Abstract
The FAS receptor—FAS ligand system is a key apoptotic pathway for cells of the immune system. Ligation of the FAS-receptor (CD95) induces apoptosis by activation of pro—caspase-8 followed by downstream events, including an increase in reactive oxygen species (ROS) and the release of proapoptotic factors from the mitochondria, leading to caspase-3 activation. We investigated the role of vitamin C in FAS-mediated apoptosis and found that intracellular accumulation of pharmacologic concentrations of vitamin C inhibited FAS-induced apoptosis in the monocytic U937 cell line and in fresh human monocytes. Cells were loaded with vitamin C by exposure to dehydroascorbic acid (DHA), thereby circumventing in vitro artifacts associated with the poor transport and pro-oxidant effects of ascorbic acid (AA). Vitamin C inhibition of FAS-mediated apoptosis was associated with reduced activity of caspase-3, -8, and -10, as well as diminished levels of ROS and preservation of mitochondrial membrane integrity. Mechanistic studies indicated that the major effect of vitamin C was inhibition of the activation of caspase-8 with no effect on it enzymatic activity. An independent action of high intracellular concentrations of vitamin C on mitochondrial membrane stabilization was also detected. These studies illuminate the nature of redox-dependent signaling in FAS-induced apoptosis of human monocytes and suggest that vitamin C can modulate the immune system by inhibiting FAS-induced monocyte death. (Blood. 2003;102:336-343)
Introduction
Apoptosis is a suicidal mode of cell death used to eliminate cells in physiologic and pathologic settings.1,2 While diverse events can induce apoptosis, such as radiation and lack of growth factors, apoptosis in the immune system is largely regulated by signaling through death receptors, including the receptor for tumor necrosis factor (TNF) and the FAS receptor (FAS-R), also known as CD95.1 Apoptosis generally occurs in 2 phases: the commitment to cell death, including activation of procaspases, followed by an execution phase resulting in cell structure alterations.3
Upon FAS-R ligation a set of intracellular signaling proteins is recruited to the death-inducing signaling complex. The cytosolic pro—caspase-8 is recruited and believed to oligomerize and autoactivate.4 Caspase-10 is closely related to caspase-8 and it has been proposed that both caspases can be recruited to the death-inducing signaling complex, where they are activated and initiate signaling pathways resulting in apoptosis.5 Active caspase-8 induces translocation of the proapoptotic protein BID to the mitochondria, causing an increase in mitochondrial permeability and a loss in mitochondrial membrane potential (Δψ).6 Oxidative phosphorylation is uncoupled and the mitochondria release reactive oxygen species (ROS), resulting in elevated levels of ROS in cells stimulated via the FAS pathway.7,8 The increase in cellular ROS is a key event in programmed cell death and oxidative molecules themselves can induce apoptosis, independent of ligation of death receptors.9,10 Release of cytochrome C (Cyt C) and other mitochondrial molecules, such as Apaf-1, leads to the downstream activation of caspase-9 and the effector caspase-3.11
Cellular ROS generation is a natural result of aerobic metabolism and is amplified by cellular activation.12 ROS are widely involved in receptor-mediated signaling,13-15 and antioxidants can inhibit important signaling pathways in immune cells.16,17 Vitamin C is a critical dietary nutrient in humans, since humans cannot synthesize it as do most nonprimates. Deficiency of vitamin C seriously impairs immune function, and there is literature pointing to the role of vitamin C in enhanced host defense and the modulation of inflammatory reactions.18-22 Vitamin C is generally thought to enhance immunity and is widely taken as a supplement. Whereas vitamin C is found in the plasma in its reduced form (ascorbic acid [AA]), it is transported by most cells in its oxidized form, dehydroascorbic acid (DHA), through facilitative glucose transporters.23 Inside the cell, DHA is reduced to AA.24 Certain specialized cells transport AA directly through cell-surface Naascorbate cotransporters.25 We sought to determine the role of vitamin C in FAS-induced apoptosis in monocytes and used DHA to load cells with vitamin C, thereby avoiding the pro-oxidant effects of AA in the presence of free transition metals ubiquitously found in tissue culture.26 We found vitamin C to be a potent inhibitor of FAS-induced apoptosis in fresh monocytes and in U937 cells. Our data indicate the primary action of vitamin C to be the inhibition of activation of caspase-8 and a separate effect on downstream oxidative events.
Materials and methods
Cells lines and monocytes
The monocytic cell line U937 was obtained from the American Type Culture Collection (Manassas, VA) and cultured in complete medium: RPMI containing 100 U penicillin, 100 U streptomycin (Gemini Bioproducts, Woodland, CA), l-glutamate, and 10% fetal calf serum (Omega Scientific, CA). Human monocytes were isolated from peripheral blood obtained from the New York Blood Center (NY). The following day, peripheral blood mononuclear cells were separated by Ficoll gradient (Accu-Prep; Accurate Chemicals and Scientific, Westbury, NY). Monocytes were immediately purified by negative selection using the magnetic-activated cell separation monocyte isolation kit (Miltenyi Biotec, Auburn, CA) following the manufacturer's instructions. Monocytes were incubated in RPMI containing 100 U penicillin, 100 U streptomycin, L-glutamate, and 20% human AB serum (Irvine Scientific, Santa Ana, CA). Purity was assessed by staining with anti-CD3 (RPE-Cy5; DAKO, Glostrup, Denmark), anti-CD56 (phycoerythrin [PE]; Becton Dickinson, San Diego, CA), anti-CD20 (peridinin chlorophyll protein [PerCP]; Becton Dickinson), and anti-CD14 (fluorescein isothiocyanate [FITC]; Becton Dickinson) fluorescent antibodies. The cells were suspended in staining buffer consisting of 1% heat-inactivated fetal calf serum and 0.1% (wt/vol) sodium azide (Sigma, St Louis, MO) in phosphate-buffered saline (PBS), pH 7.4, containing the fluorescent antibody (Ab) for 30 minutes at 4°C. After staining, the cells were washed with buffer, fixed in 1% paraformaldehyde and analyzed by flow cytometry (FACScalibur; Becton Dickinson) within 24 hours of staining. The results were analyzed using the CELLQuest software (Becton Dickinson). Monocyte preparations were always higher than 95% purity.
Detection of CD95
The antihuman CD95-RPE Ab (DAKO) was used to detect the CD95 antigen in cell lines and monocytes by flow cytometry. Cell-surface staining and analysis were performed as described in the previous section.
Induction of apoptosis
An anti-FAS Ab and soluble FAS ligand (FASL) were used to induce apoptosis. For the induction of apoptosis in monocytes, an anti-FAS Ab (immunoglobulin G1 [IgG1], clone DX2; R and D Systems, Minneapolis, MN) or an isotype control Ab (R and D Systems) were plate-immobilized in a 12—flat well plate overnight. The plate was washed twice in serum-free RPMI and blocked with RPMI containing 10% human type AB serum for 30 minutes at 37°C. The plate was then washed and the cells were added immediately. For induction of apoptosis in U937 cells, 10 μL protein G (2.5 mg/mL suspension in agarose beads; Boehringer Mannheim, Mannheim, Germany) per μg anti-FAS Ab or control Ab was mixed in cold serum-free RPMI for 5 minutes at room temperature (RT) before addition to the cell suspension. To induce apoptosis with soluble FASL, Super FASL (Alexis Biochemicals, Lausen, Switzerland) was added to U937 cells or to freshly isolated monocytes. Cells treated with anti-FAS Ab, FASL, or control cells were incubated at 37°C in an environment of 5% CO2 in air.
Detection of apoptosis
The annexin V—FITC/propidium iodine (PI) apoptosis detection kit (Pharmingen, San Diego, CA) was used to determine the frequency of apoptosis in U937 cells, following the manufacturer's instructions. Alternatively, PI staining of DNA was used to analyze the sub-G1 region by flow cytometry. For PI staining, cells were washed twice with PBS and fixed with 70% ethanol for at least 30 minutes at 4°C. Cells were then washed twice with PBS and resuspended in 50 μL of a 100 U/mL RNAse-A solution (Boehringer Mannheim) and 20 μL PI (0.1 mg/mL; Sigma) per 1 × 105 cells for 30 minutes at RT in the dark and kept at 4°C until flow cytometry acquisition. Apoptosis in monocytes was determined by analysis of the sub-G1 region by DNA staining and flow cytometry. For PI staining, the monocytes were incubated with trypsin for 5 minutes at 37°C, washed twice with PBS, and then stained as described in the previous section.
Vitamin C uptake
Vitamin C uptake was measured as intracellular accumulation after incubation of cells with DHA or AA at 37°C, as previously described.23,24 To determine the accumulation of vitamin C supplied as DHA, triplicate cell samples were incubated with several concentrations of DHA from a solution containing 0.5 μCi (0.0185 MBq) L-14C-AA (specific activity 8.0 mCi/mmol (296 MBq/mmol); Perkin-Elmer Life Sciences, MA), 1.0 to 4.0 mM AA (Sigma), and 86 U/mL ascorbate oxidase (Sigma) in incubation buffer (15.0 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 135.0 mM NaCl, 5.0 mM KCl, 1.8 mM CaCl2, and 0.8 mM MgCl2 [pH 7.4]). Cells were then washed twice with cold PBS and lysed with 0.5 mL sodium dodecyl sulfate (SDS)—lysis solution (10.0 mM Tris [tris(hydroxymethyl)aminomethane]—HCl [pH 8.0] and 0.2% SDS). Cell-associated radioactivity was quantified by scintillation spectrometry. To determine the accumulation of vitamin C supplied as AA, cells were incubated with varying concentrations of AA from a solution prepared with 0.5 μCi (0.0185 MBq) L-14C-AA, 1.0 to 4.0 mM L-AA, and 0.1 mM 1,4-dithiothreitol in incubation buffer and cell-associated radioactivity measured by scintillation spectrometry. Additionally, accumulation of vitamin C supplied as DHA was determined by high-performance liquid chromatography (HPLC) electrochemical detection (ESA, Chelmsford, MA).27 Cells incubated with DHA were washed twice in cold PBS and then lysed in 60% methanol. Lysates were centrifuged, the supernatants filtered through 0.2-μm nylon membrane filters (Nalgene, Rochester, NY) and equal amounts of each sample injected onto a modified C18 column (no. 70-4160; ESA). The column was equilibrated at 30°C with a mobile phase buffer consisting of 50 μM sodium phosphate, 50 μM sodium acetate, 189 μM dodecyltrimethylammonium chloride, and 3.66 μM tetraoctylammonium bromide in 30:70 methanol-water (vol/vol, HPLC grade; J. T. Baker, Phillipsburg, NJ), pH 4.8. The flow rate was 0.3 mL/min. Under these conditions, the retention time of AA was 4.9 minutes. Peak areas for AA were determined using a dominant potential for AA of 100 mV, and AA was quantified from a calibration curve.
Cell volume determination
Estimation of cell volume was performed as previously described.28 Briefly, 5 × 106 cells were incubated for 60 minutes at RT in 200 μL incubation buffer containing 1.0 mM 3-oxy-methyl-D-glucose and 5 μCi (0.185 MBq) 3 H-3-oxy-methyl-D-glucose. Uptake was stopped by adding 2 μL of 2.0 mM cytochalasin B (Sigma), which blocks glucose transport and prevents the efflux of trapped glucose. The cells were then washed twice in ice-cold PBS containing 20 μM cytochalasin B and cell-associated radioactivity determined by scintillation spectrometry. Glucose reached equilibrium after 60 minutes of incubation, therefore the amount of radioactivity accumulated inside the cells is in direct proportion to the intracellular volume. The cell volume estimated for monocytes was 0.15 μL per 106 cells and 1.0 μL per 106 U937 cells.
Vitamin C loading
Cells were washed twice in incubation buffer at pH 7.4, and DHA (Aldrich, Milwaukee, WI) was added. After incubation with DHA for up to 60 minutes at 37°C, the cells were washed twice in serum-free RPMI.
Detection of ROS
Intracellular ROS in U937 were estimated by 2′7′ dichlorofluorescein diacetate (DCFH-DA) fluorescence.8 Cells were washed twice in Krebs-Ringer buffer (20.0 mM HEPES, 10.0 mM dextrose, 127.0 mM NaCl, 5.5 mM KCl, 1.0 mM CaCl2, and 2.0 mM MgSO4 [pH 7.4]) and stained with 0.1 μg/mL DCFH-DA (Molecular Probes, Eugene, OR) and 1.0 mM maleic acid diethyl ester (Sigma) in Krebs-Ringer buffer. Fluorescence was determined by flow cytometry after incubation at 37°C, and the data were analyzed using CELLQuest software.
Assessment of mitochondrial membrane potential (Δψ)
U937 cells were washed twice with PBS, stained with 40 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC(6)(3); Molecular Probes) with or without 100 μM carbonyl cyanide m-chlorophenylhydrazone (Sigma) as a control for membrane potential disruption, and incubated at 37°C in the dark for 15 minutes before determination of fluorescence by flow cytometry. These experiments and the ROS quenching studies were not performed in monocytes because cell handling and the required trypsinization led to erratic results in these assays.
Cytochrome C release
Release of CytC into the cytosol was detected with a CytC enzyme-linked immunosorbent assay (ELISA) kit (MLB, Nagoya, Japan), following the manufacturer's instructions. To obtain cytosolic fractions, cells were washed twice with ice-cold PBS, resuspended in cold buffer (1.28 M NaCl, 50.0 mM KCl, 50.0 mM MgSO4, 13.0 mM CaCl2, 0.5 M HEPES, 1.0 mM phenylmethylsulfonyl fluoride, 10% vol 2.5 M sucrose, 10.0 mM 1,4-dithiothreitol, 1.0 mM reduced glutathione, and 1% glycerol) and homogenized mechanically. This total cellular extract was centrifuged at 2000 rpm for 3 minutes at 4°C and the cytosolic supernatant recovered and analyzed for the presence of cytochrome C.
Caspase assays
Caspase activity was measured using the caspase-3 (Sigma), caspase-8, or caspase-10 (R and D Systems) colorimetric assay kits, following the manufacturer's instructions. Briefly, 1 × 107 cells were lysed and equal quantities of protein loaded in MaxiSorp 96-well plates (Nunc, Roskilde, Denmark). The caspase-3 inhibitor Ac-Asp-Glu-Val-Asp-aldehyde (Sigma) or caspase-8 inhibitor benzylloxycarbonyl-Ileu-Glu-Thr-Asp-fluoromethyl ketone (Z-IEDT-FMK; BioVision Research Products, CA) was added as a control at 20-μM final concentration. The plate was incubated at 37°C and color development quantified by reading with an ELISA plate reader at 405 nm.
Recombinant caspase-8 in vitro assay
Recombinant caspase-8 (Alexis Biochemicals) was incubated with different concentrations of AA and activity measured using the caspase-8 assay kit (R and D Systems). Background was determined from triplicates of each concentration of vitamin C without the enzyme and subtracted from the experimental results. The results were expressed as the percentage of caspase activity in the absence of vitamin C.
Caspase-8 Western blot
Cells were lysed in a buffer containing 30 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, and 1% triton and protease inhibitors. Cell lysates were separated by SDS—polyacrylamide gel electrophoresis on precast Tris-HEPES-SDS-polyacrylamide gradient minigels (4%-20%; Gradipore, Frenchs Forest, Australia) according to the manufacturer's protocols. The gels were then transferred onto a nitrocellulose membrane (Bio-rad Laboratories, Hercules, CA) in a buffer containing 25 mM Tris (pH 8.3), 192 mM glycine, and 20% methanol in a semidry system (Bio-rad) for 15 minutes (15 V) at 25°C. Residual binding sites on the membrane were blocked by incubating the membrane in Tris-buffered saline (TBS; 20 mM Tris-HCl [pH 7.4] and 0.15 M NaCl), containing 5% nonfat dry milk and 0.1% Tween-20, for 2 hours at 25°C (blocking buffer). Membranes were incubated with monoclonal anti—caspase-8 (12F5; Alexis Biochemicals) or anti-βtubulin (H-235; Santa Cruz Biotechnology, Santa Cruz, CA) Abs (1:1000 dilution) for 16 hours at 4°C. The primary Ab was removed, and the blots were washed 3 times in TBS. To detect Ab reaction, the blots were incubated for one hour with anti—mouse or anti—rabbit IgG horseradish peroxidase (HRP)—conjugated Abs (Bio-rad), diluted 1:3000 in blocking buffer, washed with TBS, and the protein bands were revealed using the enhanced chemiluminescence (ECL+plus) Western blotting detection system (Amersham Pharmacia Biotech, Piscataway, NJ) before exposure to BioMax film (Kodak, Rochester, NY).
Statistical analysis
The paired 1-tail distribution Student t test was applied to compare results with a criterion for statistical significance of a P value of .05 or less.
Results
Vitamin C inhibits FAS-induced apoptosis
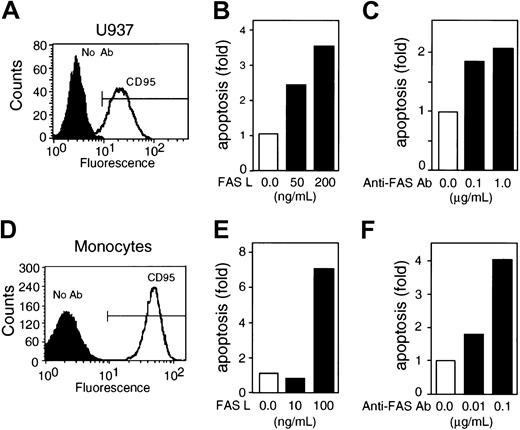
FAS-R (CD95) was expressed in U937 cells and monocytes, and both anti-FAS Ab and FASL induced apoptosis in these cells in a dose-dependent manner (Figure 1). Incubation of U937 cells for 3 hours with FASL induced up to a 3.5-fold increase in apoptosis, whereas incubation with anti-FAS Ab caused a 2-fold increase (Figure 1B-C). In monocytes, 3-hour treatment with FASL resulted in up to a 7-fold increase in apoptosis, whereas incubation with anti-FAS Ab resulted in a 4-fold increase (Figure 1E-F). An isotype control Ab did not cause apoptosis in either cell type (data not shown). FASL was more efficient than anti-FAS Ab in inducing apoptosis in both U937 cells and monocytes (Figure 1).
FAS-R ligation induces apoptosis in U937 cells and in monocytes. (A) U937 cells express the FAS-R (CD95) as determined by flow cytometry. (B-C) FASL and anti-FAS Ab induce apoptosis in U937 cells in a dose-dependent manner. (D) Fresh human monocytes express CD95 as determined by flow cytometry. (E-F) FASL and anti-FAS Ab induce apoptosis in monocytes in a dose-dependent manner. The frequency of apoptosis for U937 cells was determined by annexin V+/PI-staining; apoptosis in monocytes was determined by the frequency of events in the sub-G1 region. The results are presented as the fold increase in the frequency of apoptosis over control cells left untreated for 3 hours and are representative of at least 5 independent experiments for each cell type.
FAS-R ligation induces apoptosis in U937 cells and in monocytes. (A) U937 cells express the FAS-R (CD95) as determined by flow cytometry. (B-C) FASL and anti-FAS Ab induce apoptosis in U937 cells in a dose-dependent manner. (D) Fresh human monocytes express CD95 as determined by flow cytometry. (E-F) FASL and anti-FAS Ab induce apoptosis in monocytes in a dose-dependent manner. The frequency of apoptosis for U937 cells was determined by annexin V+/PI-staining; apoptosis in monocytes was determined by the frequency of events in the sub-G1 region. The results are presented as the fold increase in the frequency of apoptosis over control cells left untreated for 3 hours and are representative of at least 5 independent experiments for each cell type.
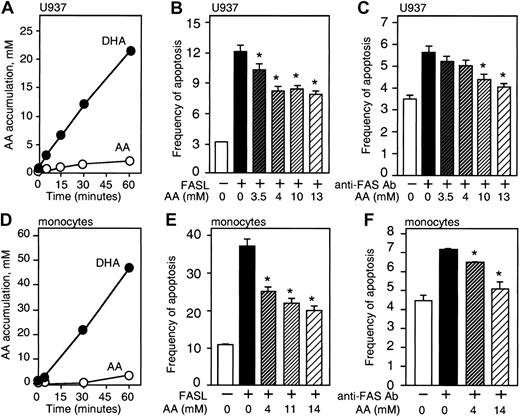
We investigated the effect of loading cells with vitamin C on FAS-R—dependent apoptosis. U937 cells and monocytes transport vitamin C in the form of DHA through facilitative glucose transporters.29,30 As measured by 14 C-substrate uptake, there was little evidence for direct transport of AA, whereas DHA was readily taken up by the cells (Figure 2A-D). We estimated the internal volume of U937 cells at 1.0 μL/106 cells and monocytes at 0.15 μL/106 cells from tritiated methylglucose equilibrium studies.23,28 Based on this internal volume and cell-associated radioactivity, the intracellular accumulation of AA in U937 cells and monocytes was estimated. The intracellular AA concentration calculated using this method was higher than values determined by HPLC electrochemical detection (Figure 2A,D). The small accumulation of vitamin C in cells incubated with AA was probably due to oxidation of AA to DHA in air.
Vitamin C decreases FAS-induced apoptosis in U937 cells and monocytes. (A) Intracellular accumulation of vitamin C in U937 cells after exposure to 1.0 mM DHA or AA. Results are presented as the intracellular concentration of accumulated AA, determined by cell-associated radioactivity. The standard deviation is smaller than the symbols in the graphs, and was always less than 10% of the values for each point. (B-C) U937 cells loaded with vitamin C were treated for 3 hours with 200 ng/mL FASL or 1 μg/mL anti-FAS Ab, and apoptosis was measured by annexin V+/PI- staining or by analysis of the sub-G1 region. The intracellular accumulation of AA was estimated by HPLC. (D) Accumulation of AA in monocytes after exposure to 0.1 mM DHA or AA, calculated by cell-associated radioactivity. (E-F) Monocytes were loaded with different amounts of vitamin C estimated by HPLC analysis before treatment with 100 ng/mL FASL or 0.1 μg/mL anti-FAS Ab for 3 hours to measure apoptosis by analysis of the sub-G1 region. Asterisks indicate statistically significant differences (P ≤ .05) using the Student t test between control and cells loaded with vitamin C before challenge with FASL. These experiments were repeated 6 times for each cell type with similar results. A representative experiment is shown. Error bars represent the SD of triplicate values.
Vitamin C decreases FAS-induced apoptosis in U937 cells and monocytes. (A) Intracellular accumulation of vitamin C in U937 cells after exposure to 1.0 mM DHA or AA. Results are presented as the intracellular concentration of accumulated AA, determined by cell-associated radioactivity. The standard deviation is smaller than the symbols in the graphs, and was always less than 10% of the values for each point. (B-C) U937 cells loaded with vitamin C were treated for 3 hours with 200 ng/mL FASL or 1 μg/mL anti-FAS Ab, and apoptosis was measured by annexin V+/PI- staining or by analysis of the sub-G1 region. The intracellular accumulation of AA was estimated by HPLC. (D) Accumulation of AA in monocytes after exposure to 0.1 mM DHA or AA, calculated by cell-associated radioactivity. (E-F) Monocytes were loaded with different amounts of vitamin C estimated by HPLC analysis before treatment with 100 ng/mL FASL or 0.1 μg/mL anti-FAS Ab for 3 hours to measure apoptosis by analysis of the sub-G1 region. Asterisks indicate statistically significant differences (P ≤ .05) using the Student t test between control and cells loaded with vitamin C before challenge with FASL. These experiments were repeated 6 times for each cell type with similar results. A representative experiment is shown. Error bars represent the SD of triplicate values.
The frequency of apoptosis induced by anti-FAS Ab or FASL in U937 cells and monocytes was prominently reduced by cellular loading with vitamin C (Figure 2). Data regarding the intracellular AA concentrations reported in these experiments were based on HPLC electrochemical detection. The frequency of apoptosis induced by FASL (200 ng/mL) in U937 cells was 12% and decreased to 7.8% in cells loaded with approximately 13 mM vitamin C (P = .01) (Figure 2B). U937 cells incubated 3 hours with 1 μg/mL anti-FAS Ab had a frequency of apoptosis of 5.8%, which decreased to 4.0% when the cells were loaded with approximately 13 mM vitamin C (P = .03) (Figure 2C). The percentage of reduction in apoptosis for U937 cells loaded with 13 mM vitamin C compared with control ranged from 22% to 27% (mean, 25%) when the cells were challenged with anti-Fas Ab and from 26% to 50% (mean 35%) when the cells were treated with FASL. Vitamin C loading did not affect the expression of CD95 in these cells (data not shown). Monocytes loaded with vitamin C also had a reduced frequency of apoptosis after treatment with anti-FAS Ab or FASL. FASL (100 ng/mL) induced 37.1% apoptosis in monocytes, and when these cells were loaded with approximately 14 mM vitamin C the frequency was reduced to 20.0% (P = .04) (Figure 2E). Monocytes treated with 0.1 μg/mL anti-FAS Ab for 3 hours showed 7.2% apoptosis, which was reduced to 5.1% in cells loaded with approximately 14 mM vitamin C (P = .047) (Figure 2F). This represents a mean reduction in apoptosis of 31% to 44%. The lowest intracellular concentration of AA used in these experiments was 4 mM, which approximates the concentration of AA reported in monocytes obtained from blood of healthy donors (3 mM).31,32 AA (4 mM) conferred significant protection from FAS-induced apoptosis in monocytes (P = .02) (Figure 2E-F).
Vitamin C quenches ROS produced by FAS-R ligation
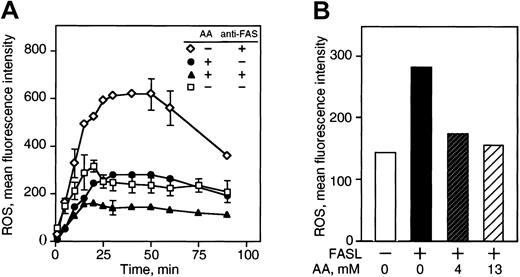
ROS levels are elevated in cells stimulated via the FAS pathway, and it has been suggested that ROS are an important component of FAS signaling.8,33,34 Since vitamin C is a potent antioxidant and quenches ROS in cells under oxidative stress,35 we examined the effect of vitamin C on ROS accumulation after FAS ligation. Incubation of U937 cells with anti-FAS Ab for 3.5 hours resulted in approximately a 2.5-fold increase in cellular ROS, whereas loading the cells with vitamin C reduced ROS accumulation in the cells (Figure 3A). FASL incubation for 3 hours induced a 2-fold increase in the levels of ROS, which were quenched by loading cells with vitamin C (Figure 3B). The production of ROS correlated with the frequency of apoptosis in cells incubated with FASL in these experiments (data not shown).
Vitamin C inhibits ROS generated by FAS-R ligation. (A) U937 cells were loaded with approximately 13 mM vitamin C prior to treatment with 1 μg/mL anti-FAS Ab for 3.5 hours and then stained with DCFH-DA to detect intracellular ROS by detection of dye fluorescence within 90 minutes by flow cytometry. (B) U937 cells were loaded with vitamin C, treated with 200 ng/mL FASL for 3 hours, and stained with DCFH-DA for 30 minutes for fluorescence measurement. The results are presented as the mean fluorescence intensity of DCFH-DA in arbitrary fluorescence units and are representative of 5 independent experiments.
Vitamin C inhibits ROS generated by FAS-R ligation. (A) U937 cells were loaded with approximately 13 mM vitamin C prior to treatment with 1 μg/mL anti-FAS Ab for 3.5 hours and then stained with DCFH-DA to detect intracellular ROS by detection of dye fluorescence within 90 minutes by flow cytometry. (B) U937 cells were loaded with vitamin C, treated with 200 ng/mL FASL for 3 hours, and stained with DCFH-DA for 30 minutes for fluorescence measurement. The results are presented as the mean fluorescence intensity of DCFH-DA in arbitrary fluorescence units and are representative of 5 independent experiments.
Vitamin C reduces mitochondrial damage induced by FAS-R ligation
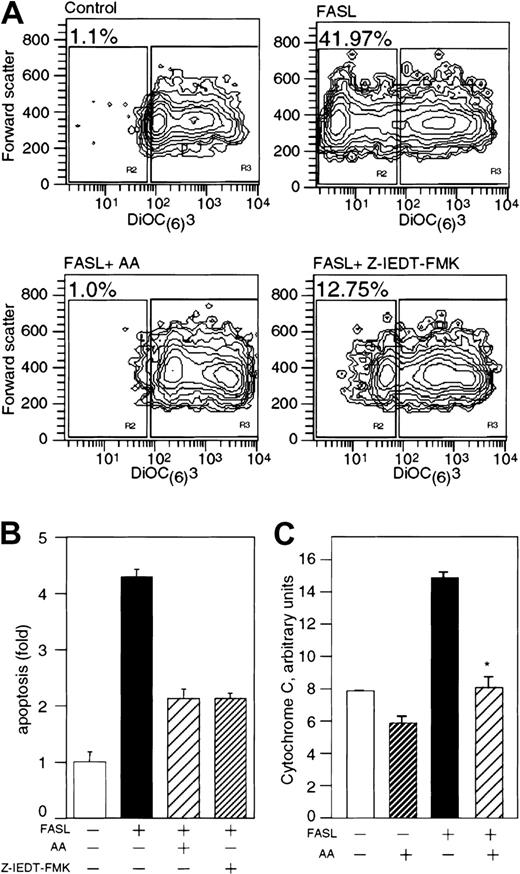
In apoptotic cells, the decrease in mitochondrial membrane differential potential (Δψ) occurs before chromatin condensation and DNA fragmentation,36 and precedes the enhanced release of ROS by the mitochondria.7 Since vitamin C prevented the increase in cellular ROS levels induced by FAS ligation, we studied the effect of vitamin C on mitochondrial apoptosis in U937 cells cultured with FASL by using the dye DiOC(6)(3). Cells with healthy mitochondria have a high uptake of DiOC(6)(3) and therefore a high fluorescence signal, whereas cells with damaged mitochondria show reduced fluorescence. Only 1.1% of control U937 cells had dull DiOC(6)(3) fluorescence (Figure 4A, upper left panel), whereas 42% of U937 cells cultured with FASL for 3 hours incorporated little DiOC(6)(3) (Figure 4A, upper right panel). We found that loading the cells with vitamin C conferred protection from FASL-induced mitochondrial damage, and these cells maintained high DiOC(6)(3) fluorescence (Figure 4A, lower left panel). Control cells treated with cyanide m-chlorophenylhydrazone showed a marked decrease in mitochondrial Δψ (data not shown). Caspase-8 activation precedes the reduction in mitochondrial Δψ and the change in mitochondrial membrane permeability.37 Thus, the mitochondrial damage and the mitochondrial collapse should be prevented by incubation with the specific caspase-8 inhibitor Z-IETD-FMK. The addition of 0.2 μM Z-IETD-FMK inhibited approximately 60% of FASL-induced mitochondrial Δψ loss compared with nearly 100% inhibited with vitamin C (Figure 4A, lower panels). We therefore reasoned that vitamin C was inhibiting events at both the mitochondrial level and upstream sites in the apoptosis cascade. The caspase-8 inhibitor Z-IETD-FMK and vitamin C, however, prevented FASL-induced apoptosis in similar proportions (Figure 4B).
Vitamin C prevents mitochondrial damage induced by FAS-R ligation.(A) U937 cells were loaded with 13 mM vitamin C prior to 3 hours of treatment with FASL (200 ng/mL) and were stained with 40 nM DiOC(6)(3) for 15 minutes at 37°C. Control cells were treated with 0.2 μM Z-IEDT-FMK. DiOC(6)(3) fluorescence was analyzed by flow cytometry. The intensity fluorescence of DiOC(6)(3) is shown on the x-axis, and the forward scatter is represented on the y-axis. The numbers in the figure indicate the frequency of the population with low intensity of DiOC(6)(3) fluorescence for each treatment and are representative of 7 independent experiments (except for the experiment with Z-IEDT-FMK, which was performed twice). (B) Cells cultured under the conditions described in panel A were stained with annexin-PI to assess frequency of apoptosis. These results are the mean of 3 independent experiments, performed in duplicates, and are presented as the fold increase in apoptosis over untreated control cells. (C) U937 cells were loaded with approximately 13 mM vitamin C prior to treatment with 200 ng/mL FASL. Cytosolic cell extracts were obtained and analyzed by ELISA for the presence of Cyt C. The results show the normalized Cyt C content for each experimental condition in arbitrary units based on the Cyt C cytosolic content of control cells and represent 2 independent experiments. Asterisk indicates statistically significant differences (P = .039) using the Student t test between control and cells loaded with vitamin C before challenge with FASL. Error bars represent the SD of triplicate values.
Vitamin C prevents mitochondrial damage induced by FAS-R ligation.(A) U937 cells were loaded with 13 mM vitamin C prior to 3 hours of treatment with FASL (200 ng/mL) and were stained with 40 nM DiOC(6)(3) for 15 minutes at 37°C. Control cells were treated with 0.2 μM Z-IEDT-FMK. DiOC(6)(3) fluorescence was analyzed by flow cytometry. The intensity fluorescence of DiOC(6)(3) is shown on the x-axis, and the forward scatter is represented on the y-axis. The numbers in the figure indicate the frequency of the population with low intensity of DiOC(6)(3) fluorescence for each treatment and are representative of 7 independent experiments (except for the experiment with Z-IEDT-FMK, which was performed twice). (B) Cells cultured under the conditions described in panel A were stained with annexin-PI to assess frequency of apoptosis. These results are the mean of 3 independent experiments, performed in duplicates, and are presented as the fold increase in apoptosis over untreated control cells. (C) U937 cells were loaded with approximately 13 mM vitamin C prior to treatment with 200 ng/mL FASL. Cytosolic cell extracts were obtained and analyzed by ELISA for the presence of Cyt C. The results show the normalized Cyt C content for each experimental condition in arbitrary units based on the Cyt C cytosolic content of control cells and represent 2 independent experiments. Asterisk indicates statistically significant differences (P = .039) using the Student t test between control and cells loaded with vitamin C before challenge with FASL. Error bars represent the SD of triplicate values.
Apoptotic cells release cytochrome C (Cyt C) from the mitochondria into the cytosol,38 and we sought to determine the effect of vitamin C on this process. The amount of Cyt C found in the cytosolic fractions of cells incubated with FASL was nearly twice that found in control cells (Figure 4C). In cells loaded with vitamin C, there was a marked reduction of Cyt C in the cytoplasmic fractions, even below the control levels. These results indicate that vitamin C loading results in inhibition of the release of Cyt C from mitochondria after FAS-R ligation.
Vitamin C reduces FAS-induced caspase-3 and caspase-10 activation
A consequence of mitochondrial damage during apoptosis is activation of caspase-3,39 and we studied the effect of vitamin C on the activation of this caspase. Activation of caspase-3 was induced by FASL incubation in U937 cells and in monocytes (Figure 5A-B). The activity of caspase-3 induced by FASL in U937 cells loaded with vitamin C was modestly reduced (30%), although the difference in caspase activity of cells with or without vitamin C was statistically significant (Figure 5A, P = .039). In contrast, caspase-3 activity induced by FASL in monocytes loaded with vitamin C was markedly reduced (Figure 5B, P = .02).
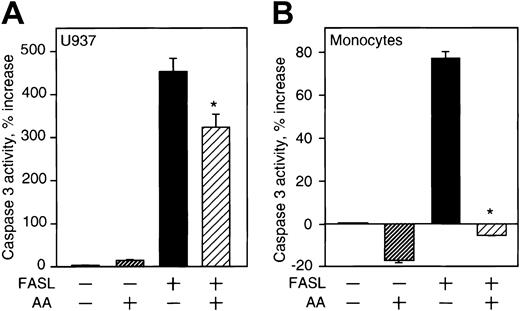
Effect of vitamin C on caspase-3 activity. (A) U937 cells loaded with approximately 13 mM vitamin C prior to treatment with FASL (200 ng/mL) were lysed, and the activity of caspase-3 in the cell lysates was determined as described in “Materials and methods.” (B) Monocytes loaded with approximately 14 mM vitamin C prior to treatment with FASL (100 ng/mL) were lysed, and the activity of caspase-3 in the cell lysates analyzed. The results are presented as the percentage increase in caspase activity compared with cells without treatment and are the mean of triplicate samples. These results are representative of 3 independent experiments for each cell type. Asterisks indicate statistically significant differences (in A, P = .03 and in B, P = .02) in caspase activity between cells loaded with vitamin C and control.
Effect of vitamin C on caspase-3 activity. (A) U937 cells loaded with approximately 13 mM vitamin C prior to treatment with FASL (200 ng/mL) were lysed, and the activity of caspase-3 in the cell lysates was determined as described in “Materials and methods.” (B) Monocytes loaded with approximately 14 mM vitamin C prior to treatment with FASL (100 ng/mL) were lysed, and the activity of caspase-3 in the cell lysates analyzed. The results are presented as the percentage increase in caspase activity compared with cells without treatment and are the mean of triplicate samples. These results are representative of 3 independent experiments for each cell type. Asterisks indicate statistically significant differences (in A, P = .03 and in B, P = .02) in caspase activity between cells loaded with vitamin C and control.
The results suggested that caspase-3 in U937 could be activated by pathways independent of mitochondrial signaling, and we therefore analyzed the effect of vitamin C on alternative mechanisms for caspase-3 activation in U937 cells. Caspase-10 is homologous to caspase-8 and is activated upstream in the apoptosis cascade and can activate caspase-3.40 We explored the effect of vitamin C on caspase-10 activity in U937 cells treated with FASL. The activity of caspase-10 increased 36% after incubation with FASL and was reduced by about 50% when the cells were loaded with vitamin C (Figure 6). We did not detect caspase-10 activity in monocytes after treatment with FASL (data not shown), and to our knowledge caspase-10 activity in monocytes has not been reported.
Effect of vitamin C on caspase-10 activity. U937 cells were loaded with 13 mM vitamin C prior to FASL treatment (200 ng/mL) and lysed to determine activity of caspase-10. The results are presented as the percentage increase in caspase activity compared with cells without treatment and are the mean of triplicate samples. These results are representative of 2 independent experiments. Asterisk indicates a statistically significant difference (P = .033) in caspase-10 activity of cells loaded with vitamin C compared with control.
Effect of vitamin C on caspase-10 activity. U937 cells were loaded with 13 mM vitamin C prior to FASL treatment (200 ng/mL) and lysed to determine activity of caspase-10. The results are presented as the percentage increase in caspase activity compared with cells without treatment and are the mean of triplicate samples. These results are representative of 2 independent experiments. Asterisk indicates a statistically significant difference (P = .033) in caspase-10 activity of cells loaded with vitamin C compared with control.
Vitamin C inhibits caspase-8 activation induced via FASL
ROS production, mitochondrial damage, and Cyt C release are downstream consequences of initiator caspases such as caspase-8.37 We therefore addressed the hypothesis that vitamin C inhibited FAS-dependent caspase-8 activation. In U937 cells incubated with FASL there was a 65% increase in caspase-8 activity, while vitamin C loading resulted in activity reduced to control levels (Figure 7A). A caspase-8 Western blot analysis indicated that cellular loading with vitamin C did not modify the expression of pro—caspase-8 in U937 cells (Figure 7B). In monocytes, FASL induced caspase-8 activity by 130%, whereas vitamin C—loaded cells showed little activation (Figure 7C).
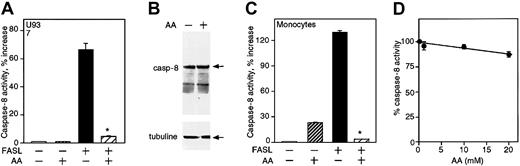
Effect of vitamin C on caspase-8 activity. (A) U937 cells were loaded with 13 mM vitamin C prior to FASL treatment. The cell lysates were analyzed for caspase-8 activity. Error bars represent the SD of triplicate values. (B) A Western blot was performed to analyze the expression of pro—caspase-8 in U937 cells loaded with 13 mM vitamin C (arrow, upper panel). Detection of β-tubulin was used as control for protein loading (arrow, lower panel). (C) Monocytes were loaded with 14 mM vitamin C prior to FASL treatment and the cell lysates were analyzed for caspase-8 activity. The results are the mean of triplicate samples expressed as the percentage increase in caspase activity compared with untreated cells and represent 3 (U937) or 2 (monocyte) experiments. (D) The activity of recombinant caspase-8 (1.25 U/well) with and without vitamin C was determined as detailed in “Materials and methods.” These results are expressed as the percentage of activity in relation to control wells (without vitamin C) and are representative of 3 independent experiments. Asterisks indicate statistically significant differences (P = .0005) in caspase activity of cells loaded with vitamin C before challenge with FASL.
Effect of vitamin C on caspase-8 activity. (A) U937 cells were loaded with 13 mM vitamin C prior to FASL treatment. The cell lysates were analyzed for caspase-8 activity. Error bars represent the SD of triplicate values. (B) A Western blot was performed to analyze the expression of pro—caspase-8 in U937 cells loaded with 13 mM vitamin C (arrow, upper panel). Detection of β-tubulin was used as control for protein loading (arrow, lower panel). (C) Monocytes were loaded with 14 mM vitamin C prior to FASL treatment and the cell lysates were analyzed for caspase-8 activity. The results are the mean of triplicate samples expressed as the percentage increase in caspase activity compared with untreated cells and represent 3 (U937) or 2 (monocyte) experiments. (D) The activity of recombinant caspase-8 (1.25 U/well) with and without vitamin C was determined as detailed in “Materials and methods.” These results are expressed as the percentage of activity in relation to control wells (without vitamin C) and are representative of 3 independent experiments. Asterisks indicate statistically significant differences (P = .0005) in caspase activity of cells loaded with vitamin C before challenge with FASL.
The inhibitory effect of vitamin C on the activity of caspase-8 in cells cultured with FASL could be due to a direct effect of vitamin C on the enzyme or to inhibition of enzyme activation. It has been reported that vitamin C can directly inhibit certain enzymes.41,42 As vitamin C is accumulated intracellulary in its reduced form, AA, we investigated if AA had a direct inhibitory effect on caspase-8 activity. The activity of recombinant caspase-8 was assayed in the presence of AA (1.0-20.0 mM). To inhibit the conversion of AA to DHA, 1,4-dithiothreitol was added to the reaction mixture. There was no significant effect of AA on the observed activity of recombinant caspase-8 (Figure 7D), while 20 μM Z-IEDT-FMK completely inhibited the activity of caspase-8 (not shown). Therefore, the data indicate that vitamin C is a potent inhibitor of the activation of caspase-8 without a direct effect on enzymatic activity.
These results indicate that vitamin C inhibits apoptosis induced by FAS-R in monocytes and U937 cells largely by preventing the activation of caspase-8. The molecular mechanisms of inhibition of the activation of caspase-8 are not known. We postulate that the activity of caspase-8 is dependent on production of ROS at the level of the FAS-R and that the antioxidant properties of vitamin C could inhibit this early step of FAS-initiated apoptosis signaling. There is support for this notion in the recent finding that preloading HL-60 cells with vitamin C inhibits the processing of pro—caspase-8 induced by hydrogen peroxide.43 Vitamin C also maintains the integrity of the mitochondrial membrane, which is disrupted during FAS signaling, and inhibits further downstream effects. Thus, the results suggest that vitamin C inhibits FAS-R—induced apoptosis in mononuclear phagocytes because of its antioxidant capabilities.
Discussion
Dietary vitamin C is critical for normal host defense, and vitamin C is widely believed to enhance immune function when used pharmacologically.18-20 Vitamin C may also have anti-inflammatory properties.21,44-46 There is now a substantial body of evidence defining a central role for oxidative reactions in cell signaling12-15,47,48 and showing that antioxidants can inhibit important signaling pathways in immune cells.16,17 For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) signaling involves ROS,17 and our evidence suggests that vitamin C inhibition of GM-CSF signaling occurs at the level of JAK-2 kinase activation.46 In this study, we undertook to understand the role of vitamin C in FAS-mediated signaling for apoptosis in human monocytes and the monocytic cell line U937.
Vitamin C circulates in the plasma as AA; however, it is generally transported into cells as DHA through the facilitative glucose transporters.23,24 Inside the cell, DHA is rapidly reduced to AA.24,28 In this way cells can accumulate high intracellular concentrations of vitamin C. For example, the intracellular concentration of AA in mononuclear cells is reported to be about 3 mM, with circulating concentrations of AA in the range of 50 μM.31,32 Specialized cells can transport AA directly through sodium/ascorbate cotransporters.25 AA is often used in in vitro experiments, leading to confounding results due to the lack of direct transport of ascorbate and because AA acts as a pro-oxidant in the presence of the free transition metals ubiquitously found in tissue culture.26 We therefore used DHA to load cells with vitamin C24 and analyzed its effect on the intracellular events mediating FAS-induced apoptosis in monocytic cells.
The central observation of this work was a significant inhibition of FAS-induced apoptosis by vitamin C loading in monocytes and U937 cells. We used pharmacologic concentrations of vitamin C to ascertain the effect of vitamin C loading on the apoptotic process. The lowest intracellular concentration of AA used in these experiments was higher than the physiologic intracellular AA reported for normal human monocytes (3 mM).31,32 Loading monocytes with 4 mM AA conferred significant protection from FAS-R—induced apoptosis. Viability of U937 cells and monocytes was not impaired by pharmacologic concentrations of vitamin C. The general cellular level of ROS increased after FAS-R ligation, and that increase was abrogated by loading cells with vitamin C. The liberation of ROS during the apoptotic process is largely due to uncoupling of oxidative metabolism in the mitochondria.7 Others have noted that N-acetyl-L-cysteine reduces ROS in monocytes after FAS ligation,8 possibly through an increase in glutathione. The release of ROS in apoptosis is preceded by a decrease in mitochondrial membrane potential (Δψ),7 and we found that the mitochondrial Δψ was maintained in U937 cells loaded with vitamin C and stimulated with FAS, suggesting that vitamin C inhibited events occurring before injury to the mitochondria.
FASL-induced aggregation of FAS-R is believed to cause caspase-8 autoactivation, presumably related to proximity. Vitamin C loading, however, prominently inhibited FASL-mediated activation of caspase-8 in U937 cells and in monocytes. In a cell-free system AA did not inhibit the enzymatic activity of recombinant caspase-8, and therefore these results suggest that vitamin C inhibits the activation of caspase-8, rather than its enzymatic activity. We postulate that local or “micro-oxidation” is required for caspase-8 activation and that vitamin C inhibits that process by quenching ROS. This thesis implies that FAS ligation leads to the local generation of ROS, which is required for caspase-8 activation. Indeed, a rapid and transient synthesis of oxygen radicals following FAS ligation has been reported that is independent of caspase activation,49,50 indicating that the initiation of FAS-mediated signaling is temporally associated with local generation of ROS. ROS production after receptor ligation has been noted for the IL-1R, TNFα-R, and other cytokine receptor systems,12 and there is abundant evidence for ROS-mediated signaling for growth factor receptors.13-15,17,51
Both vitamin C and the caspase-8 inhibitor Z-IETD-FMK inhibited apoptosis to a similar degree. The loss of mitochondrial Δψ as a consequence of FAS-R ligation was inhibited by both vitamin C and Z-IETD-FMK, although to a different extent, with almost complete protection provided by vitamin C. The greater stabilization of the mitochondrial membrane resulting from high concentrations of vitamin C in cells challenged with FAS may be due to vitamin C's capability to quench ROS generated at the receptor level and elsewhere in the cell. Nevertheless, this effect did not result in more protection from apoptosis than the selective inhibition of caspase-8. This result is consistent with the notion that the major mechanism by which vitamin C prevents apoptosis is through the inactivation of caspase-8; however, the data also point to an independent effect of vitamin C on mitochondrial membrane stabilization.
We studied the downstream consequences of inhibition of caspase-8 activation by vitamin C by measuring the liberation of Cyt C into the cytosol and the subsequent activation of caspase-3. Cyt C was not detected in the cytosol of FAS-stimulated U937 cells loaded with vitamin C, confirming that mitochondrial integrity was preserved in cells protected with vitamin C. Also, a FAS-induced increase in caspase-3 activity in monocytes was not detected in cells loaded with vitamin C. Vitamin C reduced basal levels of caspase-3 activity in these cells likely because there is some degree of oxidatively-induced apoptosis occurring in vitro.52 In U937 cells, however, vitamin C did not greatly inhibit caspase-3, maintaining 70% of FAS-induced activity. This phenomenon may be due to the activity of caspase-10, which can activate caspase-353 and which was active in U937 cells incubated with FAS (38% above basal).
Beneficial effects of vitamin C on immune function are frequently cited, and it is widely assumed that vitamin C enhances immunity via its antioxidant function. Blood monocytes migrate to tissues where some mature into macrophages, which participate importantly in the modulation of the adaptive immune system as phagocytes and antigen-presenting cells and by producing cytokines. Upon maturation into macrophages, monocytes down-regulate the expression of caspase-8 and up-regulate the expression of FAS-associated death domain—like IL-1 beta-converting enzyme-inhibitory protein, a natural caspase-8 inhibitor. Macrophages therefore are resistant to FAS-induced apoptosis.54,55 Before maturation, however, monocytes are highly susceptible to FAS-induced apoptosis while resting and during bacterial phagocytosis.52,56 Here we present evidence that the intracellular accumulation of vitamin C in monocytes causes resistance to FAS-induced apoptosis by inhibiting caspase-8 activation. A strong antioxidant such as vitamin C could prolong the active life of monocytes before they reach a FAS-insensitive stage. Maintenance of monocyte viability by vitamin C may be a physiologic mechanism of vitamin C in host defense. Further, the pharmacologic use of vitamin C administered as DHA57 could be a means to favorably affect host defense in pathologic states.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-11-3559.
Supported by a grant from the National Institutes of Health (CA 30388) and the Lebensfeld Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate the technical help of O. Borquez, C. Tat, and A. Pedraza.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal