Abstract
Hematologic stem cell rescue after high-dose cytotoxic therapy is extensively used for the treatment of many hematopoietic and solid cancers. Gene marking studies suggest that occult tumor cells within the autograft may contribute to clinical relapse. To date purging of autografts contaminated with cancer cells has been unsuccessful. The selective oncolytic property of reovirus against myriad malignant histologies in in vitro, in vivo, and ex vivo systems has been previously demonstrated. In the present study we have shown that reovirus can successfully purge cancer cells within autografts. Human monocytic and myeloma cell lines as well as enriched ex vivo lymphoma, myeloma, and Waldenström macroglobulinemia patient tumor specimens were used in an experimental purging model. Viability of the cell lines or purified ex vivo tumor cells of diffuse large B-cell lymphoma, chronic lymphocytic leukemia, Waldenström macroglobulinemia, and small lymphocytic lymphoma was significantly reduced after reovirus treatment. Further, [35S]-methionine labeling and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of cellular proteins demonstrated reovirus protein synthesis and disruption of host cell protein synthesis as early as 24 hours. Admixtures of apheresis product with the abovementioned tumor cells and cell lines treated with reovirus showed complete purging of disease. In contrast, reovirus purging of enriched ex vivo multiple myeloma, Burkitt lymphoma, and follicular lymphoma was incomplete. The oncolytic action of reovirus did not affect CD34+ stem cells or their long-term colony-forming assays even after granulocyte colony-stimulating factor (G-CSF) stimulation. Our results indicate the ex vivo use of an unattenuated oncolytic virus as an attractive purging strategy for autologous stem cell transplantations. (Blood. 2003;102:377-387)
Introduction
Autologous hematopoietic progenitor stem cell (ASC) transplantations following high-dose chemotherapy has gained extensive application as a therapeutic modality in several malignancies.1-7 Globally, the number of autologous blood and marrow transplantations now surpasses the number of allotransplantations.6-8 Despite the significant increase in ASC transplantations, controversy still exists as to the contribution of minimal residual disease to the development of relapse after high-dose chemotherapy. Evidence supported by gene marking studies indicates relapse following high-dose ablative therapy followed by ASC transplantation may be due to contaminating cancer cells within the autograft.9-11 It has been estimated that more than 30% of all autografts are contaminated with tumor cells, and this number likely will increase with better detection methodology and increasing use of ASC transplantations in patients with advanced disease.
To minimize the number of contaminating tumor cells, a variety of purging techniques to rid the graft of residual tumor cells have been used. The ideal purging technique should preferentially destroy contaminating tumor cells while preserving the number and function of the collected stem cells. Widely cited purging methods of autografts include the use of ex vivo chemotherapy, tumor targeting monoclonal antibodies linked with toxins or selected on immunocolumns, and positive (CD34+) selection.12,13 More recently, photodynamic purging processes,14-16 virus-directed enzyme prodrug therapy,17 receptor-targeted ligand toxins,18,19 and attenuated replication-competent virus-based purging techniques20 have been reported. Yet, to date no method has proved 100% successful in depleting autografts of tumor cells in the clinical setting. Although several studies have suggested that graft manipulation is of clinical benefit,21-24 until purging techniques lead to a complete eradication of contaminating tumor cells, it will be unclear whether the recurrence of the disease is the result of the contaminating tumor cells or a reflection of a resistant in vivo malignancy or both. In the present study we investigated the use of an unattenuated oncolytic virus, reovirus, as the basis for a novel purging strategy for ASC transplantation.
Reovirus is an ubiquitous double-stranded RNA virus that can be isolated from the upper respiratory and gastrointestinal tract of humans. Clinically, this virus is not considered significant because infections by reovirus are generally asymptomatic.25 It has, however, been extensively studied as a prototype for double-stranded RNA viruses. Reovirus is internalized into cells via the ubiquitous sialic acid receptor26 and uses a strategy of cell infection and lysis through exploitation of an already activated oncogenic signaling pathway in tumor cells.27 In reovirus-resistant or untransformed cells, a 65-kDa protein, identified as the double-stranded RNA activated protein kinase (PKR), is phosphorylated by the viral transcripts. The activated PKR in turn phosphorylates the α-subunit of the translation initiation factor 2 (eIF-2), leading to inhibition of viral gene translation. In Ras and probably other signal transduction pathway-transformed cells, PKR phosphorylation is inhibited, thereby allowing viral protein synthesis to ensue and ultimately a lytic infection to develop.27
We have previously reported that reovirus is oncolytic to numerous neoplasms, including several hematologic malignancies.28-33 Our results suggest that reovirus can be used as a successful ex vivo purging agent in many patients that undergo autotransplantation.
Materials and methods
Cell lines
Established cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA). U937 (monocytic) and RPMI 8226 (myeloma) cells were maintained in RPMI 1640 medium (Gibco BRL, Burlington, ON, Canada) containing 10% fetal bovine serum (FBS). U937 and RPMI 8226 cells were immunophenotyped by flow cytometry to identify markers suitable for minimal residual disease (MRD) detection. Although U937 is listed by ATCC as a histiocytic lymphoma, its immunophenotype suggests it is monocytic in origin: CD45+, CD33+, CD13+, CD15+, CD11b+, CD36+, CD11c+, CD4+, CD7+, CD19-, CD20-, CD10-, CD3-, CD2-, CD5-, CD34-, CDK-, and CDΛ-. CD33 and CD45 were used for the detection of U937 cells. CD38 and CD138 were used for the identification of RPMI 8226 myeloma cells.
Primary tumor cells
Primary tumors for which autotransplantations are performed were obtained from peripheral blood (chronic lymphocytic leukemia [CLL]), from bone marrow (Waldenström macroglobulinemia, Burkitt lymphoma, multiple myeloma), from spleen (small lymphocytic lymphoma [SLL]), or from lymph node (diffuse large B-cell lymphoma [DLBCL], follicular lymphoma) samples. Diagnosis was based on histopathology, immunohistochemistry, and immunophenotypic studies. A World Health Organization (WHO) classification protocol was followed for the classification of cases. All procedures were approved by the Human Ethics Committee at the University of Calgary, AB, Canada, and samples that were in excess of that needed for diagnostic purposes were used for experiments.
To obtain single cell suspensions, lymph node and spleen samples were mechanically disrupted in a DAKO medi machine (DAKO Diagnostics Canada, Missisauga, ON) and filtered through a 100-μm mesh. When neutrophils comprised more than 50% of peripheral blood or bone marrow samples, isolation of mononuclear cells using Ficoll-Hypaque was used to obtain single cell suspensions for further experiments. Chronic lymphocytic leukemia, follicular lymphoma, and DLBCL cells were maintained in Iscove modified Dulbecco medium (IMDM; Stem Cell Technologies, Vancouver, BC, Canada) containing 15% FBS and antibiotics (Gibco BRL). All other primary tumor samples were kept in RPMI medium with 15% FBS and antibiotics.
Reovirus
Reovirus serotype 3 (strain Dearing) was propagated in L929 cells grown in suspension in Joklik modified Eagle medium (JMEM; Gibco BRL) containing 5% FBS. Virus purification was performed according to the protocol of Smith et al34 with the exception that β-mercaptoethanol was omitted from the extraction buffer.
Apheresis product (AP)
All apheresis products used in the present study were obtained from patients registered at the Tom Baker Cancer Centre, Calgary, AB, Canada, after informed consent in accordance with the local institutional review board (IRB). AP mononuclear cells were washed in phosphate-buffered saline (PBS) prior to culturing in RPMI 1640 medium supplemented with 10% FBS or StemSpan (Stem Cell Technologies) medium for stem cell assays.
Cytopathic effect
Cells lines grown to subconfluence and purified or enriched primary tumor samples in culture media were infected with reovirus at a multiplicity of infection (MOI) of 40 plaque-forming units (PFU)/cell. To assess cytopathic effect, cells were photographed under a light microscope at 48 or 72 hours after infection.
Radiolabeling of reovirus-infected cells and preparation of lysates
Cell lines (U937 and RPMI 8226) were grown to subconfluence and infected with reovirus at an MOI of 40 PFU/cell. To evaluate whether reovirus infects AP cells, cells were cultured in RPMI medium containing 10% FBS in the presence or absence of granulocyte colony-stimulating factor (G-CSF) (10 ng/mL) and infected with reovirus at 40 MOI. At various time points after infection, the medium was replaced with media containing 0.1 μCi/mL (0.0037 MBq/mL) [35S]-methionine. After further incubation for 12 hours at 37°C, the cells were washed in PBS and lysed in lysis buffer containing 1% Triton X-100, 0.5% sodium deoxycholate, and 1 mM EDTA (ethylenediaminetetraacetic acid). The nuclei were then removed by low-speed centrifugation, and the supernatants were stored at -80°C until use. Radiolabeled lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as previously described.35
Cell counting using flow cytometry
Monocytic (U937) and myeloma (RPMI 8266) cells were cultured in the presence or absence of live virus (40 MOI) for up to 7 days. At 0, 1, 2, 3, 4, and 7 days after virus infection, cells were harvested, and 1 mL cell culture suspension containing approximately 1 × 106 cells was centrifuged at 750g for 1 minute. The cell pellet was resuspended in 1 mL of 50 μg/mL propidium iodide/RNase/Triton X-100 (Sigma Chemical, St Louis, MO), and 100 μL Flow Count Beads (Beckman Coulter, Hialeah, FL) was added to each tube. Intact cells were enumerated using Flow Count beads as an internal calibrator.
CD34+CD45+ cell enumeration
CD34-phycoerythrin (PE) (581) and CD45-fluorescein isothiocyanate (FITC) (J33) (Beckman-Coulter) antibodies were added to 100 μL diluted AP cells using a reverse pipetting technique to ensure accuracy. Samples were incubated for 10 minutes at room temperature in the dark. Flow Count beads (100 μL) were added to each tube using the same technique as in “Cell counting using flow cytometry.” Flow cytometric analysis was performed on an EPICS XL flow cytometer (Beckman Coulter) using a modified ISHAGE strategy.36,37 Data from 4 parameters were collected for analysis: forward scatter (FS), log side scatter (LSS), log fluorescence 1 (LFL1), and log fluorescence 2 (LFL2). Acquisition was halted at 100 000 CD45+ events. Hematopoietic progenitor cells were identified and counted in 2 histograms (CD45-FITC versus CD34-PE and FS versus LSS) using ISHAGE criteria: dim CD45+, bright CD34+ form a discrete cell cluster with a larger FS signal than lymphocytes. The use of a known amount of Flow Count fluorospheres allowed the determination of absolute CD34+ cell count directly from the flow cytometer.
Effect of reovirus on CD34+ stem cells
Apheresis product cells were depleted of lineage-committed cells using the StemSep immunomagnetic cell separation system (Stem Cell Technologies). The StemSep progenitor enrichment antibody cocktail (Catalog no. 14036; Stem Cell Technologies) was used to enrich for CD34+CD38- cells. The isolated cells were seeded at a density of 2 to 3 × 103/mL in StemSpan SFEM (Stem Cell Technologies) containing 40 μg/mL low-density lipoproteins (LDLs) (Sigma) and purified recombinant human Flt-3 ligand (FL; 100 ng/mL), stem cell factor (SCF; 100 ng/mL), interleukin-3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL), and thrombopoietin (Tpo, 50 ng/mL). Cultures were then incubated in the presence or absence of reovirus (40 MOI) for 5 days at 37°C in a humidified incubator with 5% CO2. Cells were harvested at days 1, 2, and 5 and assayed for CD34+ and CD45+ cells and colony-forming cells.
Colony-forming cells were evaluated by plating 103 cells in methylcellulose (MethoCult GF H4434; Stem Cell Technologies) to result in a 1:10 (vol/vol) ratio. Plates were scored for erythroid burst-forming units (BFU-Es); granulocyte-macrophage colony-forming units (CFU-GMs); and granulocyte, erythroid, macrophage, megakaryocyte, colony-forming units (CFU-GEMMs) following incubation at 37°C in a humidified 5% CO2 incubator for 2 weeks.
Contamination of apheresis product with cancer cells
U937 monocytic cells and RPMI 8226 myeloma cells were mixed with apheresis product in RPMI 1640 medium supplemented with 10% FBS to result in concentrations of 1%, 0.1%, and 0.01%. Cell admixtures were either treated with reovirus (40 MOI per total cell population) or left untreated and incubated for 3 days.
On day 0 and day 3 of purging, samples were taken from all admixed cell populations, and intact cancer cell numbers were evaluated using flow cytometry. To ensure reovirus treatment did not affect the stem cell population, admixed cell populations were analyzed for CD34+CD45+ cell counts following 3 days of reovirus treatment. The efficacy of purging was further evaluated by reculturing a portion of the purged and unpurged admixed cells in the appropriate media for each cancer cell line, and viable cancer cell outgrowth counts were enumerated using flow cytometry after 6 days of incubation.
Reovirus purging of primary human tumors contaminating apheresis product
AP cells were admixed with tumor samples to result in 10%, 5%, 1%, or 0.1% contamination and treated with 40 MOI reovirus per total cell population (AP + tumor cells). Cell admixtures were incubated in a CO2 incubator as described previously and analyzed for residual disease by flow cytometry. Minimal residual disease was detected in apheresis samples using 5-color immunophenotyping on a Cytomics FC500 flow cytometer (Beckman Coulter) using the antibodies conjugated to the following fluorochromes: FITC, PE, PE-Texas Red (ECD), PE-cyanin 5.1 (PC5), and PE-cyanin 7 (PC7). Analysis strategies included the use of lineage-gating techniques, aberrant marker expression, and enumeration of 1 × 106 cells per sample to detect rare events. Disease-free apheresis samples were run in parallel as a negative control to assess background levels.
Effect of cryopreservation and DMSO on reovirus viability
To assess whether exposure to dimethyl sulfoxide (DMSO) and/or the cryopreservation procedure affects reovirus viability, apheresis cells were exposed to reovirus (40 MOI) and incubated in a CO2 (5%) incubator at 37°C for 3 days as in purging experiments. This procedure was done to quantitate the amount of virus that would potentially be infused to the patients. Virus-treated and untreated apheresis products were then frozen as per local protocol, DMSO medium (20% DMSO [Edward Life Sciences, Irvine, CA], 60% TC199 [Stem Cell Technologies], and 20% albumin [Bayer, Elkhart, IN] vol/vol) in a 1:1 ratio.38 The DMSO-treated cells were subjected to controlled rate cooling immediately in a cryopreservation system (Planer:KRYO 10 series 11; Planer Products, Middlesex, United Kingdom) and maintained in liquid nitrogen for 2 weeks. Frozen apheresis product cells were thawed in a 37°C water bath similar to the technique used at bedside. A portion of the thawed apheresis product was washed gently in PBS once and resuspended in RPMI medium. Viral plaque titrations of the thawed products were assayed.
Results
Reovirus does not affect hematopoietic progenitors
To investigate whether reovirus would affect the number and function of stem cells, positively selected (CD34+) stem cells were challenged with reovirus at an MOI of 40 and cultured in StemSpan medium for 5 days. As shown in Figure 1A the number of CD34+CD45+ cells significantly increased with prolonged incubation and, at day 5, were 80-fold higher than at the start of the experiment. No significant difference between virus-treated and untreated stem cells was detected.
Reovirus effect on stem cells. (A) Lack of reovirus effect on cultured stem cells. CD34+ cells were isolated using negative selection columns and cultured in StemSpan medium in the presence or absence of reovirus (40 MOI). Cells were harvested 0, 1, 2, and 5 days after virus infection, and CD34+ cells were enumerated by flow cytometry. Error bars indicate the standard deviation of the mean of 3 replicates. (B) Lack of reovirus effect on stem cell progenitor assay. Stem cells (CD34+) were selected and treated with reovirus as described. Samples taken at the days indicated were cultured in methylcellulose and scored for CFU-GMs, BFU-Es, and CFU-GEMMs. NV indicates no virus; LV, live virus. Error bars indicate the standard deviation of the mean of 3 replicate plates. (C) Lack of reovirus protein synthesis detected by [35S]-methionine labeling in apheresis product primed with G-CSF. Apheresis product cells were primed with G-CSF and pulse labeled with [35S]-methionine with or without reovirus treatment (40 MOI). At time points indicated in the figure, cellular proteins were harvested and subjected to SDS-PAGE. Reovirus marker proteins (λ, μ, and σ) are indicated in lane 1. Note the absence of viral protein bands at all time points after virus infection.
Reovirus effect on stem cells. (A) Lack of reovirus effect on cultured stem cells. CD34+ cells were isolated using negative selection columns and cultured in StemSpan medium in the presence or absence of reovirus (40 MOI). Cells were harvested 0, 1, 2, and 5 days after virus infection, and CD34+ cells were enumerated by flow cytometry. Error bars indicate the standard deviation of the mean of 3 replicates. (B) Lack of reovirus effect on stem cell progenitor assay. Stem cells (CD34+) were selected and treated with reovirus as described. Samples taken at the days indicated were cultured in methylcellulose and scored for CFU-GMs, BFU-Es, and CFU-GEMMs. NV indicates no virus; LV, live virus. Error bars indicate the standard deviation of the mean of 3 replicate plates. (C) Lack of reovirus protein synthesis detected by [35S]-methionine labeling in apheresis product primed with G-CSF. Apheresis product cells were primed with G-CSF and pulse labeled with [35S]-methionine with or without reovirus treatment (40 MOI). At time points indicated in the figure, cellular proteins were harvested and subjected to SDS-PAGE. Reovirus marker proteins (λ, μ, and σ) are indicated in lane 1. Note the absence of viral protein bands at all time points after virus infection.
The preservation of the clonogenic potential of virus-treated and untreated hematopoietic progenitors was determined by culturing CD34+-enriched stem cells in StemSpan medium plated in methylcellulose medium. CFU-GMs, BFU-Es, and CFU-GEMMs were counted after 14 days of incubation. As shown in Figure 1B, no differences in the clonogenic capacity of the stem cells was detected in virus-treated or untreated stem cells.
To confirm that reovirus does not replicate in growth factor–stimulated hematopoietic progenitor cells, AP cells were primed with G-CSF, challenged with reovirus, and pulse labeled with [35S]-methionine. Cell lysates were analyzed by SDS-PAGE. As depicted in Figure 1C no viral protein bands λ, μ, and σ could be detected at any of the time points tested, even after stimulation with G-CSF. Further, host cell protein synthesis was still evident in AP cells even at 60 hours after virus infection.
Flow cytometric analysis and [35S]-methionine labeling of malignant cell lines
The susceptibility of established monocytic and myeloma cell lines to reovirus infection was tested by culturing U937 and RPMI 8226 cells in the presence (40 MOI) or absence of reovirus.
To confirm the cytopathic effect of reovirus on these cell lines samples of the cultured cells were obtained at days 0, 1, 2, 3, 4, and 7 days after virus infection and were analyzed for intact cell counts using propidium iodide. As shown in Figure 2A, cell numbers declined after virus infection, contrasting the increase in uninfected cells. These results were confirmed over multiple experiments, and the cell counts approached zero by day 7. Residual cells seen at day 7 in Figure 2A (left and right panels) are due to the fact that flow cytometry still counts membrane-intact but dying cells.
Reovirus and human cancer cell lines. (A) Effect of reovirus on human cancer cell lines. Monocytic (U937, left panel) and myeloma (RPMI 8226, right panel) cells were infected with reovirus at an MOI of 40 PFU/cell. Cells were harvested at 0, 1, 2, 3, 4, and 7 days after infection, and intact cancer cells were enumerated with propidium iodide using flow cytometry. The values depicted are the means and standard deviations of 4 replicates. ♦ indicates no virus; ▪, live virus. (B) Reovirus protein synthesis in cancer cell lines. Human monocytic (U937, left panel) and myeloma (RPMI 8226, right panel) cells were infected with reovirus at a MOI of 40 PFUs and pulse labeled with [35S]-methionine for various time points as indicated in the figures. Following labeling, the cells were harvested and lysed, and cellular proteins were subjected to SDS-PAGE. Reovirus proteins (λ, μ, and σ) are shown in lane 1. Reovirus infection and protein synthesis were found in both cell lines.
Reovirus and human cancer cell lines. (A) Effect of reovirus on human cancer cell lines. Monocytic (U937, left panel) and myeloma (RPMI 8226, right panel) cells were infected with reovirus at an MOI of 40 PFU/cell. Cells were harvested at 0, 1, 2, 3, 4, and 7 days after infection, and intact cancer cells were enumerated with propidium iodide using flow cytometry. The values depicted are the means and standard deviations of 4 replicates. ♦ indicates no virus; ▪, live virus. (B) Reovirus protein synthesis in cancer cell lines. Human monocytic (U937, left panel) and myeloma (RPMI 8226, right panel) cells were infected with reovirus at a MOI of 40 PFUs and pulse labeled with [35S]-methionine for various time points as indicated in the figures. Following labeling, the cells were harvested and lysed, and cellular proteins were subjected to SDS-PAGE. Reovirus proteins (λ, μ, and σ) are shown in lane 1. Reovirus infection and protein synthesis were found in both cell lines.
Replication of reovirus in susceptible cell lines was further confirmed by metabolic labeling with [35S]-methionine and analysis of cell lysates by SDS-PAGE. Viral protein synthesis was evident in both cell lines tested (Figure 2B). The appearance of λ, μ, and σ viral protein bands was seen as early as 12 hours after viral infection (Figure 2B, right panel). Reovirus completely shut down and took over host cell protein synthesis as judged by the replacement of host cell protein bands with viral protein bands at 48 hours in the U937 cell line. These results are in contrast to the [35S]-methionine labeling data of AP cells in which the appearance of the viral protein bands were not seen at any of the time points tested.
Purging of monocytic and myeloma cancer cells in apheresis product
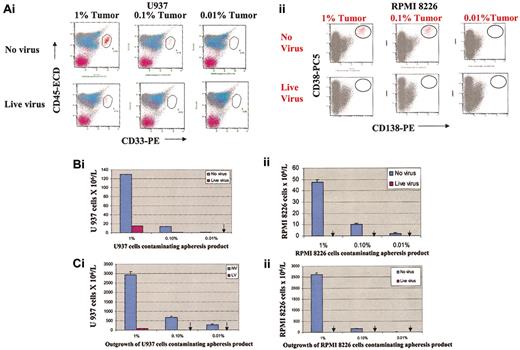
The results in “Reovirus does not affect hematopoietic progenitors” prove that exposure of hematopoietic stem cells to reovirus does not affect CD34+CD45+ cell counts or colony-forming potential of the hematopoietic progenitor cells in vitro. The monocytic and myeloma cancer cells were then mixed with apheresis product cells to result in tumor burdens of 1%, 0.1%, and 0.01% and purged with reovirus for 3 days. The purging efficacy of reovirus was evaluated using 2 different techniques: flow cytometry and cancer cell outgrowth following purging.
As depicted in Figure 3Ai,Bi, reovirus treatment and purging for 3 days resulted in significant purging of U937 cells and complete purging at 0.01% contamination. When purged and unpurged admixed samples were recultured in RPMI medium for 6 days, no tumor regrowth was detected in the 0.1% and 0.01% contaminated samples (Figure 3Ci). In contrast, U937 cell outgrowth was detected in all reovirus untreated samples.
Purging effect of reovirus on U937 monocytic cells and RPMI 8226 myeloma cells. Admixtures of apheresis product cells and U937 monocytic cells (Ai,Bi,Ci) or RPMI 8226 cells (Aii,Bii,Cii) (1%, 0.1%, and 0.01%, respectively) were treated with reovirus (40 MOI). Following 3 days of purging, CD33+/CD45+ U937 cells or CD138+/CD38+ myeloma cells were assessed by flow cytometry. (A) Flow cytometric plots of purged and unpurged samples. (B) U937 and RPMI 8226 cell numbers in purged and unpurged samples. Arrows indicate the absence of live cells. (C) Lack of out growth of U937 (i) and RPMI 8226 cells (ii) in purged samples following 6 days of incubation. Arrows indicate the absence of regrowth of U937 or RPMI 8226 cells. Error bars represent the standard deviations of the means of 3 replicates.
Purging effect of reovirus on U937 monocytic cells and RPMI 8226 myeloma cells. Admixtures of apheresis product cells and U937 monocytic cells (Ai,Bi,Ci) or RPMI 8226 cells (Aii,Bii,Cii) (1%, 0.1%, and 0.01%, respectively) were treated with reovirus (40 MOI). Following 3 days of purging, CD33+/CD45+ U937 cells or CD138+/CD38+ myeloma cells were assessed by flow cytometry. (A) Flow cytometric plots of purged and unpurged samples. (B) U937 and RPMI 8226 cell numbers in purged and unpurged samples. Arrows indicate the absence of live cells. (C) Lack of out growth of U937 (i) and RPMI 8226 cells (ii) in purged samples following 6 days of incubation. Arrows indicate the absence of regrowth of U937 or RPMI 8226 cells. Error bars represent the standard deviations of the means of 3 replicates.
Even more striking was the complete reovirus purging of RPMI 8226 myeloma cells at 1%, 0.1%, or 0.01% tumor burden as detected by flow cytometric analysis (Figure 3Aii,Bii). No tumor outgrowth was detected when purged samples were cultured in RPMI medium for 6 days and analyzed by flow cytometry (Figure 3Cii). CD34+ counts of the purged AP were analyzed by flow cytometry, assuring the purging procedure did not affect the CD34+ stem cells, confirming the results in Figure 1 (data not presented).
Reovirus successfully purges several ex vivo human tumor cells from apheresis product
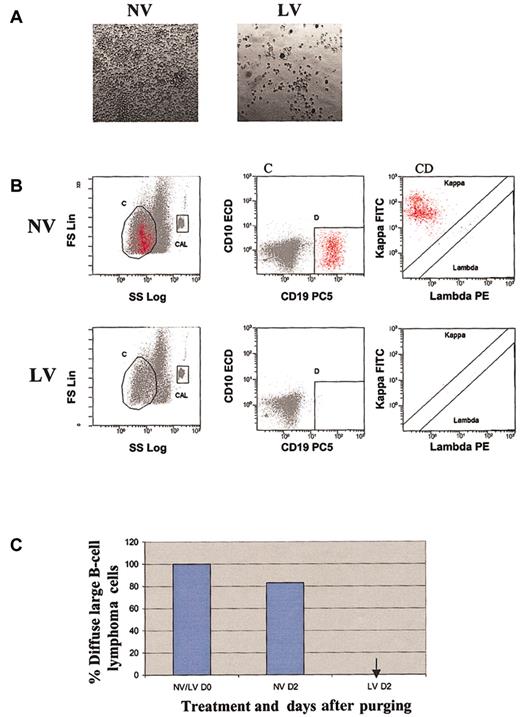
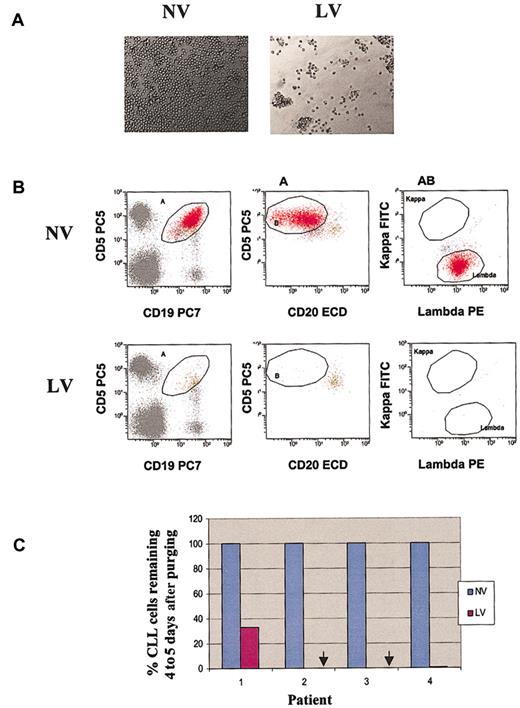
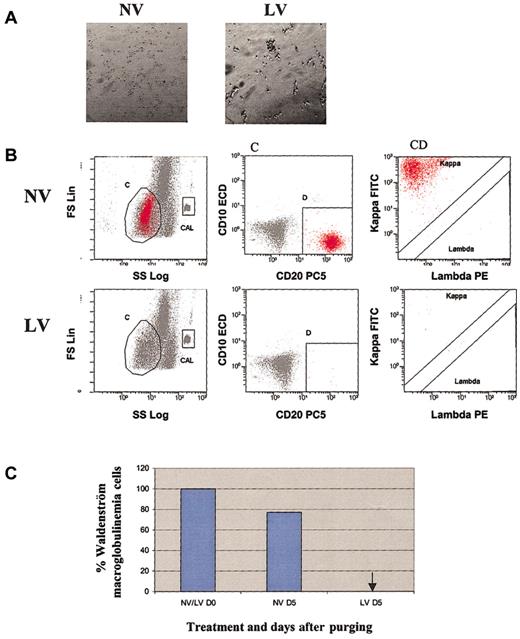
The purging ability of reovirus against 4 primary ex vivo hematopoietic and lymphoid tumors was confirmed. Tumor samples of CLL, DLBCL, Waldenström disease, and small cell lymphocytic lymphoma were initially treated with 40 MOI reovirus and observed for cytopathic effect. As depicted in Figures 4, 5, 6, and 7, significant reovirus cytopathic effect was seen. Reovirus was able to purge DLBCL, CLL, Waldenström disease, and SLL successfully after 2 to 5 days of incubation. Figure 4B-C illustrates that reovirus was able to purge DLBCL cells completely after 2 days. Of the 4 different CLL patients that were tested (Figure 5C), patients 2 and 3 exhibited complete purging at 10% contamination after reovirus treatment by days 4 and 5, respectively. Patient 4 appeared to purge completely at 1% contamination at day 4. Patient 1 appeared to be more resistant to reovirus, although tumor burden was significantly reduced by day 5. Tumors from patients with Waldenström macroglobulinemia and SLL were very sensitive, and complete purging at 10% tumor burden was attained 5 days after virus treatment (Figures 6, 7).
Purging effect of reovirus on DLBCL cells. (A) Cytopathic effect of reovirus on DLBCL cells 48 hours after infection. Purified cells were infected with 40 MOI live virus. Photomicrographs were taken at 48 hours after infection (original magnification, × 200). Significant cytopathic effect indicative of widespread killing was observed in live virus-treated but not in untreated cells. (B) Flow cytometric analysis of DLBCL following reovirus purging. Apheresis product cells were mixed with DLBCL cells (10%) and purged for 2 days with reovirus. Samples were analyzed using region C and lineage gate D (CD10-CD19+) to enumerate κ monoclonal CD10-CD19+ malignant cells (region C + D). Flow count beads were included in the CAL region to calculate absolute counts. (C) Representative histograms of viable DLBCL cells before and after purging with reovirus. Arrow indicates the absence of DLBCL cells.
Purging effect of reovirus on DLBCL cells. (A) Cytopathic effect of reovirus on DLBCL cells 48 hours after infection. Purified cells were infected with 40 MOI live virus. Photomicrographs were taken at 48 hours after infection (original magnification, × 200). Significant cytopathic effect indicative of widespread killing was observed in live virus-treated but not in untreated cells. (B) Flow cytometric analysis of DLBCL following reovirus purging. Apheresis product cells were mixed with DLBCL cells (10%) and purged for 2 days with reovirus. Samples were analyzed using region C and lineage gate D (CD10-CD19+) to enumerate κ monoclonal CD10-CD19+ malignant cells (region C + D). Flow count beads were included in the CAL region to calculate absolute counts. (C) Representative histograms of viable DLBCL cells before and after purging with reovirus. Arrow indicates the absence of DLBCL cells.
Purging effect of reovirus on CLL cells. (A) Cytopathic effect of reovirus on human CLL cells 72 hours after infection (original magnification, × 200). Purified cells were infected with 40 MOI live virus. Photomicrographs were taken at 72 hours after infection. Significant cytopathic effect was evident in reovirus-treated cells. (B) Flow cytometric analysis of CLL following reovirus purging. Apheresis product cells were mixed with CLL cells (10%) and purged for 4 to 5 days with reovirus. CLL was detected using a CD5+CD19+ region (gate A) combined with 5+ dim 20+ region (gate B) to detect monoclonal λ B cells (gate A + B). (C) Representative histograms of viable CLL cells for 4 patients following reovirus purging. Arrows indicate that CLL cells for patients 2 and 3 after virus treatment was not detected.
Purging effect of reovirus on CLL cells. (A) Cytopathic effect of reovirus on human CLL cells 72 hours after infection (original magnification, × 200). Purified cells were infected with 40 MOI live virus. Photomicrographs were taken at 72 hours after infection. Significant cytopathic effect was evident in reovirus-treated cells. (B) Flow cytometric analysis of CLL following reovirus purging. Apheresis product cells were mixed with CLL cells (10%) and purged for 4 to 5 days with reovirus. CLL was detected using a CD5+CD19+ region (gate A) combined with 5+ dim 20+ region (gate B) to detect monoclonal λ B cells (gate A + B). (C) Representative histograms of viable CLL cells for 4 patients following reovirus purging. Arrows indicate that CLL cells for patients 2 and 3 after virus treatment was not detected.
Purging effect of reovirus on Waldenström macroglobulinemia cells. (A) Cytopathic effect of reovirus on human Waldenström macroglobulinemia cells 72 hours after infection. Original magnification, × 200. Purified cells were infected with 40 MOI reovirus. Photomicrographs were taken at 72 hours after infection. Significant cytopathic effect was observed in live virus-treated but not in untreated cells. (B) Flow cytometric analysis of Waldenström macroglobulinemia following reovirus purging. Apheresis product cells were mixed with Waldenström macroglobulinemia cells (10%) and purged for 5 days with reovirus. Samples were analyzed using region C and lineage gate D (CD10-CD20+) to enumerate κ monoclonal CD10-CD20+ malignant cells (region C + D). Flow Count beads were included in the CAL region to calculate absolute counts. (C) Representative histograms of viable Waldenström macroglobulinemia cells before and after purging with reovirus. Arrow indicates that Waldenström macroglobulinemia cells were not detected.
Purging effect of reovirus on Waldenström macroglobulinemia cells. (A) Cytopathic effect of reovirus on human Waldenström macroglobulinemia cells 72 hours after infection. Original magnification, × 200. Purified cells were infected with 40 MOI reovirus. Photomicrographs were taken at 72 hours after infection. Significant cytopathic effect was observed in live virus-treated but not in untreated cells. (B) Flow cytometric analysis of Waldenström macroglobulinemia following reovirus purging. Apheresis product cells were mixed with Waldenström macroglobulinemia cells (10%) and purged for 5 days with reovirus. Samples were analyzed using region C and lineage gate D (CD10-CD20+) to enumerate κ monoclonal CD10-CD20+ malignant cells (region C + D). Flow Count beads were included in the CAL region to calculate absolute counts. (C) Representative histograms of viable Waldenström macroglobulinemia cells before and after purging with reovirus. Arrow indicates that Waldenström macroglobulinemia cells were not detected.
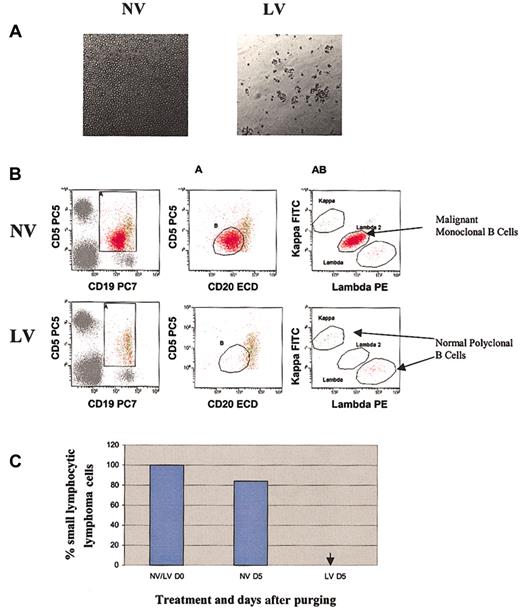
Purging effect of reovirus on SLL cells. (A) Cytopathic effect of reovirus on human SLL cells 72 hours after infection. Purified cells were infected with reovirus (40 MOI), and cells were photographed 72 hours after infection (original magnification, × 200). Cytopathic effect was seen in reovirus-infected but not in uninfected cells. (B) Flow cytometric analysis of SLL following reovirus purging. Apheresis product cells were mixed with SLL cells (10%) and purged for 5 days with reovirus. Samples were analyzed using flow cytometry. Dim CD5+CD19+CD20+ B cells were gated using 2 regions (A and B) and assessed for clonality. The λ-positive SLL cells were clearly distinguished from the normal polyclonal B cells. (C) Representative histograms of viable SLL cells before and after purging with reovirus. The arrow indicates that SLL cells were not detected.
Purging effect of reovirus on SLL cells. (A) Cytopathic effect of reovirus on human SLL cells 72 hours after infection. Purified cells were infected with reovirus (40 MOI), and cells were photographed 72 hours after infection (original magnification, × 200). Cytopathic effect was seen in reovirus-infected but not in uninfected cells. (B) Flow cytometric analysis of SLL following reovirus purging. Apheresis product cells were mixed with SLL cells (10%) and purged for 5 days with reovirus. Samples were analyzed using flow cytometry. Dim CD5+CD19+CD20+ B cells were gated using 2 regions (A and B) and assessed for clonality. The λ-positive SLL cells were clearly distinguished from the normal polyclonal B cells. (C) Representative histograms of viable SLL cells before and after purging with reovirus. The arrow indicates that SLL cells were not detected.
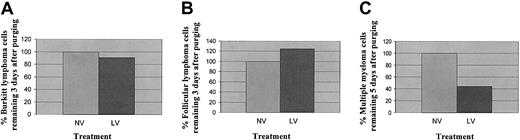
In contrast, as shown in Figure 8A-B, a Burkitt lymphoma and a follicular lymphoma appeared to be resistant to reovirus infection, and no significant differences in tumor burdens between reovirus-treated and untreated samples could be seen at 1% contamination after 3 days of reovirus treatment. Reovirus was able to purge more than 50% of the myeloma cells from one patient's apheresis product (at 5% contamination) 5 days after treatment (Figure 8C).
Purging of Burkitt lymphoma, follicular lymphoma, and multiple myeloma cells. (A) Representative histograms of viable Burkitt lymphoma cells analyzed by flow cytometry following reovirus purging. Apheresis product cells were mixed with Burkitt lymphoma cells (1%) and purged for 3 days with reovirus. Samples were analyzed by flow cytometry using Dim CD10+CD19+ B cells. The λ-positive Burkitt lymphoma cells were detected in both purged and unpurged samples. (B) Representative histograms of viable follicular lymphoma cells analyzed by flow cytometry following reovirus purging. Apheresis product cells were mixed with follicular lymphoma cells (1%) and purged for 3 days with reovirus. Samples were analyzed by flow cytometry using Dim CD10+CD19+CD20+ B cells. The λ-positive follicular lymphoma cells were detected in both purged and unpurged samples. (C) Representative histograms of viable multiple myeloma cells analyzed by flow cytometry following reovirus purging. Apheresis product cells were mixed with multiple myeloma cells (5%) and purged for 5 days with reovirus. Samples were analyzed by flow cytometry using CD138+CD38+ dimCD45+ cells. More than 50% of myeloma cells were purged by reovirus.
Purging of Burkitt lymphoma, follicular lymphoma, and multiple myeloma cells. (A) Representative histograms of viable Burkitt lymphoma cells analyzed by flow cytometry following reovirus purging. Apheresis product cells were mixed with Burkitt lymphoma cells (1%) and purged for 3 days with reovirus. Samples were analyzed by flow cytometry using Dim CD10+CD19+ B cells. The λ-positive Burkitt lymphoma cells were detected in both purged and unpurged samples. (B) Representative histograms of viable follicular lymphoma cells analyzed by flow cytometry following reovirus purging. Apheresis product cells were mixed with follicular lymphoma cells (1%) and purged for 3 days with reovirus. Samples were analyzed by flow cytometry using Dim CD10+CD19+CD20+ B cells. The λ-positive follicular lymphoma cells were detected in both purged and unpurged samples. (C) Representative histograms of viable multiple myeloma cells analyzed by flow cytometry following reovirus purging. Apheresis product cells were mixed with multiple myeloma cells (5%) and purged for 5 days with reovirus. Samples were analyzed by flow cytometry using CD138+CD38+ dimCD45+ cells. More than 50% of myeloma cells were purged by reovirus.
Effect of DMSO exposure and cryopreservation on reovirus
Because there is a theoretical concern that administration of replication-competent reovirus into an immunocompromised host poses safety issues and because cryopreservation and reinfusion of stem cells is part of the transplantation procedure, we tested the effect of DMSO exposure, cryopreservation, and thawing on reovirus viability. The original plaque titration of reovirus was 2.2 × 1011 PFU/mL. The plaque titration of reovirus treated, frozen, thawed, and washed apheresis product was 1.72 × 104 PFU/mL, thus resulting in a 7-log depletion of virus. In addition, we have shown that 5 × 109 viral PFUs administered intravenously to 25-g athymic (immunosuppressed) mice is well tolerated and that daily intravenous administration of 5 × 108 viral PFUs for 28 days in primates occurs without toxicity.
Discussion
The effect of minimal residual disease contributing to relapse following ASC transplantation is likely significant. In 3 separate studies involving patients with acute myelogenous leukemia (AML), chronic myelogenic leukemia (CML), and neuroblastoma, residual tumor cells in autografts marked by viral vectors containing the neo gene before infusion have been identified in most cases of relapse,9-11 confirming tumor cells within the autograft contribute to relapse. AML and CML are not routinely considered for autotransplantation owing to the beneficial graft-versus-leukemic effect of allotransplantation. The hematologic malignancies tested in the present study are all considered potentially treatable with autotransplantation. Malignancies such as CLL are rarely transplanted owing to the large tumor burden found in most patients'AP and peripheral blood. Only highly selected CLL patients are currently offered ASC transplantation. This number would be significantly increased if adequate purging techniques were available. In our experience, up to 20% of patients with DLBCL have flow cytometric-detectable tumor cells in their AP. The current dogma with regard to the utility of autotransplantation in multiple myeloma is thought to be survival prolonging as opposed to curative, likely in large part to contaminated autografts. The primary tumor cells tested in this article represent many of the malignancies that theoretically could benefit from purging.
In the present study we exploited a novel purging strategy involving reovirus, a benign common environmental double-stranded RNA virus that is replication competent. A major advantage of using reovirus as a purging strategy is that it selectively kills transformed cells, while sparing healthy untransformed cells. The selective oncolytic property of reovirus has been demonstrated for a variety of cancers in vitro, in vivo, and ex vivo.28-33 Coffey et al28 showed that a single intratumoral injection causes significant tumor regression in severe combined immunodeficient mice bearing tumors established from V-erbB–transformed murine NIH 3T3 cells or human U87 glioblastoma cells. Wilcox et al29 showed the susceptibility of several malignant glioma cell lines to reovirus and complete tumor regression in 2 subcutaneous and intracerebral human malignant glioma mouse models. Nine of 9 human glioma specimens tested were also susceptible to reovirus ex vivo. Similarly, the in vitro and in vivo oncolytic property of reovirus on ovarian,30 colon,30 breast,31 and prostate32 cancers has been demonstrated. More recently, Alain et al33 have shown reovirus oncolysis of several lymphoid malignancies.
It was originally hypothesized that the Ras pathway (or upstream and downstream elements) was highly correlated with reovirus susceptibility.27 We now know that reovirus infection efficiency depends on the transformed state of the cell and can involve multiple signaling pathways that channel through PKR or another pivotal molecule.27,39 Although documented evidence of a Ras mutation in human monocytic cell line U937 is lacking, it does possess a 3-fold increase in Rac, a Ras-related family of protein.40 Mutations in the N-ras and K-ras genes appear to be common in multiple myeloma,41 and the frequency of Ras mutations can vary between 10% and 40% at presentation and increase up to 70% at relapse.42,43 RPMI 8226 myeloma cell proliferation has been shown to be enhanced by GM-CSF induced by the p21-ras/mitogenacitvated protein kinase (MAPK) signaling cascade.44 Recent data from our laboratories have shown the lack of a consistent correlation between reovirus infection and Ras or MAPK activity in a majority of reovirus susceptible cell lines, suggesting an alternate signaling pathway.
In an attempt to simulate minimal residual disease in apheresis product, an ex vivo model system was used. Human apheresis product obtained from patients was admixed with monocytic leukemia and myeloma cell lines and treated with reovirus. Detection of remaining (after purge) tumor cells used 2 methodologies: flow cytometry and tumor regrowth. The results indicate that it is possible to achieve complete purging up to 1% of tumor burden. Clinically, the amount of tumor burden encountered is frequently less than 0.01%,45 well within the range of successful purging seen in our experiments. Long-term incubation with reovirus suggests that at a 1% tumor burden complete purging of cells could be obtained for U937 cells.
In this paper we demonstrate that reovirus infection and purging ability is not restricted only to cancer cell lines but also to several primary tumors of hematopoietic origin. With 10% tumor burden, reovirus was able to purge DLBCL from AP within 2 days as assessed by flow cytometry, and significant cytopathic effects supported these purging effects. This particular DLBCL specimen may have possessed a highly aberrant Ras/apoptosis signaling cascade. Consistent with our results of reovirus permissiveness of DLBCL, Alain et al33 also showed that 4 DLBCL cell lines and 2 human DLBCL specimens tested to be sensitive to reovirus. Recently, it has been reported that a protooncogene RhoH/TTF, a novel Rho guanosine triphosphate (GTP)–binding protein exclusively expressed in hematopoietic tissues, is aberrant in 46% of DLBCLs.46
Interestingly, reovirus oncolysis was detected in all 4 CLL specimens tested, and successful purging was obtained within 4 to 5 days at 1% or 10% contamination in 3 of the specimens tested. Sensitivity of reovirus to CLLs has recently been observed by others in our group as well.33 Mutations in the Ras gene itself are rare in leukemias and lymphomas. However, both the K-ras protooncogene and the insulin-like growth factor 1 receptor-encoding gene are found on chromosome 12, and trisomy of chromosome 12 is a common occurrence in CLL.47,48 It is possible that the activation of signaling pathways in CLL is autocrine in nature, as both CD40 receptor and its ligand CD154 are expressed in these cells.49,50
In addition to DLBCL and CLL purging, we demonstrated complete reovirus purging of small lymphocytic lymphoma and Waldenström macroglobulinemia within 5 days. Significant purging of a T-cell lymphoma was also observed at 3 days after purging (data not presented). Interestingly, reovirus was unable (or exhibited delayed kinetics) to purge the follicular lymphoma and the Burkitt lymphoma tested in the present study. Reovirus resistance of these malignancies has also been noted by Alain et al.33 Incomplete (50%) purging was detected in the ex vivo multiple myeloma specimen tested. Ongoing studies in our laboratories should provide further information as to the spectrum of tumors that are reovirus sensitive and to the underlying mechanisms of reovirus infection.
To ensure that the oncolytic property of reovirus did not affect stem cells, 3 methodologies were used: (1) [35S]-methionine and SDS-PAGE analysis of apheresis cells incubated in the presence of reovirus for up to 60 hours did not affect normal host (AP) protein synthesis. Nor were viral proteins detected. Even with G-CSF stimulation reovirus did not infect stem cells. (2) Isolated stem cells from patients at the time of apheresis product harvest cultured in StemSpan medium followed by exposure to reovirus at an MOI of 40 did not affect the stem cell population and terminal differentiation. (3) The inability of reovirus to affect colony formation was demonstrated by plating stem cells incubated with reovirus up to 5 days in a methylcellulose-based medium. Colony formation in methylcellulose increased with prolonged incubation irrespective of whether they had been exposed to reovirus or not. These 3 experiments confirm the fact that reovirus does not affect the function of stem cells.
Exposure to reovirus causes dose-dependent adverse effects in severe combined immunodeficiency and nonobese diabetic (SCID/NOD) mice.51 Death usually occurs within 35 to 40 days of exposure, depending on the route of administration and dose. In contrast to the SCID animals, nude mice, which are partially immunocompromised, receiving 5 × 107 PFU reovirus did not show any adverse effects, such as weight loss, lack of activity, or grooming behavior (K. Hirasawa, personal communication, April 2002). Alain et al33 did not observe adverse effects of intravenous 5 × 107 PFU injections to SCID/NOD mice bearing subcutaneous tumors of Raji cells and noted significant tumor response. Daily intravenous injections of 5 × 108 PFU for 28 days into primates was well tolerated with no adverse toxicity (M. Coffey, personal communication, October 2002). Viremia was seen in 2 of 18 patients receiving intralesional reovirus treatment during a human phase 1 clinical trial (D.G.M., unpublished observations, June 2002), and none of the 18 patients entered onto the study exhibited any grade 3/4 toxicity as defined by the National Cancer Institute Cancer Treatment Group (NCI-CTG) toxicity criteria.52 During this phase 1 trial most patients contained neutralizing antibodies at baseline, suggesting previous seroconversion. Further, preliminary data suggest that intracerebral injections of reovirus into patients with recurrent glioma on immunosuppressive steroids are well tolerated.
Because freezing, thawing, and washing of AP cells after purging before reinfusion results in a 7-fold log depletion of viral PFUs and preliminary data suggest that this concentration can be further reduced by a second wash, the amount of reovirus infused back into the patient is likely minimal. However, a potential benefit may be that residual virus might be able to purge residual disease in vivo. The possibility of in vivo purging with reovirus in patients who receive a transplant after chemotherapy is intriguing. A second potential safety concern is the possibility of nondetectable uptake by AP cells and presentation of reovirus antigens in the context of class I major histocompatibility complex (MHC). There are theoretical concerns that this possibility may lead to stem cell immune-mediated ablation. To date, we have not seen this situation in preclinical murine, canine, or primate studies.
Currently, we are investigating the role of adding immunosuppression to increase systemic efficacy in preclinical xenograft murine orthotopic model systems, as well as the role of passive immunization of antireovirus antibodies, as a possible rescue mechanism of reovirus toxicity in SCID-NOD mice. Further, the role of intravenous γ-globulin (known to contain antireovirus antibodies) as a passive immunization strategy to control reovirus replication is also being investigated. The above-mentioned investigations not withstanding, there still are theoretical risks of reinfusion of viable reovirus within the AP cells into patients. This reinfusion will ultimately need to be tested in a primate model system prior to a phase 1 trial. In addition, it is possible that in the future patients could be preimmunized to reovirus antigens before transplantation to afford a natural immunity at the time of transplantation.
Viral-mediated purging techniques have been well described in the literature. Recent reports suggest the possibility of purging breast cancer and neuroblastoma using attenuated adenoviral-based methods.53-55 Stojdl et al56 have shown the potential use of vesicular stomatitis virus (VSV), a negative strand RNA virus, as a purging agent in autologous transplantations. Because of its lack of toxicity in bone marrow cultures (ie, stem cells) and its oncolytic activity toward AML cell lines, those researchers propose the potential benefit of using this virus as an ex vivo purging agent. In the present study we demonstrate the exploitation of another unattenuated oncolytic RNA virus that has the potential to purge many hematopoietic malignancies for which autotransplantation is a viable treatment option. Our findings suggest that reovirus can be used as an ex vivo–purging agent in autologous transplantations for many malignant cancers such as DLBCL, CLL, Waldenström macroglobulinemia, and non-Hodgkin lymphomas and potentially could be extended to other cancers such as myeloma.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-08-2508.
Supported by grants from the Alberta Cancer Board and Oncolytics Biotech (D.G.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff at the Southern Alberta Bone Marrow Transplant Unit and the Apheresis Unit/Blood Bank of the Foothills Medical Centre for the support extended during this study.

![Figure 1. Reovirus effect on stem cells. (A) Lack of reovirus effect on cultured stem cells. CD34+ cells were isolated using negative selection columns and cultured in StemSpan medium in the presence or absence of reovirus (40 MOI). Cells were harvested 0, 1, 2, and 5 days after virus infection, and CD34+ cells were enumerated by flow cytometry. Error bars indicate the standard deviation of the mean of 3 replicates. (B) Lack of reovirus effect on stem cell progenitor assay. Stem cells (CD34+) were selected and treated with reovirus as described. Samples taken at the days indicated were cultured in methylcellulose and scored for CFU-GMs, BFU-Es, and CFU-GEMMs. NV indicates no virus; LV, live virus. Error bars indicate the standard deviation of the mean of 3 replicate plates. (C) Lack of reovirus protein synthesis detected by [35S]-methionine labeling in apheresis product primed with G-CSF. Apheresis product cells were primed with G-CSF and pulse labeled with [35S]-methionine with or without reovirus treatment (40 MOI). At time points indicated in the figure, cellular proteins were harvested and subjected to SDS-PAGE. Reovirus marker proteins (λ, μ, and σ) are indicated in lane 1. Note the absence of viral protein bands at all time points after virus infection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-08-2508/5/m_h81334547001.jpeg?Expires=1765922125&Signature=s8ZKKQVpqN11wbkCT~RsDYsQpMjJagG2NNTtEbSBe4V~kqd0U2eVihG3vUYGKQVS39NB7M9k2oqCuQCkisEeHA1LedqMphTy0vIiPQ7kU29PzzXT-v7pej7fgg9Ya0wmBOTQaOh4mjpr47OhEvjhSFReBgnv-1Q52s-tP3NDQWWw8J8QOVJaOO62knovs1SYxsbRxBSmJS9e3VUQ7bZYOSUp37kzIF322oYx98MUu~JBhdyez1M9InooUs37nNJ8s08Mg-NWtt6jxlGL7oWGRPugRk~9503P6-eLo~LCpYhAhldvJ0LVeheFkiFrHJREttJ6AIlRPf5d5X38tL-ydw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Reovirus and human cancer cell lines. (A) Effect of reovirus on human cancer cell lines. Monocytic (U937, left panel) and myeloma (RPMI 8226, right panel) cells were infected with reovirus at an MOI of 40 PFU/cell. Cells were harvested at 0, 1, 2, 3, 4, and 7 days after infection, and intact cancer cells were enumerated with propidium iodide using flow cytometry. The values depicted are the means and standard deviations of 4 replicates. ♦ indicates no virus; ▪, live virus. (B) Reovirus protein synthesis in cancer cell lines. Human monocytic (U937, left panel) and myeloma (RPMI 8226, right panel) cells were infected with reovirus at a MOI of 40 PFUs and pulse labeled with [35S]-methionine for various time points as indicated in the figures. Following labeling, the cells were harvested and lysed, and cellular proteins were subjected to SDS-PAGE. Reovirus proteins (λ, μ, and σ) are shown in lane 1. Reovirus infection and protein synthesis were found in both cell lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-08-2508/5/m_h81334547002.jpeg?Expires=1765922125&Signature=MEZ2y0HoAgoHxo2XliRDQnJuo8pbUTJaczwDwWKNJK2C4njccWEL0cMTIXss-JjYCcCNdvHLjCY80IrbtUrD5TaRR1urEvFVV4wyqToJoSQR4NheDYWX7uit3npCLSiMGQCS6oykRQg7pRjR9hQYIPJRP2NJg7Mb-RWE5spU5-P1T-KP4eKZ5Rh7vn~-grF0oaeelU~~qXOC4hpK0jyiZg5QTUX32aLbx~5U-xTmg36dKqkKYkJMHnu2BtqjxXxd5UYW8BvgOwvQ~mchU3IaA4qeXp-tuApNySMwxF-usSVRTGlQjUBTEPkQQNGElW3~v~Cd7wNEtQC4g~I7wXDf3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal