Granzyme M (GM) is a novel serine protease whose expression is highly restricted to natural killer (NK) cells, CD3+CD56+ T cells, and γδ T cells. Using a GM-specific monoclonal antibody, we analyzed the expression of GM in 214 mature T-cell and NK-cell lymphomas. GM was preferentially expressed in nasal NK/T-cell lymphomas (100%), γδ T-cell lymphomas (100%), and intestinal T-cell lymphomas (85%). In contrast, GM expression was present at low prevalence in mycosis fungoides/Sézary syndrome (3%), anaplastic large-cell lymphoma (6%), panniculitis-like T-cell lymphoma (11%), and angioimmunoblastic T-cell lymphoma (0%) cases. Peripheral T-cell lymphomas of unspecified subtype showed an intermediate frequency (37%) of GM expression, consistent with their heterogeneous origin. We conclude that GM expression is a distinctive feature of the nasal NK/T-cell, γδ T-cell, and intestinal T-cell lymphomas, and suggest that these tumors develop from lymphocytes involved in innate immunity.
Introduction
Target cell death induced by cytotoxic lymphocytes involves several molecular mediators, including membrane-bound proteases known as granzymes.1 To date, 5 granzymes have been demonstrated in human cells.1 These enzymes are similar in structure, but differ in their substrate specificity and chromosomal locations.1
Granzyme M (GM), a novel member of this family, has an unusual enzyme specificity, preferring cleavage after methionine, leucine, or norleucine.2 Its expression is restricted to natural killer (NK) cells, CD3+CD56+ T cells, and γδ T cells.3,4 It has been suggested that this enzyme may play a role in the effector phase of innate immune responses.4
Lymphomas arising from NK and cytotoxic T cells have been increasingly recognized over the past few years. All of these diverse lymphomas express various cytotoxic lymphocyte-associated proteins.5-10 In vivo expression of GM has not yet been reported in human lymphomas.
In order to define the expression pattern of GM and its coexpression with other cytotoxic proteins (CtxPs), we performed an immunohistochemical study in a wide variety of mature human T-cell and NK-cell lymphomas, using a GM-specific monoclonal antibody.
Study design
Formalin-fixed, paraffin-embedded samples from 214 mature T-cell and NK-cell lymphomas were retrieved from the files of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, Bethesda, MD; the Institute of Pathology, University of Wurzburg, Germany, and the Laboratory of Tumor Pathology and Molecular Diagnostics, Bay Zoltan Foundation for Applied Research, Szeged, Hungary. All cases had been previously immunophenotyped in paraffin or frozen sections and classified according to the World Health Organization classification.10
The GM-specific mouse monoclonal antibody 4H10 (1:2000 dilution) was prepared in the laboratory of M.J.S., as previously described.3 This antibody reacts with most splenic red pulp γδ T cells, with a low but variable number of lymphocytes in nonneoplastic lymph nodes (0%-10%) and weakly with scattered intramucosal and intraepithelial lymphocytes (IELs). It is of interest that the intensity of IEL GM expression is greatly increased in most reactive conditions, including celiac disease (L.K., unpublished observations, June 2001).
Additional immunostains were performed for TIA-1 and granzyme B (GB), using TIA-1/2G9 (Coulter Immunology, Hialeah, FL; 1:2000) and GB/GrB7 (Monosan, Uden, The Netherlands; 1:20).
Immunohistochemistry was performed following heat-induced antigen retrieval. Primary antibodies were applied for 60 minutes at room temperature, and developed as previously described.8 An immunoreaction was scored positive if cytoplasmic granular immunostaining occurred in at least 20% of the tumor cells, after excluding GM+ reactive small lymphocytes.
Results and discussion
GM expression was identified in 25 of 25 nasal NK/T-cell lymphomas (NK/TCLs), 5 of 5 γδ T-cell lymphomas (γδTCLs), 22 of 27 intestinal T-cell lymphomas (ITCLs), 2 of 10 primary cutaneous anaplastic large-cell lymphomas (PC-ALCLs), 1 of 39 systemic ALCLs (S-ALCLs), 2 of 18 subcutaneous panniculitic T-cell lymphomas (SPTCLs), 1 of 31 mycosis fungoides/Sézary syndrome (MF/SS) cases, 0 of 23 angioimmunoblastic T-cell lymphomas (AILTs), and in 14 of 36 peripheral T-cell lymphomas of unspecified subtype (PTCLs-NOS) (Table1). GM expression roughly segregated mature T-cell and NK-cell lymphomas into 2 broad groups, representing tumors derived from T- or NK-cells linked to innate immunity (GM+ cases), and tumors derived from lymphocytes involved in adaptive immunity (GM− cases). When evaluated with 2 other CtxPs (TIA-1 and GB), 4 categories of mature T-cell and NK-cell lymphomas could be distinguished: (1) GM+CtxP+ lymphomas; (2) GM−CtxP+ lymphomas; (3) GM−CtxP− lymphomas; and (4) lymphomas with mixed characteristics.
Expression of granzyme M and other cytotoxic proteins in cases studied
| . | TIA-1 (%) . | Granzyme B (%) . | Granzyme M (%) . | Overall* (%) . |
|---|---|---|---|---|
| GM+CtxP+lymphomas | ||||
| Nasal NK/TCL | 25/25 (100) | 25/25 (100) | 25/25 (100) | 25/25 (100) |
| γδ TCL | 5/5 (100) | 5/5 (100) | 5/5 (100) | 5/5 (100) |
| Hepatosplenic | 3/3 (100) | 3/3 (100) | 3/3 (100) | 3/3 (100) |
| Other locations | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) |
| Intestinal TCL | 26/27 (96) | 21/27 (78) | 22/27 (85) | 26/27 (96) |
| CD56− | 20/21 (95) | 19/21 (90) | 20/21 (95) | 20/21 (95) |
| CD56+ | 6/6 (100) | 2/6 (33) | 2/6 (33) | 6/6 (100) |
| GM−CtxP+lymphomas | ||||
| ALCL | 32/49 (65) | 32/49 (65) | 3/49 (6) | 39/49 (80) |
| Cutaneous | 3/10 (30) | 3/10 (30) | 2/10 (20) | 6/10 (60) |
| Systemic | 29/39 (74) | 29/39 (74) | 1/39 (3) | 33/39 (85) |
| Panniculitis-like TCL | 18/18 (100) | 18/18 (100) | 2/18 (11) | 18/18 (100) |
| CD56− | 17/17 (100) | 17/17 (100) | 1/17 (6) | 17/17 (100) |
| CD56+ | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
| GM−CtxP−lymphomas | ||||
| AILT | 1/23 (4) | 1/23 (4) | 0/23 (0) | 1/23 (4) |
| MF/SS | 2/31 (6) | 1/31 (3) | 1/31 (3) | 2/31 (6) |
| “Mixed” lymphomas | ||||
| TCL unspecified | 12/36 (33) | 13/36 (36) | 14/36 (39) | 15/36 (42) |
| Nodal | 8/27 (30) | 9/27 (33) | 9/27 (33) | 9/27 (33) |
| Extranodal | 4/9 (44) | 4/9 (44) | 5/9 (56) | 6/9 (67) |
| Total | 121/214 (57) | 116/214 (54) | 72/214 (34) | 131/214 (61) |
| . | TIA-1 (%) . | Granzyme B (%) . | Granzyme M (%) . | Overall* (%) . |
|---|---|---|---|---|
| GM+CtxP+lymphomas | ||||
| Nasal NK/TCL | 25/25 (100) | 25/25 (100) | 25/25 (100) | 25/25 (100) |
| γδ TCL | 5/5 (100) | 5/5 (100) | 5/5 (100) | 5/5 (100) |
| Hepatosplenic | 3/3 (100) | 3/3 (100) | 3/3 (100) | 3/3 (100) |
| Other locations | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) |
| Intestinal TCL | 26/27 (96) | 21/27 (78) | 22/27 (85) | 26/27 (96) |
| CD56− | 20/21 (95) | 19/21 (90) | 20/21 (95) | 20/21 (95) |
| CD56+ | 6/6 (100) | 2/6 (33) | 2/6 (33) | 6/6 (100) |
| GM−CtxP+lymphomas | ||||
| ALCL | 32/49 (65) | 32/49 (65) | 3/49 (6) | 39/49 (80) |
| Cutaneous | 3/10 (30) | 3/10 (30) | 2/10 (20) | 6/10 (60) |
| Systemic | 29/39 (74) | 29/39 (74) | 1/39 (3) | 33/39 (85) |
| Panniculitis-like TCL | 18/18 (100) | 18/18 (100) | 2/18 (11) | 18/18 (100) |
| CD56− | 17/17 (100) | 17/17 (100) | 1/17 (6) | 17/17 (100) |
| CD56+ | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
| GM−CtxP−lymphomas | ||||
| AILT | 1/23 (4) | 1/23 (4) | 0/23 (0) | 1/23 (4) |
| MF/SS | 2/31 (6) | 1/31 (3) | 1/31 (3) | 2/31 (6) |
| “Mixed” lymphomas | ||||
| TCL unspecified | 12/36 (33) | 13/36 (36) | 14/36 (39) | 15/36 (42) |
| Nodal | 8/27 (30) | 9/27 (33) | 9/27 (33) | 9/27 (33) |
| Extranodal | 4/9 (44) | 4/9 (44) | 5/9 (56) | 6/9 (67) |
| Total | 121/214 (57) | 116/214 (54) | 72/214 (34) | 131/214 (61) |
Positive for at least one cytotoxic cell-associated protein.
GM+CtxP+ lymphomas (NK/TCLs, γδ TCLs, ITCLs)
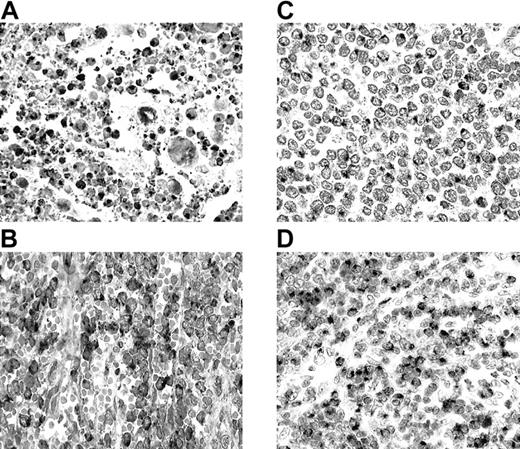
All 25 NK/TCLs were GM+ (Figure1A) and CtxP+. NK/TCLs comprise a distinct lymphoma entity characterized by CD56 and CtxP expression, presence of Epstein-Barr virus (EBV), and lack of rearranged T-cell receptor genes.6,7,10 Accordingly, 24 of our 25 cases were CD56+ and 17 of 18 were EBV+. Most cases of nasal NK/TCL are proposed to arise from NK cells.6,7,10 NK cells are crucial components of the innate immune system, representing the first line of defense. Sayers et al4 described high constitutive GM expression in NK cells, but not in purified or activated conventional αβ T cells. The uniformly high GM expression in our cases further strengthens the NK-cell origin of this lymphoma entity.
Granzyme M (GM) expression in T-cell and NK-cell lymphomas.
(A) Nasal NK/T-cell lymphoma (nasal biopsy) with exuberant apoptotic necrosis. Apoptotic and viable tumor cells are strongly GM+. (B) Hepatosplenic γδ T-cell lymphoma (splenectomy specimen). Cordal and intrasinusoidal lymphomatous cells show intense GM positivity. (C) Intestinal T-cell lymphoma with medium-sized cells (resection specimen). Lymphoma cells reveal GM expression in the Golgi region. (D) Peripheral T-cell lymphoma of unspecified subtype (lymph node biopsy). There is an infiltrate predominantly composed of medium-sized cells with strong GM positivity in most cells.
Granzyme M (GM) expression in T-cell and NK-cell lymphomas.
(A) Nasal NK/T-cell lymphoma (nasal biopsy) with exuberant apoptotic necrosis. Apoptotic and viable tumor cells are strongly GM+. (B) Hepatosplenic γδ T-cell lymphoma (splenectomy specimen). Cordal and intrasinusoidal lymphomatous cells show intense GM positivity. (C) Intestinal T-cell lymphoma with medium-sized cells (resection specimen). Lymphoma cells reveal GM expression in the Golgi region. (D) Peripheral T-cell lymphoma of unspecified subtype (lymph node biopsy). There is an infiltrate predominantly composed of medium-sized cells with strong GM positivity in most cells.
GM and CtxP expression was found in all 5 γδTCLs (Figure 1B). This is consistent with the identification of GM in peripheral blood γδ T cells.4 γδ T cells also participate in innate immune responses,11 and therefore in γδTCLs as well as in normal γδ T cells, GM expression correlates with innate immune cell derivation.
In the ITCLs, 96% expressed CtxPs and 85% were GM+ (Figure 1C). Besides lymphoma cells, IELs were also GM+. ITCL is proposed to arise from IEL. The majority of intestinal IELs are TCRαβ CD8+ cells possessing CtxPs, which suggests a cytotoxic T-cell function.12,13 A smaller number have a γδ T-cell phenotype14 and a minor subset have properties consistent with NK-cell derivation.13Intestinal αβ CD8+ IELs are distinct from conventional T cells, sharing common developmental and functional features with NK cells,13,15,16 including the expression of NK-cell receptors CD160/BY5517 and CD94.18 In addition, intestinal IEL populations represent oligoclonally or monoclonally expanded T cells19 similar to the NK-cell receptor–positive peripheral blood T cells.20 These unique features suggest that human αβ IEL cells are an integral component of the innate immune system. This idea is also supported by their GM expression, a feature shared with other effector cells of the innate immune system.4 The current study, indicating that GM is expressed in most ITCLs, suggests that a majority of these cases are tumors of intestinal αβ T cells involved in innate immunity. The expression of NK-cell receptors in some ITCL cases21,22 supports this hypothesis. One case lacking CtxP expression was CD4+ and probably represented a tumor derived from lamina propria T cells. Contrary to most ITCLs and other CD56+ lymphomas in our series, only 2 of 6 CD56+ ITCLs expressed GM. This finding corresponds with the observation that these lymphomas are morphologically and phenotypically distinct from other ITCLs,23 suggesting a derivation from a unique IEL population.
GM−CtxP+ lymphomas (PC-ALCLs, S-ALCLs, SPTCLs)
In our series, 2 PC-ALCLs and 1 S-ALCL were GM+ (6%). The latter was CD56+ and showed “null-cell” phenotype, suggesting a possible NK-cell origin of this otherwise typical ALK+ case. The 2 GM+ PC-ALCLs exhibited CD4+ and double-negative phenotypes, respectively, but were CD56−. Some ITCLs can have anaplastic features and show strong CD30 expression, mimicking S-ALCL. However, the rarity of GM expression in S-ALCL supports the concept that S-ALCL and ITCL with anaplastic features should be distinguished. This distinction has clinical implications as well, since ITCL has a much poorer prognosis than S-ALCL.10
GM−CtxP− lymphomas (MF/SS cases, AILTs)
In our MF/SS cases (7 patch, 18 plaque, and 6 tumor stage), 2 of 31 (6%) were CtxP+ and 1 (3%) was GM+. Both CtxP+ cases were plaque stage and neither was CD8+, CD56+, or of γδ T-cell origin. Of interest, most CD4+ cutaneous T-cell lymphomas have a mature αβ memory T-cell phenotype,24 consistent with our own results. Contrary to a recent report by Vermeer et al,25 we did not find a correlation between disease stage and CtxP expression. The lower percentage of CtxP+ cases in our study (6% vs 45%), and the lack of correlation between CtxP+ and disease stage may be due to our more stringent positive inclusion criterion (20% vs 10%), and/or our small number of tumor stage cases.6
Although cases of AILT showed many small nonneoplastic lymphocytes with CtxP expression, CtxP (with one exception) and GM expression were not observed in the atypical cells of these cases. This finding concurs with results from Sayers et al,4 who demonstrated no detectable GM in highly purified CD4+ T cells, which are believed to be the precursors of AILT.26
Lymphomas with mixed characteristics (PTCLs-NOS)
PTCLs-NOS displayed an intermediate prevalence of GM expression (39%), somewhat higher in extranodal (56%) than in nodal (33%) cases (Figure 1D). Most GM+ nodal cases (67%) were CD8+, whereas the majority of the extranodal cases (60%) possessed a double-negative phenotype. Only one nodal case was CD56+ and it expressed GM. This lymphoma category represents a diverse group of T-cell neoplasms that do not correspond to any of the well-defined entities.10 Its diversity is underscored by our results, showing no consistent pattern of GM expression.
Summary
Our results suggest that GM expression distinguishes 2 broad groups of lymphomas. The lymphoma entities that comprise each of these groups have characteristics of cells that belong to either the innate immune system (GM+ group), or the adaptive immune system (GM− group), respectively. Therefore, we postulate that GM+ mature T-cell and NK-cell lymphomas derive from lymphocytes involved in the innate immune system.
We thank Aniko Sarro, Maria Labdy, and Sabine Roth for their excellent technical contributions.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-09-2908.
Supported by the Janos Bolyai Research Fellowship of the Hungarian Academy of Sciences, by the Zoltan Magyary postdoctoral fellowship of the Foundation for Hungarian Higher Education and Research (Ministry of Education), Hungary; the National Health and Medical Research Council of Australia; and the Alexander von Humboldt Foundation, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark Raffeld, Specialized Diagnostics Unit, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, MD; e-mail: mraff@box-m.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal