Abstract
R115777 is a potent farnesyltransferase (FTase) inhibitor with substantial antitumor activity in preclinical models. We conducted a phase 1 study (3 + 3 design) of R115777 in patients with myelodysplastic syndrome (MDS). R115777 was administered twice daily (3-weeks-on/1-week-off schedule for 8 weeks) (starting dosage, 300 mg by mouth twice daily; total, 600 mg). Maintenance therapy at the dose/schedule tolerated during induction could be continued until toxicity or lack of benefit. Twenty-one patients with MDS were treated (median age, 66 years). Four (19%) patients had ras mutations (n-ras,3; k-ras, 1). Objective responses (hematologic improvement, 3; partial remission, 2; or complete remission, 1) were seen in 6 of 20 (30%) evaluable patients, only 2 of whom had ras mutations. Response sequences were unusual in some patients who had increases in platelet counts without intervening aplasia. Other responders demonstrated an initial, albeit modest, myelosuppressive effect. The maximum tolerated dose was 400 mg by mouth twice a day. The most frequent side effect was myelosuppression. Dose-limiting toxicities (fatigue and confusion) occurred at 900 mg by mouth total daily dose. R115777 inhibited HDJ-2 prenylation and suppressed the activity of FTase, but not of the related geranylgeranyltransferase I enzyme, in peripheral blood mononuclear cells. Modulation of Akt, Erk, and signal transducer and activator of transcription 3 (STAT3) phosphorylation was variable, and responses occurred even without their down-regulation. Reductions in serum tumor necrosis factor-α (TNF-α) levels by day 7 showed a trend toward correlation with response (P = .09). We conclude that, at doses that are well tolerated, R115777 markedly inhibits the FTase target and has antitumor activity in MDS. (Blood. 2003;102:4527-4534)
Introduction
Inhibitors of farnesyltransferase (FTase) were first developed because of the critical role this enzyme plays in the functional activation of the Ras protein.1,2 However, it is now known that farnesylation is also important to other oncoproteins,3-7 further stimulating interest in FTase inhibitors (FTIs) as novel anticancer drugs.
The significance of Ras as a tumor target is based on the fact that the ras family of genes encodes G-proteins that act as molecular switches controlling key cellular functions1,2,8-10 and on the observation that the activation of ras, either directly through mutation or indirectly through other genetic abnormalities, is one of the most frequent aberrations in cancer.1,2,11-18 For Ras to be fully functionally active, it must transfer from the cytoplasm, where it is initially made, to the inner surface of the plasma membrane.1,2,19 It follows that interference with membrane localization should suppress Ras signaling even when Ras is oncogenic because of mutations that “lock” it into an active guanosine triphosphate (GTP)-bound state. The first obligatory step in the posttranslational modification of Ras for membrane localization is accomplished through a prenylation reaction, mediated by the FTase enzyme, which involves covalent attachment of a 15-carbon isoprenyl (farnesyl) group to the sulfhydryl cysteine of the CAAX motif at the Ras carboxyl terminal.1,19,20 A closely related enzyme, geranylgeranyltransferase I (GGTase I), catalyzes the transfer of a 20-carbon lipid geranylgeranyl to proteins that end with CAAX at their carboxyl terminus.6
Interestingly, though H-, N-, and K-Ras proteins are naturally farnesylated, K-Ras and possibly N-Ras, but not H-Ras, become geranylgeranylated when human cancer cells are treated with FTIs.6 The presence of this alternative pathway has raised concerns about the ability of FTIs alone to suppress the growth of cells with k-ras or n-ras mutations. Nevertheless, in n-ras-transformed cells, the suppression of oncogenic Ras processing and the growth of tumors in nude mice can occur after treatment with FTIs alone.3,21,22 Furthermore, even in some human lung tumor cells bearing k-ras mutations (which require both FTase and GGTase I inhibitors for suppression of Ras processing), FTIs alone can suppress tumor growth.21,23 Therefore, the growth inhibitory properties of FTIs may be attributable to their effects on other farnesylated proteins. Examples of such proteins include the centromeric proteins CENP-E and CENP-F1,3,5-7 and RhoB, which is both farnesylated and geranylgeranylated but whose role as a target remains a matter of debate.6
R115777 (Zarnestra; Johnson & Johnson, Titusville, NJ) is a potent nonpeptidomimetic FTI that has shown significant antitumor activity (in vitro and in animal models) against tumors bearing mutated h-, n-, or k-ras and in those without ras mutations.3,24-27 Furthermore, in a recent phase 1 study conducted by Karp et al,28 29% of patients with refractory or relapsed acute leukemia responded to R115777; this included 2 complete remissions. Because myelodysplastic syndrome (MDS) is part of the nosologic spectrum of myeloid malignancies, investigation of this FTI in MDS appeared warranted.
We report the first clinical trial of R115777 in patients with MDS. The objectives of this trial were to assess the tolerance of patients with MDS to R115777 and to assess the impact of this agent on FTase activity, substrate prenylation, downstream effectors, and antitumor effects in the phase 1 setting.
Patients, materials, and methods
Patient eligibility and selection
Adults (18 years and older) with MDS documented by bone marrow aspirate and biopsy were treated. Eligibility criteria included diagnosis of MDS (all subtypes); patients with refractory anemia (RA) or refractory anemia with ringed sideroblasts (RARS) had to have had significant cytopenia of at least 4 weeks' duration (hematocrit less than 0.26 or requiring transfusions; absolute neutrophil count [ANC] less than 1.0 × 109/L, or platelet count less than 50× 109/L); 2 or fewer prior therapies; Zubrod performance status 2 or less; life expectancy of 12 weeks or longer; adequate renal function (creatinine level, 1.5 mg/100 mL or less); and adequate hepatic function (bilirubin concentration, 1.5 mg/100 mL or less). When the protocol was conducted, no therapy had demonstrated a survival advantage for these patients; therefore, untreated patients were eligible.
Patients had to have discontinued all therapy for at least 4 weeks. However, patients could receive hydroxyurea until 24 hours before study initiation. Other exclusion criteria were patients eligible for allogeneic bone marrow transplantation (younger than 60 with a histocompatible sibling donor and no contraindications for transplantation); pregnant or breast-feeding patients; and patients with uncontrolled or life-threatening infections.
Patient evaluation
Complete history and physical examination were performed within 7 days of the start of the drug. Baseline determinations included bone marrow aspirate, biopsy, and cytogenetics (within 2 weeks before drug initiation); complete blood count (CBC), differential, platelets, reticulocytes; renal and hepatic function tests (bilirubin, creatinine, SMA12); and computed tomography (CT) of the abdomen for patients with palpable splenomegaly.
During treatment, assessments included bone marrow aspirate/biopsy and cytogenetics at baseline and approximately every 8 weeks; physical examination weekly for 6 weeks, then every 2 to 4 weeks; complete blood counts at baseline, then 2 to 3 times per week; hepatic and renal function tests (SMA-12) at baseline, then 1 to 2 times per week; and CT of the abdomen approximately every 8 to 12 weeks for patients with palpable organomegaly.
Treatment plan
All patients signed written, informed consent in accordance with the institutional internal review board policy.
Patients were treated in cohorts of 3 per dose level. The initial cohort of patients was treated with R115777 at a dosage of 300 mg by mouth (po) twice a day (bid). For each subsequent cohort of patients, dose escalation depended on the toxicity in the previous cohort. Dose escalation was planned as follows: 200 mg by mouth twice a day (total escalation, 400 mg/d) if no toxicity was observed in any patient in the previous cohort; 100 mg by mouth twice a day (total escalation, 200 mg/d) if grade 1 toxicity was observed in any patient in the previous cohort; 100 mg/d escalation if grade 2 or greater toxicity was observed in any patient in the previous cohort. Because grade 2 toxicity was seen at the first dose level, in effect, 100-mg/d escalations were used for all cohorts. At least 3 patients were treated at each dose level. If a grade 3 or greater toxicity was seen in a cohort, that cohort was expanded to 6 patients. If no further toxicities grade 3 or higher were seen, the next cohort received a 100-mg/d dose escalation. If a second toxicity grade 3 or higher was observed, this dose was considered above the maximum tolerated dose (MTD). The next lower dose was considered the MTD. (Complete details on determining MTD are found in “Determining maximum tolerated dose.”)
Therapy was given on a 3-weeks-on/1-week-off schedule for 8 weeks (8 weeks represented 1 course). Toxicity was assessed using the Cancer Therapy Evaluation Program Common Toxicity Criteria (http://ctep.info.nih.gov). After the first 8 weeks of therapy, patients with grade 1 or less nonhematologic toxicity and stable disease or hematologic improvement could continue on treatment at the same dose or receive a dose escalation (as outlined earlier). Patients with grade 2 or less toxicity and hematologic improvement could continue on therapy at the same dose level. However, patients with persistent grade 2 toxicity (other than alopecia) could receive a 50% dose reduction. The drug was discontinued in patients with grade 3 (nonhematologic) toxicity until side effects resolved (to grade 1 or less). The drug could then be resumed with a 50% dose reduction (rounded down to the nearest 100 mg). If grade 3 toxicity recurred, the drug was discontinued. The drug was held for patients with deteriorated counts—defined as persistent (longer than 28-day) platelet count decrease by more than 50% and to less than 20 000/mm3 if baseline level was 20 000-50 000/mm3, as ANC decrease by more than 50% and to less than 500/mm3 if baseline ANC level was more than 500/mm3, or as development of transfusion dependence if the patient had been transfusion independent at baseline—but could be restarted with a 50% dose reduction (rounded down to the nearest 100 mg) after toxicity was resolved. The drug was discontinued in patients with grade 4 (nonhematologic) toxicity or with serious infection or hemorrhage in the presence of hematologic deterioration, as defined. Patients with stable disease or better could be maintained on therapy until disease progressed or for 6 months beyond complete remission.
Patients did not undergo steroid therapy or any concomitant chemotherapy that might influence the course of MDS while in the study. Patients were allowed to receive granulocyte colony-stimulating factor (G-CSF). Other growth factors were not permitted while in the study.
Definitions of response
Hematologic complete remission (CR) was defined as the achievement of normal peripheral count (ANC 1.5 × 109/L or greater and platelet count greater than 100 × 109/L) and normocellular bone marrow with less than 5% blasts, accompanied by the disappearance of organomegaly and of karyotypic abnormalities for at least 4 weeks. Hematologic partial remission (PR) was defined as the restoration of normal peripheral blood counts (ANC 1.5 × 109/L or greater and platelet count 100 × 109/L or greater) and at least a 50% decrease in organomegaly but persistent blast count elevation from 5% to 10% or less and persistent karyotypic abnormalities. Hematologic improvement (HI) was defined as (1) tripling of platelet counts and achievement of levels 20 × 109/L or higher if baseline counts were 20 × 109/L or lower, or doubling of platelet counts and achievement of counts 50 × 109/L or higher if baseline counts were higher than 20 × 109/L and 50 × 109/L or lower; (2) achievement of transfusion independence if the patient was initially transfusion dependent, or an increase in the hemoglobin level of 2 g/dL or more if the patient was not initially transfusion dependent; (3) doubling of ANC and achievement of a count 1.0 × 109/L or higher if the initial count had been 1.0 × 109/L or lower. Patients were not assessed for ANC response unless they discontinued G-CSF for at least 1 week. All responses had to last at least 4 weeks. Progressive disease was defined as any doubling of bone marrow blasts, with bone marrow blasts reaching levels of 20% or higher.
Determining maximum tolerated dose
Evaluation for toxicity in each group of 3 patients treated with different dosage levels was graded according to the Cancer Therapy Evaluation Program Common Toxicity Criteria (http://ctep.info.nih.gov). Dose-limiting toxicity was defined as any nonhematologic toxicity grade 3 or greater, except for alopecia or nausea (which may be of grade 4 severity), or any grade 4 hematologic toxicity lasting more than 28 days after the last day of therapy. Dose-limiting toxicities were evaluated during the 8-week induction period.
The plan for dose escalation relevant to determining MTD was as follows. Dose levels were escalated in cohorts of 3 untreated patients as long as no nonhematologic toxicity grade 3 or higher was observed and marrow recovery was sufficiently rapid. If one patient was observed to have grade 3 or higher nonhematologic toxicity or grade 4 hematologic toxicity (with serious infection or hemorrhage and hematologic deterioration) lasting 4 weeks or more at a particular dose level, this cohort was expanded. If no additional patients in the expanded cohort of 6 patients experienced dose-limiting toxicity, dose escalation was resumed (100-mg/d dose escalation for next cohort). If a second patient enrolled at the same dose level in a cohort of up to 6 patients experienced dose-limiting toxicity, the MTD was exceeded, and dose escalations ceased. The next lower dose level was considered the MTD. Therefore, the MTD was the dose level below that at which one third or more patients experienced dose-limiting toxicity.
Laboratory correlative studies
Baseline n-ras and k-ras mutations were assessed in all patients by sequencing. It was planned that samples would be collected for correlative studies at baseline, 24 hours, 1 week, and 4 weeks, or as close as possible to those time points, depending on the patients' availability and willingness to have samples collected on certain days. Because many patients had severe cytopenia, there was not always enough material to perform all biochemical assays. In addition, because many patients referred for study lived outside Houston, most research samples were collected during the first cycle when the patients generally remained nearby. FTase and GGTase I enzymatic activities, HDJ-2 prenylation status, and phosphorylated and total Akt, Erk1/2, and signal transducer and activator of transcription 3 (STAT3) levels were determined as described beginning in the next paragraph. Cytokine levels in serum were determined by enzyme-linked immunosorbent assay (ELISA), as described by us previously.29
FTase I and GGTase I enzyme activity assays
Patient peripheral blood mononuclear cells (PBMCs) were sonicated in 250 to 500 μL sonication buffer (50 mM Tris pH 7.5, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethyleneglycotetraacetic acid], 1 mM dithiothreitol [DTT], 2 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL aprotinin, 10 μg/mL soybean trypsin inhibitor, and 25 μg/mL leupeptin). When enough material was available, a portion of a sample was prepared for Western blotting by adding an equal volume of 2 × lysis buffer (60 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.5, 20 mM NaCl, 10 mM MgCl2, 50 mM NaF, 2 mM EGTA, 2% Triton X-100, and 20% glycerol), incubating it on ice for 30 minutes, and centrifuging it at 12 000g for 15 minutes. Supernatants were then prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) Western blotting. The other portion of sonicate was used for FTase and GGTase I enzymatic assays by spinning at 12 000g for 30 minutes. Resultant supernatants were spun at 60 000g for 1 hour, and the postmicrosomal supernatants were collected. The ability of FTase and GGTase I to transfer [3H]farnesyl and [3H]geranylgeranyl from [3H]FPP and [3H]GGPP to recombinant H-Ras-CVLS and H-Ras-CVIL, respectively, was determined as described by us previously.30 Briefly, 20 μg postmicrosomal supernatants was incubated for 30 minutes at 37°C in 50 mM Tris pH 7.5, 50 μmol/L ZnCl2, 20 mM KCl, 1 mM DTT, and 3 mM MgCl2 in the presence of either 0.5 μCi (0.0185 MBq) [3H]farnesyl pyrophosphate (FPP) and 0.5 μg/mL H-Ras-CVLS (FTase) or 0.5 μCi (0.0185 MBq) [3H]geranylgeranyl pyrophosphate (GGPP) and 0.25 μg/mL H-Ras-CVLL (GGTase I). The reaction was then stopped with 10% SDS, and the proteins were precipitated with 30% trichloroacetic acid (TCA) and collected on glass microfiber filters, as described previously.30
Determination of protein prenylation status and signaling protein level by Western immunoblotting
PBMC lysates were prepared as described above. Proteins from the lysates were then separated by SDS-PAGE and transferred to nitrocellulose membranes, and the membranes were immunoblotted with various antibodies as described previously.21 To determine the effects of R115777 on protein farnesylation, the membranes were probed with an antibody (MS-225; Neomarkers, Fremont, CA) against HDJ-2, an exclusively farnesylated protein. Prenylated proteins migrate faster than unprenylated proteins in SDS-PAGE,17 and, therefore, the effects of R115777 treatment on protein prenylation can be determined by band shift. To determine the effects of R115777 on oncogenic and tumor survival pathways, the following antibodies were used: anti-phospho-Tyr705 STAT3 (913L; Cell Signaling, Beverly, MA), anti-STAT3 (SC-483; Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-Ser473-Akt (927L; Cell Signaling), anti-Akt (SC-1619; Santa Cruz Biotechnology), anti-phospho-Thr202/Tyr204-Erk1/2 (9101L; Cell Signaling), and anti-Erk1/2 (9102; Cell Signaling).
Determination of serum cytokine levels
Serum was assayed for the cytokines tumor necrosis factor-alpha (TNF-α), endostatin, interleukin-10 (IL-10), and IL-6. Commercially available ELISA kits were used. The kits were purchased from R&D Systems (Minneapolis, MN) (for IL-6 and TNF-α), Endogen (Woburn, MA) (for IL-10), and CytImmune Sciences (College Park, MD) (for endostatin). Lower limits of sensitivity were 4.4 pg/mL for TNF-α, 1.95 ng/mL for endostatin, 3 pg/mL for IL-10, and 0.7 pg/mL for IL-6. The appropriate recombinant cytokine was used to create a standard curve for each cytokine, and serum level was ascertained using the appropriate standard curve. All values represent the means of 3 sets of duplicate experiments.
Results
Patient characteristics
Twenty-one patients with MDS participated in this phase 1 study. One patient failed to take the drug as directed and was not evaluable for response. All patients were evaluable for toxicity. Only some patients were evaluable for biochemical correlates. Patient demographics and disease characteristics are shown in Table 1. Median age was 66 years (range, 50-83 years), and 71% (15 of 21) of the patients were men. All subtypes of MDS were included, with chronic myelomonocytic leukemia (CMMoL) and refractory anemia with excess blasts (RAEB) the most heavily represented. Median disease duration was 10 months (range, 2-25 months). Results of cytogenetic analyses were normal in 10 patients. The other patients showed a variety of karyotypic abnormalities, as shown in Table 1. In 19% (4 of 21) of the patients, ras mutations were detected (k-ras, 1; n-ras, 3). Fifteen (71%) of 21 patients had received prior treatment: 9 patients had received 1 prior treatment, and 6 patients had received 2 prior treatments. Those treatments are shown in Table 1.
Patient characteristics
Characteristics . | . |
|---|---|
| General | |
| No. patients | 21 |
| Male/female ratio | 15:6 |
| Median age, y (range) | 66 (50-83) |
| Evaluable for toxicity | 21 |
| Evaluable for response | 20 |
| Median disease duration, mo (range) | 10 (2-25) |
| Diagnosis* | |
| RA | 2 |
| RAEB | 7 |
| RAEB-T | 2 |
| CMMoL | 10 |
| IPSS score† | |
| Low | 2 |
| Intermediate-1 | 8 |
| Intermediate-2 | 5 |
| High | 6 |
| ras Mutations | |
| n-ras | 3 |
| k-ras | 1 |
| Cytogenetics | |
| Diploid | 10 |
| Monosomy 7 | 4 |
| −5/5q- | 3 |
| 13q- | 1 |
| Trisomy 8 | 1 |
| Other | 5 |
| Prior therapy | |
| Topotecan | 5 |
| Ara-C | 5 |
| Cytoxan | 2 |
| Thalidomide | 3 |
| EPO | 4 |
| IL-11 | 4 |
| Fludarabine | 2 |
| ATG/CSA | 1 |
| SCF | 1 |
| None | 6 |
Characteristics . | . |
|---|---|
| General | |
| No. patients | 21 |
| Male/female ratio | 15:6 |
| Median age, y (range) | 66 (50-83) |
| Evaluable for toxicity | 21 |
| Evaluable for response | 20 |
| Median disease duration, mo (range) | 10 (2-25) |
| Diagnosis* | |
| RA | 2 |
| RAEB | 7 |
| RAEB-T | 2 |
| CMMoL | 10 |
| IPSS score† | |
| Low | 2 |
| Intermediate-1 | 8 |
| Intermediate-2 | 5 |
| High | 6 |
| ras Mutations | |
| n-ras | 3 |
| k-ras | 1 |
| Cytogenetics | |
| Diploid | 10 |
| Monosomy 7 | 4 |
| −5/5q- | 3 |
| 13q- | 1 |
| Trisomy 8 | 1 |
| Other | 5 |
| Prior therapy | |
| Topotecan | 5 |
| Ara-C | 5 |
| Cytoxan | 2 |
| Thalidomide | 3 |
| EPO | 4 |
| IL-11 | 4 |
| Fludarabine | 2 |
| ATG/CSA | 1 |
| SCF | 1 |
| None | 6 |
Ara-C indicates cytosine arabinoside; ATG/CSA, antithymocyte globulin/cyclosporine; EPO, erythropoietin; IPSS, International Prognosis Scoring System; and SCF, stem cell factor.
Study initiation predated the new World Health Organization (WHO) criteria for MDS classification.31 French-American-British (FAB) criteria were used.32
IPSS was used.33
Dose levels
Six patients received R115777 at a starting dose of 300 mg by mouth twice a day (600 mg/d total), 3 patients received 700 mg/d total dose, 6 patients received 800 mg/d total dose, and 6 patients received 900 mg per day total dose. (Cohorts of patients were expanded from 3 to 6 if a single toxicity of grade 3 or higher occurred at that dose level.) In addition, 2 patients were given escalated doses during their drug courses (to 800 mg/d total daily dose from 600 and 700 mg/d total, respectively), but only after the initial 8-week induction period.
Toxicities/side effects
All 21 patients were evaluable for toxicity. Overall, R115777 was well tolerated (Table 2). The major side effect was myelosuppression (19 of 21 patients). Most side effects were grades 1 and 2 and included rash, nausea, bilirubin concentration increase, constipation, diarrhea, bone pain, creatinine increase, visual changes, and headache. Cumulative, significant myelosuppression developed in 3 patients with repeated courses. This was generally reversible within 2 weeks of the discontinuation of R115777.
Side effects of R115777
Toxicity . | No. patients (%) . | No. with dose-limiting toxicity (%)* . |
|---|---|---|
| Myelosuppression | 19 (91) | 1 (5) |
| Fatigue | 11 (52) | 3 (14) |
| Rash | 7 (33) | 0 |
| Nausea | 7 (33) | 0 |
| Bilirubin increase | 5 (24) | 0 |
| Constipation | 4 (19) | 0 |
| Diarrhea | 3 (14) | 0 |
| Bone pain | 3 (14) | 0 |
| Myalgia | 2 (10) | 1 (5) |
| Creatinine increase | 2 (10) | 0 |
| Visual changes | 2 (10) | 0 |
| Confusion | 1 (5) | 1 (5) |
| Headache | 1 (5) | 0 |
Toxicity . | No. patients (%) . | No. with dose-limiting toxicity (%)* . |
|---|---|---|
| Myelosuppression | 19 (91) | 1 (5) |
| Fatigue | 11 (52) | 3 (14) |
| Rash | 7 (33) | 0 |
| Nausea | 7 (33) | 0 |
| Bilirubin increase | 5 (24) | 0 |
| Constipation | 4 (19) | 0 |
| Diarrhea | 3 (14) | 0 |
| Bone pain | 3 (14) | 0 |
| Myalgia | 2 (10) | 1 (5) |
| Creatinine increase | 2 (10) | 0 |
| Visual changes | 2 (10) | 0 |
| Confusion | 1 (5) | 1 (5) |
| Headache | 1 (5) | 0 |
Twenty-one patients were evaluable for toxicity.
Dose-limiting toxicity was defined as grade 3 or 4 toxicity, except for hematologic toxicity, for which the criterion was grade 4 toxicity lasting more than 28 days after R115777 administration. Dose-limiting toxicity was assessed during the first 8 weeks of therapy.
Maximum tolerated dose
Because myelosuppression is often an integral part of the response in hematologic disorders, grade 3 lowering of blood counts was not considered a dose-limiting toxicity. Grade 4 hematologic toxicity, lasting more than 28 days after the drug was discontinued, was included in the criteria for dose-limiting toxicity.
During the 8-week induction period, at an R115777 dose of 900 mg/d (500 mg in the morning and 400 mg in the evening), 5 of 6 patients required dose reduction. Three patients had grade 3 toxicity (fatigue, 2; confusion, 1). Two patients had persistent grade 2 toxicity (fatigue, 1; headache, 1). Of note, 5 of 6 patients treated at this dosage were elderly (age range, 71-83 years).
At an R115777 dose of 800 mg/d (400 mg po bid), 0 of 6 patients had grade 3 nonhematologic toxicity or grade 4 hematologic toxicity, meeting the criteria for dose-limiting toxicity. One patient's dose was reduced because of grade 2 rash. At a lower dose (300 mg/po, bid), a grade 2 rash also developed in another patient, and this patient requested dose reduction. (The rash did not recur at the lower dose.) Therefore, the MTD for R115777 was 800 mg/d (400 mg po bid).
Responses
Six of 20 evaluable patients showed responses (Table 3). Examples of responses are shown in Figures 1 and 2. Thirteen patients showed no response, and 1 patient had progressive disease. Although 10 patients received G-CSF at some point during the trial (mainly for severe neutropenia with a history of infection), ANC responses were not assessed until 7 or more days after G-CSF was discontinued. Responses (HI, 3; PR, 2; CR, 1) occurred at all dose levels (range, 300-900 mg/d). Reduced dose levels of 300 mg/d were given to patients who experienced toxicity according to the criteria previously described. For instance, 1 patient started treatment at 600 mg/d dose, but the dose was reduced to 300 mg/d on day 15; response occurred at this dose level and lasted for 16 months (Figure 1). Only 2 of the responders had ras mutations. Responses lasted 2, 3, 6, 9+, 16, and 16+ months. Two response sequences were noted. Some patients had increases in platelet counts without intervening aplasia (Figure 1); others demonstrated an initial myelosuppressive effect, albeit to a much lesser extent than seen after induction chemotherapy with cytosine-arabinoside-based regimens (Figure 2). Occasionally, patients with early increases in platelet counts showed cumulative myelosuppression after repeated dosing. Finally, 1 patient with proliferative CMMoL showed an early rapid increase in WBC count, probably because of the discontinuation of hydroxyurea. Leukapheresis was required to keep the patient in the study. By the third week, the WBC count decreased, leukapheresis was stopped, and the patient attained PR that lasted 6 months.
Response to R115777 in MDS patients
Patient . | Diagnosis . | Response . | Duration, mo . | Total daily dose, mg . | ras Mutation . |
|---|---|---|---|---|---|
| 2 | RAEB | HI | 16 | 300* | No |
| 3 | CMMoL | HI | 2 | 600 | Yes (k-ras) |
| 6 | CMMoL | PR | 6 | 600 | Yes (n-ras) |
| 12 | CMMoL | PR | 16+ | 800 | No |
| 16 | RAEB | HI | 3 | 900 | No |
| 21 | RAEB-T | CR | 9+ | 800 | No |
Patient . | Diagnosis . | Response . | Duration, mo . | Total daily dose, mg . | ras Mutation . |
|---|---|---|---|---|---|
| 2 | RAEB | HI | 16 | 300* | No |
| 3 | CMMoL | HI | 2 | 600 | Yes (k-ras) |
| 6 | CMMoL | PR | 6 | 600 | Yes (n-ras) |
| 12 | CMMoL | PR | 16+ | 800 | No |
| 16 | RAEB | HI | 3 | 900 | No |
| 21 | RAEB-T | CR | 9+ | 800 | No |
Time of initiation of the protocol predated the publication of the international response criteria.34 However, reanalysis of the data according to those criteria does not change the response designation.
Patient started at a total daily dose of 600 mg/d, but dose was reduced after 2 weeks.
Hematologic improvement (diagnosis, RAEB). Platelet response is shown. Arrows at the bottom show courses of R115777. Total daily dose is shown below the graph. BM indicates bone marrow; Plt txn, platelet transfusion.
Hematologic improvement (diagnosis, RAEB). Platelet response is shown. Arrows at the bottom show courses of R115777. Total daily dose is shown below the graph. BM indicates bone marrow; Plt txn, platelet transfusion.
Hematologic response (complete remission) in patient 21 (diagnosis, RAEB-T). Absolute neutrophil count, platelet count, and hemoglobin level are shown. Arrows at the bottom show courses of R115777. Total daily dose is shown below the graph. Hb indicates hemoglobin; plt, platelets.
Hematologic response (complete remission) in patient 21 (diagnosis, RAEB-T). Absolute neutrophil count, platelet count, and hemoglobin level are shown. Arrows at the bottom show courses of R115777. Total daily dose is shown below the graph. Hb indicates hemoglobin; plt, platelets.
Laboratory correlative studies
R115777 treatment inhibited FTase but not GGTase I enzymatic activity in PBMCs from patients with MDS. Of the 21 patients enrolled in the study, FTase activity levels were assessed in 17 patients. In all patients evaluated, 24-hour R115777 treatment significantly inhibited FTase activity. No dose-response effect was discernible at the doses used in this study. In 12 of 17 patients evaluated, FTase enzymatic activity was maximally inhibited by more than 75% on treatment with R115777. For the other 5 patients, maximal inhibition rates of FTase were 60%, 60%, 48%, 45%, and 22%. Figure 3A shows FTase activity levels at baseline and several days after the initiation of R115777 treatment. The data show that, in some patients, clinical response did not occur despite the substantial inhibition of FTase enzymatic activity. For example, FTase activity was inhibited by more than 80% in patients who did not respond (patients 9 and 14) and in those who responded (patient 12, PR; patient 3, HI). In 2 patients, partial inhibition of FTase was maintained during the rest period (not shown). In addition, in 1 patient tested as far out as the seventh cycle, FTase inhibition was maintained (data not shown).
R115777 treatment results in the inhibition of FTase but not GGTase I enzymatic activity in PBMCs from patients with MDS. Blood was collected from patients with MDS before R115777 treatment (baseline) and at various days after the initiation of treatment. PBMCs were then prepared and processed for FTase (A) or GGTase I (B) activity assays, as described in “Patients, materials and methods.” FTase and GGTase I activities are reported as percentages relative to control. Baseline counts per minute were 2100 to 5500 per 20 μg for FTase and 370 to 1100 for GGTase I. NR indicates no response.
R115777 treatment results in the inhibition of FTase but not GGTase I enzymatic activity in PBMCs from patients with MDS. Blood was collected from patients with MDS before R115777 treatment (baseline) and at various days after the initiation of treatment. PBMCs were then prepared and processed for FTase (A) or GGTase I (B) activity assays, as described in “Patients, materials and methods.” FTase and GGTase I activities are reported as percentages relative to control. Baseline counts per minute were 2100 to 5500 per 20 μg for FTase and 370 to 1100 for GGTase I. NR indicates no response.
To determine whether R115777 was selective for FTase over GGTase I, we analyzed GGTase I activity in PBMCs from 9 patients. R115777 treatment increased GGTase I activity in 4 patients, had no significant effect in 4 patients, and partially inhibited GGTase I activity in 1 patient (Figure 3B).
R115777 treatment inhibited HDJ-2 farnesylation in PBMCs of patients with MDS. We next evaluated the ability of R115777 to inhibit the farnesylation of HDJ-2, a protein that is farnesylated and easily detectable and hence is a good surrogate biochemical marker for FTase activity. To this end, PBMCs from 10 patients were processed for SDS-PAGE immunoblotting with anti-HDJ-2 antibody, as described in “Patients, materials and methods.” Although HDJ-2 farnesylation was inhibited on R115777 treatment in all patients analyzed, the degree of inhibition varied (Figure 4). There is no direct correlation between degree of inhibition of HDJ-2 farnesylation by R115777 and clinical response. For example, patient 21, who had weak inhibition, achieved CR, whereas patient 18, who had strong inhibition, showed no response.
R115777 inhibits HDJ-2 farnesylation in PBMCs from patients with MDS. PBMCs were processed for SDS-PAGE Western immunoblotting with anti-HDJ-2 antibody, as described in “Patients, materials and methods.” BL, D6, D7, D8, and D9 indicate baseline pretreatment levels and days 6, 7, 8, and 9 after initiation of R115777 treatment, respectively. U indicates unprocessed HDJ-2; P, processed HDJ-2.
R115777 inhibits HDJ-2 farnesylation in PBMCs from patients with MDS. PBMCs were processed for SDS-PAGE Western immunoblotting with anti-HDJ-2 antibody, as described in “Patients, materials and methods.” BL, D6, D7, D8, and D9 indicate baseline pretreatment levels and days 6, 7, 8, and 9 after initiation of R115777 treatment, respectively. U indicates unprocessed HDJ-2; P, processed HDJ-2.
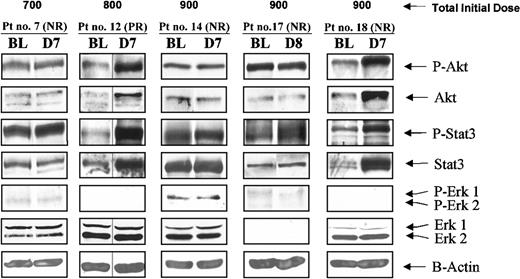
R115777 treatment has differential effects on oncogenic and tumor survival pathways. The observations described indicate that inhibiting farnesylation is insufficient for responses in many patients, suggesting that the oncogenic and tumor survival pathways that drive the MDS malignant cells are dependent on protein farnesylation in only some patients. Previously, we showed in cultured human cancer cells that FTI-induced apoptosis depends on the ability of these agents to suppress the phosphatidylinositol 3 kinase (PI3K)/Akt pathway.35 We, therefore, determined the ability of R115777 to modulate PI3K/Akt phosphorylation and other signaling pathways. To this end, we processed PBMCs for SDS-PAGE immunoblotting with antibodies against total and phosphorylated forms of Akt, Erk1/2, STAT3, and β-actin (see “Patients, materials and methods”). Although attempts were made to analyze the effects of R115777 on these pathways in all patients, quantity or quality of the processed samples allowed us to report on only 5 patients, probably because many had very low blood counts. The results showed that all 3 PBMC pathways were not activated in many patients. In addition, R115777 treatment affected these pathways differentially. In patients 7, 14, and 17, R115777 treatment did not affect phospho-Akt or phospho-STAT3 levels (Figure 5). Phospho-Erk1/2 levels were low to undetectable. Figure 5 also shows that in patients 12 and 18, R115777 treatment resulted in an increase in phospho-Akt, total Akt, phospho-STAT3, and total STAT3 but did not alter total Erk1/2 and β-actin levels. The increase in phosphorylated Akt and STAT3 was proportional to and, therefore, probably the result of the increase in total Akt and STAT3. As with FTase activity and HDJ-2 farnesylation, there was no obvious correlation between R115777 clinical activity and its ability to modulate these pathways.
Effects of R115777 on the levels of P-Akt/Akt, P-STAT3/STAT3, P-Erk1/2/Erk1/2, and β-actin in PBMCs from patients with MDS. PBMCs were processed for Western immunoblotting and probed with antibodies against phosphorylated and total Akt, STAT3, and Erk1/2, as described in “Patients, materials and methods.” β-Actin was used as loading control. BL, D7, and D8 indicate baseline and days 7 and 8 after R115777 treatment initiation.
Effects of R115777 on the levels of P-Akt/Akt, P-STAT3/STAT3, P-Erk1/2/Erk1/2, and β-actin in PBMCs from patients with MDS. PBMCs were processed for Western immunoblotting and probed with antibodies against phosphorylated and total Akt, STAT3, and Erk1/2, as described in “Patients, materials and methods.” β-Actin was used as loading control. BL, D7, and D8 indicate baseline and days 7 and 8 after R115777 treatment initiation.
R115777 and cytokine levels
Serum cytokine levels were assessed in 19 patients (13 nonresponders, 6 responders). Cytokines chosen for measurement (TNF-α, endostatin, IL-6, and IL-10) were those that we had previously found to be elevated in MDS and acute myelogenous leukemia (AML) and to correlate with a poor outcome (R.K., manuscript in preparation). There was no significant difference in baseline TNF-α levels in nonresponders (median, 4.6 pg/mL; range, undetectable to 19.5 pg/mL) compared with responders (median, 8.3 pg/mL; range, undetectable to 15.6 pg/mL) (P = .2). There was a trend toward a greater decrease (by day 7) of TNF-α levels in responders (P = .09), but this did not reach statistical significance. Levels in nonresponders increased by a median of 10%; levels in responders decreased by a median of 42%. Median baseline endostatin levels in responders and nonresponders were not significantly different (67 ng/mL vs 74 ng/mL), nor was the percentage change by day 7 in these 2 groups different (-7% vs +8%) (P = .3). Similarly, neither baseline nor posttherapy levels of IL-6 or IL-10 correlated with responses.
Discussion
Major milestones have been achieved in the development of FTIs for clinical use.1,36,37 In vitro and animal model studies suggest that these molecules effectively inhibit the growth of murine and human tumors and induce tumor regression in oncomouse models.1,21,23,38-42 In addition, it appears that the growth of ras-transformed cells is more sensitive to FTIs than normal parental cells,42,43 a result consistent with the lack of toxicity in animals. These results have prompted several early-phase human studies of FTIs used alone or in combination with traditional cytotoxic chemotherapy.4,44-50
Myelodysplastic syndrome refers to a group of interrelated “preleukemic” disorders characterized by profound cytopenias and increasing risk for transformation to AML.51 Patients succumb to infection or bleeding, usually within 6 months to 2 years, or to MDS progression to AML, which is often therapy resistant.52 Novel therapeutic approaches are needed for this disorder.51,52 ras mutations (generally involving n-ras) occur in approximately 25% of patients with MDS or AML.2,12,13,15,16 The published literature may underestimate the frequency of ras mutations because some series did not test all 3 ras genes. Even in the absence of ras mutations, alternative mechanisms of Ras activation may be operative.53,54
This phase 1 study is the first clinical trial of the FTase inhibitor R115777 for the treatment of MDS. Thirty percent of the patients with MDS in this study responded (HI, 3; PR, 2; CR, 1) to R115777. Responses occurred in 3 of 10 patients with CMMoL, 1 of 2 patients with refractory anemia with excess blasts in transformation (RAEB-T), and 2 of 7 patients with RAEB. Responses were observed at total doses ranging from 300 to 900 mg daily. (Although the starting dose was 600 mg po daily, 1 patient's dose was reduced to 300 mg/d because of side effects soon after starting (on day 15). This patient then had a 16-month response to this low dose). Only 2 responders had ras mutations (k-ras, 1; n-ras, 1). These observations were similar to those noted in an earlier trial of R115777 in relapsed/refractory AML.28 In that trial, 29% of patients responded, and, as in the current study, there was no correlation between response and dose level or the presence of ras mutations.
Two response patterns were noted in our patients with MDS. The first pattern was myelosuppression followed by blood count recovery/response. Peripheral blood myelosuppression, however, was generally mild compared with what might be expected with induction chemotherapy, and patients were followed up as outpatients. For instance, patient 21 was an 80-year-old woman with RAEB-T whose blood counts decreased during the first 3 weeks of therapy, followed by CR (Figure 2). Remission induction was accomplished on an outpatient basis without serious toxicity. The second response pattern was distinct from that after chemotherapy. Patients showed increased blood counts, usually platelets, soon after starting R115777 therapy. In some patients, platelet responses continued with ongoing therapy (Figure 1); in others, initial platelet responses were followed by decreases in blood counts, suggesting cumulative myelosuppression with prolonged dosing. Maximal response was noted in some patients after the first course (eg, patient 21, who achieved CR after 4 weeks; Figure 2). In other patients, maximal response was not evident until multiple cycles of therapy were given (patient 2; Figure 1). Responses were short-lived in 2 patients, lasted 6 and 16 months in 2 others, and are ongoing at 9 and 16 months in 2 patients.
R115777 toxicity was dose related and consisted primarily of transient myelosuppression. Dose-limiting toxicity occurred at total doses of 900 mg/d, and the MTD was 400 mg by mouth twice daily (3 weeks on/1 week off). Dose-limiting side effects consisted of fatigue, myalgia, and confusion. In an earlier study of this molecule in relapsed/refractory acute leukemia,28 dose-limiting toxicity (primarily neurotoxicity) was seen at doses of 1200 mg by mouth twice daily (total, 2400 mg/d). The reason the MTD in our study was lower than that in the AML trial may relate to the advanced age of many patients with MDS in our study or to unknown biologic factors.
Regardless of dose level, R115777 inhibited FTase enzymatic activity and HDJ-2 farnesylation in PBMCs in all patients evaluated but 1 (Figures 3, 4). This inhibition was selective; in 8 of 9 patients evaluated, R115777 either increased GGTase I activity or had no effect. Furthermore, FTase inhibition occurred within 24 hours of initiation of R115777 treatment. It was important to demonstrate the ability of R115777 to reach and suppress its biochemical target, FTase. However, suppression of farnesylation was not sufficient for clinical response in all patients, indicating that farnesylated HDJ-2 proteins are not required for tumor survival in some patients with MDS.
We also evaluated the ability of R115777 to affect 3 well-characterized oncogenic and tumor survival pathways—PI3K/Akt, Janus kinase (JAK)/STAT3, and mitogen-activated protein kinase kinase (MEK)/Erk. We found R115777 to have variable effects on the levels of phosphorylated Akt, STAT3, and Erk1/2, but there was no obvious correlation between these effects and R115777 clinical activity. Surprisingly, after treatment, 2 patients showed increased levels of steady state and phosphorylated STAT3 and Akt, including a patient who was a responder (Figure 5). The small number of patients assessed makes these results preliminary; they require confirmation by other investigators. Finally, we have previously shown that elevated serum levels of several cytokines (TNF-α, endostatin, IL-6, IL-10) are found in patients with MDS and AML and that some of these (in particular TNF-α and endostatin) correlate with poor event-free and overall survival.55 We, therefore, evaluated the baseline and posttherapy (day 7) levels of these cytokines in our patients. There was no difference in baseline serum levels in responders compared with nonresponders. However, a decrease in TNF-α levels by day 7 showed a trend, albeit without reaching statistical significance, toward correlation with response (P = .09). Changes in other cytokine levels were not significant. The small number of patients, their heterogeneity within the MDS diagnostic framework, and the different doses administered preclude a definitive analysis of the impact of these laboratory correlates.
We conclude that, in the phase 1 setting, R115777 shows antitumor activity in MDS at doses that are well tolerated, even in elderly patients. Substantial inhibition of the FTase target is consistently observed and suggests that FTase-independent pathways drive the disease in nonresponders. The presence of RAS mutations does not correlate with response, and responses are seen at a variety of dose levels. The latter results are similar to those previously reported in AML.28 Because Ras can be activated indirectly, even in the absence of mutation, the lack of correlation between response and the presence of ras mutations does not, in of itself, rule out a role for Ras. On the other hand, the observations that the important downstream effector, Erk1/2, is not phosphorylated in most patients and that R115777 can induce responses even without a decrease in phosphorylation of other downstream effectors, such as Akt or STAT3, suggest that R115777 responses are mediated by the interruption of other FTase-sensitive pathways, at least in some patients. The MTD in this trial was 400 mg by mouth twice daily. This dose is well tolerated and may, therefore, be appropriate for phase 2 trials. However, it may be reasonable and perhaps preferable to use lower doses (300 mg po bid) because they consistently inhibit FTase activity and can result in response. Additional trials are needed to determine dosing regimens that maximize clinical responses and minimize myelosuppression, perhaps by intermittent administration, a lower starting dose, or both. In addition, larger phase 2 clinical/correlative studies are warranted to better determine response rates, and combining R115777 with other agents, such as low-dose cytosine arabinoside or azacitidine, merits consideration.56,57 Finally, evaluating biologic correlates of response and hence identification, perhaps by microarray analysis, of subsets of patients more likely to respond should be an important objective of future investigations.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2002-11-3359.
Supported by National Cancer Institute grant 1 R21 CA91518-1 and in part by grant 2057-01 from the Leukemia and Lymphoma Society. J.E.C. is a Clinical Research Scholar for the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal