).
RosA does not suppress the TCR-induced tyrosine/threonine phosphorylation of Erk.
Jurkat cells were preincubated with the indicated concentrations of RosA for 4 hours and kept unstimulated or stimulated for 2 minutes as indicated in “Materials and methods.” Cells approximately of 2 × 106 were lysed in lysis buffer and resolved by 12% SDS-PAGE. The blot was probed with antiphospho-Erk1/2 mAb or rabbit polyclonal anti-ERK1/2 antibody. Erk1/2 of p44 and p42 are indicated by arrows. Results are representative of 2 separate experiments.
RosA does not suppress the TCR-induced tyrosine/threonine phosphorylation of Erk.
Jurkat cells were preincubated with the indicated concentrations of RosA for 4 hours and kept unstimulated or stimulated for 2 minutes as indicated in “Materials and methods.” Cells approximately of 2 × 106 were lysed in lysis buffer and resolved by 12% SDS-PAGE. The blot was probed with antiphospho-Erk1/2 mAb or rabbit polyclonal anti-ERK1/2 antibody. Erk1/2 of p44 and p42 are indicated by arrows. Results are representative of 2 separate experiments.
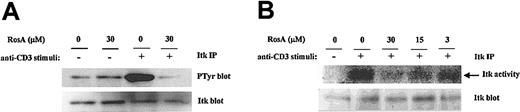
RosA suppresses the TCR-induced tyrosine phosphorylation of Itk and subsequent Itk activation
Itk is involved in the generation of critical second messengers (Ca2+, PKC) by performing tyrosine phosphorylation of PLC-γ1. Given the ability of RosA to inhibit the TCR-induced tyrosine phosphorylation of PLC-γ1 and Ca2+ mobilization in Jurkat T cells, we also assessed the ability of RosA to inhibit the TCR-stimulated tyrosine phosphorylation and subsequent activation of Itk. The tyrosine phosphorylation status of Itk from RosA-treated or -untreated Jurkat cells was monitored by immunoprecipitation (IP)–Western. Previously, it was shown that Itk was rapidly tyrosine phosphorylated within 2 minutes and returns to basal level by 15 minutes in response to TCR stimulus.54 In our experiments, Itk was maximally phosphorylated at 3 minutes; therefore, we chose a 3-minute stimulus for determining Itk activation status. As shown in Figure 8 (top panel), stimulation of Jurkat cells with OKT3 greatly induced the tyrosine phosphorylation of Itk, whereas preincubation of Jurkat cells with 30 μM RosA for 4 hours prior to stimulation significantly inhibited the TCR-stimulated tyrosine phosphorylation of Itk. The nitrocellulose membrane was subsequently stripped and reprobed with anti-Itk antibody to confirm that approximately equal amounts of Itk had been immunoprecipitated from each sample (Figure 8A, bottom panel).
RosA inhibits the TCR-induced tyrosine phosphorylation and subsequent activation of Itk.
(A) RosA inhibits TCR-induced tyrosine phosphorylation of Itk. Jurkat cells were preincubated with the indicated concentrations of RosA for 4 hours and stimulated for 3 minutes as described in “Materials and methods.” Supernatants of TCR-stimulated Jurkat cell lysates (5 × 107) were precleared with mouse serum agarose and immunoprecipitated with anti-Itk mAb (5 μg/mL). Immunoprecipitates were resolved by 12% SDS-PAGE, and the blot was subsequently probed with antiphosphotyrosine mAb (0.5 μg/mL) or rabbit polyclonal anti-Itk antibody (1 μg/mL). Representative data from more than 2 similar experiments are shown. (B) RosA inhibits TCR-induced activation of Itk kinase activity. Jurkat cells were left unstimulated or stimulated with OKT3 cross-linking and then lysed. Total cell lysates were precipitated with anti-Itk mAb. Itk immunoprecipitates were divided into 2 parts, one for in vitro kinase assay (top panel) and the other for Western blotting (bottom panel). Itk kinase assay was performed as described in “Materials and methods.” Equivalent immunoprecipitation of Itk (bottom panel) was confirmed by Western blotting. Data are representative of 2 independent experiments.
RosA inhibits the TCR-induced tyrosine phosphorylation and subsequent activation of Itk.
(A) RosA inhibits TCR-induced tyrosine phosphorylation of Itk. Jurkat cells were preincubated with the indicated concentrations of RosA for 4 hours and stimulated for 3 minutes as described in “Materials and methods.” Supernatants of TCR-stimulated Jurkat cell lysates (5 × 107) were precleared with mouse serum agarose and immunoprecipitated with anti-Itk mAb (5 μg/mL). Immunoprecipitates were resolved by 12% SDS-PAGE, and the blot was subsequently probed with antiphosphotyrosine mAb (0.5 μg/mL) or rabbit polyclonal anti-Itk antibody (1 μg/mL). Representative data from more than 2 similar experiments are shown. (B) RosA inhibits TCR-induced activation of Itk kinase activity. Jurkat cells were left unstimulated or stimulated with OKT3 cross-linking and then lysed. Total cell lysates were precipitated with anti-Itk mAb. Itk immunoprecipitates were divided into 2 parts, one for in vitro kinase assay (top panel) and the other for Western blotting (bottom panel). Itk kinase assay was performed as described in “Materials and methods.” Equivalent immunoprecipitation of Itk (bottom panel) was confirmed by Western blotting. Data are representative of 2 independent experiments.
To evaluate the concomitant change in the specific activity of Itk, we also determined Itk kinase activity in vitro (Figure 8B). Because there is no known substrate available for evaluating Itk kinase activity,49 we determined autophosphorylation activity of Itk. As can be seen in Figure 8B, the Itk immunoprecipitates from the stimulated Jurkat cells showed strong autophosphorylating activity. However, the Itk immunoprecipitates from RosA-treated Jurkat cells showed remarkably reduced kinase activity. A half of the Itk immunoprecipitates was used for Western blotting to quantify the amount of Itk, and according to Western blot fairly equal amounts of Itk were immunoprecipitated (Figure 8B, bottom panel). Taken together, RosA inhibits TCR-induced tyrosine phosphorylation of Itk and subsequent Itk activation. We conclude that RosA inhibits PLC-γ1 activity by down-regulating Itk activity. However, it is not clear how RosA inhibits the TCR-induced tyrosine phosphorylation of Itk.
RosA equally inhibits TCR-induced intracellular Ca2+mobilization and the tyrosine phosphorylation of Itk but not ZAP-70 in human peripheral blood lymphocytes
We demonstrated that RosA inhibits Ca2+-dependent TCR signaling by acting upstream of Itk and PLC-γ1 in Jurkat cells. To determine whether this inhibitory mechanism of RosA is a universal feature in T cells, we evaluated several key signaling events, including Ca2+ mobilization, tyrosine phosphorylation of Itk and ZAP-70 in normal hPBLs. Because anti-CD3 antibody was used for stimulation, we assumed that our observation in hPBLs is mostly ascribed to T cells. Consistent with the effect in Jurkat T cells (Figures 2A,8A), RosA inhibited TCR-induced Ca2+ flux and tyrosine phosphorylation of Itk in a dose-dependent manner in hPBLs (Figure 9A-B). However, tyrosine phosphorylation of ZAP-70 was not affected by up to 30 μM RosA (Figure 9C). Therefore, we conclude that RosA inhibits TCR-signaling of hPBLs and Jurkat cells in a similar mechanism.
RosA inhibits TCR-induced intracellular calcium mobilization and the tyrosine phosphorylation of Itk but not ZAP-70 in human peripheral blood lymphocytes.
Human PBLs from healthy volunteers were isolated as described in “Materials and methods.” (A) hPBLs were loaded with calcium-sensitive dye fura-2/am and then treated with varying concentrations of RosA for 15 minutes. Cells were stimulated, as indicated by arrows, with OKT3 ascites (1:1000) and cross-linking antibodies, and results are representative of 2 separate experiments. (B-C) hPBLs were pretreated with indicated concentrations of RosA for 4 hours and then either left unstimulated or stimulated for 3 minutes with OKT3. hPBLs were either subjected to immunoprecipitation with the rabbit polyclonal anti-Itk (B) or anti–ZAP-70 mAb (C), separated on SDS-PAGE, and immunoblotted with antiphosphotyrosine mAb (top panels). The membranes were stripped and reblotted with anti-Itk or ZAP-70 mAb (bottom panels).
RosA inhibits TCR-induced intracellular calcium mobilization and the tyrosine phosphorylation of Itk but not ZAP-70 in human peripheral blood lymphocytes.
Human PBLs from healthy volunteers were isolated as described in “Materials and methods.” (A) hPBLs were loaded with calcium-sensitive dye fura-2/am and then treated with varying concentrations of RosA for 15 minutes. Cells were stimulated, as indicated by arrows, with OKT3 ascites (1:1000) and cross-linking antibodies, and results are representative of 2 separate experiments. (B-C) hPBLs were pretreated with indicated concentrations of RosA for 4 hours and then either left unstimulated or stimulated for 3 minutes with OKT3. hPBLs were either subjected to immunoprecipitation with the rabbit polyclonal anti-Itk (B) or anti–ZAP-70 mAb (C), separated on SDS-PAGE, and immunoblotted with antiphosphotyrosine mAb (top panels). The membranes were stripped and reblotted with anti-Itk or ZAP-70 mAb (bottom panels).
Discussion
In this study, we further clarified the inhibition mechanism of RosA on TCR signaling. Because RosA specifically inhibited NF-AT reporter activation but not AP-1 (Figure 1), it is likely that RosA suppresses IL-2 promoter activation by blocking Ca2+-dependent TCR-signaling events. This notion was confirmed by the observation whereby signaling events, such as the IP3 increase and Ca2+ mobilization, required for NF-AT activation were efficiently inhibited by RosA treatment (Figure 2). Furthermore, the failure of RosA to inhibit the TCR-induced phosphorylation of Erk1/2 explains (Figure 7) why RosA does not inhibit AP-1 activation. Our previous study suggested that RosA works in the membrane proximal point of TCR signaling, based on the fact that the RosA-mediated inhibition of IL-2 promoter activation is restricted only to TCR stimulation and not to PMA/ionomycin-mediated stimulation. This led us to determine the phosphorylation status of PLC-γ1 activity, and, interestingly, we found that the TCR-induced tyrosine phosphorylation of PLC-γ1 was completely abrogated by RosA (15-30 μM) treatment for 4 hours (Figure 3).
PLC-γ1 activity is known to be controlled by both PTK and non-PTK factors.48 Although the mechanism of the TCR-induced tyrosine phosphorylation of PLC-γ1 is still unclear, biochemical and genetic data indicate that tyrosine phosphorylation of PLC-γ1 occurs by the activation of Lck, ZAP-70, and Itk.48 ZAP-70 essentially provides the opportunity for PLC-γ1 to meet SLP-76–associated Itk by phosphorylating tyrosine residues in LAT and SLP-76. Tyrosine phosphorylation of SLP-76 and LAT induces the binding of Itk to SLP-76 and of Gad/SLP-76/Itk complex to PLC-γ1–associated LAT. The pivotal roles of LAT and SLP-76 for PLC-γ1 activation and in the Ras pathway have been demonstrated.19,52,53Importantly, recruitment of SLP-76 to phosphorylated LAT seems essential for signaling downstream of PLC-γ1. After recruitment, Itk activated by Lck directly phosphorylates PLC-γ1. Because Lck is responsible for phosphorylating ZAP-70 and Itk, Lck seems to govern all initial TCR-signaling steps leading to PLC-γ1 activation. In our study, RosA was not found to inhibit either the Lck-mediated tyrosine phosphorylation of ZAP-70 or the ZAP-70–mediated phosphorylation and association of LAT and SLP-76 (Figures 5-6). RosA, however, inhibited TCR-induced tyrosine phosphorylation and subsequent activation of Itk (Figure 8); therefore, it is likely that RosA inhibits PLC-γ1 activity by down-regulating Itk activity. Previous reports indicated that T cells from Itk-deficient mice show markedly reduced tyrosine phosphorylation of PLCγ-1 and Ca2+ mobilization in response to TCR stimulus.55,56 However, tyrosine phosphorylation status of ζ-chain and ZAP-70 was not affected in Itk−/− mice. Along with other reports,27this suggests that Itk activity is critical for PLCγ-1 activation, and inhibition of PLCγ-1 activation is possible in a ZAP-70–independent manner by specifically inhibiting Itk activity. RosA inhibited TCR-induced activation of Itk and PLCγ-1 in a ZAP-70–independent manner, and this supports a previously proposed hypothesis that coordinate activation of multiple PTK is required for full activation of PLCγ-1.
Exactly how RosA down-regulates Itk activity has not been clarified. In the present study, we only know that RosA inhibits Itk activity in a ZAP-70–independent manner. Heyeck et al49 reported that Lck directly phosphorylates and activates Itk. However, RosA did not inhibit Lck kinase activity, given that (1) Lck kinase activity was intact in in vitro kinase assay (Figure 4A) and (2) Lck substrates, eg the ζ-chain and ZAP-70, were phosphorylated normally (Figure 5). Whether this inhibition is made by interruption of the Lck recruitment to Itk/SLP76/LAT/PLCγ-1 complex or by suppression of another unidentified kinase activity responsible for Itk phosphorylation is not known.
It is known that the Ca2+-dependent pathway and the Ras/Erk pathway cooperate in T cells to activate the IL-2 promoter through the activation of the transcription factors NF-AT and AP-1.35Although inhibiting NF-AT activation, RosA displayed no inhibition of AP-1–mediated transcription on TCR stimulation (Figure 1). In conjunction with the failure of RosA to inhibit the TCR-induced phosphorylation of Erk1/2, this result indicates that RosA inhibits the Ca2+ signaling pathway but not the Ras/Erk pathway. PLC-γ1 generally controls both NF-AT and AP-1 activation through the IP3 increase/Ca2+ flux and the DAG increase/PKC/Ras/Raf/MEK/Erk pathway, respectively.36,40,57-59 Because RosA seems to block PLC-γ1 activity, it is paradoxical that RosA inhibits only one side of the PLC-γ1–dependent pathways, eg, IP3 release and Ca2+ flux. TCR-induced Ras/Raf/Erk activation is well delineated and various suggestions have been made. Besides Ras activation by DAG-activated PKC, one of the major mechanisms reported to be responsible for Erk pathway activation, other ways of activating Ras have been proposed. LAT has many potential Grb2 binding sites, and it was proposed that the Grb2-SOS complex might be able to recruit Ras to the membrane and thus activate the Ras/Raf/Erk pathway.25,28-30,44 Because RosA did not disturb the LAT-Grb2 association (Figure 6B), the observed Erk pathway might be activated through the LAT/Grb2/SOS/Ras/Raf route. In reconstitution experiments with various LAT mutants, Lin and Weiss60demonstrated that Grb2-binding sites, including Tyr110 and Tyr226, are required for LAT to fully reconstitute Erk activity, in addition to the tyrosine residues Tyr132, Tyr171, and Tyr191, which are required for PLC-γ1 binding, phosphorylation, and Ca2+flux.60 This indicates that the association of the Grb2-SOS complex with LAT is also important for Ras/Raf activity leading to Erk activation. However, single mutation at Tyr132 that is mostly responsible for PLC-γ1 binding and Ca2+ flux abrogates Erk phosphorylation significantly without affecting Grb2 binding to LAT. Therefore, hypothetical activation of Ras/Raf/Erk pathway by LAT-associated Grb2-SOS complex cannot fully explain why Erk activity is not inhibited by RosA treatment. Additional mechanisms of MAPK regulation in T cells have been suggested, including the direct recruitment of Shc-Grb2-SOS complexes to the phosphorylated TCR ζ-chain,61,62 direct interactions of Lck with MAPK,63 modulation by nitric oxide, and the regulation of phosphatases.64 Clearly, regulation of the Ras-Erk pathway in T cells is complex.
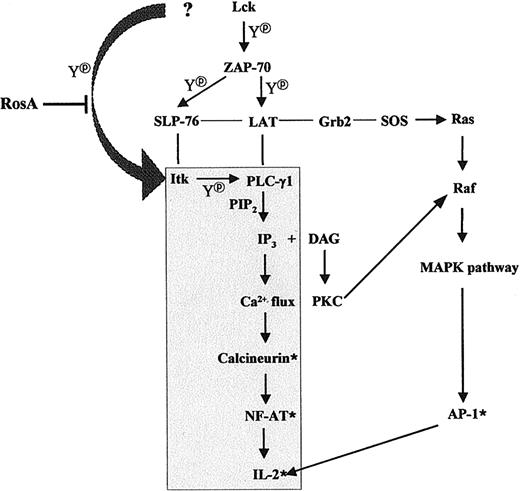
In summary, we propose that RosA inhibits PLC-γ1/IP3release/Ca2+ flux/NF-AT activation by interrupting upstream signaling events, eg, tyrosine phosphorylation of Itk. RosA did not inhibit Lck kinase activity mostly responsible for Itk phosphorylation, and it is not known whether RosA inhibits recruitment of Lck to Itk or whether RosA inhibits another unknown PTK responsible for Itk phosphorylation. RosA did not inhibit ZAP-70–dependent TCR signaling, such as the tyrosine phosphorylation of LAT and SLP-76, the association of Grb2 to LAT, and subsequent Ras/Raf/Erk activation (Figure10). This is the first case, which shows that Lck-controlled recruitment of Itk to LAT/SLP-76/PLCγ-1 complexes and Itk phosphorylation can be separately inhibited. This also supports previous reports in terms of the required coordinated action of 3 PTKs, namely, Lck, ZAP-70, and Itk, for full PLCγ-1 activation.
Schematic diagram of the TCR-signaling pathway suppressed by RosA treatment.
The shaded area indicates the route of the TCR-signaling pathway that is affected by RosA treatment. Thin lines indicate protein-protein interactions and arrows indicate the directions of signaling. *Denotes the activated status and Y denotes tyrosine phosphorylation. The figure schematically depicts 2 pathways, which are RosA susceptible, namely, the Ca2+-dependent pathway and the RosA-resistant, Ca2+-independent pathway.
denotes tyrosine phosphorylation. The figure schematically depicts 2 pathways, which are RosA susceptible, namely, the Ca2+-dependent pathway and the RosA-resistant, Ca2+-independent pathway.
Schematic diagram of the TCR-signaling pathway suppressed by RosA treatment.
The shaded area indicates the route of the TCR-signaling pathway that is affected by RosA treatment. Thin lines indicate protein-protein interactions and arrows indicate the directions of signaling. *Denotes the activated status and Y denotes tyrosine phosphorylation. The figure schematically depicts 2 pathways, which are RosA susceptible, namely, the Ca2+-dependent pathway and the RosA-resistant, Ca2+-independent pathway.
denotes tyrosine phosphorylation. The figure schematically depicts 2 pathways, which are RosA susceptible, namely, the Ca2+-dependent pathway and the RosA-resistant, Ca2+-independent pathway.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-07-1992.
Supported by the Korea Green Cross Company and grant M1-9808-00-0036 from the Korean Ministry of Science and Technology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jonghwa Won, Signal Transduction Laboratory, Mogam Biotechnology Research Institute, Yonginsi, Gyunggido, 449-913, Korea; e-mail: agnes@greencross.com.

![Fig. 2. RosA suppresses TCR-induced elevation of intracellular Ca2+ and IP3 production in Jurkat T cells. / (A) Reduced TCR-induced elevation of intracellular Ca2+release by RosA. Jurkat cells were loaded with the indicator dye fura-2/am for 45 minutes, as described in “Materials and methods.” Fura-2–loaded Jurkat cells (5 × 106 cells/3 mL/sample) were preincubated in the absence or presence of various concentrations of RosA for 15 minutes and stimulated with anti-CD3 mAb (UCHT1) followed by cross-linking with antimouse IgG antibody. The arrows indicate time points for the addition of anti-CD3 mAb and antimouse IgG antibody. The Rmax/Rmin ratios of Ca2+ are representative of 4 independent experiments. (B) Inhibition of TCR-induced IP3 production by RosA. Jurkat cells were preincubated with or without the indicated concentrations of RosA. RosA-treated Jurkat T cells were either unstimulated or stimulated as indicated earlier for 3 minutes. The amount of IP3 was quantified using a competitive [3H] IP3 binding assay kit. The graph shows the average and the standard error of at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-07-1992/4/m_h80934231002.jpeg?Expires=1769150181&Signature=zJDgWedHlStuuPNHJ67S9N~yMfpp9DowlaMkIMlg5UA-RnmFIIgQS4J~EV51u8RkOIFeDgd8V~FKaWUHIXtAEyACAwAj5a~X2qfLnznkIpXLDx0TL0QaeIZgbB5NmmBJiqAwS4sQ~knCjJjUjx8KeLrPAgJueMiy0LdOZGwyUbFmeCY7kmW6XL-5JNFc4itf6593HMNGT95C3kmfAEDTUtRaupoImSkKCcxEx-HmlTltK9zzy4MdkMqN3WCRK8OfqgKdAw2YzJO6d2y6CWm2Ur08yugNA-LxHGW1Ht6TvNSrykABm9JlGoYIIq3~bQU5HgHI4mCqcstqM8iS2mwGdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal