Abstract
Recent preclinical and clinical trials have demonstrated the therapeutic potential of T lymphocytes redirected with genetically engineered T-cell receptor (TCR) surrogates against infected, cancerous, or autoreactive cells. These surrogate TCRs link a ligand-recognition domain to signaling regions from the TCR. We previously compared the function of surrogate TCRs that include TCR or TCR and CD28 signaling regions. We found that primary murine T cells modified to specifically target Kb-restricted CD8+ T cells using either Kb-ζ or Kb-CD28-ζ receptors had similar functional activities, although the CD28-ζ receptor showed a 2-fold to 4-fold decreased expression. We have now identified a previously unrecognized dileucine motif in the murine CD28 signaling domain that accounts for this reduced expression. Inactivation of this motif increased chimeric receptor surface expression 2- to 5-fold. T cells expressing the dileucine-mutated CD28-ζ chimeric receptor demonstrated enhanced proliferation, cytokine production, and cytolytic activities. Further, cells expressing this dileucine-mutated receptor were highly effective in eliminating antigen-specific CD8+ T lymphocytes in vivo. These results therefore identify a critical motif limiting the function of receptor-modified T lymphocytes, demonstrate that inactivation of this motif enhances chimeric receptor function, and illustrate a potential novel application of receptor-modified T lymphocytes in the induction of immune tolerance. (Blood. 2003;102:4320-4325)
Introduction
T cells transgenically modified to express genetically engineered chimeric receptors (receptor-modified T cells [RMTCs]) can target antigens not normally recognized by the immune system.1-3 The chimeric receptors that redirect these RMTCs against their targets functionally substitute for the T-cell receptor (TCR). They recognize target antigen through an extracellular antigen-recognition domain, such as a single-chain Fv fragment, and signal the RMTCs through a linked TCR-derived signal transduction domain, typically from the TCR ζ chain. RMTCs have shown therapeutic potency in model systems, selectively targeting cancerous, infected, and autoreactive T cells, and have not shown significant toxicity in phase 1 clinical trials.4-7
Although engineered surrogate receptors can redirect therapeutic T cells, their effectiveness in doing this may be limited by the limited signal they can transduce. Coreceptor and costimulatory signals, normally provided to T cells when they interact with a “professional” antigen-presenting cell, will often not be available to RMTCs engaging a ligand on a tumor or other target cell with a chimeric receptor.8,9 These signals can promote T-cell survival, proliferation, and effector function and may therefore be critical for RMTC function. To overcome this limitation, we and others have developed single-chain chimeric receptors that incorporate modular signal transduction subunits derived from the TCR, costimulatory, and/or coreceptor molecules.5,10-13 The receptor structure most commonly analyzed includes the signaling domain of the CD28 costimulatory molecule genetically linked to the cytoplasmic tail of the TCR ζ chain. In several studies, RMTCs that expressed chimeric receptors including a CD28-ζ signaling region, when compared with those including only ζ, showed improved functional responses in vitro and in vivo.
We have been interested in using RMTCs to specifically target T lymphocytes that are pathologic in transplantation or other settings. The defining feature of a pathologic T lymphocyte is the specificity of its TCR. In transplantation, these TCRs are generally directed against allogeneic major histocompatibility complex (MHC) or syngeneic MHC coupled with minor histocompatability antigens.14,15 To specifically redirect RMTCs against pathologic class I MHC-restricted T lymphocytes in murine models of transplantation, we developed chimeric receptors that include the class I MHC Kb molecule extracellular and transmembrane domains linked to either a murine ζ or CD28-ζ signaling tail.10 The Kb extracellular region serves as bait for Kb-restricted pathologic T cells, whereas the signaling domain activates the RMTCs, inducing effector functions. Biochemical analysis of receptor-mediated signal transduction in Kb-CD28-ζ-transduced T-cell hybridomas, when compared with Kb-ζ-transduced cells, demonstrated enhanced receptor phosphorylation and calcium flux. Further, the added CD28 domain allowed direct receptor association with the src kinase p56lck, which is critically involved in initiating and sustaining receptor-mediated signal transduction. T-cell hybridomas expressing the Kb-CD28-ζ receptor also showed increased interleukin-2 (IL-2) production and signaling sensitivity.
In contrast to the enhanced function of the Kb-CD28-ζ receptor in immortalized T-cell hybridomas, when we transduced primary T lymphocytes with this or the Kb-ζ receptor, we did not observe significant differences in chimeric receptor-mediated functional responses.16 We further observed a 2-fold to 4-fold decreased expression level of the Kb-CD28-ζ receptor in primary T cells when compared with the Kb-ζ receptor. Interestingly, decreased expression of CD28-ζ-containing receptors when compared with otherwise identical ζ-containing receptors is also apparent in reports of others, although this feature has not previously been quantitatively analyzed.11-13 The inclusion of the CD28 costimulatory region in chimeric receptor signal transduction units therefore has conflicting effects. It provides an enhanced signal into RMTCs but simultaneously diminishes receptor surface expression and thereby limits the magnitude and/or duration of this signal. This decreased expression may be particularly significant with the Kb-CD28-ζ receptor, which engages its cognate TCR ligand in a low-affinity interaction.17
To determine potential sources for the reduced expression of the Kb-CD28-ζ receptor, we analyzed the sequence of the murine CD28 cytoplasmic tail. We noticed there a noncanonical dileucine internalization motif.18 This motif had not been previously studied in regard to CD28 function, although dileucine motifs have been well characterized in other proteins. Dileucine motifs bind AP or GGA adaptor proteins and thereby promote receptor internalization. To clarify the role of the CD28 dileucine motif in CD28-ζ chimeric receptor function, we inactivated it in the Kb-CD28-ζ receptor. We found that mutating the essential leucines in the motif to glycines increases surface expression of the Kb-CD28-ζ receptor 2-fold to 5-fold when compared with the unmutated receptor. Further Kb-CD28[L→G]-ζ-modified T cells showed increased sensitivity in proliferation, cytokine production, and cytolysis studies when compared with Kb-CD28-ζ-modified T cells, and were highly effective in eliminating antigen-specific target T lymphocytes in vivo. This study therefore identifies a previously undescribed dileucine motif within the murine CD28 tail, demonstrates a specific role for this dileucine motif in limiting chimeric receptor function in RMTCs, and illustrates how protein engineering may be used to modify specific motifs within multidomain chimeric receptors to optimize their expression and function. We additionally provide the first evidence that RMTCs may be used to target antigen-specific CD8+ T cells in vivo, suggesting a potential new use for RMTCs in the generation of transplant tolerance.
Materials and methods
Constructs
Synthesis and sequences of the chimeric constructs are as described.10 Briefly, cDNA fragments encoding the extracellular and transmembrane domain of the H-2Kb molecule and the cytoplasmic tails of murine CD28 and ζ were isolated by polymerase chain reaction (PCR) from cDNA clones or splenic cDNA. The dileucine mutation was introduced by PCR mutagenesis. Flanking restriction sites were added to the fragments by PCR and the fragments linked. Assembled constructs were subcloned into the murine stem cell virus-internal ribosome entry site (IRES)-green fluorescence protein (MSCV-I-GFP) retroviral vector (gift from E. Vanin, St Jude Children's Research Hospital).19 Fidelity of construct DNA sequences was confirmed by sequencing at the St Jude Hartwell Center for Biotechnology.
Mice, cells, and antibodies
TG-B mice,20 transgenic for a rearranged SV40-T/H-2Kk-restricted TCR, were bred more than 20 generations with B10.BR mice and used as a source of CD8+ T cells for transducing constructs. OT-1 mice (Jackson Laboratories, Bar Harbor, ME), transgenic for a rearranged ovalbumin 257-264/H-2Kb-restricted TCR, were used as a source of target cells. C57BL/6J-Prkdcscid/SzJ mice (Jackson Laboratories) were used as adoptive transfer recipients. Antibodies used include clone B20.1 antimouse Vα2 (Pharmingen, San Diego, CA); clone 2C11 antimouse CD3ϵ (gift from M. Blackman, Trudeau Institute, Saranac, NY); clone AF6-88.5 antimouse H-2Kb (Pharmingen and gift from M. Blackman); goat antimouse immunoglobulin G (IgG) (Jackson Laboratories); and goat antirat IgG (Jackson Laboratories).
Retroviral transduction and T-cell culture
Retrovirus was produced as described.21 Briefly, 10 μg of chimeric receptor constructs and 10 μg of the retrovirus helper DNA construct PEQPAM (gift from J. Cleveland) were cotransfected into 293 T cells by calcium phosphate precipitation. At 16 hours the cells were washed and cultured in Dulbecco modified Eagle medium/10% fetal calf serum (DMEM/10% FCS) for 48 hours. Supernatant was collected twice daily and used to infect GP+E86 retroviral producer cells in the presence of 8 μg/mL polybrene. Transduced GP+E86 cells were flow cytometrically sorted for the presence of green fluorescence protein (GFP) and expanded. To transduce T lymphocytes, isolated lymph node cells were stimulated with soluble CD3- and CD28-specific antibodies in the presence of 2 ng/mL recombinant murine IL-2 (rmIL-2) (R&D Systems, Minneapolis, MN) for 2 days. Medium was removed, replaced with cleared supernatant from the GP+E86 retroviral producer cells and 8 μg/mL polybrene, and the cells were spun at 1800 rpm for 90 minutes in a Jouan CR422 tabletop centrifuge (Winchester, VA). On day 4, transduced T cells were sorted for expression of GFP and CD8 and expanded by culturing in EHAA medium (Biosource International, Camarillo, CA) in the presence of rmIL-2 for up to 5 days. The cells were restimulated every 7 to 10 days using 2 μg/mL concanavalin A (conA; Sigma, St Louis, MO), 2 × 106/mL 30 Gy (3000 rad) irradiated syngeneic splenocytes, and 2 ng/mL rmIL-2. Transduced cells were washed and assayed 5 to 6 days after stimulation. Assays were performed in the absence of exogenously added IL-2.
Proliferation
The designated concentration of purified AF6-88.5 antibody was loaded onto goat antimouse IgG-coated wells in 96-well plates. Then, 5 × 104 transduced T cells and 2.5 × 105 25 Gy (2500 rad) irradiated syngeneic B10.BR splenocytes were added per well. After 2 days, the cells were pulsed with 37 KBq (1 μCi) 3H-thymidine for 16 hours and harvested onto filtermats. Proliferation was measured by liquid scintillation counting of incorporated 3H. All samples were analyzed in triplicate and means plotted.
Cytotoxicity assay
Receptor-modified T cells, day 5 or 6 after stimulation, were incubated overnight in medium to which was added 50 μg/mL or the designated concentration of ovalbumin 257-264 peptide (St Jude Hartwell Center for Biotechnology) in phosphate-buffered saline (PBS) or control PBS diluent, washed 3 times, and resuspended in medium. Effector cells were incubated with approximately 105 OT-1 target T cells at the designated ratio. Primary T-cell targets were isolated from OT-1 TCR transgenic lymph node cells or splenocytes by panning on goat antimouse IgG-coated plates or by nylon wool. After 5 to 6 hours of coincubation, 5000 to 10 000 6-μm fluorescent TruCount beads (Becton Dickinson, Franklin Lakes, NJ) were added. Samples were stained for Vα2, washed once, and analyzed by flow cytometry as described.16 Viable target T cells could be distinguished from effector T cells by the presence of Vα2 and the absence of GFP. Absolute target cell numbers were determined by normalization of cellular events with the TruCount bead events. Percent specific cytotoxicity was determined as 100 × (1 - viable target cell count after incubation with peptide-pulsed effectors/viable target cell count after incubation with unpulsed effectors). In all experiments parallel cultures of target cells in the absence of effector cells were simultaneously performed. Essentially identical results were obtained when cytotoxicity was alternatively calculated as 100 × (1 - viable target cell count after incubation with peptide-pulsed effectors/viable target cell count after incubation without effectors). All samples were analyzed in quintuplicate.
Cytokine analysis
Interferon-γ (IFN-γ) was analyzed using a Bioplex assay (Bio-Rad, Hercules, CA). A 96-well filter plate was prewet and approximately 3000 analytical beads added per well. Dilutions of standards or experimental samples were added to the beads and incubated for 1 hour at room temperature (RT). Supernatant was aspirated, beads washed, and then incubated for 1 hour with biotinylated anti-IFN-γ detection antibody. After washing, detection was performed by staining with streptavidin-phycoerythrin (PE) and fluorescence analysis with a Bioplex plate reader (Bio-Rad).
In vivo cytotoxicity
Peripheral lymph nodes from OT-1 mice were harvested, labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR), and washed 3 times with Hanks balanced salt solution (HBSS). Approximately 107 cells were injected intravenously into the retro-orbital plexus of a recipient mouse. Shortly after, approximately 107 RMTCs were injected into the alternate retro-orbital plexus. Twenty-four hours after injection, spleen and lymph nodes were harvested, and a single-cell suspension prepared, stained with PE-labeled anti-Vα2, and analyzed by flow cytometry.
Statistics
Standard deviations and paired 2-sided t tests were calculated using Excel spreadsheet software (Microsoft, Redmond, WA). Error bars correspond to ± 1 standard deviation.
Results
Design and expression of chimeric receptors
The wild-type Kb-CD28-ζ and dileucine-mutated Kb-CD28[L→G]-ζ receptors included the H-2Kb extracellular and transmembrane domains, linked to the cytoplasmic domains of CD28 and ζ (Figure 1). Constructs were subcloned into the MSCV-I-GFP retroviral vector, which includes an IRES-linked GFP gene. Retrovirus-rich supernatant was produced and used to transduce primary CD8+ T lymphocytes. Transduction efficiencies of 15% to 50% were typically observed.
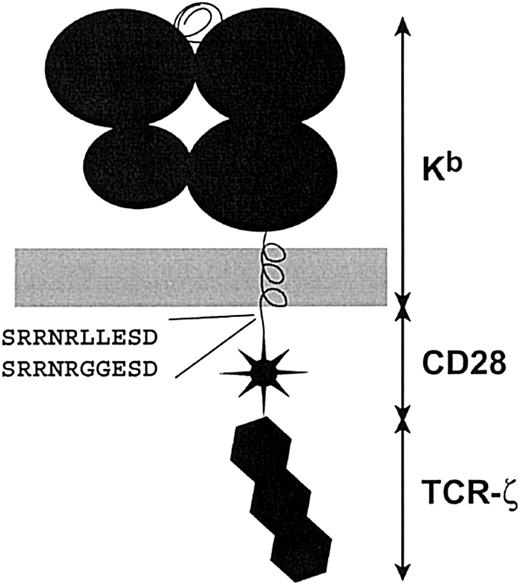
Chimeric receptor structure and sequence of the dileucine motif. Chimeric constructs were created by linking components in a cassette fashion. Extracellular and transmembrane domains are derived from the MHC class I H-2Kb molecule. The murine CD28 and TCR-ζ cytoplasmic tails were attached as described.10 PCR mutagenesis was used to introduce the leucine to glycine change in the CD28 tail. This corresponds to an L184G and L185G conversion in the CD28 sequence (GenBank accession NP_031668).
Chimeric receptor structure and sequence of the dileucine motif. Chimeric constructs were created by linking components in a cassette fashion. Extracellular and transmembrane domains are derived from the MHC class I H-2Kb molecule. The murine CD28 and TCR-ζ cytoplasmic tails were attached as described.10 PCR mutagenesis was used to introduce the leucine to glycine change in the CD28 tail. This corresponds to an L184G and L185G conversion in the CD28 sequence (GenBank accession NP_031668).
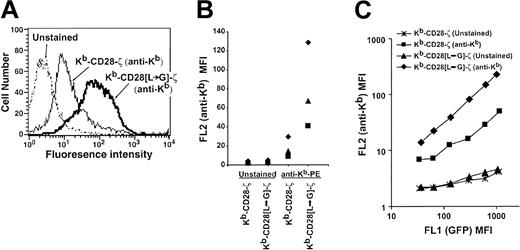
To determine the role of the dileucine motif in chimeric receptor expression, we flow cytometrically sorted CD8+GFP+ cells transduced with either the Kb-CD28-ζ or Kb-CD28[L→G]-ζ receptor and stained them with a Kb-specific antibody. Cells bearing the receptor mutated for the dileucine motif showed a 2-fold to 5-fold increase in receptor level compared with wild-type receptor in several independent transduction experiments (Figure 2A-B). This increased receptor expression did not result from increased transcription of the mutated when compared with the wild-type receptor. When chimeric receptor expression level was analyzed as a function of the level of linked and cotranscribed GFP, this 2-fold to 5-fold increase in expression level was consistently seen regardless of the amount of GFP present in individual cells (Figure 2C). These results therefore demonstrate that the CD28 dileucine motif significantly restricts the level of surface chimeric receptor, while its disruption enhances surface expression.
Increased surface expression of dileucine-mutated chimeric receptor. (A) Overlay histogram plots showing fluorescence intensity of transduced TG-B T cells that are unstained or stained with PE-labeled H-2Kb-specific antibody. Viable cells were gated based on forward and side scatter. Control basal fluorescence was identical for cells transduced with either the mutated or unmutated receptor (data not shown). Control MSCV vector-transduced cells also did not detectably stain with H-2Kb-specific antibody (data not shown). (B) Staining results from 3 independent transduction experiments, each denoted by a unique symbol (♦, ▴, ▪), are compared. In each experiment an increase in the mean fluorescence intensity (MFI) of the dileucine-mutated receptor versus unmutated receptor is apparent. (C) Chimeric receptor (anti-Kb) expression is plotted as a function of cotranscribed GFP level. Transduced cell populations were analyzed for GFP (FL1) expression level and gated into regions comprising approximately 0.2 to 0.3 log fluorescence using Cellquest software (BD Biosciences, San Jose, CA). For cells within each of these gated regions, FL1 MFI (GFP) and FL2 MFI (anti-Kb staining or control unstained) was calculated and plotted.
Increased surface expression of dileucine-mutated chimeric receptor. (A) Overlay histogram plots showing fluorescence intensity of transduced TG-B T cells that are unstained or stained with PE-labeled H-2Kb-specific antibody. Viable cells were gated based on forward and side scatter. Control basal fluorescence was identical for cells transduced with either the mutated or unmutated receptor (data not shown). Control MSCV vector-transduced cells also did not detectably stain with H-2Kb-specific antibody (data not shown). (B) Staining results from 3 independent transduction experiments, each denoted by a unique symbol (♦, ▴, ▪), are compared. In each experiment an increase in the mean fluorescence intensity (MFI) of the dileucine-mutated receptor versus unmutated receptor is apparent. (C) Chimeric receptor (anti-Kb) expression is plotted as a function of cotranscribed GFP level. Transduced cell populations were analyzed for GFP (FL1) expression level and gated into regions comprising approximately 0.2 to 0.3 log fluorescence using Cellquest software (BD Biosciences, San Jose, CA). For cells within each of these gated regions, FL1 MFI (GFP) and FL2 MFI (anti-Kb staining or control unstained) was calculated and plotted.
Functional response of RMTCs
A substantial body of data has demonstrated that the T-cell response to stimulation will vary with the intensity and duration of the stimulus received.22,23 This implies that the increased expression of dileucine-mutated chimeric receptors should result in improved signaling compared with unmutated receptors. However, the role of the dileucine motif in CD28 signaling has not been established, and it was possible that disruption of this motif would cripple signal transduction. To determine the functional impact of the dileucine mutation on T-cell functional responses, we first measured T-cell proliferation after stimulation through the Kb-CD28-ζ or Kb-CD28[L→G]-ζ receptors. Kb-CD28-ζ, Kb-CD28[L→G]-ζ, and retroviral vector-modified T cells responded equivalently to a control, nonspecific mitogen, concanavalin A, demonstrating that receptor expression did not adversely impact the ability of the transduced cells to proliferate (Figure 3). In contrast, T cells transduced with the dileucine-mutated receptor proliferated significantly better than wild-type receptor-transduced T cells in response to chimeric receptor-specific stimulation. Therefore, the CD28 dileucine motif functionally restricts chimeric receptor activity, and this restriction is alleviated by the L→G mutation.
Proliferative response of Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. GFP-sorted CD8+ RMTCs were stimulated 7 days after transduction with irradiated splenocyte feeders on plates coated with AF6-88.5 anti-H-2Kb or in the presence of the nonspecific mitogen conA. After 2 days the cultures were pulsed with 3H-thymidine and harvested 16 hours later. Data points are means of triplicate samples. Error bars show ± 1 SD. One of 3 essentially identical experiments is shown.
Proliferative response of Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. GFP-sorted CD8+ RMTCs were stimulated 7 days after transduction with irradiated splenocyte feeders on plates coated with AF6-88.5 anti-H-2Kb or in the presence of the nonspecific mitogen conA. After 2 days the cultures were pulsed with 3H-thymidine and harvested 16 hours later. Data points are means of triplicate samples. Error bars show ± 1 SD. One of 3 essentially identical experiments is shown.
To determine whether the enhanced function of RMTCs expressing the mutated receptor extended to the production of effector cytokines, we analyzed IFN-γ release by RMTCs. Stimulation with chimeric receptor-specific antibody induced more than 3.5-fold more IFN-γ in Kb-CD28[L→G]-ζ RMTCs than in Kb-CD28-ζ RMTCs (Figure 4). Thus, disabling the dileucine motif improves RMTC effector cytokine response.
IFN-γ production by Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. GFP-sorted CD8+ RMTCs were stimulated 7 days after transduction in the presence of splenocyte feeders on plates coated with 5 μg/mL AF6-88.5 anti-Kb, with conA, or cultured in the absence of stimulation. Stimulation-induced IFN-γ production was measured by Bioplex assay using anti-IFN-γ-coated beads. Data points are means of triplicate samples. Error bars show ± 1 SD; *, less than 1 ng/mL.
IFN-γ production by Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. GFP-sorted CD8+ RMTCs were stimulated 7 days after transduction in the presence of splenocyte feeders on plates coated with 5 μg/mL AF6-88.5 anti-Kb, with conA, or cultured in the absence of stimulation. Stimulation-induced IFN-γ production was measured by Bioplex assay using anti-IFN-γ-coated beads. Data points are means of triplicate samples. Error bars show ± 1 SD; *, less than 1 ng/mL.
The effector function of RMTCs most often required for immunotherapy is target cell cytolysis. We have previously demonstrated that Kb-CD28-ζ-modified RMTCs antigen-specifically kill Kb-restricted target T cells with a similar efficiency as Kb-ζ-modified cells. To compare the cytolysis efficiency of Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs we analyzed their ability to lyse transgenic OT-1 T cells. The OT-1 TCR recognizes the 257-264 peptide of ovalbumin (SIINFEKL) complexed with the chimeric receptor's extracellular Kb domain.16 We pulsed RMTCs with this peptide or diluent, washed them, and coincubated the peptide- or control-pulsed RMTCs with purified OT-1 TCR transgenic T lymphocytes to analyze specific cytolysis.
We first examined the relationship of RMTC dose to cytolytic response. Equivalent specific lysis of OT-1 T cells occurred with approximately 3-fold to 9-fold fewer peptide-pulsed Kb-CD28[L→G]-ζ RMTCs compared with Kb-CD28-ζ RMTCs (Figure 5A and data not shown). This demonstrates an increased efficiency of lysis by RMTCs transduced with the dileucine-mutated chimeric receptor. To better define how limitations in the quantity of chimeric receptor ligand present on individual RMTCs influence lytic potency, we varied the concentration of antigenic peptide used to peptide-pulse the RMTCs (Figure 5B). Fewer chimeric receptors would be expected to incorporate the ovalbumin peptide when lower antigen concentrations are used for pulsing. Our analysis showed that the Kb-CD28[L→G]-ζ RMTCs lysed OT-1 target cells equivalently to the Kb-CD28-ζ RMTCs when pulsed with 5-fold to 20-fold lower concentrations of antigenic peptide. This further demonstrates that the chimeric receptor dileucine mutation significantly increases the lytic potency of effector RMTCs.
Cytolysis of antigen-specific T cells by Kb-CD28-ζ and Kb-CD28[L→G]- ζ RMTCs. (A) RMTCs were pulsed with 50 μg/mL ovalbumin 257-264 peptide or saline diluent, washed, and cultured for 6 hours with OT-1 TCR transgenic T lymphocytes at the designated effector-target ratio. Target cell survival was determined using quantitative flow cytometry. Specific cytolysis was calculated from the number of residual viable target cells in wells containing target cells pulsed with peptide compared with that in otherwise identical control wells including unpulsed effectors. (B) Similar to panel A, except experimental RMTCs were pulsed with the designated concentration of ovalbumin peptide. All samples were cultured at an effector-target ratio of 1. Data points are means of quintuplicate samples. Error bars show ± 1 SD. Plots are representative of 3 independent experiments.
Cytolysis of antigen-specific T cells by Kb-CD28-ζ and Kb-CD28[L→G]- ζ RMTCs. (A) RMTCs were pulsed with 50 μg/mL ovalbumin 257-264 peptide or saline diluent, washed, and cultured for 6 hours with OT-1 TCR transgenic T lymphocytes at the designated effector-target ratio. Target cell survival was determined using quantitative flow cytometry. Specific cytolysis was calculated from the number of residual viable target cells in wells containing target cells pulsed with peptide compared with that in otherwise identical control wells including unpulsed effectors. (B) Similar to panel A, except experimental RMTCs were pulsed with the designated concentration of ovalbumin peptide. All samples were cultured at an effector-target ratio of 1. Data points are means of quintuplicate samples. Error bars show ± 1 SD. Plots are representative of 3 independent experiments.
In vivo targeting of antigen-specific T cells by RMTCs
Peptide-pulsed Kb-CD28[L→G]-ζ or Kb-CD28-ζ RMTCs efficiently and antigen-specifically lysed target OT-1 T cells in vitro. To determine the feasibility of similarly targeting these cells in vivo, we labeled lymph node cells from OT-1 TCR transgenic mice with the fluorescent marker CFSE and adoptively transferred them into severe combined immunodeficiency (SCID) mice prior to the transfer of peptide-pulsed or unpulsed Kb-CD28[L→G]-ζ or Kb-CD28-ζ RMTCs. Twenty-four hours later we determined by flow cytometry the number of residual OT-1 cells present in the spleens and lymph nodes (LNs) of treated mice (Figure 6). The CFSE-positive OT-1 target cells were readily distinguished from the GFP-positive RMTCs by their fluorescence intensity and scatter characteristics. To distinguish ovalbumin-specific T cells from B, nonspecific T, and other cell types present among the CFSE-positive transferred cells, we stained the posttreatment splenocytes or LN cells with an anti-Vα2 antibody that recognizes ovalbumin-specific OT-1 T cells. The Vα2-negative CFSE-positive cell population, which is not specific for ovalbumin and should not be targeted by the RTMCs, was, as expected, not significantly affected by peptide-pulsed compared with unpulsed Kb-CD28[L→G]-ζ or Kb-CD28-ζ RMTCs (Figure 6A-B). In contrast, a significant loss of Vα2-positive CFSE-positive OT-1 target T cells in both spleen and LNs was seen in mice treated with peptide-pulsed compared with unpulsed RMTCs. To control for the efficiency of adoptive transfer, we normalized the number of Vα2-positive CFSE-positive cells detected to the number of unaffected Vα2-negative CFSE-positive cells (Figure 6C-D). With this normalization we could calculate that the peptide-pulsed Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs depleted 93% ± 4% and 98% ± 0.4% of Vα2+ OT-1 cells from the LNs and 96% ± 3% and 99% ± 0.1% from the spleen, respectively. The Kb-CD28[L→G]-ζ RMTCs consistently performed better than the Kb-CD28-ζ RMTCs in 3 of 3 independent experiments in which splenocytes were analyzed and in 2 of 2 independent experiments analyzing lymph node cells. Cumulative data from these experiments, however, only showed a statistically significant difference for splenocytes (P = .01, n = 9 per treatment group for splenocytes; P = .27, n = 6 per treatment group for LN cells). These results therefore demonstrate that both Kb-CD28[L→G]-ζ and Kb-CD28-ζ RMTCs are effective in depleting CD8+ antigen-specific T cells in vivo, show that RMTCs expressing the dileucine-mutated receptor are more active in vivo, and suggest that RMTCs may be effective in inducing transplant tolerance by eliminating pathologic alloreactive T cells.
In vivo killing of antigen-specific T lymphocytes using Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. A total of 107 CFSE-labeled OT-1 lymph node cells were adoptively transferred intravenously into SCID mice; 107 Kb-CD28-ζ or Kb-CD28[L→G]-ζ peptide-pulsed or unpulsed RMTCs were then adoptively transferred intravenously at an anatomically separate location. Twenty-four hours after transfer, spleen and mixed lymph nodes (mesenteric, cervical, axillary, inguinal) were isolated and single-cell suspensions prepared, stained with Vα2-specific antibody, and analyzed by flow cytometry. (A) Percentage of transferred CFSE-positive OT-1 cells that are Vα2-positive (ovalbumin-specific) or Vα2-negative (nontarget cells) in the spleens of mice receiving different RMTC treatments is shown. RMTC effector and ovalbumin peptide pulsing is indicated on the abscissa. For each RMTC effector and peptide-pulse combination, data from individual mice are indicated with a unique symbol (♦, ▴, ▪). Mean percentages are indicated by a horizontal bar. (B) As in panel A, but LNs of recipient animals were analyzed. (C) Normalized numbers of target cells in the spleens of treated animals. The ratio of residual transferred (CFSE-positive) RMTC targets (Vα2-positive) to nontargets (Vα2-negative) was calculated to control for the efficiency of adoptive transfer in mice treated with peptide-pulsed or control unpulsed effectors. (D) Analysis of LN cells. Error bars show ± 1 SD. Results are representative of 3 independent experiments.
In vivo killing of antigen-specific T lymphocytes using Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. A total of 107 CFSE-labeled OT-1 lymph node cells were adoptively transferred intravenously into SCID mice; 107 Kb-CD28-ζ or Kb-CD28[L→G]-ζ peptide-pulsed or unpulsed RMTCs were then adoptively transferred intravenously at an anatomically separate location. Twenty-four hours after transfer, spleen and mixed lymph nodes (mesenteric, cervical, axillary, inguinal) were isolated and single-cell suspensions prepared, stained with Vα2-specific antibody, and analyzed by flow cytometry. (A) Percentage of transferred CFSE-positive OT-1 cells that are Vα2-positive (ovalbumin-specific) or Vα2-negative (nontarget cells) in the spleens of mice receiving different RMTC treatments is shown. RMTC effector and ovalbumin peptide pulsing is indicated on the abscissa. For each RMTC effector and peptide-pulse combination, data from individual mice are indicated with a unique symbol (♦, ▴, ▪). Mean percentages are indicated by a horizontal bar. (B) As in panel A, but LNs of recipient animals were analyzed. (C) Normalized numbers of target cells in the spleens of treated animals. The ratio of residual transferred (CFSE-positive) RMTC targets (Vα2-positive) to nontargets (Vα2-negative) was calculated to control for the efficiency of adoptive transfer in mice treated with peptide-pulsed or control unpulsed effectors. (D) Analysis of LN cells. Error bars show ± 1 SD. Results are representative of 3 independent experiments.
Discussion
Immunotherapeutically targeting T lymphocytes against pathologic cell types is a primary objective of cellular immunotherapy. Adoptively transferred therapeutic T lymphocytes are long-lived, can migrate throughout the body, and express a variety of therapeutically useful effector functions. Further, clinically administered antigen-specific T cells have shown promise in the treatment of infectious diseases and cancer.24-26 Chimeric receptors linking antigen-recognition domains, such as scFv or scTCR, to signaling domains derived from the TCR have proven straightforward to construct and highly effective at redirecting therapeutic T cells against desired targets.27,28 First-generation receptors generally included the cytoplasmic tail of the TCR ζ chain or the structurally and functionally similar FcϵRI γ chain for signal transduction. However, these receptors proved limited by their inability to transduce supplementary costimulatory signals into T lymphocytes. As a result a newer generation of receptors that include the cytoplasmic tail of CD28 linked to ζ has been constructed.
Data from several groups have proven CD28-ζ receptors superior to otherwise identical ζ-containing receptors.5,11-13 Although we observed enhanced function of a Kb-CD28-ζ receptor when compared with a Kb-ζ receptor in immortalized T-cell hybridomas, we saw no functional difference when primary T lymphocytes were transduced with these receptors. We hypothesized that this resulted from the poor expression we observed with the Kb-CD28-ζ receptors. We now identify a dileucine motif in the cytoplasmic tail of murine CD28 that limits chimeric receptor expression and function.
Two classes of dileucine motifs have been characterized, containing either [DE]XXXL[LI] or DXXLL canonical sequences.18 The sequence we identified in the CD28 tail, SRRNRLL, lacks the upstream negative charge typical of these motifs. DXXLL motifs bind to the GGA family of ADP-ribosylation factor (ARF)-dependent clathrin adaptors and are intolerant of mutations of the upstream aspartic acid or of the twin leucines. In contrast the [DE]XXXL[LI] motif, which binds the adaptor protein-1 (AP-1), AP-2, and/or AP-3 family of adaptors, has more varied sequences at the amino end and may also contain either a leucine or isoleucine at the carboxyl end. The CD28 dileucine motif therefore falls into this latter class. Indeed, the SRRNRLL CD28 motif resembles the positively charged RRTPSLL, PRGSRLL, and SERRNLL motifs of insulin-responsive glucose transporter (GLUT4), insulin-regulated aminopeptidase (IRAP), and vesicle-associated membrane protein 4 (VAMP4), respectively.18 The positive charge provided by the arginine residues in these dileucine motifs contrasts with the canonical negatively charged glutamic or aspartic acid residue and is believed to influence the destination of these proteins with internalization.29
A positively charged dileucine motif, SKRSRLL, homologous to the murine sequence that we studied is present in the cytoplasmic tail of human CD28. We would therefore expect that mutations in the human motif would similarly improve the expression of human chimeric receptors that include the CD28 signaling chain. However, further study of humanized chimeric receptors will be important to definitively establish this.
Our results with chimeric receptors imply that the noncanonical dileucine motif in CD28 is biologically functional. The role of this dileucine motif in CD28 itself, however, is unclear. Although with our chimeric receptors we observe enhanced basal expression after disrupting the dileucine motif, in many other molecules dileucine-mediated internalization is only apparent in restricted circumstances. For example, the SDKQTLL sequence of CD3γ requires serine phosphorylation to mediate internalization of the T-cell receptor complex.30 Potentially this motif is only accessible to membrane-associated sorting proteins after a conformational shift induced by phosphorylation. It seems likely that the dileucine motif of native CD28 is likewise normally inaccessible, only mediating internalization in select circumstances, such as after activation when CD28 rapidly down-modulates. Placement of the CD28 dileucine motif outside of its native context, such as in chimeric receptors, may inadvertently expose it to the protein-sorting machinery and thereby constitutively activate its function.
The presence of a functional dileucine motif would be expected to limit signaling through chimeric receptors. Indeed, similar motifs present in the tails of CD3γ and CD4 are known to restrict TCR activity.31-33 Our results clearly demonstrate that inactivation of the CD28 dileucine motif increases expression of and strongly up-regulates chimeric receptor-mediated proliferation, cytokine production, and cytolysis. Although the location of the dileucine motif is important for its function, dileucine motifs may be located either membrane proximally or more C-terminal. Switching the CD28 and ζ modular domains of the CD28-ζ receptor may therefore not be adequate to inactivate it. Indeed, we and others have shown even poorer expression of receptors that include a ζ-CD28 tail compared with the CD28-ζ tail present in the receptors studied here.10,11 In contrast to moving the CD28 domain, mutagenically inactivating it should more uniformly improve receptor potency.
In addition to demonstrating improved functional characteristics of dileucine-mutated receptors, we for the first time show that RMTCs may be used to selectively target antigen-specific CD8+ T cells in vivo. Approaches to selectively tolerize the alloantigen-specific or minor histocompatability antigen-specific T cells that mediate transplant rejection or graft versus host disease, such as the veto effect or the use of tolerizing regimens of antigen, are limited and have yet to be clinically validated.34-36 Retargeting RMTCs against pathologic T cells represents a promising new therapeutic possibility. We observed improved in vivo cytolysis using Kb-CD28[L→G]-ζ compared with Kb-CD28-ζ RMTCs, demonstrating that the mutated receptor is more potent and would likely be preferable for therapeutic use. Further studies, however, will be required to determine how these RMTC may be optimally applied to induce transplant tolerance.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-04-1255.
Supported by the National Institutes of Health (NIH) grants AI49872 and CA21765 (T.L.G.) and the American Lebanese Syrian Associated Charities/St Jude Children's Research Hospital (T.L.G., P.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Richard Cross, Dick Ashmun, and Jennifer Hoffage for their assistance with flow cytometric sorting and Janet Gatewood for her assistance with cytokine analysis.

![Figure 3. Proliferative response of Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. GFP-sorted CD8+ RMTCs were stimulated 7 days after transduction with irradiated splenocyte feeders on plates coated with AF6-88.5 anti-H-2Kb or in the presence of the nonspecific mitogen conA. After 2 days the cultures were pulsed with 3H-thymidine and harvested 16 hours later. Data points are means of triplicate samples. Error bars show ± 1 SD. One of 3 essentially identical experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-04-1255/6/m_h82435385003.jpeg?Expires=1765886351&Signature=pHTl-b3Okb3fkF-whPUBDeZAmsNF4s0aKVX-ES6A~DnrPpYCrM1cQVa-z7Qo7fn7MOoXNoDOzTsaz9AmOgcitNn-9hpj9FJYrJvGQkQLGZNgZ3baNJTmJ7HJ83j0bagTXGdHMTpajDG7pMAepaXkw3SurzW2zanK9HgzV30wNmJ-bFUP91RaBWkLwDz9BCNxSzdVBi5JQkKfkNjetPYqyxqt42iR29-QuJWgjcE6mCfD4bsiAFlOgxQyDEtzC7n7vvlbdn1vgJLb8jfmdcsFqSONpHkU0VlR1QfNgRUP58ZGk~XQ7W9dzJTGCHuJGIgaLEHmnjYtmV6vQT2zr0gyyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. IFN-γ production by Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. GFP-sorted CD8+ RMTCs were stimulated 7 days after transduction in the presence of splenocyte feeders on plates coated with 5 μg/mL AF6-88.5 anti-Kb, with conA, or cultured in the absence of stimulation. Stimulation-induced IFN-γ production was measured by Bioplex assay using anti-IFN-γ-coated beads. Data points are means of triplicate samples. Error bars show ± 1 SD; *, less than 1 ng/mL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-04-1255/6/m_h82435385004.jpeg?Expires=1765886351&Signature=q8t0PZxyYoDCRjkuePkO0Ll7X-ad6OetOiv9AE0jro72tMywLRhZBblI4OPqs1GXga0u2FIJCxWZ7awAfPlVJGwzYVMf0GCKRd0fUC9l2e~KV7zm1qcNgBA5OkwXphIpM3OH8XaZY7bqXXeaCT9xbFHonf-sKH0HldczGrVenZtXzjTmSnjsYER91GpZVmc1IsU8AJJBUiQ6-0ELsnWTxhn2T7-DJcENQHOZ4447RhyAYIj2U95rbGb7R2wpwYsAXQfxK2kTRsGks3xZxF-wf-biaTNW8j4hiZml7sUmM7ElbuoD9kyc0bYeZj8R8-9Q2ZMezOK91iiTxOtiW4tUuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Cytolysis of antigen-specific T cells by Kb-CD28-ζ and Kb-CD28[L→G]- ζ RMTCs. (A) RMTCs were pulsed with 50 μg/mL ovalbumin 257-264 peptide or saline diluent, washed, and cultured for 6 hours with OT-1 TCR transgenic T lymphocytes at the designated effector-target ratio. Target cell survival was determined using quantitative flow cytometry. Specific cytolysis was calculated from the number of residual viable target cells in wells containing target cells pulsed with peptide compared with that in otherwise identical control wells including unpulsed effectors. (B) Similar to panel A, except experimental RMTCs were pulsed with the designated concentration of ovalbumin peptide. All samples were cultured at an effector-target ratio of 1. Data points are means of quintuplicate samples. Error bars show ± 1 SD. Plots are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-04-1255/6/m_h82435385005.jpeg?Expires=1765886351&Signature=EK1NXvrTS2wLQfqq6v1hyYAYmoDudk7XK-7lrL7YCP09itfH~oL-wrVQU6LhDZEYnV5z~ZNo4be8jzVblJ3zQw1KBlKi0IhpCD8PUgvLUatkfFnsYaOBHvVQC75HkATfNK3OtKUEUZiP6CEew-hxCs-SNux4i4Dqbp5ESFbWwPrLM3KSAEVjPlmECHMN1Valnr1WTS0Z8N0YQeKTpT0EUVqckLinHKcjXy0CYiUPT4mKY-rTnHcI9I6FXOFUnobB0Ryp5~YafCoVrZatBHx44fkY0o1cjiTfTDDBmubNj6dSiev-XyVUynnylsX43eEvRZg5WmWy-dQX7PWbKgVVEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. In vivo killing of antigen-specific T lymphocytes using Kb-CD28-ζ and Kb-CD28[L→G]-ζ RMTCs. A total of 107 CFSE-labeled OT-1 lymph node cells were adoptively transferred intravenously into SCID mice; 107 Kb-CD28-ζ or Kb-CD28[L→G]-ζ peptide-pulsed or unpulsed RMTCs were then adoptively transferred intravenously at an anatomically separate location. Twenty-four hours after transfer, spleen and mixed lymph nodes (mesenteric, cervical, axillary, inguinal) were isolated and single-cell suspensions prepared, stained with Vα2-specific antibody, and analyzed by flow cytometry. (A) Percentage of transferred CFSE-positive OT-1 cells that are Vα2-positive (ovalbumin-specific) or Vα2-negative (nontarget cells) in the spleens of mice receiving different RMTC treatments is shown. RMTC effector and ovalbumin peptide pulsing is indicated on the abscissa. For each RMTC effector and peptide-pulse combination, data from individual mice are indicated with a unique symbol (♦, ▴, ▪). Mean percentages are indicated by a horizontal bar. (B) As in panel A, but LNs of recipient animals were analyzed. (C) Normalized numbers of target cells in the spleens of treated animals. The ratio of residual transferred (CFSE-positive) RMTC targets (Vα2-positive) to nontargets (Vα2-negative) was calculated to control for the efficiency of adoptive transfer in mice treated with peptide-pulsed or control unpulsed effectors. (D) Analysis of LN cells. Error bars show ± 1 SD. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-04-1255/6/m_h82435385006.jpeg?Expires=1765886351&Signature=jjnt~xnRXh2jKk1euMGVSSqs4QP~T0ExeCj-daUPm2weo92w13CkzOFqTI4~sqi0lgtBc~yuX1Wn9A8IIxpfc650Mjorj59XVLx7stMDt8wR0fygWCikMHwpo2rs0imONHHDBmu6EGZx4YRUtWJBsrjuJCghNDpEzvhtJEGwobiRPfuruh--i-ggP79POUW83t1HGyB8dUDov~uMVZr7AeB0kxM7hxMHotc6JsB4nAD-N-scCZrE13j79rbWbYNn3WDsLSa~B5BQVufX-bvheizXYl5UJFynk9U--UQEyU6-ja3efug7LIhtMEUoBksMYK0TVpQ1ZxXzO1TVjjtnmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal