Abstract
The feasibility of combining gemtuzumab ozogamicin (GO) with intensive chemotherapy as first-line treatment of acute myeloid leukemia (AML) was assessed in 72 patients, aged 17 to 59 years, as a prelude to the United Kingdom Medical Research Council (MRC) AML15 trial. Sixty-four patients received induction chemotherapy (DAT [daunorubicin, ara-C, thioguanine], DA [daunorubicin, ara-C], or FLAG-Ida [fludarabine, ara-C, G-CSF, idarubicin]) with GO on day 1. It was possible to give GO 3 mg/m2 with course 1, but 6 mg/m2 with course 1 or GO in a dose of 3 mg/m2 with consecutive courses was not feasible because of hepatotoxicity and delayed hematopoietic recovery. Thirty-one patients who were treated in consolidation with MACE (amsacrine, ara-C, etoposide) or HidAC (HidAC) and GO (3 mg/m2), and 23 in induction and consolidation, tolerated GO (3 mg/m2) well. Grade 4 liver toxicity and sinusoidal obstructive syndrome was more common in thioguanine-containing schedules (P = .007). Remission with course 1 was seen in 86% of patients. DA or FLAG-Ida with GO in induction achieved complete remission in 91% of patients and 78% of these patients are in continuous complete remission at 8 months. GO given with induction (DA or FLAG-Ida) and consolidation (MACE or HidAC) was well tolerated. These schedules are now being compared in the MRC AML15 trial in patients younger than 60 years. (Blood. 2003; 102:4277-4283)
Introduction
Although evidence indicates that intensification of chemotherapy used in the treatment of acute myeloid leukemia (AML) has, in recent years, improved survival in younger patients,1-6 further intensification5,7,8 is limited by toxicity and compromised by reduced compliance. Stem cell transplantation studies, both allograft and autograft, have demonstrated that, although it is not always possible to improve overall survival, the rate of relapse can be reduced.2-4,9,10 This supports the contention that more antileukemic treatment can reduce the risk of relapse. One approach to this problem is to target treatment using immunologically directed treatment either with antibody alone or antibody-directed chemotherapy or radiation therapy.
The immunotoxin gemtuzumab ozogamicin (GO [Mylotarg; Wyeth Pharmaceuticals, Collegeville, PA]) is a humanized IgG4 monoclonal antibody directed against the CD33 epitope, which is chemically linked to calicheamicin, a highly potent antitumor antibiotic.11 As a single agent it has been shown to be an effective agent in the treatment of relapsed AML with a tolerable toxicity profile compared to what is expected using intensive chemotherapy.12-14 One strategy for expanding its place in the treatment of AML is to administer it simultaneously with chemotherapy in situations where intensification has been shown to be effective, specifically, in younger patients. Where this has been attempted in patients with advanced disease and in combination with various chemotherapy schedules in older patients, liver toxicity was the limiting toxicity,15 and the potential for unacceptably prolonged myelosuppression is not known.
The United Kingdom Medical Research Council (MRC) AML15 trial has been designed to evaluate the effect of adding GO to each course of intensive induction or consolidation chemotherapy in patients younger than 60 years as first-line treatment. It was originally intended that patients would receive 2 courses of induction chemotherapy with or without GO in each course and would then be assigned to receive 2 consolidation courses with or without GO in each course. GO was to be given on day 1 of each treatment course. No studies are available to assess the safety of such a strategy, so to assess the feasibility of this approach, we conducted a pilot trial in which the antibody-directed chemotherapy was added to each chemotherapy component course of the proposed AML15 trial to evaluate safety and feasibility.
Patients, materials, and methods
Induction treatment plan
It was intended to recruit patients to sequential cohorts of each component of the proposed AML15 trial treatment. The GO doses were planned to be escalated from 3 mg/m2 to 6 mg/m2 and to 9 mg/m2 expressed as protein, in successive cohorts of patients treated with each chemotherapy course. GO was administered on day 1 of each course. Escalation of the GO dose to the 6 mg/m2 and 9 mg/m2 cohorts could occur only if safety criteria were met in at least 3 of 4 patients in each cohort. If more than 25% of cases experienced grade 4 toxicity or a grade 3 nonhematologic toxicity that did not recover by day 28, the cohort was expanded to 8 patients to assess toxicity before progression to the next dose level or treatment course. If the safety criteria were then not met, that is, more than 25% grade 3 or 4 toxicity was seen, in the expanded cohort, the previous dose level was accepted as the study dose. Once the study dose was established with course 1, patients were to be recruited into subsequent cohorts to receive course 1 with GO at the study dose and course 2 with dose escalations of GO. Patients who received induction courses 1 and 2 then received consolidation course 3 (MACE [amsacrine, ara-C, etoposide] or HidAC [HidAC]) and 4 (MidAC [mitoxantrone ara-C] or HidAC) without GO, to determine whether GO treatment in induction predisposed patients to excess toxicity with the consolidation chemotherapy alone. During the course of the study 9 patients were treated with stem cell transplantation as consolidation.
Consolidation treatment
Patients who were treated with induction chemotherapy courses 1 and 2 without GO were recruited into cohorts that combined the proposed consolidation chemotherapy (MACE and MidAC or 2 courses of HidAC) with GO starting at 3 mg/m2. A similar approach to dose escalation of GO was planned, as in the induction plan.
Induction and consolidation
After a study dose had been separately established for induction and consolidation, cohorts of patients were recruited to receive the induction and consolidation chemotherapy with GO in both phases, where the dose of GO was the previously established study dose.
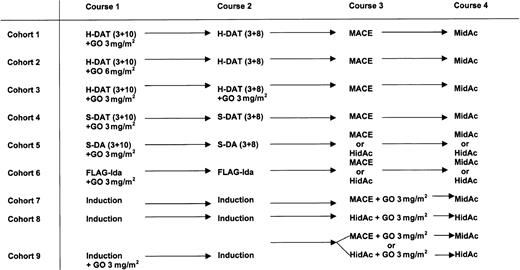
The requirement to expand cohorts due to toxicity in the initial patients in each cohort or the need to expand the final study cohort, or to recruit additional patients to feed the consolidation cohorts explains why the final numbers in each cohort are different as shown in Tables 5 and 6 and Figure 1.
Outcome of induction cohorts
Cohort . | Treatment . | No. . | ANC to 1.0 × 109/L, d median (range) . | Platelets to 100 × 109/L, d median (range) . | Grade 3 or 4 hepatotoxicity . | Deaths . | CR course 1, N (%) . |
|---|---|---|---|---|---|---|---|
| 1 | H-DAT + 3 mg/m2 GO | 22 | 30.5 (23-41) | 35 (26-48) | 6 | 2 | 20 (91) |
| 2 | H-DAT + 6 mg/m2 GO | 9 | 24 (22-43) | 36 (30-43) | 5 | 1 | 8 (89) |
| 3 | H-DAT + 3 mg/m2 GO and H-DAT + 3 mg/m2 GO | 15 | 33 (23-50)* | 50 (35-68)*† | 5* | 5* | NA |
| 4 | S-DAT + 3 mg/m2 GO | 10 | 26 (21-54) | 27 (22-34) | 4‡ | 2 | 6 (60) |
| 5 | S-DA + 3 mg/m2 GO | 8 | 28 (19-35) | 25 (21-41) | 2 | 0 | 8 (100) |
| 6 | FLAG-Ida + 3 mg/m2 GO | 15 | 24 (22-38) | 26 (21-60) | 3 | 1 | 13 (87) |
Cohort . | Treatment . | No. . | ANC to 1.0 × 109/L, d median (range) . | Platelets to 100 × 109/L, d median (range) . | Grade 3 or 4 hepatotoxicity . | Deaths . | CR course 1, N (%) . |
|---|---|---|---|---|---|---|---|
| 1 | H-DAT + 3 mg/m2 GO | 22 | 30.5 (23-41) | 35 (26-48) | 6 | 2 | 20 (91) |
| 2 | H-DAT + 6 mg/m2 GO | 9 | 24 (22-43) | 36 (30-43) | 5 | 1 | 8 (89) |
| 3 | H-DAT + 3 mg/m2 GO and H-DAT + 3 mg/m2 GO | 15 | 33 (23-50)* | 50 (35-68)*† | 5* | 5* | NA |
| 4 | S-DAT + 3 mg/m2 GO | 10 | 26 (21-54) | 27 (22-34) | 4‡ | 2 | 6 (60) |
| 5 | S-DA + 3 mg/m2 GO | 8 | 28 (19-35) | 25 (21-41) | 2 | 0 | 8 (100) |
| 6 | FLAG-Ida + 3 mg/m2 GO | 15 | 24 (22-38) | 26 (21-60) | 3 | 1 | 13 (87) |
NA indicates not applicable.
After course 2.
Five cases of SOS.
Two cases of SOS.
Outcome of consolidation cohorts
Cohort . | Treatment . | No. . | ANC to 1.0 × 109/L, d median (range) . | Platelets to 100 × 109/L, d median (range) . | Grade 3 or 4 hepatotoxicity . | Deaths . |
|---|---|---|---|---|---|---|
| 7 | MACE and 3 mg/m2 GO | 18 | 27.5 (17-55) | 45 (19-70) | 3 | 2 |
| 8 | HidAC and 3 mg/m2 GO | 13 | 28.5 (21-51) | 45 (21-59) | 0 | 0 |
| 9 | Course 1 with 3 mg/m2 GO and Course 3 (either MACE or HidAC) with 3 mg/m2 GO | 23 | 28 (17-55) | 44.5 (19-70) | 1 | 0 |
Cohort . | Treatment . | No. . | ANC to 1.0 × 109/L, d median (range) . | Platelets to 100 × 109/L, d median (range) . | Grade 3 or 4 hepatotoxicity . | Deaths . |
|---|---|---|---|---|---|---|
| 7 | MACE and 3 mg/m2 GO | 18 | 27.5 (17-55) | 45 (19-70) | 3 | 2 |
| 8 | HidAC and 3 mg/m2 GO | 13 | 28.5 (21-51) | 45 (21-59) | 0 | 0 |
| 9 | Course 1 with 3 mg/m2 GO and Course 3 (either MACE or HidAC) with 3 mg/m2 GO | 23 | 28 (17-55) | 44.5 (19-70) | 1 | 0 |
Study treatment plan by cohort. The treatment planned for each of the 9 cohorts is shown. Cohorts were recruited sequentially, that is, when enough patients entered a cohort and were assessed a new cohort was opened. Cohort 7 and 8 included patients who had different induction chemotherapy schedules without GO. In cohort 7, 3 patients received H-DAT, 8 received S-DAT, 4 received DA, and 3 received FLAG-Ida. In cohort 8, 7 patients received FLAG-Ida, 4 received DA, and 2 received S-DAT. In cohort 9, 7 patients received DA, 10 received FLAG-Ida, and 6 received S-DAT as induction with GO 3 mg/m2. Of these 23 patients, 13 received MACE with GO and 10 received HidAC with GO as course 3.
Study treatment plan by cohort. The treatment planned for each of the 9 cohorts is shown. Cohorts were recruited sequentially, that is, when enough patients entered a cohort and were assessed a new cohort was opened. Cohort 7 and 8 included patients who had different induction chemotherapy schedules without GO. In cohort 7, 3 patients received H-DAT, 8 received S-DAT, 4 received DA, and 3 received FLAG-Ida. In cohort 8, 7 patients received FLAG-Ida, 4 received DA, and 2 received S-DAT. In cohort 9, 7 patients received DA, 10 received FLAG-Ida, and 6 received S-DAT as induction with GO 3 mg/m2. Of these 23 patients, 13 received MACE with GO and 10 received HidAC with GO as course 3.
Chemotherapy
The induction treatments used were those planned for courses 1 and 2 in the AML15 trial: H-DAT 3 + 10 followed by H-DAT 3 + 8 or S-DAT 3 + 10 followed by S-DAT 3 + 8 or FLAG-Ida (fludarabine, ara-C, G-CSF, idarubicin) followed by FLAG-Ida. During the study, thioguanine became unavailable in the United Kingdom so a cohort of 8 patients received S-DA 3 + 10 followed by S-DA 3 + 8. The consolidation chemotherapy comprised MACE and MidAC or 2 courses of HidAC. Treatment details are given in Table 1.
Chemotherapy schedules
Induction chemotherapy | |
| H-DAT schedule | |
| Course 1 (DAT 3+10) | Daunorubicin 50 mg/m2 daily IV, d 1, 3, and 5 |
| Cytosine arabinoside 200 mg/m2 twice a day, d 1-10 | |
| Thioguanine 100 mg/m2 twice a day, d 1-10 | |
| Course 2 (DAT 3+8) | Daunorubicin 50 mg/m2 daily IV, d 1, 3, and 5 |
| Cytosine arabinoside 200 mg/m2 twice a day, d 1-8 | |
| Thioguanine 100 mg/m2 twice a day, d 1-8 | |
| FLAG-Ida schedule | |
| Courses 1 and 2 | Fludarabine 30 mg/m2 IV, d 2-6 |
| Cytosine arabinoside 2 g/m2 d 2-6 | |
| G-CSF 263 μg SC, d 1-7 | |
| Idarubicin 10 mg/m2 IV, d 4-6 | |
| Consolidation chemotherapy | |
| Course 3 (MACE) | Amsacrine 100 mg/m2 IV, d 1-5 |
| Cytosine arabinoside 200 mg/m2 Cl, d 1-5 | |
| Etoposide 100 mg/m2 IV, d 1-5 | |
| Course 4 (MidAC) | Mitoxantrone 10 mg/m2 d 1-5 |
| Cytosine arabinoside 1 g/m2 twice a day, d 1-3 | |
| Courses 3 and 4 (HidAC) | Cytosine arabinoside 3 g/m2 twice a day, d 1, 3, and 5 |
Induction chemotherapy | |
| H-DAT schedule | |
| Course 1 (DAT 3+10) | Daunorubicin 50 mg/m2 daily IV, d 1, 3, and 5 |
| Cytosine arabinoside 200 mg/m2 twice a day, d 1-10 | |
| Thioguanine 100 mg/m2 twice a day, d 1-10 | |
| Course 2 (DAT 3+8) | Daunorubicin 50 mg/m2 daily IV, d 1, 3, and 5 |
| Cytosine arabinoside 200 mg/m2 twice a day, d 1-8 | |
| Thioguanine 100 mg/m2 twice a day, d 1-8 | |
| FLAG-Ida schedule | |
| Courses 1 and 2 | Fludarabine 30 mg/m2 IV, d 2-6 |
| Cytosine arabinoside 2 g/m2 d 2-6 | |
| G-CSF 263 μg SC, d 1-7 | |
| Idarubicin 10 mg/m2 IV, d 4-6 | |
| Consolidation chemotherapy | |
| Course 3 (MACE) | Amsacrine 100 mg/m2 IV, d 1-5 |
| Cytosine arabinoside 200 mg/m2 Cl, d 1-5 | |
| Etoposide 100 mg/m2 IV, d 1-5 | |
| Course 4 (MidAC) | Mitoxantrone 10 mg/m2 d 1-5 |
| Cytosine arabinoside 1 g/m2 twice a day, d 1-3 | |
| Courses 3 and 4 (HidAC) | Cytosine arabinoside 3 g/m2 twice a day, d 1, 3, and 5 |
S-DAT was identical to H-DAT except the cytosine arabinoside dose was 100 mg/m2 twice a day; DA was identical to S-DAT with the exclusion of thioguanine.
IV indicates intravenously; G-CSF, granulocyte colony-stimulating factor; SC, subcutaneously, and CI, continuous infusion.
Toxicity assessment and treatment dose escalation
Toxicity was assessed using the National Cancer Institute common toxicity criteria.16 Each patient was scored on the basis of the highest grade seen in the period between starting the study course and either day 28 or the day of starting the subsequent treatment course. A GO dose increase was permitted if 25% or less of cases experienced grade 4 toxicity or a grade 3 toxicity that did not resolve by day 28. The initial size of each cohort was 4 patients, so if 3 patients avoided toxicity this was accepted as the study dose or enabled escalation of the GO dose for the next cohort.
The main feasibility assessments focused on hepatic or hematologic toxicity (Tables 2 and 3). The grades are summarized in Table 2 where the upper limit of normal was that of each investigator's own institution.
Liver toxicity criteria
Liver criteria . | Grade 0 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Bilirubin | WNL | WNL | ULN to 1.5 × ULN | > 1.5-3.0 × ULN | > 3.0 × ULN |
| AST | WNL | ULN to 2.5 × ULN | > 2.5-5.0 × ULN | 5.0-20.0 × ULN | > 20.0 × ULN |
| ALT | WNL | ULN to 2.5 × ULN | > 2.5-5.0 × ULN | 5.0-20.0 × ULN | > 20.0 × ULN |
Liver criteria . | Grade 0 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Bilirubin | WNL | WNL | ULN to 1.5 × ULN | > 1.5-3.0 × ULN | > 3.0 × ULN |
| AST | WNL | ULN to 2.5 × ULN | > 2.5-5.0 × ULN | 5.0-20.0 × ULN | > 20.0 × ULN |
| ALT | WNL | ULN to 2.5 × ULN | > 2.5-5.0 × ULN | 5.0-20.0 × ULN | > 20.0 × ULN |
WNL indicates within normal limits; ULN, upper limit of normal.
Hematopoietic toxicity criteria
Hematopoietic criteria . | Course 1 . | Course 2 . | Course 3 . |
|---|---|---|---|
| ANC to 1.0 × 109/L* | 39 | 36 | 41 |
| Platelets to 100 × 109/L* | 40 | 42 | 60 |
Hematopoietic criteria . | Course 1 . | Course 2 . | Course 3 . |
|---|---|---|---|
| ANC to 1.0 × 109/L* | 39 | 36 | 41 |
| Platelets to 100 × 109/L* | 40 | 42 | 60 |
These data represent the recovery times from the end of treatment for 97.5% of historical control patients treated on the H-DAT schedule.
Sinusoidal obstruction syndrome (SOS) was defined clinically as the syndrome of weight gain of more than 10% of baseline, right upper quadrant pain or tender hepatomegaly, jaundice, and edema or ascites.17 Ultrasonic imaging of hepatic vein blood flow was not required but was used to support a diagnosis of SOS in the clinical setting. Liver biopsy was not routinely undertaken.
Because grades 3 and 4 hematologic toxicity was expected in all cases and delayed hematopoietic recovery was a relevant end point, hematopoietic recovery criteria were developed for each treatment course based on an historical group of 1000 patients in the MRC AML12 trial who received H-DAT chemotherapy. For each course, dose-limiting hematologic toxicity was defined as recovery of absolute neutrophil counts (ANCs) to 1.0 × 109/L and platelet counts to 100 × 109/L, delayed beyond that expected for 97.5% of historical controls who received the same treatment course. The values for each course are shown in Table 3. If more than 25% of patients exceed these recovery times dose escalation could not take place.
By aiming to test each component of the proposed phase 3 trial it was expected to conclude if a combination of chemotherapy with GO was feasible, and what dose level of GO was acceptable. To be acceptable for prospective use the hematologic and hepatotoxicity criteria set out above had to be achieved in a group of at least 8 patients. This approach followed the standard phase 1 dose escalation approach with a traditional Fibonacci design.18
Response assessment
Complete remission (CR) was defined as less than 5% blasts on a bone marrow aspirate of adequate cellularity showing evidence of trilineage regeneration with a peripheral ANC to 1.0 × 109/L and platelet count to 100 × 109/L. Partial remission (PR) was defined as 5% to 15% blasts in a bone marrow of adequate cellularity with evidence of trilineage regeneration. Bone marrow assessment was undertaken 18 to 23 days after the end of course 1 and repeated after course 2 if patients were not in CR. Central review was provided by an experienced hematologist who was not involved in the study and who examined the marrow smears in a blinded manner.
The study was approved at the national level by the Wales Multicentre Research Ethics Committee and by each participating institution's local research ethics committee. All patients received written information and provided written consent before entry to the study.
Patients
Seventy-two patients with untreated AML were recruited and treated in 9 centers in the United Kingdom. Patients were eligible if they were 16 to 59 years old inclusive, and had AML (de novo or secondary) diagnosed by French-American-British criteria on bone marrow aspirate or trephine biopsy together with relevant immunophenotyping or cytochemistry as required. The European Cooperative Oncology Group (ECOG) performance score was required to be 2 or less with normal liver function (aspartate aminotransferase/alanine aminotransferase [AST/ALT], alkaline phosphatase [ALP], and bilirubin), a negative pregnancy test where relevant, and no previous chemotherapy or other experimental agent within 30 days. CD33 positivity on leukemic blast cells was defined as more than 20% positive blast cells but was not a requirement for study entry. Patients with acute promyelocytic leukemia or blast transformation of chronic myeloid leukemia were not eligible.
Sixty-four patients were recruited to the induction phase of the study. Of these, 23 were treated in the combined induction and consolidation phase. Eight patients were recruited to the consolidation courses with GO. These patients had received courses 1 and 2 of chemotherapy without GO. The characteristics of all patients are shown in Table 4. Nine patients in the study underwent stem cell transplantation in consolidation (6 allogeneic, including 2 nonmyeloablative transplants, and 3 autografts).
Patient demographics
. | Induction . | Consolidation . |
|---|---|---|
| No. | 64 | 31* |
| Age, y (range) | 46.5 (18-59) | 46 (17-58) |
| Sex, M/F | 37/27 | 17/14 |
| De novo/secondary | 61/3 | 30/1 |
| Presenting WBC count, × 109/L (range) | 9.5 (0.54-305.8) | 9.19 (0-305.8) |
| Cytogenetic risk group, 19 favorable/standard/unfavorable/not available | 13/39/7/5 | 5/20/4/2 |
. | Induction . | Consolidation . |
|---|---|---|
| No. | 64 | 31* |
| Age, y (range) | 46.5 (18-59) | 46 (17-58) |
| Sex, M/F | 37/27 | 17/14 |
| De novo/secondary | 61/3 | 30/1 |
| Presenting WBC count, × 109/L (range) | 9.5 (0.54-305.8) | 9.19 (0-305.8) |
| Cytogenetic risk group, 19 favorable/standard/unfavorable/not available | 13/39/7/5 | 5/20/4/2 |
WBC indicates white blood cell.
A total of 23 patients were also treated with GO induction.
The median patient age was 46.5 years; 13 were good cytogenetic risk, 47 were standard, 7 were unfavorable, and 5 were undetermined cytogenetic risk by the MRC criteria.19 Sixty cases were CD33+, 5 were CD33- (< 20%), and CD33 status was unknown in 7.
Patients received prophylaxis with 500 to 1000 mg/m2 paracetamol (acetaminophen) and 50 mg diphenhydramine orally or 50 mg methylprednisolone intravenously 1 hour prior to receiving GO. Standard antiemetic schedules were given and itraconazole or other antifungal prophylaxis was withheld until day 5 following GO because of concern about a possible contribution to hepatotoxicity. All other medications that were clinically indicated were permitted with the exception of immunosuppressive or cytotoxic chemotherapy except as provided in the chemotherapy schedules. Growth factors were not routinely used except in the FLAG-Ida regimen.
GO was given as 3 mg/m2 or 6 mg/m2 (expressed as dose equivalent protein) over 2 hours via intravenous pump infusion and allowing a 2-hour gap between chemotherapy. GO was light protected throughout preparation and infusion. Full blood count and biochemical data were collected at least thrice weekly and reported to a central database.
Remission status was assessed 18 to 23 days after course 1 induction by examination of the bone marrow and hematopoietic recovery. CR required normalization of the peripheral blood counts with less than 5% blasts in the bone marrow.
Results
In summary, the first cohort with 3 mg/m2 GO and H-DAT fulfilled the GO dose escalation criteria so a dose of 6 mg/m2 with course 1 (DAT) was evaluated. This resulted in excess hepatic toxicity and some delay in hematopoietic recovery so it was concluded after an expanded cohort that 6 mg/m2 was not possible and that the study dose with course 1 should be 3 mg/m2. Patients were then recruited to receive GO (3 mg/m2) with course 1 and course 2. This was found not to be feasible because of hepatic and hematologic toxicity in the second course. It was, therefore, decided to limit the induction combination to GO (3 mg/m2) with course 1 only, and cohorts were recruited to S-DAT or FLAG-Ida induction chemotherapy each with GO (3 mg/m2). At this stage of the study, thioguanine became unavailable in the United Kingdom so a cohort of patients received S-DA with GO (3 mg/m2). For consolidation evaluation 2 cohorts of patients, who had received 2 courses of DAT without GO in induction, received MACE or HidAC, respectively, as course 3 with GO (3 mg/m2). In view of the induction experience at the 6-mg/m2 dose, a GO dose beyond 3 mg/m2 was not attempted, nor was a sequential course 4 with GO. The 3-mg/m2 dose fulfilled the study criteria so subsequent patients who had received induction course 1 with GO 3 mg/m2 were recruited to the final phase of testing where GO was given with course 1 and course 3 each at the 3-mg/m2 dose. The results are presented for the final total of patients who contributed to the different cohorts, which are shown in Table 5. No other significant toxicity was reported.
Induction cohorts
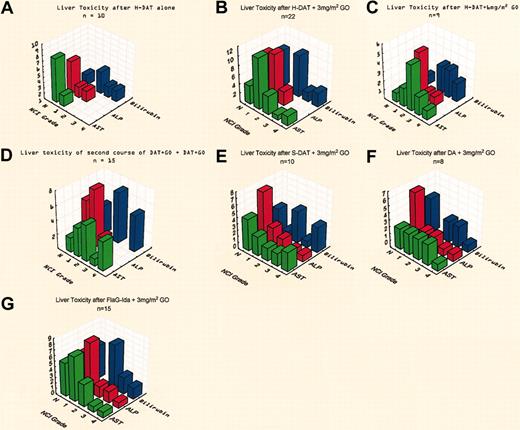
Cohort 1: H-DAT with GO 3 mg/m2. Twenty-two patients in total received H-DAT with GO (3 mg/m2) as course 1. They had a median age of 39 years (range, 19-56 years). Six patients experienced grade 3 or 4 hepatotoxicity of at least one liver parameter, which was more than that seen in a group of patients treated with H-DAT without GO (Figure 2A-B). Four patients recovered and progressed to receive the remaining chemotherapy courses without further toxicity. Two patients died, one with toxic megacolon and sepsis and another from sepsis after having developed diffuse pulmonary hemorrhage 5 days after GO therapy to which it was thought to be possibly related. Twenty (91%) of the 22 patients achieved CR with one treatment course. Nineteen of the 20 patients fulfilled the hematopoietic recovery targets (Table 5).
Assessment of liver toxicity in induction.Panels demonstrate the maximum liver toxicity grade seen for each induction cohort. (A) Historical group of patients receiving H-DAT treatment alone; (B) H-DAT with GO (3 mg/m2) in 22 patients; (C) H-DAT with GO (6 mg/m2) in 9 patients; (D) DAT with GO in 2 courses showing the toxicity after course 2 in 15 patients; (E) S-DAT with GO (3 mg/m2) in 10 patients; (F) after DA and GO (3 mg/m2) in 8 patients; and (G) after FLAG-Ida and GO (3 mg/m2) in 15 patients.
Assessment of liver toxicity in induction.Panels demonstrate the maximum liver toxicity grade seen for each induction cohort. (A) Historical group of patients receiving H-DAT treatment alone; (B) H-DAT with GO (3 mg/m2) in 22 patients; (C) H-DAT with GO (6 mg/m2) in 9 patients; (D) DAT with GO in 2 courses showing the toxicity after course 2 in 15 patients; (E) S-DAT with GO (3 mg/m2) in 10 patients; (F) after DA and GO (3 mg/m2) in 8 patients; and (G) after FLAG-Ida and GO (3 mg/m2) in 15 patients.
Cohort 2: H-DAT with GO 6 mg/m2. Because the first 4 recipients of GO (3 mg/m2) with course 1 fulfilled the escalation criteria, cohort 2 recruited patients to receive H-DAT with 6 mg/m2 GO. This group was eventually expanded to 9 patients. Five patients developed grade 3 or 4 hepatotoxicity, 2 of whom had grade 3 bilirubinemia only (Figure 2C). No patients developed SOS. Hematopoietic recovery was delayed in platelet recovery in 4 patients, one of whom also failed to recover neutrophils by the target. Eight patients (89%) achieved CR. One patient died of sepsis and intracerebral hemorrhage. This cohort therefore did not achieve the protocol requirements for the study dose based on liver toxicity.
Cohort 3: H-DAT with GO 3 mg/m2 with courses 1 and 2.Fifteen patients who entered cohort 1 were also treated with GO (3 mg/m2) in course 2 of H-DAT. Five patients experienced grade 3 or 4 liver toxicity, all of whom had clinical or ultrasonic features of SOS (Figure 2D). Complete hematopoietic recovery was not achieved within the target time in 13 patients, although with longer follow up 7 achieved recovery. It was therefore concluded that it was not possible to give GO, even at 3 mg/m2, on consecutive courses of H-DAT therapy in view of combined hematopoietic and liver toxicity.
Cohort 4: S-DAT with GO 3 mg/m2. It had become clear from our analysis of the MRC AML12 trial, which compared the daily dose of ara-C (H-DAT 400 mg/m2 ara-C versus S-DAT 200 mg/m2 ara-C), that there was no difference in efficacy between the schedules.20 Consequently a cohort of patients was treated with S-DAT, that is, with the lower ara-C dose level, aiming to determine if less toxicity occurred when combined with GO. Ten patients were treated, 4 of whom developed grade 3 or 4 hepatotoxicity including 2 with SOS (Figure 2E). Hematopoietic recovery occurred by the target date. Two patients died, one from SOS and the other from a cerebral bleed and sepsis.
Cohort 5: S-DA with GO 3 mg/m2. Serendipitously, thioguanine became unavailable in the United Kingdom as interest was developing about its potential contribution to liver toxicity. Consequentially, 8 patients were treated with S-DA and GO 3 mg/m2. Hematopoietic recovery was satisfactory. Two patients developed grade 3 toxicity only. No patients developed grade 4 toxicity or SOS (Figure 2F). All patients achieved CR and tolerated subsequent chemotherapy, including GO with course 3 (see “Cohort 9”).
Cohort 6: FLAG-Ida with GO 3 mg/m2. Fifteen patients received FLAG-Ida with GO on day 1 of the course. Three patients had grade 3 or 4 hyperbilirubinemia, which was attributed to disseminated Aspergillus infection (Figure 2G). No patient developed SOS. Ten patients later received HidAC (n = 7) or MACE (n = 3) with GO in the consolidation phase without further problems. Hematopoietic recovery was within target after course 1 (Table 5). One patient died of sepsis and a second patient did not achieve CR with course 1. Overall, 14 patients (93%) achieved CR, 13 of them after course 1. Fourteen patients received a second course of FLAG-Ida without GO. Three of these patients had delayed platelet recovery and 2 patients had delayed neutrophil recovery. Thus, 5 of 14 patients had delayed hematopoietic recovery after course 2. In a subsequent experience using 2 consecutive courses of FLAG-Ida without GO patients showed a similar delay in recovery, suggesting that the use of GO was not the cause of delayed recovery.
The role of thioguanine
The overall experience in these patients suggested that grade 3 or 4 liver toxicity was more likely in patients who received a thioguanine-containing schedule (grade 3: 10% versus 15%, P = .39; grade 4: 5% versus 22%, P = .005; and grades 3 and 4: 15% versus 37%, P = .007).
Consolidation treatment
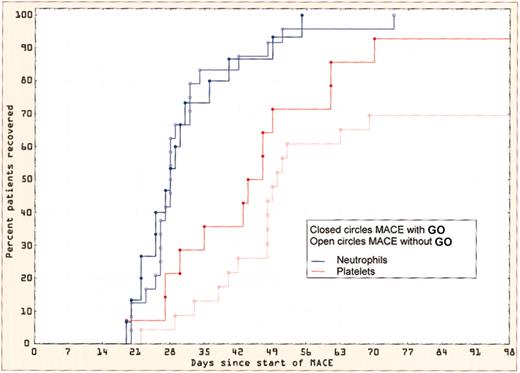
Cohort 7: MACE with GO 3 mg/m2. Eighteen patients received MACE with GO (3 mg/m2). Of these, 5 had received courses 1 and 2 of chemotherapy without GO and 13 had received GO with course 1. There was no difference in hepatotoxicity or hematopoietic recovery whether or not GO was used in course 1. Three patients developed grade 3 (n = 1) or 4 (n = 2) hepatotoxicity, in one of whom it was attributed to sepsis. When compared with an historical group of patients treated with MACE, there was no evidence of delayed hematopoietic recovery (Figure 3).
Hematologic recovery after MACE with and without GO. The neutrophil and platelet recovery is shown in patients receiving MACE with or without GO.
Hematologic recovery after MACE with and without GO. The neutrophil and platelet recovery is shown in patients receiving MACE with or without GO.
Cohort 8: HidAC with GO 3 mg/m2. Thirteen patients received HidAC with GO. Ten patients had received GO with course 1 (7 FLAG-Ida, 3 S-DA). Hematopoietic recovery was satisfactory (Table 6) and no patient developed grade 3 or 4 hepatotoxicity.
Cohort 9: induction and consolidation with GO 3 mg/m2.Twenty-three patients received GO (3 mg/m2) with both course 1 and course 3. Only 1 patient developed grade 3/4 toxicity and hematopoietic recovery targets were met.
Outcome of liver toxicity
Overall, 29 patients experienced grade 3 or 4 liver toxicity. In one case this related to a raised ALP concentration alone, 2 to AST levels, and 9 to bilirubin values alone. The abnormalities occurred a median of 15 days (range, 8-40 days) after the GO therapy. In 3 patients the cause was thought to be due to sepsis, from which 2 patients died. In 11 patients hepatotoxicity was thought to be related to GO therapy, but historical experience of H-DAT therapy suggests that grades 3 and 4 hepatotoxicity will occur in 10% to 20% of patients (Figure 2A). The median duration of grade 3 or 4 toxicity was 6 days (range, 1-26 days) and tended to be longer in patients with hyperbilirubinemia. Seven patients developed SOS, 5 from cohort 3 (DAT + GO × 2) and 2 from cohort 4 (S-DAT with GO course 1). This was defined clinically and supported by ultrasonography in all cases. Six of these patients were treated with defibrotide of whom 5 recovered and were able to receive subsequent chemotherapy without further toxicity. One other patient received GO with course 1 and 2 with no significant toxicity and received 2 further courses of treatment. An SOS-like syndrome developed 28 days after the fourth (MidAC) course from which the patient died. Postmortem findings were consistent with SOS. Nine patients received an allogeneic (n = 6 including 2 nonablative) or autologous (n = 3) stem cell transplant at a median of 122 days (range, 64-149 days) after exposure to GO. No patient developed unexpected hepatotoxicity or SOS.
Deaths
Twenty-four patients have died. Two patients failed to achieve CR and 10 patients who achieved CR had a relapse after a median of 244 days. Progressive leukemia was the main cause of death in these patients. Twelve patients died of documented infection (5 aspergillosis; 7 bacterial) with organ failure. One patient died of a paralytic ileus that was assumed to be due to infection. None of these deaths were thought to be linked to GO therapy. One patient died after diffuse pulmonary hemorrhage that developed on day 5 of course 1 in a patient treated with H-DAT + 3 mg/m2. This was an unusual complication and a link with GO could not be excluded. The remaining 3 deaths were attributed to a SOS-like syndrome as described (see “Outcome of liver toxicity”).
Disease response
Overall, of 64 patients treated in induction, 54 patients (84.4%) achieved CR, 53 (83%) following course 1. One patient had refractory disease and 6 patients died before disease response assessment could be carried out. Within the risk groups, 10 (77%) of 13 good risk, 34 (89%) of 38 standard risk, and 5 (71%) of 7 poor risk achieved CR with course 1.
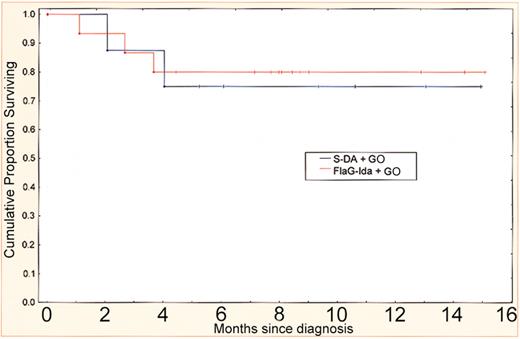
The 2 arms considered most feasible as a result of this pilot trial were DA plus GO and FLAG-Ida plus GO (cohorts 5 and 6). The overall survival in these patients at a median follow-up of 8 months is 78% (Figure 4). These arms have been adopted for prospective comparison in the AML15 trial.
Survival after S-DA and FLAG-Ida with GO. Survival from diagnosis in cohorts 5 and 6, which have been adopted for prospective comparison with or without GO in the MRC AML15 trial.
Survival after S-DA and FLAG-Ida with GO. Survival from diagnosis in cohorts 5 and 6, which have been adopted for prospective comparison with or without GO in the MRC AML15 trial.
Discussion
Intensification of treatment has improved the cure rate in patients under the age of 60 years with AML. Remission rates of 75% to 80% can now be expected with several treatment combinations. In the experience of the MRC group of more than 3500 patients aged 16 to 60 years over the last 15 years 83% of patients achieved CR overall and 65% with course 1.1,20 Forty percent of patients remain alive at 5 years. We have not been able to demonstrate an improved CR rate or quality of remission, as demonstrated by relapse rate or improved disease-free survival, in any of the induction schedules used. Incorporation of HidAC into induction treatment has met with mixed results5,7,8 and the MRC12 trial evaluated an intermediate dose escalation of ara-C (H-DAT versus S-DAT) without demonstrating a difference.20 Further intensification is compromised by toxicity or reduced compliance with subsequent treatment.
The development of antibody-directed chemotherapy with more specificity against leukemic cells has been a goal of cancer treatment for several years. The lack of a leukemia-specific antigen in AML reduced the prospect of success. CD33 has emerged as a favored target epitope because it is expressed in over 90% of cases of AML21 and, as far as is known, expression is limited to hematopoietic precursor cells but not stem cells. Another potentially advantageous feature is that the antigen-antibody complex is rapidly internalized suggesting that this would be a convenient drug delivery system to cells. The use of unconjugated humanized antibodies has met with little success in relapsed disease, but has been shown to be effective in acute promyelocytic leukemia in converting molecularly positive hematologic remissions to molecular negativity.22,23 The ability chemically to link a potent antitumor agent (calicheamicin) to a humanized anti-CD33 monoclonal antibody of the IgG4 subclass in a way that ensures only intracellular drug activity now makes such an approach to antibody-directed therapy feasible in AML. Calicheamicin is an extremely potent antitumor antibiotic with considerably more antileukemic potency in vitro than currently available agents.24 It is, however, considered too toxic to develop as a single agent. The drug delivery mechanism available through the CD33 complex represents a potentially powerful and safe immunotoxin. Feasibility and efficacy have been demonstrated in phase 1 and phase 2 trials, which led to approval as a single agent for relapsed disease in patients older than 60 years.3,14
Several groups are now evaluating the potential of GO in different situations in the treatment of AML. The MRC group has focused on its potential to augment intensive chemotherapy in induction and consolidation in patients younger than 60 years. In these patients, intensification whether by stem cell transplantation or intensification of chemotherapy appears to increase response and reduce relapse, but at the cost of increasing toxicity. It is not clear whether it is essential to limit the approach of immunotherapy with GO to patients whose leukemia is CD33+ as was done in the phase 1 and 2 trials. The minimum CD33 expression compatible with internalization of the drug is not known. For this reason we decided to include all AML cases regardless of CD33 expression. Four of the 6 cases known to be CD33- achieved CR with course 1.
Of some concern in the development of GO in AML is the risk of hepatotoxicity. As a single agent grade 3 or 4 liver toxicity was seen in 3% of cases,14 typically occurring 10 days after exposure, and resolving within a few days. Patients who are treated for relapse after stem cell transplantation appear to be at greater risk of developing a SOS-like syndrome,25,26 as are patients who received GO treatment before transplantation. The risk may be related to the interval between GO treatment and stem cell transplantation.27
The effects of combining GO with chemotherapy were unknown. The risks were that liver toxicity could be exacerbated or hematopoietic recovery, particularly of platelets, would be compromised to such an extent that combination therapy would not be feasible. This was suggested by a study of full-dose GO (9 mg/m2) with various chemotherapy and cytokine schedules in older patients with advanced disease where 13% developed a veno-occlusive disease (VOD) syndrome.15
In our study the VOD-like syndrome, now called sinusoidal obstruction syndrome (SOS),17 was observed in 7 patients (11%), all of whom had received thioguanine. Five of the 6 patients treated with defibrotide recovered and were able to receive subsequent chemotherapy. Other potential contributory factors to liver toxicity were considered such as the use of prophylactic azole antifungal agents, but this does not seem to be borne out in the phase 1 or 2 trials (M. Berger, personal written communication, February 1992).
Our experience from this pilot study suggests that combining 6 mg/m2 GO with the DAT schedule is not feasible for a multicenter trial. Similarly, the use of a lower dose of 3 mg/m2 in consecutive courses was limited both by liver and hematopoietic toxicity. Nonthioguanine-containing chemotherapy was associated with less frequent and less severe liver toxicity and was felt to be manageable in a multicenter context. It is possible that a higher dose of GO could be used in nonthioguanine-containing chemotherapy; this was not tested in our study but has been found to be possible in a phase 2 study in a dose of 6 mg/m2 given on day 4 of a DA schedule.26,28 Virtually no increased liver toxicity was seen when GO was given with MACE or HidAC in the consolidation phase. It also proved to be feasible for patients who had received GO with course 1 to receive it with course 3 combined with either MACE or HidAC. Delayed hematopoietic recovery occurs normally in consolidation, and we found no evidence that the addition of GO makes this expected pattern of recovery worse.
These observations taken together enabled us to initiate the AML15 trial, which will evaluate the role of GO (3 mg/m2) in course 1 (induction) or course 3 (consolidation) using a 2 × 2 design. If there is a benefit, this design will enable us to conclude whether GO is advantageous in induction or consolidation, or both. In a preliminary experience of 90 patients treated in the trial serious hepatotoxicity has not been reported.
Another possible problem is that the use of GO may increase the toxicity of subsequent treatment. This was not generally the case in our study. Even patients who had grade 3 or 4 liver toxicity did not usually develop further toxicity after subsequent chemotherapy courses. One patient who received GO with courses 1 and 2 with grade 3 liver toxicity subsequently received 2 further courses of chemotherapy. One month after the last course she developed an SOS-like syndrome and died. The relationship to prior GO therapy is unclear. Of particular interest is that none of the 9 patients who went on to stem cell transplantation developed liver toxicity after the transplantation. Although not designed formally to assess efficacy, it was reassuring to note that 54 (84%) of 64 patients achieved CR. All but one achieved CR after course 1 including 5 of 7 high-risk patients. Historically the H-DAT schedule achieved a CR rate of 65% with course 1, and because patients who achieve CR with the first course have better survival, this provides some justification for proceeding with a reduced GO dose of 3 mg/m2 into a major randomized trial. Because of the trend for more liver toxicity in thioguanine-containing chemotherapy, the trial will use DA versus FLAG-Ida in combination with GO. In this pilot study 91% of patients treated with these schedules enter remission with course 1 and 78% remain in CCR at a median of 8 months (range, 4-15 months) follow-up.
Supported by a grant form the United Kingdom Leukaemia Research Fund and John Wyeth, Inc.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1620.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Jay Feingold for his advice and support, our research nurses for meticulous participation, Ms Sian Edwards for preparing the manuscript, and Dr A. I. Al-Sabah for central morphologic review.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal