Abstract
Antibody-secreting plasma cells represent the critical end-stage effector cells of the humoral immune response. Here, we show that several distinct plasma cell subsets are concurrently present in the lymph nodes, spleen, and bone marrow of mice deficient in both E- and P-selectin. One of these subsets was a B220-negative immunoglobulin g (IgG) plasma cell population expressing low to negative surface levels of syndecan-1. Examination of the chemotactic responsiveness of IgG plasma cell subsets revealed that migration toward stromal cell-derived factor 1/CXC ligand 12 (SDF-1/CXCL12) was primarily limited to the B220-lo subset regardless of tissue source. Although B220-negative plasma cells did not migrate efficiently in response to CXCL12 or to other chemokines for which receptor mRNA was expressed, these cells expressed substantial surface CXC chemokine receptor-4 (CXCR4), and CXCL12 stimulation rapidly induced extracellular signal regulated kinase 1 (ERK1)/ERK2 phosphorylation, demonstrating that CXCR4 retained signaling capacity. Therefore, B220-negative plasma cells exhibit a selective uncoupling of chemokine receptor expression and signaling from migration. Taken together, our findings document the presence of significant heterogeneity within the plasma cell compartment, which suggests a complex step-wise scheme of plasma cell differentiation in which the degree of differentiation and tissue location can influence the chemotactic responsiveness of IgG plasma cells. (Blood. 2003;102:4076-4083)
Introduction
Antibody production by long-lived plasma cells is an important component of protective long-term immunity. Although formed in secondary lymphoid organs, such as the spleen and peripheral lymph nodes (LNs), most of the long-lived plasma cells eventually reside in the bone marrow (BM).1-3 Recent work has suggested that plasma cells likely represent a heterogeneous population with distinct survival, adhesion, and migratory characteristics.4-7 For example, following immunization with tetanus toxoid, human plasma cells exhibited phenotypes consistent with increasing stages of maturity from the tonsil, to the blood, and finally in the BM.4 Furthermore, precursors of plasma cells have also been demonstrated to display long-lived potential as well as heterogeneous properties, such as the capacity of different precursor subsets to differentiate into plasma cells with varying life spans.8 Further analysis into the complexity of the plasma cell compartment will provide important information regarding pathways of plasma cell differentiation and the possible distinct roles of plasma cell subsets.
Several chemokines have recently been implicated in the regulation of plasma cell migration.5,6,9-11 For example, splenic plasma cells 6 days following immunization migrated in response to CXCL12 (CXC ligand 12, or stromal cell-derived factor 1 [SDF-1]), and plasma cells genetically lacking CXC chemokine receptor-4 (CXCR4), the receptor for CXCL12, exhibited an altered positioning in the spleen and reduced accumulation in the BM.9 Interestingly, BM plasma cells were also shown to display a chemotactic response toward CXCL12, as well as toward another chemokine, CXCL9 (Mig), at 6 days but not at 12 days following immunization, demonstrating that plasma cell responsiveness to these chemokines is a transient component of the immune response.5 In addition, immunoglobulin A (IgA)-secreting plasma cells but not IgM- or IgG-secreting plasma cells were shown to migrate efficiently in response to CC chemokine ligand 25 (CCL25, or TECK) as well as CCL28 (MEC), 2 chemokines that are abundantly expressed in mucosal tissues.6,12 Taken together, these data demonstrate that plasma cells display heterogeneous migratory capacities in response to various chemokines.
We have previously illustrated that mice deficient in both E- and P-selectin (E/P-/-) represent a model to investigate terminal B-cell differentiation.13 These mice spontaneously display an expanded cervical LN B-cell compartment consistent with a persistent polyclonal immune response. The B-cell compartment of these LN comprises several subsets, including IgM+ B cells, germinal center and memory B cells, as well as IgG-secreting plasma cells. We have previously described the purification of B220-negative terminally differentiated IgG-secreting plasma cells from these cervical LN and reported the unique adhesion and gene expression profile of these cells.13,14 In addition to LN, the E/P-/- mice exhibit an increased frequency of IgG-secreting plasma cells compared with wild-type cells in other tissues as well, including spleen, BM, and peripheral blood.13 Consequently, the concurrent presence of significant numbers of plasma cells in multiple tissues suggests that further analysis of the distribution and functional properties of plasma cells in E/P-/- mice could provide substantial information concerning key aspects of plasma cell differentiation. In this report, we show that multiple plasma cell subsets are detectable in cervical LN, spleen, as well as BM, of E/P-/- mice, suggesting that later stages of plasma cell differentiation can occur in several tissues. Additionally, we demonstrate that these subsets exhibit distinct chemotactic responses to CXCL12 associated with stage of differentiation and anatomical location.
Materials and methods
Mice
The E-selectin/P-selectin double-deficient (E/P-/-) mice, backcrossed 5 generations to C57BL/6, were provided by Dr Dan Bullard (University of Alabama-Birmingham). Wild-type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in our facility.
ELISPOT analysis
The quantification of antibody-secreting plasma cells in a given sample was performed by using enzyme-linked immunospot (ELISPOT) analysis as previously described.13 Briefly, a monoclonal antibody (mAb) specific for κ-light chain was used as the capture antibody and was plated in 96-well Unifilter plates (Whatman, Clifton, NJ). Prior to adding cells, the plates were washed with phosphate-buffered saline (PBS), and then blocked with Dulbecco modified Eagle medium/2% bovine serum albumin (DMEM/BSA). The cells of interest were then incubated on the plates for 3 to 4 hours at 37°C. All samples were analyzed in triplicate at several cellular concentrations. Following washes, the appropriate detecting antibody was added and incubated overnight at 4°C. For the detection of IgM-secreting cells a biotinylated anti-IgM monoclonal antibody (Pharmingen, San Diego, CA) was used. For the detection of IgG-secreting cells biotinylated monoclonal antibodies specific for IgG1, IgG2a, IgG2b, and IgG3 (Pharmingen) were used either separately or in combination. The plates were then washed and incubated with an alkaline phosphatase-conjugated antibiotin antibody (Vector Labs, Burlingame, CA). Subsequently, the plates were developed as described13 and counted by using an ELISPOT plate reader and software (Cellular Technologies, Cleveland, OH).
Cell isolation
For purification of B220-defined subsets cervical LN, spleen, and BM cells were labeled with anti-B220 microbeads (Miltenyi Biotec, Auburn, CA) and separated into B220-positive and B220-negative (neg) fractions using lymphocyte separation (LS)-positive selection columns according to the manufacturer's protocol (Miltenyi Biotec). A purity of more than 98% was achieved. The total number of plasma cells in each fraction for each tissue was determined by scaling the frequency of plasma cells (determined by ELISPOT, described in “ELISPOT analysis”) by the total cell counts and the overall percentage of B220-expressing cells in the unseparated populations as determined by flow cytometry. In addition, total BM plasma cell numbers were calculated, assuming that both femurs and both tibias contain 13.4% and 5.7% of total BM cells, respectively.15 For isolation of the B-cell compartment of E/P-/- LN, cervical LN cells from pooled mice were depleted of non-B cells by using anti-CD5 and anti-Mac-1 microbeads and lymphocyte depletion (LD) columns (Miltenyi Biotec). Further enrichment was also performed where indicated by adding anti-IgM microbeads to the cocktail to deplete surface IgM+ B cells. Purification of B220-neg plasma cells was performed as previously described in detail,13 using anti-CD5, anti-Mac-1, and anti-B220 microbeads and LD columns (Miltenyi Biotec). Subsequent separation of the syndecan-1-hi and syndecan-1-lo/negative fractions was performed by labeling the B220-neg plasma cells with biotinylated anti-syndecan-1(CD138), clone 281.2 (Pharmingen), followed by an incubation with streptavidin-conjugated microbeads (Miltenyi Biotec), and separated using LD columns. Wild-type IgM+ B cells were isolated from wild-type C57BL/6 spleens by using anti-IgM microbeads and LS+ columns.
Flow cytometry
Flow cytometry was performed as described elsewhere.16 For comparison of lineage marker and adhesion molecule expression between B220-lo and B220-neg plasma cells, 3-color flow cytometry analysis was performed on the purified B-cell (CD5/Mac-1-negative) compartment of E/P-/- cervical LN by using anti-B220 and anti-IgM in combination with a series of monoclonal antibodies specific for the marker of interest as described in detail previously.13 For analysis of the CD5/Mac-1/IgM-negative LN compartment, correlated B220 and syndecan-1 expression was examined by using allophycocyanin (APC)-conjugated anti-B220, clone RA3/6B2, and phycoerythrin (PE)-conjugated anti-syndecan-1 (CD138), clone 281.2, both purchased from Pharmingen. Examination of the surface expression of CXCR4 by plasma cell subsets was performed by using APC-anti-B220, PE-anti-syndecan-1, and fluorescein isothiocyanate (FITC)-conjugated anti-CXCR4, clone 2B11 (Pharmingen).
Chemotaxis assay
Recombinant murine CXCL12 (SDF-1α) was purchased from Peprotech (Rocky Hill, NJ). Chemotaxis assays were performed by using 24-well plates with 6.5-mm diameter Transwell inserts (5-μm pores; Corning, Corning, NY). The lower chamber of the Transwell was loaded with chemokine at the appropriate concentration in 600 μL RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), penicillin, streptomycin, and l-glutamine. Between 0.5 × 106 and 2.5 × 106 of the cells of interest were then added to the upper chamber of the Transwell in 100 μL. Cells were allowed to migrate for 3 hours at 37°C, and migrated cells from replicate wells were pooled and counted. The fraction of IgG- or IgM-secreting cells in the input and migrated populations was determined by ELISPOT, and the percentage of migrated antibody-secreting cells was calculated. For experiments using the CD5/Mac-1/IgM-depleted LN population, the input and migrated cells were additionally analyzed by flow cytometry as described earlier for the expression of B220 and syndecan-1.
RT-PCR
Reverse transcription-polymerase chain reaction (RT-PCR) analysis, as previously described,17 was used to examine the expression of a panel of chemokine receptors, including CCR1-CCR9, CXCR2-CXCR5, D6, XCR1, and CX3CR1. Total cellular RNA was isolated by using Trizol (Invitrogen, Carlsbad, CA) from an equal number of B220-neg plasma cells from E/P-/- cervical lymph nodes (CLNs), wild-type splenic IgM+ B cells, and wild-type whole splenic cells and used as a template in a 20-μL RT reaction. Reactions performed in the absence of reverse-transcriptase served as negative controls. Wild-type whole splenic cells were used as the positive control for all genes, and a 50-μL PCR reaction with conditions optimized for each gene was performed. Primer sequences were kindly provided by A. Wagers (Stanford University, Stanford, CA) and B. Fife (Northwestern University, Chicago, IL) and were described previously.18 For D6 the following primers were used: sense, 5′-GTGCAGATCCACCAGACCTTAGATG-3′, and antisense, 5′-CCTTCAGGTACCGGCGGAAGC-3′. Specific PCR products were visualized on 1.4% agarose gels with ethidium bromide. For semiquantitative experiments, 2-fold serial dilutions of the input cDNA were used as templates for the PCR reactions.
SDS-PAGE and Western blot analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were performed as described.19 B220-neg plasma cells purified from E/P-/- CLNs were incubated at 37°C for 30 minutes in unsupplemented RPMI. Cells were then stimulated with CXCL12 (100 ng/mL) or phorbol 12-myristate 13-acetate (PMA; 10 nM) for the indicated times at 37°C. Cell lysates were made from equal numbers of cells with the following lysis buffer: 1% Triton X-100, 10 mM Tris (tris(hydroxymethyl)aminomethane)-Cl, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 1 mM pepstatin A, 1 mM leupeptin, 50 mM β-glycerophosphate, 50 mM NaF, and 0.5 mM Na3VO4. Rabbit anti-p44/p42 mitogen-activated protein (MAP) kinase (extracellular signal regulated kinase 1[ERK1]/ERK2) and rabbit antiphospho-p44/p42 antibodies were purchased from Cell Signaling Technology (Beverly, MA), and horseradish peroxidase-conjugated antirabbit IgG was purchased from Biosource (Camarillo, CA).
Statistical analysis
Statistical differences were determined using the Student t test, with a P value of less than .05 considered statistically significant.
Results
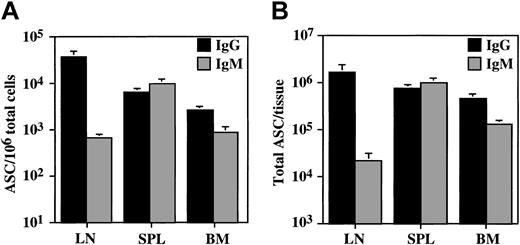
We previously focused our analysis of the plasma cell compartment of E/P-/- mice on the B220-negative IgG-secreting plasma cells present in the cervical lymph nodes (LN).13 However, in addition to the cervical LN, IgG plasma cells are also present in elevated numbers in other E/P-/- lymphoid tissues, including spleen and bone marrow (BM) (Figure 1). Furthermore, although most plasma cells in cervical LNs secrete IgG, there is a small but detectable number of IgM-secreting plasma cells in these LNs. Spleen and BM also contained significant numbers of IgM plasma cells (Figure 1). Overall, IgG and IgM plasma cells combined represented 3.67%, 1.56%, and 0.35% of total cervical LN, spleen, and BM cells, respectively.
IgM and IgG plasma cells are found in most E/P-/- lymphoid tissues. The density and total number of IgG (▪) and IgM (▦) plasma cells (ASC) in the cervical lymph nodes (LNs), spleen (SPL), and bone marrow (BM) of E/P-/- mice was determined by ELISPOT. The data are represented as the mean ± SEM. For IgM, n = 8 mice, and for IgG, n = 10 to 12 mice. (A) ASC/106 total cells. (B) Total ASC/tissue.
IgM and IgG plasma cells are found in most E/P-/- lymphoid tissues. The density and total number of IgG (▪) and IgM (▦) plasma cells (ASC) in the cervical lymph nodes (LNs), spleen (SPL), and bone marrow (BM) of E/P-/- mice was determined by ELISPOT. The data are represented as the mean ± SEM. For IgM, n = 8 mice, and for IgG, n = 10 to 12 mice. (A) ASC/106 total cells. (B) Total ASC/tissue.
Antibody-secreting cells either lacking B220 or expressing B220 at low levels have been described previously in several models.6,11,20,21 We previously observed, following depletion of non-B (CD5+/Mac-1+) cells from the cervical LNs of E/P-/- mice, a B220-negative (neg) population of IgG plasma cells, as well as a surface IgM-negative population expressing an intermediate level of B220 (B220-lo).13 Further investigation of the phenotype of these B220-lo cells illustrated that they shared several characteristics with B220-neg plasma cells, including the elevated expression of CD49d, CD18, CD11a, P-selectin glycoprotein ligand-1 (PSGL-1), CD44, and 2 CD43 epitopes, S7 and S11 (Table 1). However, the B220-lo population contained a significantly increased percentage of cells expressing the B-cell markers CD19, CD38, and major histocompatibility complex (MHC) class II, as well as L-selectin (Table 1), suggesting that these cells may be less differentiated and may represent precursors of the B220-neg plasma cell population.
Expression of lineage markers and adhesion molecules by B220-lo and B220-neg plasma cells
Lineage marker/adhesion molecule . | B220-lo, %positive . | B220-neg, %positive . | MFI fold change, B220-neg/B220-lo . |
|---|---|---|---|
| CD19 | 61.70 ± 12.76 * | 21.63 ± 13.96 * | 0.39 ± 0.05 |
| CD38 | 49.22 ± 14.41 * | 21.18 ± 7.90 * | 0.45 ± 0.09 |
| MHC class II | 57.08 ± 12.64 * | 19.96 ± 11.24 * | 0.34 ± 0.11 |
| L-selectin | 18.27 ± 0.59 * | 3.08 ± 0.55 * | 0.33 ± 0.10 |
| CD43 (S7) | 87.48 ± 8.39 | 88.32 ± 3.73 | 0.97 ± 0.20 |
| CD43 (S11) | 95.62 ± 2.15 | 97.80 ± 0.98 | 1.20 ± 0.14 |
| CD18 | 96.19 ± 4.71 | 97.08 ± 1.61 | 0.95 ± 0.15 |
| CD11a | 93.43 ± 8.55 | 96.85 ± 1.78 | 1.05 ± 0.14 |
| CD49d | 74.87 ± 11.13 | 81.90 ± 8.60 | 1.05 ± 0.19 |
| PSGL-1 | 77.43 ± 7.17 | 79.81 ± 4.16 | 0.99 ± 0.34 |
| CD44 | 99.64 ± 0.06 | 99.63 ± 0.22 | 1.35 ± 0.42 |
Lineage marker/adhesion molecule . | B220-lo, %positive . | B220-neg, %positive . | MFI fold change, B220-neg/B220-lo . |
|---|---|---|---|
| CD19 | 61.70 ± 12.76 * | 21.63 ± 13.96 * | 0.39 ± 0.05 |
| CD38 | 49.22 ± 14.41 * | 21.18 ± 7.90 * | 0.45 ± 0.09 |
| MHC class II | 57.08 ± 12.64 * | 19.96 ± 11.24 * | 0.34 ± 0.11 |
| L-selectin | 18.27 ± 0.59 * | 3.08 ± 0.55 * | 0.33 ± 0.10 |
| CD43 (S7) | 87.48 ± 8.39 | 88.32 ± 3.73 | 0.97 ± 0.20 |
| CD43 (S11) | 95.62 ± 2.15 | 97.80 ± 0.98 | 1.20 ± 0.14 |
| CD18 | 96.19 ± 4.71 | 97.08 ± 1.61 | 0.95 ± 0.15 |
| CD11a | 93.43 ± 8.55 | 96.85 ± 1.78 | 1.05 ± 0.14 |
| CD49d | 74.87 ± 11.13 | 81.90 ± 8.60 | 1.05 ± 0.19 |
| PSGL-1 | 77.43 ± 7.17 | 79.81 ± 4.16 | 0.99 ± 0.34 |
| CD44 | 99.64 ± 0.06 | 99.63 ± 0.22 | 1.35 ± 0.42 |
Percentage (%) positive and MFI fold change are represented as the mean ± SD determined from 2 to 6 independent experiments for each marker. MFI indicates mean fluorescence intensity.
Statistically significant difference for % positive between B220-lo and B220-neg populations.
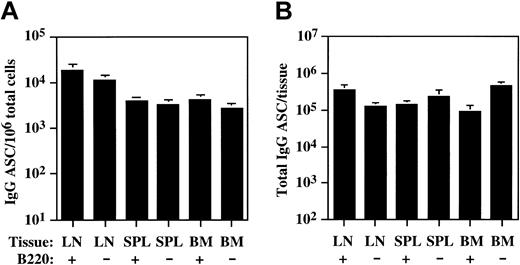
Consequently, to determine if the presence of B220-lo plasma cells was restricted to the LN in E/P-/- mice, we separated LN, spleen, and BM cells into B220-positive and B220-negative fractions and quantified IgG-secreting plasma cell numbers in each fraction by ELISPOT (Figure 2). In addition to the B220-neg plasma cells, significant numbers of IgG plasma cells were also detected in the B220-positive fraction from the LNs, consistent with the presence of a B220-expressing plasma cell population. Similarly, significant numbers of plasma cells were detected in both the B220-positive and B220-negative fractions of both spleen and BM (Figure 2), demonstrating that both B220-positive and B220-negative subsets of plasma cells can be found in each of these lymphoid organs.
B220-lo and B220-neg plasma cells are found in multiple lymphoid organs. Cervical lymph node (LN), spleen (SPL), and bone marrow (BM) cells were separated into B220-positive (+) and B220-negative (-) fractions, and the density and total number of IgG plasma cells (ASC) per tissue in each fraction was determined by ELISPOT as described in “Materials and methods.” The data are represented as the mean ± SEM. For LN, n = 5 mice, and for SPL and BM, n = 6 mice. (A) IgG ASC/106 total cells. (B) Total IgG ASC/tissue.
B220-lo and B220-neg plasma cells are found in multiple lymphoid organs. Cervical lymph node (LN), spleen (SPL), and bone marrow (BM) cells were separated into B220-positive (+) and B220-negative (-) fractions, and the density and total number of IgG plasma cells (ASC) per tissue in each fraction was determined by ELISPOT as described in “Materials and methods.” The data are represented as the mean ± SEM. For LN, n = 5 mice, and for SPL and BM, n = 6 mice. (A) IgG ASC/106 total cells. (B) Total IgG ASC/tissue.
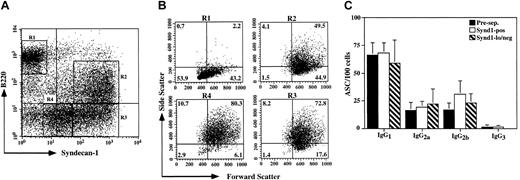
To further investigate the nature and diversity of plasma cell subsets, we enriched for the more differentiated B-cell compartment by depleting LN cells of IgM+ B cells as well as non-B (CD5+/Mac-1+) cells and examined the expression of B220 and syndecan-1 on this plasma cell-enriched population (Figure 3A). The B220-hi/syndecan-1-neg population exhibited a light scatter and surface marker phenotype consistent with a combination of memory and germinal center B cells (Figure 3B and data not shown). A minor subset of B220-hi/syndecan-1-positive cells was also observed, consistent with the previously reported partial plasma cell phenotype exhibited by a small fraction of germinal center cells.22 In addition, 3 plasma cell populations were evident, including a B220-lo/syndecan-1-hi population, representing the plasma cells detected by ELISPOT in the B220-positive fraction of these LNs in Figure 2, as well as 2 distinct subpopulations of B220-neg plasma cells exhibiting differing levels of syndecan-1 (CD138) expression (Figure 3A). Interestingly, a stepwise increase in side light scatter was observed going from the B220-lo/syndecan-1-hi cells to the B220-neg/syndecan-1-hi cells (1.19-fold ± 0.12-fold, n = 4) and further from the B220-neg/syndecan-1-hi cells to the B220-neg/syndecan-1-lo/neg population (1.15-fold ± 0.11-fold, n = 4) (Figure 3B). In contrast, on average the change in forward light scatter between any 2 of these populations was 1.03-fold ± 0.01-fold (n = 4). These data suggest that, although cell size does not change significantly between these populations, the number and/or density of cytoplasmic organelles and possibly antibody production is increased.
Novel plasma cell subsets defined by B220 and syndecan-1 expression. (A) E/P-/- cervical lymph node cells from pooled mice were depleted of CD5, Mac-1, and IgM+ cells and the expression of B220 and syndecan-1 (CD138) was determined by flow cytometry. (B) The forward and side light scatter profiles of the 4 major populations present in panel A are displayed as indicated. A representative experiment of 4 is shown. (C) E/P-/- cervical lymph node cells were initially depleted of CD5, Mac-1, and B220 expressing cells (Pre-sep.; ▪). This population was subsequently separated into syndecan-1-positive (Synd1-pos; □) and syndecan-1-lo/negative (Synd1-lo/neg; ▧) fractions as described in “Materials and methods.” The number of cells secreting each of the IgG subclasses (mean ± SD) was determined by ELISPOT for the syndecan-1-separated populations in addition to the unseparated CD5/Mac-1/B220-negative population. A representative experiment of 2 is shown.
Novel plasma cell subsets defined by B220 and syndecan-1 expression. (A) E/P-/- cervical lymph node cells from pooled mice were depleted of CD5, Mac-1, and IgM+ cells and the expression of B220 and syndecan-1 (CD138) was determined by flow cytometry. (B) The forward and side light scatter profiles of the 4 major populations present in panel A are displayed as indicated. A representative experiment of 4 is shown. (C) E/P-/- cervical lymph node cells were initially depleted of CD5, Mac-1, and B220 expressing cells (Pre-sep.; ▪). This population was subsequently separated into syndecan-1-positive (Synd1-pos; □) and syndecan-1-lo/negative (Synd1-lo/neg; ▧) fractions as described in “Materials and methods.” The number of cells secreting each of the IgG subclasses (mean ± SD) was determined by ELISPOT for the syndecan-1-separated populations in addition to the unseparated CD5/Mac-1/B220-negative population. A representative experiment of 2 is shown.
Taken together, the distinct surface marker expression profiles and stepwise increases in side light scatter suggest a pathway of plasma cell differentiation from B220-hi B cells to B220-hi/syndecan-1-positive putative plasma cell precursors,22 to B220-lo plasma cells, to B220-neg plasma cells, and within the B220-neg subset, further differentiation from syndecan-1-hi to syndecan-1-lo/neg Additionally, at least 2 of these subsets, B220-lo and B220-neg, are present in all lymphoid organs examined. By using the identical depletion strategy as we used for LNs, we were unable to obtain adequate enrichment of the plasma cell compartment from spleen or BM because of significant Mac-1-negative myeloid progenitor cell contamination (data not shown).
To confirm that the cells expressing low to negative levels of syndecan-1 in the LNs were in fact plasma cells, the population in Figure 3A was depleted of B220-expressing cells, separated into syndecan-1-positive and syndecan-1-negative fractions, and the number of IgG-secreting cells was quantified by ELISPOT. For each of the IgG subclasses, the syndecan-1-lo/neg population exhibited a similar frequency of antibody-secreting cells compared with either the unfractionated B220-neg or syndecan-1-positive populations (Figure 3C). Within the limits of the dilution error inherent in the assay, the fraction of syndecan-1-lo/neg cells that spontaneously secreted IgG approached 100% (Figure 3C). Additionally, consistent with the ELISPOT results, 89.6% (844 of 942 counted) of the cells in the B220-neg/syndecan-1-lo/neg fraction displayed pronounced typical plasma cell morphology, with abundant cytoplasm and an eccentrically placed nucleus, and these cells exhibited a higher cytoplasmic-nuclear ratio than B220-neg cells as a whole (data not shown). These data demonstrate that plasma cell subsets defined by differential syndecan-1 expression display indistinguishable isotype expression profiles. Furthermore, the syndecan-1-lo/neg plasma cells represent a unique plasma cell subpopulation, and the presence of these cells illustrates that high syndecan-1 expression is not a universal marker of plasma cells in all settings.
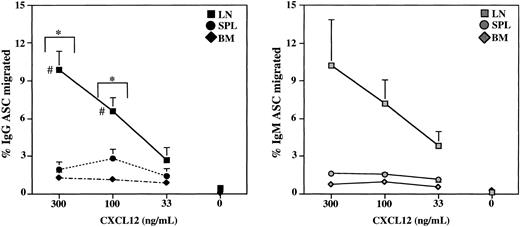
Chemokines have been recently demonstrated to play a role in regulating plasma cell migration.5,6,9-11 The presence and diversity of plasma cells in multiple E/P-/- tissues enabled an investigation of the chemotactic responsiveness of distinct plasma cell subsets in several anatomical sites. Unfractionated total LN IgG plasma cells displayed a significant chemotactic response toward graded concentrations of CXCL12/SDF-1 (Figure 4), consistent with previous reports of plasma cells from spleen.5,9 In addition, IgG plasma cells within the spleen and BM of E/P-/- mice also migrated toward CXCL12, although the magnitude of the response was decreased in these tissues relative to lymph node (Figure 4). A similar response profile was observed for IgM-secreting plasma cells (Figure 4), demonstrating that CXCL12 can induce the migration of plasma cells of both of these isotypes with equal efficiency. To determine whether the chemotactic response was limited to a distinct plasma cell subset, we depleted LN cells of non-B and IgM+ B cells, as in Figure 3, and performed chemotaxis assays using this plasma cell-enriched population. Basal plasma cell migration toward media alone was minimal (Figure 5A). In response to CXCL12, the B220-lo/syndecan-1-hi plasma cells exhibited significantly enhanced migration. In contrast, the B220-neg plasma cells, present at substantial frequencies in the input population, failed to migrate efficiently toward CXCL12 (Figure 5A). The failure of B220-neg plasma cells from LN to migrate was also observed when the pore size of the transwell inserts was increased from 5 μm to 8 μm (data not shown), indicating that cell size was not the limiting factor. To determine the migratory capacity of B220-lo versus B220-neg plasma cell subsets in other tissues we separated spleen, BM, as well as LN, into B220-positive and B220-negative fractions prior to the chemotaxis analysis. Consistent with the results from the enriched population, IgG plasma cells within the B220-positive fraction of LN migrated with a significantly enhanced efficiency versus the B220-neg plasma cells (Figure 5B). An analogous profile was observed in the spleen, where plasma cells in the B220-positive fraction migrated nearly as well as B220-positive plasma cells in LN. Interestingly, although the efficiency was decreased in the BM, the migrating plasma cells in the BM were similarly contained within the B220-positive fraction. Furthermore, the migration efficiencies of the separated fractions were consistent with the migration observed for the unfractionated populations (Figure 4), when the relative input frequencies of B220-lo and B220-neg plasma cells in each tissue are taken into account (Figure 2). Taken together, these data demonstrate that B220-lo plasma cells migrate substantially better than B220-neg plasma cells in response to CXCL12 irrespective of anatomical source.
Chemotaxis efficiency of total IgG and IgM plasma cells from E/P-/- tissues. Chemotaxis assays were performed with unfractionated cervical lymph node (LN), spleen (SPL), and bone marrow (BM) cells in response to graded concentrations of CXCL12 (SDF-1). The frequencies of IgG (left) and IgM (right) plasma cells in the input and migrated samples were determined by ELISPOT, and the percentages of plasma cells migrated were calculated. The data are represented as the mean ± SEM of several independent experiments. For IgG, n = 4 to 9, and for IgM, n = 2 to 3. The (*) indicates statistically significant migration of LN, SPL, and BM IgG plasma cells compared with basal migration in each tissue. The (#) indicates statistical difference between response of LN compared with SPL or BM at 300 and 100 ng/mL, respectively.
Chemotaxis efficiency of total IgG and IgM plasma cells from E/P-/- tissues. Chemotaxis assays were performed with unfractionated cervical lymph node (LN), spleen (SPL), and bone marrow (BM) cells in response to graded concentrations of CXCL12 (SDF-1). The frequencies of IgG (left) and IgM (right) plasma cells in the input and migrated samples were determined by ELISPOT, and the percentages of plasma cells migrated were calculated. The data are represented as the mean ± SEM of several independent experiments. For IgG, n = 4 to 9, and for IgM, n = 2 to 3. The (*) indicates statistically significant migration of LN, SPL, and BM IgG plasma cells compared with basal migration in each tissue. The (#) indicates statistical difference between response of LN compared with SPL or BM at 300 and 100 ng/mL, respectively.
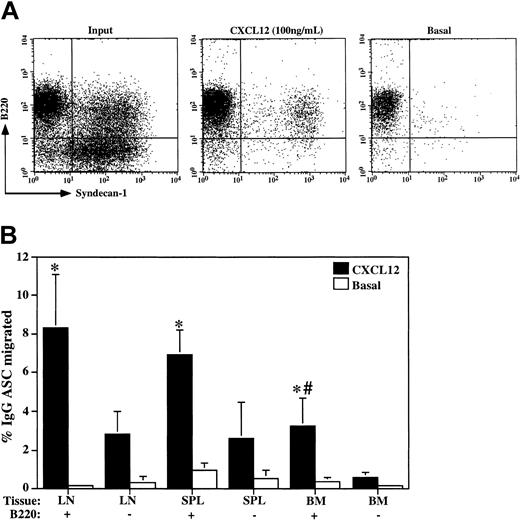
Chemotaxis of IgG plasma cells in response to CXCL12 is limited to B220-positive plasma cells. (A) Chemotaxis assays in response to CXCL12 (100 ng/mL) were performed with CD5/Mac-1/IgM-depleted cervical lymph node cells. Overall, 7.53% ± 2.04% (n = 4) of input cells migrated toward CXCL12, whereas only 0.81% ± 0.49% (n = 4) migrated toward media alone (Basal). Input and migrated cells were analyzed by flow cytometry for the expression of B220 and syndecan-1 to identify plasma cell subsets. A representative experiment of 4 is shown. (B) Cervical lymph node (LN), spleen (SPL), and bone marrow (BM) cells were separated into B220-positive (+) and B220-negative (-) fractions as in Figure 1B prior to the CXCL12 (100 ng/mL) chemotaxis assays. The number of IgG plasma cells in the input and migrated populations for each fraction was determined by ELISPOT (n = 6), and the percentage migrated was calculated (mean ± SD). The (*) indicates statistical difference between B220-positive and B220-negative within each tissue. The (#) indicates statistical difference between the BM B220-positive fraction and B220-positive fractions from LN or SPL. A representative experiment of 3 is shown.
Chemotaxis of IgG plasma cells in response to CXCL12 is limited to B220-positive plasma cells. (A) Chemotaxis assays in response to CXCL12 (100 ng/mL) were performed with CD5/Mac-1/IgM-depleted cervical lymph node cells. Overall, 7.53% ± 2.04% (n = 4) of input cells migrated toward CXCL12, whereas only 0.81% ± 0.49% (n = 4) migrated toward media alone (Basal). Input and migrated cells were analyzed by flow cytometry for the expression of B220 and syndecan-1 to identify plasma cell subsets. A representative experiment of 4 is shown. (B) Cervical lymph node (LN), spleen (SPL), and bone marrow (BM) cells were separated into B220-positive (+) and B220-negative (-) fractions as in Figure 1B prior to the CXCL12 (100 ng/mL) chemotaxis assays. The number of IgG plasma cells in the input and migrated populations for each fraction was determined by ELISPOT (n = 6), and the percentage migrated was calculated (mean ± SD). The (*) indicates statistical difference between B220-positive and B220-negative within each tissue. The (#) indicates statistical difference between the BM B220-positive fraction and B220-positive fractions from LN or SPL. A representative experiment of 3 is shown.
To ascertain the mechanism underlying the reduced migration of the B220-neg subset, we first analyzed the expression of CXCR4 on the plasma cell-enriched population by flow cytometry. The B220-lo plasma cells and the B220-neg plasma cells expressed equivalent surface CXCR4 (Figure 6A). Furthermore, examination of the correlated expression of syndecan-1 and CXCR4 also demonstrated that most of the syndecan-1-expressing cells, including the syndecan-1-lo/neg plasma cells, exhibited surface expression of the receptor (Figure 6A). Expression of CXCR4, in the absence of migration to CXCL12, has been previously demonstrated for germinal center B cells.23 These data described here are also consistent with the previously reported maintenance of CXCR4 surface expression by nonmigrating antigen-specific plasma cells in the BM 12 days following secondary immunization.5
CXCR4 surface expression and signaling are maintained in B220-negative plasma cells. (A) CD5/Mac-1/IgM-depleted cervical lymph node cells were examined by flow cytometry for surface expression of CXCR4 versus B220 and syndecan-1. A representative experiment of 2 is shown. (B) Purified B220-negative plasma cells from E/P-/- cervical lymph nodes were stimulated with CXCL12 (100 ng/mL) or PMA (10 nM) for the indicated times in unsupplemented RPMI at 37°C, and the presence of phosphorylated ERK1/ERK2 (p44/p42 MAP kinase) was then determined by Western blot as described in “Materials and methods.” Subsequently, the nitrocellulose membrane was stripped and reprobed to determine the presence of total ERK1/ERK2 protein. A representative experiment of 2 is shown.
CXCR4 surface expression and signaling are maintained in B220-negative plasma cells. (A) CD5/Mac-1/IgM-depleted cervical lymph node cells were examined by flow cytometry for surface expression of CXCR4 versus B220 and syndecan-1. A representative experiment of 2 is shown. (B) Purified B220-negative plasma cells from E/P-/- cervical lymph nodes were stimulated with CXCL12 (100 ng/mL) or PMA (10 nM) for the indicated times in unsupplemented RPMI at 37°C, and the presence of phosphorylated ERK1/ERK2 (p44/p42 MAP kinase) was then determined by Western blot as described in “Materials and methods.” Subsequently, the nitrocellulose membrane was stripped and reprobed to determine the presence of total ERK1/ERK2 protein. A representative experiment of 2 is shown.
To determine whether CXCR4 expressed by the B220-neg plasma cells was competent for signaling, we analyzed ERK1/ERK2 (p44/p42) phosphorylation in purified B220-neg plasma cells in response to CXCL12 stimulation. CXCL12 induced a rapid and robust increase in ERK1/ERK2 phosphorylation (Figure 6B), and the magnitude of phosphorylation was similar to that induced by PMA (Figure 6B), a known strong activator of MAP kinase signaling.24 These data demonstrate that the greatly reduced chemotactic response to CXCL12 exhibited by B220-neg plasma cells is not due to a global defect in CXCR4 signaling.
The nearly absent migration of the B220-neg plasma cells toward CXCL12 despite retention of CXCR4 prompted us to examine the expression of other chemokine receptors by this subset. The mRNA expression profile determined by RT-PCR of a panel of chemokine receptors in B220-neg plasma cells compared with IgM+ B cells is summarized in Table 2. IgM+ B cells were used as a baseline comparison to identify any receptor that was specifically up-regulated or down-regulated as a function of plasma cell differentiation. In agreement with previous results,9,11 the B220-neg plasma cells had lost expression of CCR7 and CXCR5 (Figure 7). Surprisingly, the plasma cells maintained expression of mRNA encoding several other chemokine receptors in addition to CXCR4, including CCR1, CCR2, CCR5, and CCR6 (Table 2). The plasma cells also displayed a down-regulation of CCR3, CXCR2, and D6, but an up-regulation of CCR4 and CCR9 mRNA (Figure 7). The expression of CCR4, CCR9, and the other receptors retained by the B220-neg plasma cells suggests that other chemokines besides CXCL12 could potentially participate in various aspects of IgG plasma cell function. However, so far we have not observed chemotaxis of B220-lo or B220-neg IgG plasma cells from E/P-/- mice in response to TECK/CCL25 (CCR9 ligand), TARC/CCL17 (CCR4 ligand), JE/CCL2 (CCR2 ligand), or macrophage inflammatory protein-1α (MIP-1α)/CCL3 (CCR1 and CCR5 ligand) (data not shown). Furthermore, the combination of CCL2 or CCL3 with CXCL12 did not alter the responsiveness of either plasma cell subset to CXCL12 (data not shown). Taken together, these data suggest a generalized lack of chemotactic responsiveness by B220-neg plasma cells, and the function of these receptors for which mRNA was expressed remains to be determined.
Summary of B220-neg plasma cell chemokine receptor mRNA expression
Receptor . | B220-neg PC . | IgM+ B . | Spleen . |
|---|---|---|---|
| PC > B | |||
| CCR4 | + | − | + |
| CCR9 | + | +/− | + |
| PC < B | |||
| CCR3 | +/− | + | + |
| CCR7 | − | + | + |
| CXCR2 | − | +/− | + |
| CXCR5 | − | + | + |
| D6 | − | + | + |
| PC and B (+) | |||
| CCR1 | + | + | + |
| CCR2 | + | + | + |
| CCR5 | + | + | + |
| CCR6 | + | + | + |
| CXCR4 | + | + | + |
| PC and B (Io/−) | |||
| CCR8 | +/− | +/− | + |
| CXCR3 | +/− | +/− | + |
| XCR1 | +/− | +/− | + |
| CX3CR1 | − | − | + |
Receptor . | B220-neg PC . | IgM+ B . | Spleen . |
|---|---|---|---|
| PC > B | |||
| CCR4 | + | − | + |
| CCR9 | + | +/− | + |
| PC < B | |||
| CCR3 | +/− | + | + |
| CCR7 | − | + | + |
| CXCR2 | − | +/− | + |
| CXCR5 | − | + | + |
| D6 | − | + | + |
| PC and B (+) | |||
| CCR1 | + | + | + |
| CCR2 | + | + | + |
| CCR5 | + | + | + |
| CCR6 | + | + | + |
| CXCR4 | + | + | + |
| PC and B (Io/−) | |||
| CCR8 | +/− | +/− | + |
| CXCR3 | +/− | +/− | + |
| XCR1 | +/− | +/− | + |
| CX3CR1 | − | − | + |
Wild-type whole spleen cells served as a positive control for all genes. (+) represents strong signal, (−) represents no signal, and (+/−) represents intermediate signal. The results are representative of 4 plasma cell and 6 IgM+ B-cell isolations. PC indicates plasma cell.
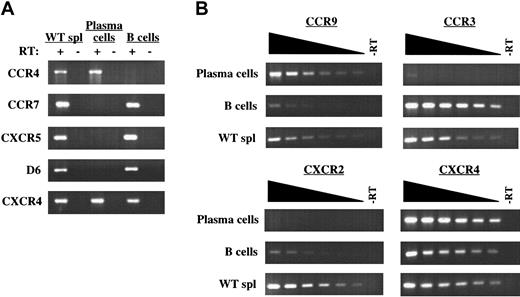
B220-negative plasma cells display a unique chemokine receptor mRNA expression profile. (A) mRNA expression for CCR4, CCR7, CXCR5, D6, and CXCR4 by B220-negative plasma cells from E/P-/- cervical lymph nodes and wild-type IgM+ B cells was determined by RT-PCR. The presence or absence of reverse transcriptase (RT) in the RT-PCR reactions is indicated. (B) Semiquantitative RT-PCR using 2-fold serial dilutions of input cDNA was used to determine the relative expression of CCR9, CCR3, and CXCR2 for plasma cells and IgM+ B cells. CXCR4 was included as a control for each cell type. WT whole spleen cells were used as a positive control for all genes in panels A and B. The complete mRNA expression profile of chemokine receptors examined is shown in Table 2. The results are representative of 4 plasma cell and 6 IgM+ B-cell isolations.
B220-negative plasma cells display a unique chemokine receptor mRNA expression profile. (A) mRNA expression for CCR4, CCR7, CXCR5, D6, and CXCR4 by B220-negative plasma cells from E/P-/- cervical lymph nodes and wild-type IgM+ B cells was determined by RT-PCR. The presence or absence of reverse transcriptase (RT) in the RT-PCR reactions is indicated. (B) Semiquantitative RT-PCR using 2-fold serial dilutions of input cDNA was used to determine the relative expression of CCR9, CCR3, and CXCR2 for plasma cells and IgM+ B cells. CXCR4 was included as a control for each cell type. WT whole spleen cells were used as a positive control for all genes in panels A and B. The complete mRNA expression profile of chemokine receptors examined is shown in Table 2. The results are representative of 4 plasma cell and 6 IgM+ B-cell isolations.
Discussion
In this study we examined in detail heterogeneity within the plasma cell compartment in mice deficient in both E- and P-selectin. Phenotypically and functionally distinct subsets of plasma cells were clearly present in these mice and were found in multiple lymphoid tissues. B220-lo and B220-neg IgG plasma cells were detected in significant numbers in the LNs, as well as spleen and BM, although the fraction of total plasma cells that were B220-neg was increased in the spleen compared with LNs and further increased in BM (Figure 2). Comparison of the surface phenotype of the B220-lo and B220-neg subsets in LNs demonstrated that the B220-lo and B220-neg plasma cells exhibited similar expression of several adhesion molecules, including α4 integrin (CD49d), lymphocyte function-associated antigen-1 (LFA-1) integrin (CD11a/CD18), PSGL-1, and CD44. However, the B220-lo plasma cells displayed an increased percentage of cells expressing B-cell markers such as CD19, CD38, and MHC class II (Table 1). The expression of markers normally associated with naive or memory B cells by the B220-lo plasma cells, as well as the presence of a higher percentage of B220-neg plasma cells in the BM, where long-lived terminally differentiated plasma cells are primarily localized,1-3 suggests that the B220-neg plasma cells may be a more fully differentiated population. The higher side light scatter (Figure 3), indicative of increased cytoplasmic constituents, by the B220-neg compared with B220-lo plasma cells, also implies a scheme of differentiation from B220-lo to B220-neg. Notably, the equal expression of α4 integrin and LFA-1 integrin by the B220-lo and B220-neg plasma cells (Table 1) demonstrates that this profile of integrin expression is a general phenotype for plasma cells formed in LNs, and, although there are clear subsets defined by B220 expression, these subsets may share similar mechanisms for localization within the LN or homing to subsequent sites.
Members of the syndecan family of proteoglycans have been demonstrated to function as adhesion molecules as well as modulators of growth factor signaling.25 Although B220-lo plasma cells in LNs expressed a relatively high level of syndecan-1 (CD138), consistent with previous reports for B220-lo plasma cells in several other models,9,11,20 syndecan-1 expression among the B220-neg plasma cells was heterogeneous (Figure 3A). Furthermore, side light scatter was increased for the syndecan-1-lo/B220-neg plasma cells compared with syndecan-1-hi/B220-neg plasma cells (Figure 3B), suggesting that these subsets could represent distinct points in the differentiation profile. Separation of the B220-neg plasma cells into syndecan-1-hi and syndecan-1-lo/neg fractions demonstrated that virtually all of the cells expressing low to negative levels of syndecan-1 spontaneously secreted IgG (Figure 3C), similar to syndecan-1-hi cells, confirming their identity as plasma cells. To our knowledge, this is the first description and purification of a syndecan-1-lo/neg population of primary plasma cells. Although syndecan-1 is often used as a marker for primary plasma cells and myeloma cells, there have been several previous studies suggesting syndecan-1 heterogeneity. For example, human plasma cells in the blood following immunization were shown to express a variable level of syndecan-1.4 In addition, members of the syndecan family can be shed from the cell surface,26,27 and soluble syndecan-1 was recently demonstrated to play a role in promoting the growth of multiple myeloma tumors as well as enhancing the dissemination of myeloma cells to other organs.28 The possible role of decreased syndecan-1 surface expression or syndecan-1 shedding in primary plasma cell function is as yet unknown, as is the function of syndecan-1 on plasma cells generally. Regardless, these findings show that high syndecan-1 expression is not characteristic of plasma cells at all stages of differentiation. Investigation of possible distinct functional properties of the syndecan-1-hi and syndecan-1-lo/neg subsets in the E/P-/- mice could provide further information regarding the interaction of plasma cells with other related cell types as well as extracellular matrix components.
Recent work has illustrated that several chemokines play a role in plasma cell migration and, specifically, that the chemotactic responsiveness of plasma cells is a function of isotype and the time after immunization.5,6 In the analysis of the E/P-/- mice described here, we demonstrated that plasma cells concurrently present within the LNs, spleen, and BM migrated in response to a gradient of CXCL12 (Figure 4). This overall migration was most pronounced in LNs, was decreased in the spleen, and further decreased in the BM. Furthermore, in each of these tissues, IgM and IgG plasma cells displayed similar migration efficiency. The presence of significant numbers of plasma cells, including plasma cell subsets, in several E/P-/- tissues enabled the further investigation into the identity of the CXCL12 responsive plasma cell subset. We found that in all tissues examined, migration of IgG plasma cells toward CXCL12 was primarily restricted to the B220-lo subset (Figure 5). B220-neg plasma cells also failed to migrate in response to multiple other chemokines tested, or to combinations of CXCL12 with CCL2 or CCL3 (data not shown). These data suggest that a distinct property of B220-neg plasma cells may be the down-regulation of migratory responsiveness to CXCL12 and other chemokines, regardless of anatomical location. This observation for IgG plasma cells is in agreement with a previous report for IgA plasma cells, which demonstrated that only IgA plasma cells expressing an intermediate level of B220 could migrate in response to CCL25, CCL28, and CXCL12.6,12 In addition, the diminished migratory capacity of B220-expressing plasma cells in BM compared with LN or spleen (Figure 5B) is consistent with BM representing the terminal destination of long-lived plasma cells, and the previously reported decrease in chemotactic responsiveness of BM plasma cells at later stages of an immune response.5
Interestingly, although the B220-neg plasma cells displayed significantly reduced migration toward CXCL12, these cells maintained surface expression of CXCR4 (Figure 6A), and, furthermore, CXCR4 signaling remained intact. Specifically, CXCL12 induced ERK1/ERK2 phosphorylation (Figure 6B). ERK1/ERK2 phosphorylation has been observed in response to CXCR4 signaling in several cell types.29-32 However, to our knowledge, this is the first demonstration of CXCR4 signaling in plasma cells. The retention of CXCR4 signaling despite absent migration suggests that the mechanism underlying the failure to migrate is a selective uncoupling of CXCR4 from chemotaxis. CXCL12 and CXCR4, therefore, likely play a role in other aspects of plasma cell function, for example, regulating processes such as adhesion and/or survival.
In addition to the loss of CCR7 and CXCR5, consistent with previous reports,9,11 the B220-neg plasma cells also showed a loss of expression of other receptors, including CCR3, CXCR2, and D6 (Figure 7). In contrast, the B220-neg plasma cells maintained expression of several other chemokine receptors and up-regulated mRNA encoding CCR4 and CCR9 (Table 2; Figure 7). The unavailability of antibodies to many of these other receptors, except for CXCR4, prevented the investigation of surface expression. However, as mentioned earlier, so far we have not observed migration of B220-neg or B220-lo plasma cells toward the respective ligands for many of these receptors, including CCR4, CCR9, CCR1, CCR2, and CCR5 (data not shown). Consequently, the lack of migration of B220-neg plasma cells to all chemokines tested thus far suggests that as part of the differentiation program B220-neg plasma cells have down-regulated factor(s) downstream of all chemokine receptors that are important in motility and chemotaxis following chemokine receptor engagement. CCR9 is expressed significantly by IgA plasma cells, and B220-lo IgA plasma cells exhibited a migratory response to CCL25.6 However, hematopoietic stem cells express CCR9 mRNA, but these cells do not migrate to CCL25,18 similar to the E/P-/- B220-neg plasma cells described here. In addition, CCR9 signaling has also been shown to protect some cells from cycloheximide-induced apoptosis.33 Further investigation will provide insight into the expression of CCR9 by IgG plasma cells in different contexts and the possible role of CCR9 in the biology of cells, such as stem cells and plasma cells, which similarly exhibit primary localization within the BM.
Taken together, the surface phenotype, side scatter profile, and retention of CXCL12-induced migration imply that the B220-lo plasma cells may be the direct precursors of the B220-neg plasma cells. It is particularly interesting that significant numbers of both B220-lo and B220-neg plasma cells were present in LNs, spleen, and BM of E/P-/- mice (Figure 2), suggesting that plasma cell differentiation from B220-lo to B220-neg may occur in multiple tissues. Conceivably the B220-lo plasma cells in the BM could represent B220-lo plasma cells formed in LN or spleen that have recently arrived in the bone marrow environment, have since partially lost migratory responsiveness to CXCL12, and will soon further differentiate into B220-neg plasma cells. However, the data described here do not specifically eliminate the possibility that the B220-lo and B220-neg plasma cell populations could be stable, distinct, plasma cell compartments.
The heterogeneous expression of syndecan-1 by the B220-neg plasma cells in the LN of E/P-/- mice (Figure 3) demonstrates that there are additional subpopulations within the B220-defined fractions. It is likely that a fraction of the B220-neg plasma cells in LNs and spleen represent fully differentiated but short-lived plasma cells that are destined for apoptosis within these tissues. Potentially, the syndecan-1-lo/neg subset could represent these short-lived cells. Accordingly, previous work has associated the loss of syndecan-1 surface expression with apoptotic myeloma cells.34 However, it is clear that some B220-neg plasma cells from the E/P-/- LNs can exhibit long-term survival in vitro when cocultured with BM stromal cells,35 suggesting that a subset of the B220-neg plasma cells are potentially long lived, although the syndecan-1 phenotype of these cells was not determined. Furthermore, gene expression profiling of these B220-neg plasma cells demonstrated that they up-regulated numerous factors associated with protection against apoptosis.14 Determination of the survival capacities of subsets defined by differential syndecan-1 expression could potentially provide important information regarding plasma cell survival mechanisms.
It is also possible that some B220-neg plasma cells in the spleen and LNs use a mechanism for homing to or localization within BM independent of migration to CXCL12. The presence of detectable, albeit reduced, numbers of CXCR4-/- plasma cells in BM suggests the possible existence of CXCR4/CXCL12 independent pathways.9 The recruitment into the BM of plasma cells that do and do not migrate in response to CXCL12 or other chemokines could provide a mechanism for the segregation of plasma cells at varied levels of differentiation into distinct microenvironments. Taken together, our findings suggest a scheme of plasma cell differentiation from B220-lo to B220-neg, and within the B220-neg subpopulation, from syndecan-1-hi to syndecan-1-lo/neg, which can proceed to completion within any lymphoid tissue, or begin in one tissue (ie, LN or spleen) and end in another (ie, BM). Further investigation of the different functional properties of B220-lo and B220-neg plasma cells, including their capacity for homing and interaction with BM stromal cells, will provide more insight into pathways of plasma cell differentiation and localization.
In summary, we have shown that multiple distinct plasma cell subsets are detectable in significant numbers in the lymph nodes, spleen, and bone marrow of mice deficient in both E- and P-selectin. Furthermore, we identified a unique subpopulation of primary plasma cells expressing a low to negative level of surface syndecan-1. Our findings also illustrate that stage of differentiation is critical in defining the chemotactic responsiveness of IgG plasma cells toward CXCL12 and other chemokines. The examination of plasma cell subsets present in the E/P-/- tissues described here further highlights the complexity of the plasma cell compartment. Additional analysis using this system will aid in deciphering the physiologic implications of functional heterogeneity within the plasma cell compartment.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-03-0947.
Supported by a grant from the National Institutes of Health (HL58710).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal