CD28 is the quintessential costimulatory molecule expressed on CD4+ and CD8+ T cells. During chronic infections and the normal aging process, CD28 expression is lost, compromising the functional activity of T cells. CD28 loss is promoted by replicative stress, particularly in the presence of tumor necrosis factor–α, owing to an inoperative CD28 initiator element. It is currently unknown whether CD28 loss is irreversible. The present study examined cytokines for their ability to reinduce CD28 expression. CD4+CD28null T cells constitutively expressed interleukin-12 (IL-12) α and β receptors, which were functional and allowed for the up-regulation of the signal transducer and activator of transcription–4 (STAT-4)–dependent gene CD161. Costimulation of the T-cell and IL-12 receptors induced the transcription of CD28 in approximately 50% of CD4+CD28null T-cell clones and lines. IL-12 by itself did not restore CD28 expression. Up-regulation of CD28 after IL-12 exposure correlated with the reassembly of the CD28–initiator protein complex. The re-expressed CD28 was functional and restored the ability of CD4+CD28null T cells to express CD25 and CD40 ligand. Our data suggest that IL-12 may, in part, functionally rescue senescent CD4+ T cells.
Introduction
T-cell activation requires specific antigen recognition by the T-cell receptor (TCR) and a second signal from costimulatory molecules. The classic costimulatory molecule is CD28.1 CD28 provides signals that promote T-cell survival, interleukin-2 (IL-2) production, metabolic activity, and T-cell clonal expansion.2-5 This central role of CD28 suggests that modulation of the levels of CD28 expression profoundly alters T-cell function. Indeed, targeted deletion of the CD28 gene in mice results in an immunocompromised phenotype, typified by reduced helper T-cell activity and impaired immunoglobulin production.6
Although CD28 is constitutively expressed on the surface of human T cells, its expression can be down-regulated. A progressive increase in the percentage of T cells that lack CD28 expression is common with increasing age in healthy individuals7,8 and in patients with chronic infections.9CD4+CD28null T cells can comprise up to 50% of the total CD4+ T-cell compartment in some individuals older than 65 years.10-12 CD28null T cells have a memory (CD45RO+) phenotype, are long lived in vivo, and form large oligoclonal populations.8,13 In vitro, successive lymphocyte replication is accompanied by CD28 loss and telomeric shortening.11,12,14,15 CD28null T cells have reduced division potential and delayed cell cycle kinetics. CD4+CD28null T cells are resistant to apoptotic signals, while the data for CD8+CD28null T cells are conflicting, and increased and decreased susceptibility to apoptosis has been described for CD8+ T cells.12,16 Taken together, CD28null T cells have features that are hallmarks of an aged immune system. Indeed, an increased frequency of CD28null T cells has been found to be the best predictor of humoral incompetence in the elderly.17
Several additional phenotypic and functional characteristics distinguish CD4+CD28null T cells from classic CD4+CD28+ helper T cells. Expression of the IL-2 receptor (CD25) after activation is short lived compared with CD4+CD28+ T cells.18CD4+CD28null T cells also lack CD40 ligand (CD40L) expression, which renders the cells incapable of promoting B-cell differentiation and immunoglobulin secretion.19CD4+CD28null T cells have acquired receptors that are typically associated with natural killer (NK) cells. In contrast to CD4+CD28+ T cells, they express CD8-αα homodimers and major histocompatibility complex (MHC) class I–recognizing receptors of the killer-immunoglobulin receptor (KIR) and C-type lectin families (CD161, CD94, NKG2).20 Also in common with NK cells, CD4+CD28null T cells express perforin and granzyme B, which impart cytotoxic ability.21 Thus, CD4+CD28null T cells integrate functional properties of rapid, nonspecific immune responses with antigen-specific immunity.
CD4+ T cells may also completely lose CD28 surface expression during chronic inflammatory conditions, such as rheumatoid arthritis (RA),22 Wegener granulomatosis,23and multiple sclerosis.24 In these diseases, CD4+CD28null T cells are proinflammatory and have been implicated in the autoimmune pathogenesis.22,24,25 CD4+CD28nullT cells isolated from patients with RA are autoreactive13and produce large amounts of interferon-γ (IFN-γ),18which is typical of a T-helper 1 (Th1)–like immune response.
We have hypothesized that modulation of CD28 expression on senescent CD4+CD28null T cells influences immune responses. In the context of an aging immune system, re-expression of CD28 may be desirable to restore immunocompetence. In contrast, induction of CD28 on CD28null T cells in autoimmune diseases may augment the autoimmune response. Mechanisms that restore CD28 expression are, therefore, potential therapeutic targets.
Here, we describe the IL-12 influence on the phenotype and function of CD4+CD28null T cells. Our study shows that CD4+CD28null T cells express a functional IL-12 receptor and that IL-12 modulates expression of the IL-12–responsive gene, CD161, on these cells. More importantly, IL-12 induces the surface expression of functional CD28 molecules on CD4+CD28null T cells.
Materials and methods
T-cell cloning
This protocol was approved by the Mayo Foundation Institutional Review Board, and all study participants gave informed consent. Peripheral blood mononuclear cells (PBMCs) were sorted into CD4+CD28+ and CD4+CD28null subsets on a FACSVantage flow cytometer (Becton Dickinson, San Jose, CA). Sorted cells were stimulated with irradiated PBMCs and anti-CD3 (OrthoClone OKT3; Ortho Diagnostics, Raritan, NJ) monoclonal antibody (mAb) to establish short-term T-cell lines, or they were cloned by limiting dilution in the presence of irradiated Epstein-Barr virus (EBV)–transformed B-lymphoblastoid lines and 20 U/mL recombinant human IL-2 (rhIL-2) (Proleukin; Chiron, Emeryville, CA) to generate T-cell clones.13 Established T-cell clones were maintained with biweekly restimulation and supplementation of rhIL-2.
T-cell activation
T-cell lines and clones were activated with soluble anti-CD3 mAb (5 ng/mL) and irradiated promonocytic U937 cells (American Type Culture Collection, Manassas, VA) with or without 10 ng/mL rhIL-12 (R&D Systems, Minneapolis, MN). In other studies, T-cell lines and clones were incubated with plate-immobilized anti-CD3 (100 ng/mL OKT3) and anti-CD28 (300 ng/mL) (28-2; BD-Pharmingen, San Diego, CA) mAbs.
Flow cytometry
Flow cytometry was performed on freshly purified PBMCs and on CD4+ T-cell clones and lines with the following mAbs: mouse anti-CD4 (fluorescein isothiocyanate [FITC]), mouse anti-CD4 (peridinin chlorophyll A protein [PerCP]), and mouse anti-CD28 (phycoerythrin [PE]) (all Becton Dickinson); mouse anti-CD4 (allophycocyanin [APC]), mouse anti-CD25 (APC), mouse anti-CD154 (FITC), mouse anti–interferon-γ (IFN-γ), rat anti–IL-12Rβ1, and rat anti–IL-12Rβ2 (all BD-Pharmingen); and mouse antiperforin (Ancell, Bayport, MN). Mouse myeloma immunoglobulin G1 (IgG1) (ICN Pharmaceuticals, Costa Mesa, CA) and Simultest Control (Becton Dickinson) were used as isotype controls. Secondary antibodies were goat antirat immunoglobulin (APC), goat antimouse immunoglobulin (FITC) (both Becton Dickinson), and rat antimouse immunoglobulin (PerCP) (BD-Pharmingen). Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson), and the frequencies of T-cell subsets were calculated by means of WinMDI software (Joseph Trotter, Scripps Research Institute, LaJolla, CA).
Reverse-transcribed PCR
Total RNA was extracted from T-cell clones by means of TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA), and cDNA was synthesized by means of oligodeoxythymidine (oligo(dT)) and avian myeloblastosis virus (AMV) reverse transcriptase (Roche Molecular Biochemicals, Indianapolis, IN). CD28 transcripts were amplified by polymerase chain reaction (PCR) (5′-CGCCCATGCTTGTAGCGTACG-3′ and 5′-GATAGGCTGCGAAGTCGCGTG-3′), and products were electrophoresed on 2% agarose gels.
Electrophoretic mobility shift assays
Nuclear extracts were prepared10 from T-cell clones and lines 5 to 8 days after incubation in IL-12 alone, activation with immobilized anti-CD3 mAb alone, or activation with immobilized anti-CD3 mAb with IL-12. Resting T-cell lines were harvested 3 weeks after the last stimulation. Briefly, 5 × 106 to 1 × 107 cells were lysed in cold HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) hypotonic buffer, and the nuclei were isolated by centrifugation. Nuclear proteins were extracted in 50 μL high-salt buffer, and protein concentration was determined by means of a protein assay kit (BioRad, Richmond, CA). Nuclear extracts (20 μg) were combined with 30 μL binding buffer containing 10 μg polydoxyinosine-polydeoxycytidine (poly(dI-dC)) (ICN Pharmaceuticals) and 5 μg nonspecific oligonucleotide. To this mixture, 5 μL wash buffer was added. The total reaction volume was adjusted to 50 μL with binding buffer and incubated on ice for 30 minutes. Approximately 40 fmol radiolabeled probe corresponding to the α site of the CD28 initiator element10 26 was added and incubated for an additional 30 minutes at room temperature. Protein-DNA complexes were resolved on 6% nondenaturing polyacrylamide gels and were visualized by autoradiography.
As a system control, parallel DNA-binding assays were performed by means of oligonucleotide probes specific for specificity protein–1 (SP1),10 a ubiquitous transcription factor complex.
Results
Constitutive expression of the IL-12 receptor on CD4+CD28null T cells
We have previously shown that CD4+CD28nullT cells have phenotypic and functional properties in common with NK cells.20 In particular, CD4+CD28null T cells express CD8-αα homodimers; several types of human leukocyte antigen (HLA) class I–recognizing receptors; and CD161, a C-type lectin receptor. A critical cytokine in the regulation of NK cells is IL-12, which can induce IFN-γ secretion and enhance NK-cell cytotoxicity.
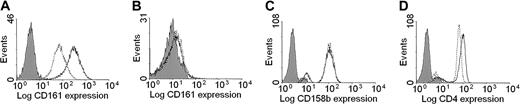
To examine whether CD4+CD28null T cells are responsive to IL-12, we analyzed PBMCs from donors who had an expansion of CD4+CD28null T cells (greater than 1% of the CD4+ population) for the expression of the IL-12 receptor. Antibodies specific for the IL-12Rβ1 and IL-12Rβ2 subunits were used to determine the cellular distribution of the receptor. As expected, IL-12Rβ1 was constitutively expressed on resting CD16+ NK cells. More interestingly, IL-12Rβ1 was also expressed on the majority of resting CD4+CD28null T cells; CD4+CD28+ T cells expressed only low levels of the receptor subunit (Figure 1A). This finding was consistent in all individuals examined (Figure 1B). The IL-12Rβ2 subunit was not detectable on resting CD4+ T cells of either the CD28+ or the CD28nullsubset (Figure 1C). However, IL-12β2 mRNA could be detected by reverse-transcribed polymerase chain reaction (RT-PCR) (data not shown) in CD4+CD28null PBMCs, and cell surface expression was up-regulated after in vitro stimulation. Cultured CD4+CD28null T-cell lines and clones expressed IL-12Rβ1 and IL-12β2, implying that these cells expressed the functional heterodimeric IL-12 receptor (Figure 1D-E).
IL-12R expression by CD4+CD28null T cells.
CD4+CD28null T cells constitutively express high levels of the IL-12 receptor. (A) The expression of the IL-12R on PBMCs was determined by 3-color flow cytometry. A representative histogram gated on CD4+CD28+ T cells (dotted line) and CD4+CD28null T cells (solid line) is shown. (B) The mean fluorescence intensity of IL-12Rβ1 staining was significantly higher on the CD4+CD28null T-cell subset. This result was consistent in all 10 individuals analyzed (P < .001). (C) IL-12Rβ2 cell surface expression was absent on resting CD4+ T cells, on both the CD4+CD28+ (dotted line) and the CD4+CD28null T-cell subsets (solid line). (D-E) Cultured CD4+CD28null T-cell clones and polyclonal CD4+CD28null T-cell lines were examined by 4-color flow cytometry. A representative histogram of 13 clones (panel D) and 11 lines (panel E) is shown for IL-12Rβ1 (dotted line) and IL-12Rβ2 (solid line) expression. All CD4+CD28null T-cell lines and clones expressed both receptor chains. In all histograms, the shaded area represents staining with an isotype control mAb.
IL-12R expression by CD4+CD28null T cells.
CD4+CD28null T cells constitutively express high levels of the IL-12 receptor. (A) The expression of the IL-12R on PBMCs was determined by 3-color flow cytometry. A representative histogram gated on CD4+CD28+ T cells (dotted line) and CD4+CD28null T cells (solid line) is shown. (B) The mean fluorescence intensity of IL-12Rβ1 staining was significantly higher on the CD4+CD28null T-cell subset. This result was consistent in all 10 individuals analyzed (P < .001). (C) IL-12Rβ2 cell surface expression was absent on resting CD4+ T cells, on both the CD4+CD28+ (dotted line) and the CD4+CD28null T-cell subsets (solid line). (D-E) Cultured CD4+CD28null T-cell clones and polyclonal CD4+CD28null T-cell lines were examined by 4-color flow cytometry. A representative histogram of 13 clones (panel D) and 11 lines (panel E) is shown for IL-12Rβ1 (dotted line) and IL-12Rβ2 (solid line) expression. All CD4+CD28null T-cell lines and clones expressed both receptor chains. In all histograms, the shaded area represents staining with an isotype control mAb.
CD4+CD28null T-cells clones are IL-12 responsive
To confirm that CD4+CD28null T cells express a functional IL-12 receptor, we examined the capacity of IL-12 to modulate the expression of CD161, an IL-12–responsive gene.20 CD161 was constitutively expressed on CD4+CD28null T-cell clones and was up-regulated after exposure to IL-12 (Figure 2A). In contrast, CD161 on CD4+CD28+ T-cell clones, which express low levels of the molecule, was not inducible by IL-12 exposure (Figure 2B). Exposure of CD4+CD28nullT-cell clones to IL-12 had no effect on the expression of members of the KIR family, such as the CD158b receptor (Figure 2C). The expression of CD4 also did not appreciably change after stimulation with IL-12 (Figure 2D).
Effect of CD161 on CD4+CD28nullT-cell clones in the presence of IL-12.
CD161 on CD4+CD28null T-cell clones can be induced by IL-12. CD4+CD28null and CD4+CD28+ T-cell clones were cultured in the presence (solid line) or absence (dotted line) of 10 ng/mL IL-12 and tested for the expression of the IL-12–responsive gene, CD161, by flow cytometry. CD161 was up-regulated on CD4+CD28null (panel A) but not CD4+CD28+ (panel B) T-cell clones. The expression of CD158b (panel C) and CD4 (panel D) on CD4+CD28null T-cell clones was not significantly changed by exposure to IL-12. Representative histograms of 3 experiments are shown. In all histograms, the shaded area represents staining with an isotype control mAb.
Effect of CD161 on CD4+CD28nullT-cell clones in the presence of IL-12.
CD161 on CD4+CD28null T-cell clones can be induced by IL-12. CD4+CD28null and CD4+CD28+ T-cell clones were cultured in the presence (solid line) or absence (dotted line) of 10 ng/mL IL-12 and tested for the expression of the IL-12–responsive gene, CD161, by flow cytometry. CD161 was up-regulated on CD4+CD28null (panel A) but not CD4+CD28+ (panel B) T-cell clones. The expression of CD158b (panel C) and CD4 (panel D) on CD4+CD28null T-cell clones was not significantly changed by exposure to IL-12. Representative histograms of 3 experiments are shown. In all histograms, the shaded area represents staining with an isotype control mAb.
IL-12 induces expression of CD28 on CD4+CD28null T-cells
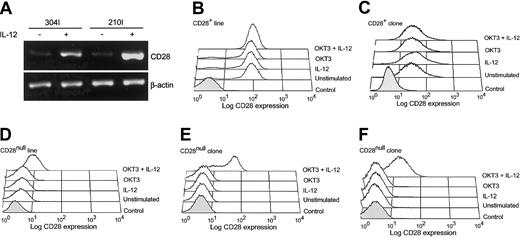
CD4+CD28null T cells lack expression of CD28 owing to a transcriptional block of the CD28 gene.10 26 To determine whether IL-12 could modulate expression of CD28, CD4+CD28null T-cell lines and clones were stimulated with IL-12. Exposure to IL-12 alone did not induce CD28 transcription although CD161 was up-regulated (Figure 2A and data not shown). Activation with anti-CD3 mAb in the presence of IL-12 restored CD28 transcription, and CD28 mRNA could be detected in IL-12–stimulated clones (Figure 3A). Cell surface re-expression of the CD28 molecule after IL-12 stimulation was confirmed by flow cytometry. Stimulation of resting CD4+CD28null T-cell clones with anti-CD3 alone did not up-regulate CD28 expression. In contrast, activation with anti-CD3 mAb in the presence of IL-12 markedly enhanced CD28 surface expression on 7 of 15 CD28null T-cell clones tested (Figure 3E-F). CD28 expression on CD28+ T-cell clones was unchanged regardless of the mode of stimulation (Figure 3C). There was no obvious difference between the T-cell clones that re-expressed CD28 and those that did not. The 2 types of clones had similar levels of expression of a functional IL-12 receptor and equivalently induced CD161 transcription after IL-12 stimulation. For some of the clones, IL-12 consistently restored CD28 expression in only a fraction of all cells, indicating clonal heterogeneity (Figure 3E).
Effect of activation in the presence of IL-12 on transcription and re-expression of CD28 in CD4+CD28null T cells.
CD4+CD28null T cells transcribe and re-express CD28 when activated in the presence of IL-12. (A) CD4+CD28null clones were activated with anti-CD3 mAb in the presence (+) or absence (−) of IL-12 (10 ng/mL), and the transcription of CD28 was analyzed by RT-PCR (clones 304I and 210I are shown). (B-F) Re-expression of CD28 was confirmed by flow cytometry. CD4+CD28null T-cell clones (panels E-F) and lines (panel D) were stimulated with anti-CD3 mAb, IL-12, or a combination of anti-CD3 mAb and IL-12. Cells were analyzed by 4-color flow cytometry after 5 to 6 days; representative histograms are shown. The combination of anti-CD3 mAb and IL-12 induced CD28 expression in 5 of 11 lines and 6 of 13 clones. In contrast, the levels of CD28 expression were not affected in CD4+CD28+ T-cell clones or lines (panels B-C). In all histograms, the shaded areas represent staining with an isotype control.
Effect of activation in the presence of IL-12 on transcription and re-expression of CD28 in CD4+CD28null T cells.
CD4+CD28null T cells transcribe and re-express CD28 when activated in the presence of IL-12. (A) CD4+CD28null clones were activated with anti-CD3 mAb in the presence (+) or absence (−) of IL-12 (10 ng/mL), and the transcription of CD28 was analyzed by RT-PCR (clones 304I and 210I are shown). (B-F) Re-expression of CD28 was confirmed by flow cytometry. CD4+CD28null T-cell clones (panels E-F) and lines (panel D) were stimulated with anti-CD3 mAb, IL-12, or a combination of anti-CD3 mAb and IL-12. Cells were analyzed by 4-color flow cytometry after 5 to 6 days; representative histograms are shown. The combination of anti-CD3 mAb and IL-12 induced CD28 expression in 5 of 11 lines and 6 of 13 clones. In contrast, the levels of CD28 expression were not affected in CD4+CD28+ T-cell clones or lines (panels B-C). In all histograms, the shaded areas represent staining with an isotype control.
To determine whether the results on the T-cell clones were representative of the in vivo situation, polyclonal CD4+CD28null T-cell lines were examined. The results were very similar to what was observed on the T-cell clones. CD28 was reinducible by IL-12 in 5 of 11 CD4+CD28null T-cell lines tested (Figure 3D). These results were reproducible and did not depend on the culture conditions or the duration of the T cells in culture, but they were characteristic for the individuals from whom the line or clone was established. CD28 levels were unaffected on CD4+CD28+ T-cell lines following IL-12 exposure (Figure 3B).
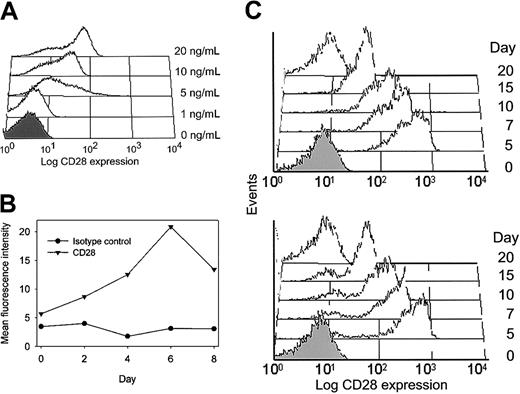
The effect of IL-12 on CD28 expression was seen to be variable at low doses of 1 ng/mL, and optimal induction was achieved by 10 to 20 ng/mL IL-12 (Figure 4A). CD28 re-expression was generally not seen early after T-cell receptor and IL-12 stimulation but was a delayed response. To determine the time course for CD28 expression, CD4+CD28null T-cell clones were stimulated with IL-12 and evaluated by serial flow cytometric analysis. Results are expressed as the mean fluorescence intensity of CD28 expression (Figure 4B). CD28 expression peaked at day 6 following activation, suggesting that IL-12 had an indirect effect on the de novo transcription and translation of the CD28 gene. Therefore, all experiments shown in Figure 3B-F were done at day 5 to 6 after stimulation with an optimal concentration of 20 ng/mL IL-12.
CD28 induction by IL-12.
(A) CD4+CD28null clones were stimulated with anti-CD3 mAb alone or in the presence of increasing concentrations of IL-12. Histograms of a representative experiment are shown. IL-12 at 20 ng/mL was optimal to induce CD28 expression. (B) The time course for CD28 expression was analyzed by serial flow cytometric analysis of an anti-CD3 mAb/IL-12–stimulated T-cell clone. Results are expressed as mean fluorescence intensity of CD28 expression (▾) compared with the isotype control (●) at each time point. CD28 expression was maximal after 6 days of culture in IL-12. (C) To examine whether re-expression of CD28 was transient, T cells were stimulated with anti-CD3 mAb and IL-12 for 5 days and then maintained in the absence of IL-12. CD28 expression decreased again and was absent at day 20 after initial stimulation. A representative example of 3 experiments is shown for a clone (upper panel) and a line (lower panel). In all histograms, the shaded areas represent staining with an isotype control.
CD28 induction by IL-12.
(A) CD4+CD28null clones were stimulated with anti-CD3 mAb alone or in the presence of increasing concentrations of IL-12. Histograms of a representative experiment are shown. IL-12 at 20 ng/mL was optimal to induce CD28 expression. (B) The time course for CD28 expression was analyzed by serial flow cytometric analysis of an anti-CD3 mAb/IL-12–stimulated T-cell clone. Results are expressed as mean fluorescence intensity of CD28 expression (▾) compared with the isotype control (●) at each time point. CD28 expression was maximal after 6 days of culture in IL-12. (C) To examine whether re-expression of CD28 was transient, T cells were stimulated with anti-CD3 mAb and IL-12 for 5 days and then maintained in the absence of IL-12. CD28 expression decreased again and was absent at day 20 after initial stimulation. A representative example of 3 experiments is shown for a clone (upper panel) and a line (lower panel). In all histograms, the shaded areas represent staining with an isotype control.
Restoration of CD28 expression on CD4+CD28nullT-cell lines and clones was a temporary phenomenon. Representative results are shown in Figure 4C. CD28 expression was maximal 5 days after T-cell receptor and IL-12 stimulation. At that time, IL-12 was removed from the culture medium, and the clones or lines were maintained in only IL-2. Cells started to lose CD28 again between days 7 and 15 after stimulation and were completely negative by day 20.
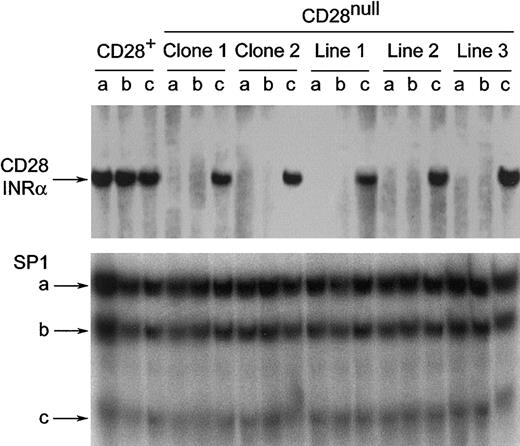
Activation of CD4+CD28null T cells restores DNA-binding activity to the CD28 initiator region
Transcription of the CD28 gene is controlled at the level of the CD28 initiator region (INR).26 Loss of DNA-binding activities to CD28 INR sequences is associated with a transcriptional block that leads to the CD28null phenotype. IL-12 stimulation may reconstitute the initiator protein complex, thereby restoring CD28 expression. Alternatively, IL-12 may directly enhance/up-regulate CD28 transcription. To address this question, electrophoretic mobility shift assays with INR-specific probes were performed on nuclear extracts of CD4+CD28nullT-cell lines and T-cell clones activated with anti-CD3 mAb in the presence or absence of IL-12 for 5 to 8 days. As expected, the anti-CD3–stimulated and the IL-12–stimulated CD4+CD28null T-cell clones and lines did not yield any binding activity (Figure 5, upper panel). A full restoration of the DNA-binding activity was seen with anti-CD3 mAb and IL-12 in those CD28null T cells that could be induced to express CD28 on the cell surface. In contrast, T-cell clones that could not be induced to express CD28 did not yield any binding activity (data not shown).
How stimulation of CD4+CD28nullT cells in the presence of IL-12 affects the CD28 transcriptional initiator protein complex.
Stimulation of CD4+CD28null T cells in the presence of IL-12 restores the CD28 transcriptional initiator protein complex. Nuclear extracts from CD4+CD28nullT-cell clones and lines were analyzed by electrophoretic mobility shift assays for the presence of a protein complex binding to the INRα site of the CD28 gene.26 33 Binding activities were absent in anti-CD3 mAb– (a) or IL-12– (b) stimulated CD4+CD28null T cells. The DNA binding activity was fully restored by costimulation with anti-CD3 mAb and IL-12 (c) and reached the level seen in CD28+ T cells. SP1 binding was used as a system control. One representative experiment of 4 is shown.
How stimulation of CD4+CD28nullT cells in the presence of IL-12 affects the CD28 transcriptional initiator protein complex.
Stimulation of CD4+CD28null T cells in the presence of IL-12 restores the CD28 transcriptional initiator protein complex. Nuclear extracts from CD4+CD28nullT-cell clones and lines were analyzed by electrophoretic mobility shift assays for the presence of a protein complex binding to the INRα site of the CD28 gene.26 33 Binding activities were absent in anti-CD3 mAb– (a) or IL-12– (b) stimulated CD4+CD28null T cells. The DNA binding activity was fully restored by costimulation with anti-CD3 mAb and IL-12 (c) and reached the level seen in CD28+ T cells. SP1 binding was used as a system control. One representative experiment of 4 is shown.
The reinduction of CD28 INR–specific transcription factors by IL-12 was a specific response. The level of INR-binding activities in CD28+ T cells was unaffected by T-cell receptor triggering with or without IL-12 (Figure 5, upper panel). Moreover, DNA-binding activities of the SP1 transcription factor complex were also unaffected (Figure 5, lower panel).
IL-12 induction of CD28 expression restores costimulatory function
To determine whether IL-12 restores functional competence of CD4+CD28null T cells, costimulatory activity of the re-expressed CD28 was assessed for the up-regulation of the T-cell activation markers, CD25 and CD40L.
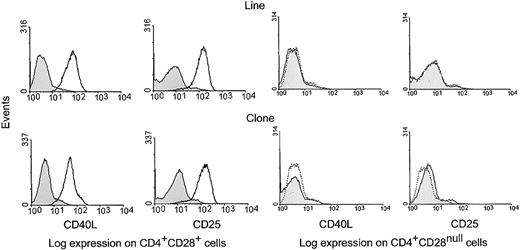
CD4+CD28null T-cell clones and lines were cultured with anti-CD3 mAb and IL-12 for 6 days to induce CD28 expression. Cells were harvested and activated for 24 hours with anti-CD3 mAb in the presence or absence of anti-CD28 mAb. Under these conditions, at least 50% of all cells expressed CD28. Stimulated cells were then analyzed by 4-color flow cytometry for the expression of CD25 and CD40L on CD4+CD28null and CD4+CD28+ T cells. Stimulation with anti-CD3 and anti-CD28 mAbs, but not with anti-CD3 mAb alone, resulted in increased CD25 and CD40L expression on the fraction of T cells that expressed CD28 (Figure 6, left panel). No costimulatory activity was seen on CD4+ T cells that failed to express CD28 (Figure 6, right panel).
Functionality of CD28 molecules induced on CD4+CD28null T-cell clones.
CD28 molecules induced on CD4+CD28null T-cell clones are functional. CD4+CD28null T-cell lines and clones were cultured with anti-CD3 mAb and IL-12 for 6 days to induce CD28 expression (Figure 4). Cells were then harvested and subsequently activated for 24 hours with anti-CD3 mAb or with anti-CD3 and anti-CD28 mAbs together. The cells were then analyzed by 4-color flow cytometry for the expression of the IL-2R α-chain (CD25) and CD40L. Histograms are shown for gated CD4+CD28+ and CD4+CD28null cell fractions; results are representative of 5 experiments with different T-cell clones and lines. Costimulation with anti-CD3 and anti-CD28 mAbs (solid line), but not with anti-CD3 mAb alone (dotted line), resulted in up-regulation of CD25 and CD40L expression on cells that re-expressed CD28; however, expression of CD25 and CD40L was not induced on cells that failed to express CD28. In all histograms, the shaded areas represent staining with an isotype control.
Functionality of CD28 molecules induced on CD4+CD28null T-cell clones.
CD28 molecules induced on CD4+CD28null T-cell clones are functional. CD4+CD28null T-cell lines and clones were cultured with anti-CD3 mAb and IL-12 for 6 days to induce CD28 expression (Figure 4). Cells were then harvested and subsequently activated for 24 hours with anti-CD3 mAb or with anti-CD3 and anti-CD28 mAbs together. The cells were then analyzed by 4-color flow cytometry for the expression of the IL-2R α-chain (CD25) and CD40L. Histograms are shown for gated CD4+CD28+ and CD4+CD28null cell fractions; results are representative of 5 experiments with different T-cell clones and lines. Costimulation with anti-CD3 and anti-CD28 mAbs (solid line), but not with anti-CD3 mAb alone (dotted line), resulted in up-regulation of CD25 and CD40L expression on cells that re-expressed CD28; however, expression of CD25 and CD40L was not induced on cells that failed to express CD28. In all histograms, the shaded areas represent staining with an isotype control.
Discussion
The present studies demonstrate that the function of CD4+CD28null T-cells is profoundly influenced by exposure to IL-12. CD4+CD28null T cells express IL-12 receptors and respond by up-regulating a known IL-12–responsive gene, CD161 (Figures 1-2). More interestingly, activation in the presence of IL-12 results in the restoration of CD28 gene transcription and the cell surface appearance of a functional CD28 molecule (Figures 3-4). CD28 gene transcription is mediated by reassembly of a protein complex binding to the initiator element of the CD28 gene promoter (Figure 5). In cells that re-express CD28, the molecule is functional and is able to provide a costimulatory signal that enhances expression of CD40L and CD25 (Figure 6).
The IL-12 receptor is a heterodimeric protein composed of β1 and β2 subunits; the IL-12Rβ2 chain is essential for IL-12 signaling.27 CD4+CD28null T cells constitutively express high levels of IL-12Rβ1, a feature shared with NK cells.27 Cell surface expression of IL-12Rβ2 is not detected on peripheral blood CD4+CD28null T cells; however, these cells express IL-12Rβ2 mRNA, and the receptor is readily up-regulated following T-cell activation. Expression of IL-12Rβ2 on CD4+CD28null T cells provides further evidence of the Th1 commitment of this T-cell subset and suggests that these cells are IL-12 responsive. Indeed, CD4+CD28null T cells exposed to IL-12 without concurrent TCR triggering up-regulate surface expression of the IL-12–inducible protein, CD161. CD161 expression on T cells has been suggested as a means to enhance the transendothelial migration of cells independently of chemotactic stimuli.28 CD161-expressing γδ T-cell clones isolated from patients with multiple sclerosis have been reported to migrate across an endothelial layer more effectively than CD161− clones, and this migratory potential is enhanced by IL-12.29 Therefore, IL-12–mediated modulation of CD161 expression on CD4+CD28null T cells may convey tissue-invasive properties to these autoreactive T cells.13 Indeed, CD4+CD28nullCD161+ T cells have been identified within lymphoid follicles in synovial tissue of patients with RA.20
The origin of CD4+CD28null T cells is not known. The resemblance of CD4+CD28null T cells to NK T cells suggests that they derive from a lineage that is separate from that of classic CD4+ T cells. However, CD4+CD28null T cells do not express the invariant TCR that is characteristic of CD1d-restricted NK T cells.22 Additionally, recent work suggests that CD4+CD28null T cells successively acquire NK receptors after completion of TCR rearrangement.30 In vitro, T-cell replicative senescence is associated with down-regulation of CD28 expression,7,11,14 and CD4+CD28null T cells are expanded in the elderly8-10 and in patients with autoimmune diseases.22-25 CD4+CD28null T cells, therefore, are likely to derive from CD28+precursors.
CD28 expression can be influenced by cytokines. In vitro, tumor necrosis factor–α (TNF-α) down-regulates the expression of CD28 on CD4+ T cells by directly influencing CD28 gene transcription.31 Additional evidence that CD4+T cells have variable CD28 expression that is dictated by their environment comes from studies of RA. TCR sequences of CD4+T-cell clones that uniformly lacked CD28 expression in the peripheral blood were detected in both the CD28null and the CD28+ compartments when isolated from joint synovial fluid.13 This suggests that an inflammatory microenvironment induces CD28 expression. Our study shows that IL-12, which is elevated in the joints of patients with RA,32 is important in modulating expression of CD28 on T cells.
Stimulation with IL-12 alone was insufficient to induce CD28 transcription and required concurrent triggering of the TCR. This was in contrast to the induction of the signal transducer and activator of transcription–4 (STAT-4)–dependent gene, CD161. The CD28 promoter has a putative STAT-4–binding site. It is, therefore, possible that re-expression of CD28 requires the concerted action of several transcription factors, including STAT-4. Alternatively, TCR- and IL-12–triggered signaling may act on the CD28 promoter only indirectly. We have previously shown that CD4+CD28null T cells lack CD28 expression owing to a transcriptional block of the CD28 gene and that these cells uniformly lack binding activity of specific nuclear proteins to the CD28 promoter.10,14 It has also been shown that the expression of these nuclear protein complexes correlates with the cell surface expression of CD28 on T cells.14 In transcription assays, this nuclear protein complex is able to drive a CD28 INR–driven promoter construct, suggesting that CD28 loss is regulated at the level of transcription initiation.26 We now demonstrate that activation of CD4+CD28null T cells through the TCR in the presence of IL-12 results in restoration of this initiator complex and consequently reverses the transcriptional block of the CD28 gene. Recent studies suggest that the assembly of this initiator complex requires the posttranslational modification of the common nuclear proteins nucleolin and heterogeneous nuclear ribonucleoprotein–D (hnRNPD).33 How IL-12 stimulation induces this modification is not known; however, such an indirect mechanism is consistent with the delayed effect of IL-12 expression.
Loss of CD28 expression is one of several developmental changes that occur in T cells during replicative senescence.7,11,14Other changes are the loss of CD40L expression and the expression of several genes that are generally associated with NK cells, such as KIRs, CD161 and other C-type lectin receptors, perforin, and granzyme B.19-21 This raises the question of whether IL-12 can reverse the entire process or whether it selectively reactivates the transcription of the CD28 gene. Indeed, IL-12 restored the ability to express CD40L after optimal stimulation (Figure 6). In some clones and lines, IL-12 also down-regulates perforin expression (data not shown). Although these results indicate a broader action of IL-12, the major effect was on CD28 expression. IL-12 is unable to down-regulate KIR expression, but it induces CD161 expression instead of repressing it (Figure 2). The rather selective activity of IL-12 on CD28 expression is consistent with the finding that the CD28 INR sequence motifs have not been identified in other genes.26 Ultimately, defining the IL-12–mediated modification of the CD28 initiator complex will be necessary to determine whether and how perforin down-regulation is related to CD28 re-expression.
Re-expression of CD28 upon IL-12/TCR costimulation is not universal for all CD4+CD28null T cells. Approximately 50% of T-cell clones and T-cell lines are completely refractory to the action of IL-12 although they express a functional IL-12 receptor. Some clones and cell lines were heterogeneous with respect to their ability to re-express CD28. The ability or inability of each T-cell clone to activate the transcription of CD28 is reproducible. These results are consistent with a model in which CD28 loss is a stepwise process that is initially reversible upon IL-12 exposure but is ultimately permanent. Both stages correlate with the formation, and eventually the loss, of the CD28 initiator complex.14,26 31 In CD4+ clones with reversible CD28 expression, the loss of the nuclear CD28 initiator complex is reversible. In T cells with permanent CD28 loss, the CD28 initiator complex cannot be formed.
Loss of CD28 expression on CD4+ and CD8+ T cells is generally considered to be a feature of immunosenescence,34 and the frequency of CD28null T cells has been shown to be a predictor of humoral incompetence to vaccination in the elderly.17 The lack of CD28 expression may not only be a surrogate marker for immunosenescence but could be of direct functional importance. Among the several defects of CD4+CD28null T cells, the lack of CD40L expression after TCR stimulation is most striking. As a consequence, CD4+CD28null T cells cannot support the differentiation of B cells to produce immunoglobulins.19 Our results demonstrate that IL-12–mediated restoration of CD28 signaling enhances the up-regulation of CD40L expression. Restoration of the helper cell phenotype in immunosenescent T-cell populations may, in part, reverse the age-related decline in immune function.
CD4+CD28null T cells have low and unstable expression of CD25,12,18 which contributes to the decreased replicative potential of senescent T cells and to the prolonged survival and accumulation of CD28null T cells in the aging immune system. The increased resistance of CD4+CD28null T cells to apoptosis-inducing signals has been linked to dysregulation of the FLICE (Fas-association death domain [FADD]–like IL-1–converting enzyme)–like inhibitory protein, which is in part controlled by IL-2–mediated signals.12 CD25 expression is dependent on CD28-mediated signals, and restoration of CD28 expression enhances the expression of CD25. Thus, re-expression of CD28 may restore T-cell homeostasis, reduce the expansion of the CD28null compartment, and generate space for naive T-cells.35
In addition to the beneficial effects of restoring immunocompetence, re-expression of CD28 induced by IL-12 and TCR costimulation may be detrimental in some chronic inflammatory diseases by enhancing autoimmune responses.13,24,36CD4+CD28null T cells expanded in patients with RA, acute coronary syndromes, multiple sclerosis, and some vasculitides are potent producers of IFN-γ and other cytokines, and they respond to a low threshold of T-cell activation. In these cases, loss of CD28 may be a protective mechanism to control these cells. Previous studies have shown that CD4+CD28null T cells regain expression of CD28 in inflammatory lesions.13 This re-expression of CD28 may be initiated by locally produced IL-12 and will enhance the effector function and the cytokine production by these cells in the lesion. Here, targeting IL-12 production may prevent CD28 expression in the inflammatory lesion and could be beneficial in controlling the autoimmune response.
We thank James W. Fulbright for assistance in preparing this manuscript and Linda H. Arneson for secretarial support.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/ blood-2002-08-2574.
Supported by grants from the National Institutes of Health (R01-AG15043, R21-GM58604, R01-AR42527, and R01-AR41974) and the Mayo Foundation.
K.J.W. and A.N.V. contributed equally and are regarded as co–first authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jörg J. Goronzy, Guggenheim 401, 200 First St SW, Mayo Clinic, Rochester, MN 55905; e-mail:goronzy.jorg@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal