Abstract
Sickle erythrocytes have increased ferritin and increased molecular iron on the inner membrane leaflet, and we postulated that cytosolic labile iron is also elevated. We used the fluorescent metal-losensor, calcein, and a permeant Fe2+ chelator to estimate labile cytoslic Fe2+, and calcein plus an Fe3+ chelator to estimate total cytosolic labile iron (Fe2+ + Fe3+). We measured membrane nonheme iron by its reactivity with ferrozine. As estimated by calcein and Fe2+ chelator, the mean ± SD labile Fe2+ concentration was significantly lower in hemoglobin (Hb) SS (n = 29) than hemoglobin AA (n = 17) erythrocytes (0.56 ± 0.35 μM versus 1.25 ± 0.65 μM; P < .001). In contrast, as estimated by calcein and Fe3+ chelator, total erythrocyte labile iron was similar in hemoglobin SS (n = 12) and hemoglobin AA (n = 10) participants (1.75 ± 0.41 μM versus 2.14 ± 0.93 μM; P = .2). Mean membrane nonheme iron levels were higher in hemoglobin SS cells than hemoglobin AA cells (0.0016 × 10-4 versus 0.0004 × 10-4 fmol/cell; P = .01), but much lower than the mean amounts of total labile iron (1.6-1.8 × 10-4 fmol/cell) or hemoglobin iron (18 000-19 000 × 10-4 fmol/cell). Both membrane iron and total labile iron were much less than the mean amount of iron potentially present in erythrocyte ferritin as calculated from results of other investigators (15 × 10-4 versus 34 × 10-4 fmol/cell in HbAA versus HbSS erythrocytes). We conclude that cytosolic labile iron is not elevated in hemoglobin SS erythrocytes and that elemental membrane iron is present in only trace amounts. (Blood. 2003;102:357-364)
Introduction
In sickle cell disease (SCD) valine is substituted for glutamic acid at position 6 of the β-globin chain of hemoglobin. The mutated hemoglobin S polymerizes under low oxygen tension1-3 and causes the red blood cells (RBCs) to lose their deformability and become dehydrated and rigid.4-6 Furthermore, hemoglobin S has an increased tendency to undergo auto-oxidation of the heme-bound iron to form methemoglobin,7,8 to undergo further oxidation of the globin subunits to form hemichromes,7,9 to dissociate the heme and globin moieties, and to interact and precipitate on the inner cytoplasmic surface of the red blood cell membrane.10 As a result of these processes, the cytoplasmic leaflet of the sickle red blood cell membrane contains abnormal insoluble iron aggregates that contain both heme and nonheme iron.11,12 The membrane binds nonheme ferric iron with great avidity.13,14 In fact the amount of nonheme iron exceeds the amount of heme iron in these membrane aggregates.11,13 This membrane-associated nonheme iron is reported to participate in oxidative reactions,11,15 thus contributing to dysfunction and premature demise of red blood cells and possibly to the pathophysiology of sickle cell disease.15,16 In addition to changes in the membrane, hemoglobin SS erythrocytes have increased ferritin content compared with hemoglobin AA erythrocytes.17,18
Despite the increased amount of membrane-associated iron and the increased ferritin content, it is not clear whether the cytosolic low-molecular-weight (or labile) iron concentration is elevated in hemoglobin SS erythrocytes. Since intracellular iron not associated with ferritin, heme proteins, and other enzymes is potentially toxic, elevated cytosolic labile iron might represent a means of damage to the cell membrane. It has been reported that hemoglobin-free cytosolic preparations of hemoglobin SS and β-thalassemic red blood cells contain no detectable iron as measured by atomic absorption spectrometry (limit of detection 2.5 μM),14 but “free” iron might have been lost in the preparation of hemoglobin-free cytosolic solutions. It has also been suggested that the cytoplasm of sickle red blood cells, unlike that of normal red blood cells, may contain low-molecular-weight Fe3+ chelates.8
Low-molecular-weight iron chelates or cytosolic labile iron in cells has been difficult to assess reliably as analytical techniques have depended on cell-disruptive procedures, which might themselves alter the cytosolic iron pool.19 Recently, Epsztejn et al described a methodology for estimating the labile iron levels in intact nonerythroid and erythroid cell lines using the iron-sensing fluorescent probe, calcein (CA).20 Subsequently we extended this methodology to human red blood cells in a study of iron chelation in erythrocytes infected with Plasmodium falciparum.21 In the present study we have used calcein methodology to estimate the cytosolic iron concentrations in intact erythrocytes from individuals with sickle cell disease (hemoglobins SS, SC, and Sβ-thalassemia), sickle cell trait (hemoglobin AS), and normal hemoglobin (hemoglobin AA). We hypothesized that hemoglobin SS erythrocytes, which are subject to denaturation of hemoglobin S and possible release of free iron from heme,11,12 would contain increased levels of labile iron compared with hemoglobin AA and hemoglobin AS red blood cells.
Patients, materials, and methods
Human subjects
The study was approved by the Howard University institutional review board, and informed consent was obtained from all participants before drawing blood. The research subjects consisted of sickle cell patients attending the pediatric and adult sickle cell clinics of Howard University Hospital, relatives of patients, and healthy control participants. Venous blood was drawn into EDTA-2Na (disodium ethylenediaminetetraacetic acid, 1 μg/mL) Vacutainer tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ). We studied 41 pediatric and adult sickle cell anemia (hemoglobin SS) patients, 2 patients with hemoglobin SC disease, 3 patients with hemoglobin Sβ-thalassemia, 7 healthy individuals with sickle cell trait (hemoglobin AS), and 24 healthy control individuals with hemoglobin AA. All sickle cell disease participants were in a steady state (not in pain crisis) and had not been transfused for at least 3 months before blood donation.
Laboratory determinations
Complete blood counts with differential and red blood cell indices were performed using a Coulter Gems automated cell counter (Beckman-Coulter, Miami, FL). Hemoglobin phenotypes of the participants were verified by electrophoresis on cellulose acetate (pH 8.4) and citrate agar (pH 6.4).22 Plasma iron was measured by a method modified from that recommended by the International Committee for Standardization in Haematology.23 Plasma ferritin was measured by a quantitative enzyme immunoassay procedure as described by Alfrey24 using a commercial kit (Ramco Laboratories, Stafford, TX). The amount of iron contained in hemoglobin per erythrocyte was calculated from the following equation:
Chemicals for determination of labile iron
Calcein (CA) and calcein-acetomethoxy (calcein/AM) were obtained from Molecular Probes (Eugene, OR). The Fe2+ chelator, 2′,2′-bipyridyl (BIP; 99+% pure) was purchased from Acros Organics, Summerville, NJ. Anticalcein antibody was a gift of Dr Z. I. Cabantchik (Department of Biological Sciences, Institute of Life Sciences, Hebrew University of Jerusalem, Jerusalem, Israel). The Fe3+ chelator, salicylaldehyde isonicotinoyl hydrazone (SIH), was a gift from Dr Prem Ponka (Lady Davis Institute for Medical Research, Jewish General Hospital, McGill University, Montreal). All other reagents were purchased from Fisher Scientific (Fair Lawn, NJ) or Sigma Chemical (St Louis, MO).
Preparation of erythrocytes for determination of labile iron
Free-flowing venous blood from the study participants was drawn into sodium EDTA tubes. Cell-free plasma was obtained by centrifuging the blood at 5000g for 20 minutes. The buffy coat of white blood cells was aspirated and discarded. The packed erythrocytes were resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-NaCl buffer with glucose (125 mM NaCl, 20 mM HEPES, 5 mM glucose, 295 mOsm, pH 7.35), (1:1, vol/vol) and layered on 3 mL of Ficoll-Paque (LKB-Pharmacia, Camden, NJ, and Mediatech CellGro, Fairfax, VA). The red blood cells were separated from the remaining platelets, mononuclear cells, and granulocytes by density centrifugation through the Ficoll-Paque at 1500 rpm for 15 minutes as previously described.5 The leukocyte-depleted red blood cells were then washed by centrifuging 4 times at 200g for 10 minutes at 22°C with 5 volumes of HEPES-saline-glucose buffer to remove the Ficoll-Paque. The washed erythrocytes were resuspended to a hematocrit of 25% in 5 mL HEPES-saline-glucose buffer to determine cell concentration. Cell concentration, cell volume, and other red blood cell indices were measured using the Coulter-Gems automatic cell counter (Beckman-Coulter).
Principles and rationale of the fluorescence measurements of labile iron in erythrocytes
Labile iron, which includes low-molecular-weight iron complexes, comprises the chelatable and rapidly exchangeable forms of cell iron. The liganded forms of cell labile iron exist in dynamic equilibrium as Fe2+ and Fe3+ salts, of which Fe2+ is the only soluble free form of the metal. When calcein (CA) enters the cell, some of it binds the free and soluble forms of iron and exists in the iron-bound (quenched) form [CA-Fe], while part remains free and unbound [CA] and provides the basal fluorescence (F1). This basal fluorescence varies among different cells and cells from different individuals depending on the intracellular concentration of labile iron and other intracellular factors that can quench the fluorescence such as pH, hemoglobin, and other pigmented molecules. Epsztejn et al,20 Breuer et al,25 and Espósito et al26 determined that the Fe-bound form of calcein [CA-Fe] is in rapid equilibrium with Fe2+ and therefore exists as follows:
Therefore, the formation of [CA-Fe] in the erythrocyte depends on the relative concentrations of “free” or labile Fe and free CA and the apparent dissociation constant, Kd, which can be expressed in terms of the relative concentrations of the reactants as follows:
Addition of excess amounts of a membrane-permeant iron chelator rapidly shifts the equilibrium to the right (Eq 2), toward the unbound forms, [CA] + [Fe2+], releasing the CA that was bound to the labile iron and resulting in a rise in free CA fluorescence intensity, which is denoted as (F2). The magnitude of the absolute change in fluorescence (F2 - F1) is equivalent to the change in calcein concentration or the amount of cell iron originally bound to CA (ie, [CA-Fe]). Thus, the changes in CA fluorescence intensity rapidly reflect changes of the soluble Fe concentration in the cytosol due to the stoichiometric (1:1) binding of Fe to CA. At equilibrium the relative concentrations of free calcein and calcein bound to Fe2+ depend on the dissociation constant, Kd (Eq 3). The fractional increase in fluorescence (Eq 4) therefore reflects labile iron concentration and can be used to calculate and compare labile iron concentrations in different cells:
This fractional increase in fluorescence intensity after the addition of the chelator is proportional to the change in calcein concentration in the cytosol and correlates with the concentration of labile iron within erythrocytes. Addition of an Fe2+-specific chelator such as BIP tends to measure the Fe2+ fraction of the labile iron pool, while addition of an Fe3+ chelator such as SIH tends to measure the total labile iron pool (ie, both Fe2+ and Fe3+).26 In our previous study, we demonstrated that the addition of hemoglobin (as red cell hemolysate), β-hematin (a synthetic analog of hemozoin), and hematin, at high nonphysiologic concentrations to solutions of calcein in buffer only slightly affected the fractional increases in calcein fluorescence.21 This experiment validated the calcein method, suggesting that it can be used to determine the labile iron status of red blood cells despite the presence of hemoglobin when changes in fluorescence intensities are examined as relative fractional increases (ΔF) rather than absolute values.21
Loading of erythrocytes with calcein-acetomethoxy and measurements of fluorescence
The labile iron in erythrocytes of the study cohort was determined as described by Epsztejn et al,20 Breuer et al,25 and Loyevsky et al.21 Leukocyte-depleted erythrocyte suspensions (2 × 109 cells/mL) from the participants were incubated with 500 μM calcein/AM for 30 minutes at 37°C in NaCl medium with 20 mM HEPES, 5 mM glucose, pH 7.35, 290 mOsm/kg, and at a final calcein/AM concentration of 250 nM. Following the incubation, the cells were washed 4 times with HEPES-saline-glucose buffer to remove the extracellular CA, and the cell suspension was adjusted to 107 cells/mL. One milliliter of the calcein-loaded erythrocytes (107cells/mL) was pipetted into a spectrofluorometer cuvette, an aliquot of anticalcein antibody (1:1000 final titer) was added, and the intracellular calcein fluorescence was recorded on a Perkin Elmer LS-50B Luminescence Spectrometer station (Perkin Elmer Instruments, Shelton, CT). The addition of anticalcein antibodies to the erythrocyte suspensions quenched any extracellular fluorescence in the suspension (which was < 1% of the initial signal) and ensured that all recorded fluorescence signals originated from within the red blood cells.21
Calcein fluorescence was monitored at an excitation wavelength of 488 nm and an emission wavelength of 512 nm. After a stable basal fluorescence signal (F1) was observed, BIP or SIH (100 μM final) was added to the suspension, which caused a rise in the fluorescence signal (F2). The addition of the chelator led to its competitive binding of intracellular labile iron, subsequent release of calcein-bound iron, and an increase in fluorescence intensity. The fractional increase in fluorescence (ΔF) therefore reflected labile iron concentration and was used, together with the measured values of Kd and the total intracellular calcein concentration, to calculate and compare the labile iron concentrations in the different erythrocytes.
Loading of dehydrated normal erythrocytes with calcein-acetomethoxy and measurements of fluorescence
Some sickle red blood cells are partially dehydrated and have high mean corpuscular hemoglobin concentration (MCHC). We examined the effect of red cell dehydration on calcein fluorescence methodology by measuring cytosolic labile Fe2+ in nondehydrated and dehydrated hemoglobin AA erythrocytes. Leukocyte-depleted hemoglobin AA erythrocytes were resuspended (2 × 109 cells/mL) in hypertonic medium (160 mM NaCl with 20 mM HEPES, 5 mM glucose, pH 7.35 yielding 350 mOsm/kg) for 10 minutes (including 3 washings with the hypertonic medium to maintain hyperosmolarity) and then loaded with calcein/AM. An aliquot of the same cells was treated in the same way with isotonic buffer. The mean corpuscular hemoglobin concentration (MCHC) of the nondehydrated and dehydrated cells was determined using the nonaqueous phthalate ester density technique as previously described.5 With this technique, the measured change in median density of the cells is due to the increase in intracellular viscosity and proportional to the change in MCHC. The labile Fe2+ concentrations in nondehydrated and dehydrated erythrocytes were then determined with the calcein and BIP.
Estimation of dissociation constant (Kd) of calcein-metal complexes in various types of erythrocytes
The apparent dissociation constant (Kd) of calcein-Fe complexes at equilibrium in red blood cells was determined independently for the healthy control, sickle trait, and sickle erythrocytes as described in our previous report21 by substituting cobalt for iron. The Kd for calcein-Fe2+ and calcein-Co2+ complexes are identical but Co2+, unlike Fe2+, does not undergo oxidation and provides a better estimation of Kd.20 Briefly, control and sickle erythrocytes were loaded with calcein/AM. After washing the cells to remove the remaining extracellular calcein/AM, the cell suspensions were supplemented with 1 μM A23187 ionophore (final concentration) to permit equilibration of the intracellular and extracellular concentrations of Co2+ during the fluorometric titration to determine Kd. Cobalt chloride solution was added to the cell suspension in aliquots to attain 0.01 μM to 0.50 μM of metal concentration. The quenching of fluorescence was continuously recorded for about 3 minutes after each addition of cobalt chloride. The best fit of the relationship between calcein fluorescence and cobalt concentration represents the nonlinear least-squares best fit, and was obtained by using the following equation for the equilibrium in the association reaction of Co2+ with calcein to form CA-Co complex:
where [CA] is the concentration of calcein that was added to the red cell suspension; [Co] is the cumulative concentration of cobalt that was added to the cell suspension; and [CA-Co] is the concentration of calcein-cobalt complexes present in the cell suspensions upon additions of Co. [CA-Co] was derived from the relationship between measured fluorescence values and the initial fluorescence of calcein in the red blood cell suspensions before the addition of cobalt. The equation was solved for Kass, the association constant for the calcein-cobalt complexes in the suspension. The apparent dissociation constant Kd is 1/Kass.
Estimation of intracellular calcein concentration
The total intracellular calcein concentration, [CA]t, that provided the measured fluorescence was determined as follows: CA-loaded erythrocyte (107 cells/mL) suspension in 1 mL HEPES-buffered NaCl containing anticalcein antibodies was used to measure basal fluorescence signal (F1) and the increase in fluorescence intensity (F2) after addition of 100 μM BIP or SIH. The calcein concentration in the cell suspension, [CA]s, was determined using a suspension of calcein-loaded red blood cells treated with BIP or SIH (100 μM), but without the calcein antibody, and then by adding increasing 1-nM aliquots of calcein solution sequentially to the suspension. The changes in fluorescence signals (F1) and (F2) and cumulative increases in the fluorescence signal after addition of each 1-nM solution of calcein were recorded. The cumulative fractional increase in fluorescence (ΔF) was calculated and used to establish a calibration curve of ΔF versus calcein in solution, [CA]s (where [CA]s was 1 nM to 5 nM), for every sample of red blood cell suspension of every subject in the study cohort. Using the measured mean corpuscular volume of the erythrocyte for each subject's sample (Vc), and the number of red blood cells per milliliter of suspension (Nc), the total intracellular [CA]t was calculated from the relationship [CA]t = [CA]s ÷ (Nc × Vc) as previously described.20,21
Estimation of the labile iron pools in erythrocytes
The calculations of erythrocyte labile iron concentration or pool (LIP) for every measured sample of the study subjects in the 4 groups were then performed using the following equation:
where ΔF represents the fractional increase in fluorescence intensity after addition of BIP or SIH to the erythrocyte suspensions in a spectrofluorometer cuvette (Eq 4); Kd is the dissociation constant of calcein and iron for human erythrocytes as determined for the cells of the hemoglobin AA, hemoglobin AS, and hemoglobin SS participants; and [CA]t is the total intracellular concentration of calcein.
Measurement of RBC ghost membrane-associated nonheme iron
Ghosts from hemoglobin AA and hemoglobin SS erythrocytes were prepared by 1:40 dilution of a known number of erythrocytes into ice-cold solution of 5 mM NaHPO, pH 8.0. Ghosts were washed with cold buffer until white (at 14 000g, 40 minutes at 4°C; usually 5 wash cycles with 5-minute incubation on ice between washes). White ghosts were suspended in 0.2 M HEPES, pH 7, to a concentration of 50 to 80 × 108 ghosts/mL. The ghosts were incubated for 10 minutes at 37°C with 1 mL of 0.5 mM diethylenetriamine pentaacetic acid (DTPA) to chelate all the free iron from the solution and washed 3 times in 1 mL of HEPES buffer to remove the residual DTPA. A known amount of the ghosts was suspended in 3 mL of HEPES buffer to a concentration of 8 to 10 × 108/mL and used for the measurement of total nonheme iron associated with the cell membrane.27 Briefly, 200 μL of the ghost suspension was dissolved with 500 μL of 0.6% sodium dodecyl sulfate (SDS) in 0.2 M sodium acetate, pH 4.5. A 400-μL aliquot of the SDS-dissolved ghosts was taken into 500 μL of reductants solution (0.2% ascorbic acid and 0.2% sodium dithionite dissolved in 0.2 M sodium acetate, pH 4.5) and incubated for 5 minutes at room temperature. Then, 100 μL of the color-developing solution was added (200 mg ferrozine and 1.25 g thiourea in 50 mL water). After 5 minutes of incubation at room temperature, the absorbance was measured at 562 nm. A standard curve was developed using known standard solutions of ferrous chloride (0 to 4 μM) and used to determine the concentration of iron in the unknown samples.
Statistical analysis of the data
The results of the data are expressed as means ± standard deviation or median and ranges. We used the Student t test to compare the results for absolute (F2 - F1) and fractional (ΔF) increases in fluorescence intensities, total intracellular calcein concentrations, and estimated erythrocyte labile iron concentrations between each of 3 groups of participants with variant hemoglobins (hemoglobins SS, SC or Sβ-thalassemia, and AS) and the control hemoglobin AA participants. All tests were 2-tailed, and a significance level of .05 was used. Linear regression was used to correlate hemoglobin concentrations, leukocytes counts, and plasma ferritin levels with erythrocyte labile Fe2+ concentrations among subjects with hemoglobins SS and SC or Sβ-thalassemia.
Results
Demographic and clinical characteristics of participants
Some demographic and clinical characteristics of the study participants are summarized in Table 1. Of the 41 hemoglobin SS patients, 28 were receiving hydroxyurea therapy at the time of labile iron analysis, as were 1 of 5 subjects with hemoglobins SC or Sβ-thalassemia. At least once in their lifetime, 30 of 41 hemoglobin SS subjects and 2 of 5 subjects with hemoglobins SC or Sβ-thalassemia had been transfused, but hemoglobin electrophoresis of the patients' blood samples did not show the presence hemoglobin A at the time of the study. Plasma iron and serum ferritin concentrations tended to be higher in the patients with sickle cell disease than the individuals with sickle cell trait (hemoglobin AS) or normal hemoglobin AA (Table 1).
Clinical and laboratory characteristics of the study cohort
Variable . | Hemoglobin SS, n = 41 . | Hemoglobin SC, Sβthal, n = 5 . | Hemoglobin AS, n = 7 . | Hemoglobin AA, n = 24 . |
|---|---|---|---|---|
| Median age, y | 22 ± 16 | 27 ± 14 | 37 ± 14 | 41 ± 19* |
| Sex, M/F | 23/18 | 1/4 | 2/5 | 14/10 |
| Number (%) receiving hydroxyurea treatment | 28 (68) | 1 (20) | 0 (0) | 0 (0) |
| Hemoglobin, g/dL | 9.0 ± 1.6* | 10.4 ± 2.7 | 12.4 ± 0.9† | 13.3 ± 1.2‡ |
| White blood cells, 103/μL | 11.7 ± 5.6* | 13.8 ± 5.0 | 6.5 ± 1.0§ | 5.9 ± 1.4‡ |
| Absolute neutrophils, 103/μL | 6.1 ± 3.9∥ | 8.6 ± 3.2 | 3.6 ± 1.2§ | 2.9 ± 0.9# |
| Absolute lymphocytes, 103/μL | 3.8 ± 1.9∥ | 4.3 ± 2.2 | 2.3 ± 0.9§ | 1.3 ± 1.3# |
| Absolute monocytes, 103/μL | 1.3 ± 1.6∥ | 0.8 ± 0.4 | 0.4 ± 0.1§ | 0.4 ± 0.1** |
| Platelets, 103/μL | 397 ± 143* | 392 ± 145 | 280 ± 98§ | 222 ± 61‡ |
| Plasma iron, μg/dL | 164 ± 90∥ | 162 ± 55§ | 139 ± 75 | 118 ± 66# |
| Ferritin, ng/mL in median (range) | 262†† (16-5523) | 413‡‡ (128-2224) | 30§§ (6-483) | 46∥∥ (10-1468) |
Variable . | Hemoglobin SS, n = 41 . | Hemoglobin SC, Sβthal, n = 5 . | Hemoglobin AS, n = 7 . | Hemoglobin AA, n = 24 . |
|---|---|---|---|---|
| Median age, y | 22 ± 16 | 27 ± 14 | 37 ± 14 | 41 ± 19* |
| Sex, M/F | 23/18 | 1/4 | 2/5 | 14/10 |
| Number (%) receiving hydroxyurea treatment | 28 (68) | 1 (20) | 0 (0) | 0 (0) |
| Hemoglobin, g/dL | 9.0 ± 1.6* | 10.4 ± 2.7 | 12.4 ± 0.9† | 13.3 ± 1.2‡ |
| White blood cells, 103/μL | 11.7 ± 5.6* | 13.8 ± 5.0 | 6.5 ± 1.0§ | 5.9 ± 1.4‡ |
| Absolute neutrophils, 103/μL | 6.1 ± 3.9∥ | 8.6 ± 3.2 | 3.6 ± 1.2§ | 2.9 ± 0.9# |
| Absolute lymphocytes, 103/μL | 3.8 ± 1.9∥ | 4.3 ± 2.2 | 2.3 ± 0.9§ | 1.3 ± 1.3# |
| Absolute monocytes, 103/μL | 1.3 ± 1.6∥ | 0.8 ± 0.4 | 0.4 ± 0.1§ | 0.4 ± 0.1** |
| Platelets, 103/μL | 397 ± 143* | 392 ± 145 | 280 ± 98§ | 222 ± 61‡ |
| Plasma iron, μg/dL | 164 ± 90∥ | 162 ± 55§ | 139 ± 75 | 118 ± 66# |
| Ferritin, ng/mL in median (range) | 262†† (16-5523) | 413‡‡ (128-2224) | 30§§ (6-483) | 46∥∥ (10-1468) |
Results are given as mean ± standard deviation unless otherwise stated. Conversion to Sl units for white blood cells, absolute neutrophils, absolute lymphocytes, absolute monocytes, and platelets: 103/μL × 1 = × 109/L; ferritin: ng/mL × 1 = μg/L.
n = 38.
n = 5.
n = 10.
n = 4.
n = 34.
n = 9.
n = 8.
n = 20.
n = 3.
n = 6.
n = 6.
Estimated dissociation constant (Kd) of calcein-metal complexes in erythrocytes
A Kd of 0.32 ± 0.03 μM was estimated for hemoglobin AA erythrocytes, 0.30 ± 0.03 μM for hemoglobin AS erythrocytes, and 0.25 ± 0.02 μM for hemoglobin SS erythrocytes.
Erythrocyte labile Fe2+ concentrations as measured by calcein and Fe2+ chelator
Table 2 presents the means and standard deviations of the absolute and fractional (ΔF) increases in fluorescence intensities following addition of the ferrous iron chelator, BIP, to the erythrocyte suspensions. The table also shows the estimated erythrocyte concentrations of calcein and labile Fe2+ for the various groups of study participants. The absolute and fractional increases in fluorescence intensities and the estimated labile Fe2+ concentrations of the patients with sickle cell disease (hemoglobin SS or hemoglobins SC or Sβ-thalassemia) were significantly lower than the comparable values from the control hemoglobin AA individuals. In contrast, the fractional changes in fluorescence and labile Fe2+ concentrations did not differ significantly between the hemoglobin AS individuals and the hemoglobin AA controls. The estimated total intracellular calcein concentrations in erythrocytes of hemoglobin SS patients were significantly lower than the concentrations in the hemoglobin AA erythrocytes, but these values for the erythrocytes of hemoglobins SC or Sβ-thalassemia individuals did not differ significantly from the control values. A subset analysis comparing the mean labile Fe2+ concentrations for hemoglobin SS patient who were on hydroxyurea therapy with those not on therapy showed no significant differences.
Intracellular calcein fluorescence, estimated calcein, and labile iron (Fe2+) values in erythrocytes
Erythrocyte hemoglobin genotype . | Absolute fluorescence, au . | Fractional rise in fluorescence, ΔF . | RBC calcein [CA]t, μM . | Erythrocyte labile iron (II), μM . |
|---|---|---|---|---|
| HbSS RBC, n = 29 | 38.70 ± 23.30† | 0.22 ± 0.08‡ | 2.03 ± 1.02§ | 0.56 ± 0.35§ |
| HbSC, Sβthal RBC, n = 5 | 36.55 ± 9.17† | 0.26 ± 0.05* | 2.71 ± 0.55 | 0.81 ± 0.24† |
| HbAS RBC, n = 7 | 34.14 ± 13.44† | 0.30 ± 0.13 | 3.02 ± 1.23 | 1.09 ± 0.70 |
| HbAA RBC, n = 17 | 55.50 ± 27.13 | 0.32 ± 0.11 | 3.24 ± 1.12 | 1.25 ± 0.65 |
Erythrocyte hemoglobin genotype . | Absolute fluorescence, au . | Fractional rise in fluorescence, ΔF . | RBC calcein [CA]t, μM . | Erythrocyte labile iron (II), μM . |
|---|---|---|---|---|
| HbSS RBC, n = 29 | 38.70 ± 23.30† | 0.22 ± 0.08‡ | 2.03 ± 1.02§ | 0.56 ± 0.35§ |
| HbSC, Sβthal RBC, n = 5 | 36.55 ± 9.17† | 0.26 ± 0.05* | 2.71 ± 0.55 | 0.81 ± 0.24† |
| HbAS RBC, n = 7 | 34.14 ± 13.44† | 0.30 ± 0.13 | 3.02 ± 1.23 | 1.09 ± 0.70 |
| HbAA RBC, n = 17 | 55.50 ± 27.13 | 0.32 ± 0.11 | 3.24 ± 1.12 | 1.25 ± 0.65 |
Results are mean ± standard deviation. au indicates arbitary units.
P < .1 compared with Hb AA group.
P < .05 compared with HbAA group.
P < .01 compared with HbAA group.
P < .001 compared with HbAA group.
Estimation of labile iron concentrations using calcein and Fe2+ chelator versus calcein and Fe3+chelator
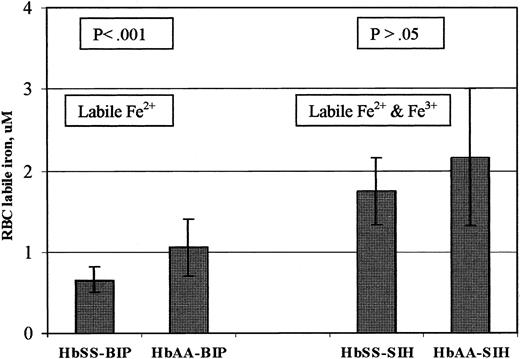
Since cytosolic labile iron is in dynamic equilibrium as Fe2+ ↔ Fe3+ and since hemoglobin SS erythrocytes have increased oxidative potential compared with hemoglobin AA erythrocytes,7,8 it is conceivable that using an Fe3+ chelator in the calcein-based assay for cytosolic labile iron could give a different result from using an Fe2+ chelator. We performed parallel measurements of erythrocyte labile iron concentration in the same sample using the Fe2+ or Fe3+ chelator. As estimated by the calcein and Fe2+ chelator system, the labile Fe2+ pools for 12 hemoglobin SS patients (mean ± SD of 0.65 ± 0.16 μM) was significantly lower than those in 10 hemoglobin AA participants (1.09 ± 0.36 μM) (P < .001). As estimated by the calcein and Fe3+ chelator system, the labile iron pools for 12 hemoglobin SS patients (1.75 ± 0.41 μM) were not significantly different compared with those estimated in 10 hemoglobin AA participants (2.14 ± 0.93 μM) (P = .199) (Table 3 and Figure 1).
Intracellular calcein fluorescence, estimated calcein, and labile iron values in hemoglobin AA and hemoglobin SS erythrocytes measured using BIP or SIH
. | Absolute fluorescence rise, au . | . | . | Fractional fluorescence rise, ΔF . | . | . | Erythrocyte, calcein [CA]t, μM . | . | . | Erythrocyte labile iron pool, μM . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythrocyte hemoglobin genotype . | BIP . | SIH . | P* . | BIP . | SIH . | P* . | BIP . | SIH . | P* . | BIP . | SIH . | P* . | ||||||||
| HbSS erythrocytes, n = 12 | 25.49 ± 7.67† | 75.27 ± 16.82† | < .001 | 0.18 ± 0.03‡ | 0.40 ± 0.03† | < .001 | 3.24 ± 0.62§ | 3.95 ± 0.93† | < .037 | 0.65 ± 0.16∥ | 1.75 ± 0.41† | < .001 | ||||||||
| HbAA erythrocytes, n = 10 | 32.60 ± 10.09 | 65.96 ± 15.54 | < .001 | 0.25 ± 0.06 | 0.40 ± 0.10 | < .003 | 3.94 ± 0.54 | 4.48 ± 1.16 | .202 | 1.09 ± 0.36 | 2.14 ± 0.93 | < .004 | ||||||||
. | Absolute fluorescence rise, au . | . | . | Fractional fluorescence rise, ΔF . | . | . | Erythrocyte, calcein [CA]t, μM . | . | . | Erythrocyte labile iron pool, μM . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythrocyte hemoglobin genotype . | BIP . | SIH . | P* . | BIP . | SIH . | P* . | BIP . | SIH . | P* . | BIP . | SIH . | P* . | ||||||||
| HbSS erythrocytes, n = 12 | 25.49 ± 7.67† | 75.27 ± 16.82† | < .001 | 0.18 ± 0.03‡ | 0.40 ± 0.03† | < .001 | 3.24 ± 0.62§ | 3.95 ± 0.93† | < .037 | 0.65 ± 0.16∥ | 1.75 ± 0.41† | < .001 | ||||||||
| HbAA erythrocytes, n = 10 | 32.60 ± 10.09 | 65.96 ± 15.54 | < .001 | 0.25 ± 0.06 | 0.40 ± 0.10 | < .003 | 3.94 ± 0.54 | 4.48 ± 1.16 | .202 | 1.09 ± 0.36 | 2.14 ± 0.93 | < .004 | ||||||||
Results are mean ± standard deviation. P value determined at .05 level comparing the mean values of each variable for hemoglobin SS versus hemoglobin AA. BIP indicates 2′2′-bipyridyl, iron (Fe2+) chelator; SIH, salicylaldehyde isonicotinoyl hydrazone, iron (Fe3+) chelator.
P value, BIP vs SIH for HbSS or HbAA erythrocytes.
P > .05 compared with HbAA group.
P < .05 compared with HbAA group.
P < .01 compared with HbAA group.
P < .001 compared with HbAA group.
Erythrocyte labile iron concentrations using calcein and Fe2+ chelator versus calcein and Fe3+ chelator. Erythrocytes were loaded with calcein using acetomethoxyl-calcein, and fluorescence was measured before and after addition of 100 μM permeant Fe2+ chelator (BIP) or Fe3+ chelator (SIH) to separate identical cell suspensions from the same subject. Erythrocyte labile iron was then calculated based on the fractional increase in fluorescence, the intracellular calcein concentration, and the dissociation constant, Kd, of calcein and iron, as described in “Patients, materials, and methods.” The bar graphs show mean ± SD of erythrocyte labile iron concentration of 10 hemoglobin AA control individuals and 12 patients with hemoglobin SS.
Erythrocyte labile iron concentrations using calcein and Fe2+ chelator versus calcein and Fe3+ chelator. Erythrocytes were loaded with calcein using acetomethoxyl-calcein, and fluorescence was measured before and after addition of 100 μM permeant Fe2+ chelator (BIP) or Fe3+ chelator (SIH) to separate identical cell suspensions from the same subject. Erythrocyte labile iron was then calculated based on the fractional increase in fluorescence, the intracellular calcein concentration, and the dissociation constant, Kd, of calcein and iron, as described in “Patients, materials, and methods.” The bar graphs show mean ± SD of erythrocyte labile iron concentration of 10 hemoglobin AA control individuals and 12 patients with hemoglobin SS.
Labile Fe2+ concentration in nondehydrated and dehydrated normal erythrocytes
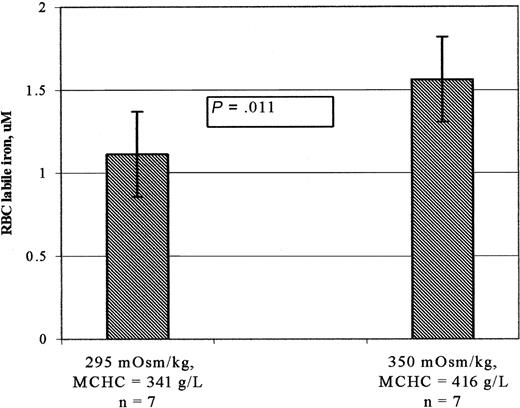
Dehydrating hemoglobin AA erythrocytes from 7 control participants increased the MCHC from a mean value of 341 ± 8 g/L to 416 ± 16 (P < .005). As shown in Figure 2, mean ± SD labile cytosolic Fe2+ concentrations increased from 1.11 ± 0.29 μM in cells maintained under isotonic conditions (295 mOsm/kg) to 1.56 ± 0.26 μM in cells maintained under hypertonic conditions (350 mOsm/kg) (P = .011). As to be expected, the average rise in the labile Fe2+ concentration (41%) was greater than the average rise in the MCHC (22%).
Labile iron concentrations of normal hemoglobin AA erythrocytes in isotonic and hypertonic buffers. Erythrocytes suspended in isotonic or hypertonic buffers were loaded with calcein using calcein acetomethoxy, and fluorescence was measured before and after addition of the 100 μM permeant Fe2+ chelator (BIP). Erythrocyte labile iron was then calculated based on the fractional increase in fluorescence, the intracellular calcein concentration, and the dissociation constant, Kd, of calcein and iron, as described in “Patients, materials, and methods.” The bar graphs show mean ± SD of erythrocyte labile iron concentration of 7 hemoglobin AA (HbAA) control individuals. The increase in MCHC of erythrocytes suspended in hypertonic buffer was determined using the phthalate ester technique as described in ”Patients, materials, and methods.” The average increase in the labile Fe2+ concentration (41%) of cells in hypertonic buffer was greater than the average rise in the MCHC (22%).
Labile iron concentrations of normal hemoglobin AA erythrocytes in isotonic and hypertonic buffers. Erythrocytes suspended in isotonic or hypertonic buffers were loaded with calcein using calcein acetomethoxy, and fluorescence was measured before and after addition of the 100 μM permeant Fe2+ chelator (BIP). Erythrocyte labile iron was then calculated based on the fractional increase in fluorescence, the intracellular calcein concentration, and the dissociation constant, Kd, of calcein and iron, as described in “Patients, materials, and methods.” The bar graphs show mean ± SD of erythrocyte labile iron concentration of 7 hemoglobin AA (HbAA) control individuals. The increase in MCHC of erythrocytes suspended in hypertonic buffer was determined using the phthalate ester technique as described in ”Patients, materials, and methods.” The average increase in the labile Fe2+ concentration (41%) of cells in hypertonic buffer was greater than the average rise in the MCHC (22%).
Erythrocyte ghost membrane-associated nonheme iron
The membranes of hemoglobin SS erythrocytes (n = 6) contained significantly higher amounts of nonheme iron compared with hemoglobin AA erythrocytes (n = 6) (0.0016 ± 0.0009 versus 0.0004 ± 0.0001 × 10-4 fmol/cell; P = .01). However, as shown in Table 4, in both hemoglobin SS and hemoglobin AA erythrocytes, these were only trace amounts of iron when compared with the amounts of hemoglobin iron and cytosolic iron measured in the same cells. These amounts of membrane iron are also miniscule compared with the amounts of iron potentially present in intracellular ferritin as calculated from the results of other investigators (34 × 10-4 fmol iron/cell in hemoglobin SS erythrocytes and 15 × 10-4 fmol iron/cell in hemoglobin AA erythrocytes).18
Average intracellullar amounts per cell of hemoglobin iron, cytosolic labile iron, and membrane-associated iron in hemoglobin AA and hemoglobin SS erythrocytes
Variable . | HbAA, n = 6 . | HbSS, n = 6 . | P* . |
|---|---|---|---|
| Hemoglobin iron, × 10−4 fmol/cell | 17 873 ± 1 348 | 18 763 ± 3 207 | .65 |
| RBC labile Fe2+ (with calcein & BIP), × 10−4 fmol/cell | 1.08 ± 0.34 | 0.66 ± 0.22 | .03 |
| RBC total labile Fe2+ and Fe3+ (with calcein & SIH), × 10−4 fmol/cell | 1.58 ± 0.71 | 1.78 ± 0.48 | .59 |
| RBC membrane iron, × 10−4 fmol/cell | 0.0004 ± 0.0001 | 0.0016 ± 0.0009 | .01 |
Variable . | HbAA, n = 6 . | HbSS, n = 6 . | P* . |
|---|---|---|---|
| Hemoglobin iron, × 10−4 fmol/cell | 17 873 ± 1 348 | 18 763 ± 3 207 | .65 |
| RBC labile Fe2+ (with calcein & BIP), × 10−4 fmol/cell | 1.08 ± 0.34 | 0.66 ± 0.22 | .03 |
| RBC total labile Fe2+ and Fe3+ (with calcein & SIH), × 10−4 fmol/cell | 1.58 ± 0.71 | 1.78 ± 0.48 | .59 |
| RBC membrane iron, × 10−4 fmol/cell | 0.0004 ± 0.0001 | 0.0016 ± 0.0009 | .01 |
Results are mean ± standard deviation. BIP indicates 2′2′-bipyridyl, iron (Fe2+) chelator; SIH, salicylaldehyde isonicotinoyl hydrazone, iron (Fe3+) chelator.
P value HbSS compared with HbAA group.
Correlation of erythrocyte labile iron with hemoglobin and other measured variables
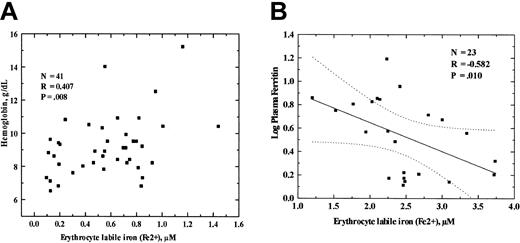
We examined the relationship of certain hematologic and plasma iron measurements with the erythrocyte labile iron concentrations as measured by the calcein and Fe2+ chelator system in sickle cell disease by combining the results for patients with hemoglobin SS and patients with hemoglobins SC or Sβ-thalassemia. Spearman rank correlation analysis revealed that erythrocyte labile Fe2+ concentrations of the sickle cell patients had a direct correlation with hemoglobin concentration (p = 0.407, P = .008), and an inverse correlation with plasma ferritin concentration (p = - 0.582, P = .010), leukocyte concentration (p = - 0.329, P = .10), and absolute monocyte count (p = - 0.379, P = .05). Erythrocyte labile Fe2+ concentrations did not correlate significantly with mean corpuscular volumes, absolute lymphocyte counts, absolute neutrophil counts, or plasma iron levels. The linear relationships of erythrocyte labile Fe2+ concentrations with hemoglobin concentrations and serum ferritin levels are shown in Figure 3.
Statistical correlation of the erythrocytes' labile iron concentration with hemoglobin and total serum ferritin. (A) Erythrocyte labile iron was measured by the calcein methodology and the permeant Fe2+-specific chelator, BIP, as described in “Patients, materials, and methods.” Hemoglobins of patients with HbSS, HbSC, and HbSβ-thalassemia were measured by standard technique. The results were analyzed by linear regression. The p and P values are shown in the inserts. Dotted lines indicate the 95% confidence limits. (B) Correlation with the erythrocyte labile Fe2+ concentration with logarithm of total serum ferritin concentration of patients with hemoglobin SS, hemoglobin SC, and Sβ-thalassemia genotypes. The p and P values are shown in the inserts. Dotted lines indicate the 95% confidence limits.
Statistical correlation of the erythrocytes' labile iron concentration with hemoglobin and total serum ferritin. (A) Erythrocyte labile iron was measured by the calcein methodology and the permeant Fe2+-specific chelator, BIP, as described in “Patients, materials, and methods.” Hemoglobins of patients with HbSS, HbSC, and HbSβ-thalassemia were measured by standard technique. The results were analyzed by linear regression. The p and P values are shown in the inserts. Dotted lines indicate the 95% confidence limits. (B) Correlation with the erythrocyte labile Fe2+ concentration with logarithm of total serum ferritin concentration of patients with hemoglobin SS, hemoglobin SC, and Sβ-thalassemia genotypes. The p and P values are shown in the inserts. Dotted lines indicate the 95% confidence limits.
Discussion
In this study we used the iron-sensing fluorescence probe, calcein, to determine the labile iron concentrations in intact erythrocytes of 4 groups of participants: (1) patients with hemoglobin SS, (2) patients with hemoglobins SC or Sβ-thalassemia, (3) participants with sickle cell trait (hemoglobin AS), and (4) control individuals (hemoglobin AA). Using the calcein and Fe2+ chelator system, we found significant differences in the estimated erythrocyte labile ferrous iron concentrations of the 2 groups of patients with sickle cell disease compared with the controls but not the sickle cell trait participants and the controls. On the other hand, using the calcein and Fe3+ chelator system, total cytosolic labile iron concentrations did not differ significantly between hemoglobin SS and hemoglobin AA erythrocytes (Figure 1).
Erythrocytes contain about 20 000 μM ferrous iron (Fe2+) coordinately bound to the protoporphyrin moiety of heme, but the concentration of “free” cytosolic ferrous iron in the erythrocyte is very low28,29 and has been difficult to measure in intact cells. In our previous study21 we demonstrated that calcein methodology can be applied to measure labile iron concentrations in solutions containing hemoglobin and heme and in intact erythrocytes, even though hemoglobin partially quenches the fluorescence of calcein. These observations laid the groundwork for the present study in which calcein methodology was used to assess the cytosolic iron status of erythrocytes from sickle cell disease patients compared with control participants with hemoglobin AA. Although hemoglobin SS cells tend to become dehydrated, Figure 2 provides evidence that the calcein methodology for determining erythrocyte labile iron concentration is valid in both nondehydrated and dehydrated erythrocytes.
The mean (± SD) erythrocyte cytosolic labile Fe2+ concentration of healthy control individuals of 1.25 ± 0.65 μM measured in the present study with calcein and BIP is lower than the median value of 2.96 μM (range of 2.53-4.73 μM) measured with calcein and BIP in our previous study.21 We believe that we have improved our technique since the previous study. Our present results are comparable with labile iron concentrations of human erythrocyte hemoglobin-free lysates analyzed by gel filtration (mean ± SD of 2.37 ± 1.23 μM)28 and measurements of deferrioxamine-chelatable iron in intact mouse erythrocytes (mean ± SD values of 1.7 ± 0.6 and 2.0 ± 0.2 μM).29
Our results for labile iron concentrations in hemoglobin SS and hemoglobin AA erythrocytes are contrary to our initial hypothesis that hemoglobin SS erythrocytes would contain the higher labile iron levels. However, our observation is consistent with the report of Shalev and Hebbel, in that they were not able to measure molecular iron in the hemoglobin-free lysates of sickle red blood cells by atomic absorption spectrometry.14 On the other hand, Sheng et al also suggested that the sickle cell cytoplasm may have low-molecular-weight Fe3+ chelates not present in normal red blood cells.8
Fe2+ ion is the major cytosolic metal that reacts with the fluorescent calcein in several cell systems other than erythrocytes,25,26,30 and this is likely the case with normal erythrocytes as well. Hemoglobin SS erythrocytes have increased oxidative potential compared with hemoglobin AA erythrocytes.7,8 Therefore, hemoglobin SS erythrocyte labile iron may be expected to have a higher Fe3+ fraction and a lower Fe2+ fraction than hemoglobin AA erythrocyte labile iron. The results of our present investigation are consistent with this expectation. Our observations of lower ferrous cytosolic labile iron but similar total cytosolic labile iron (Fe2+ and Fe3+) in hemoglobin SS compared with hemoglobin AA erythrocytes implies that hemoglobin SS erythrocytes have a higher ferric labile iron fraction.
To place the amounts of cytosolic labile iron measured in perspective, we determined the amounts of nonheme iron associated with the membrane in both hemoglobin SS and AA erythrocytes. The average amounts per cell of nonheme membrane-associated iron and of cytosolic labile iron are summarized in Table 4, along with the amounts of hemoglobin iron. Mean membrane nonheme iron levels were higher in hemoglobin SS cells than hemoglobin AA cells (0.0004 × 10-4 versus 0.0016 × 10-4 fmol/cell; P = .01), but much lower than the mean amounts of labile iron (1.6-1.8 × 10-4 fmol/cell) or hemoglobin iron (18 000-19 000 × 10-4 fmol/cell). The amounts of membrane iron we measured in hemoglobin SS erythrocytes are very similar to the findings of Kurros and Hebbel11 and Kurros et al.12 To facilitate comparisons of our data with those of other investigators for erythrocyte ferritin,18,31 we expressed published results in terms of the amount of iron potentially present in ferritin within erythrocytes. Using the assumptions of (1) mean corpuscular volume of 90 fL, (2) one ferritin molecule able to bind up to 4500 atoms of iron,32 and (3) 5.0 × 10-8 μg protein per erythrocyte membrane33,34 yields estimates of 34 × 10-4 fmol per cell for hemoglobin SS erythrocytes versus 15 × 10-4 fmol per cell for hemoglobin AA erythrocytes.18 Whether compared with potential or measured amounts of hemoglobin iron, ferritin iron, or labile iron, membrane nonheme iron appears to exist only in trace amounts in both hemoglobin SS and AA erythrocytes. In fact, one questions whether the slight membrane-associated iron of hemoglobin SS erythrocytes is pathologically important. The presence of trace amounts of nonheme iron associated with hemoglobin SS erythrocyte membranes could easily be explained by an uptake from the much larger cytosolic labile iron pool, without requiring breakdown of the heme molecule due to oxidative stress as others have proposed.11,12 Several model experiments have demonstrated that the polar head groups of aminophospholipids in sickle erythrocyte membranes have a high affinity for molecular iron compared with polar head groups in the hemoglobin AA erythrocyte membrane.14 It seems possible that the trace molecular iron detected in hemoglobin SS erythrocyte membranes could derive from the cytosolic labile iron pool as a result of increased affinity of the inner leaflet phospholipids for iron.35
We observed a positive correlation of erythrocyte labile iron concentration (as determined with the calcein and Fe2+ chelator system) with hemoglobin concentrations in SS patients and a negative correlation with white blood cell counts, raising the possibility that low cytosolic labile ferrous concentrations in hemoglobin SS erythrocytes might be a reflection of a more severe clinical course.36
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-03-0914.
Supported by National Institutes of Health (NIH) research grant no. UH1HL 03679 funded by the National Heart, Lung, and Blood Institute and the Office of Research on Minority Health, and by Howard University General Clinical Research Center grant from the NIH no. 2MO1 RR10284.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tiffany Johnson and Bak C. Kim for technical assistance; Selma Roberson, RN, for clinical assistance; and the Clinical Hematology Laboratories, Howard University Hospital, for laboratory assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal