Abstract
Allogeneic hematopoietic stem cell transplantation (AHSCT) leads to a prolonged state of immunodeficiency characterized by low peripheral naive T-cell counts. To identify the mechanisms leading to this defect we quantitatively and qualitatively analyzed thymic function through quantification of T-cell receptor excision circle (TREC) frequencies (both the signal-joint TREC [sjTREC] and 6 different DβJβ TRECs, by-products of T-cell receptor [TCR] α and β gene rearrangement, respectively), in conjunction with immunophenotype and spectratype analyses in a cohort of patients sampled from 1 to 10 years following AHSCT. In this cohort, reduced thymic function was associated only with ongoing clinical chronic graft-versus-host disease (cGVHD). Nonetheless, the diversity of thymic production remained unchanged irrespective of the patient's cGVHD status. Interestingly, increased homeostatic proliferation was found in the naive T-cell compartment of cGVHD- patients who underwent transplantation. However, reduced expression of both the interleukin-7 receptor α (IL-7Rα) (CD127) chain and the antiapoptotic protein Bcl-2 was observed. Taken together, these data indicate that the inability to reconstitute the naive T-cell compartment for several years after AHSCT, in the absence of cGVHD, is a consequence of impaired naive T-cell survival rather than thymic dysfunction. (Blood. 2003;102:4600-4607)
Introduction
Allogeneic hematopoietic stem cell transplantation (AHSCT) is often the only available treatment for several hematologic disorders such as severe aplastic anemia (SAA), acute lymphocytic/myelogenous leukemia (ALL, AML), chronic myelogenous leukemia (CML), and multiple myeloma (MM).1,2 The transplantation procedure/conditioning regimen generally leads to a profound and long-lasting state of immunodeficiency characterized by persisting low levels of naive T cells.3 Pioneer studies aimed at better understanding the mechanisms responsible for the regeneration of the T-cell compartment demonstrated that T cells can be generated through both thymus-dependent and thymus-independent pathways.4-9 However, adequate broadening of the naive T-cell receptor (TCR) repertoire is ensured by the thymus.10,11 Nowadays, the magnitude of thymic function is best determined through the peripheral blood quantification of T-cell receptor excision circles (TRECs), by-products of TCR gene rearrangement events that occur during thymocyte ontogeny.12 TREC molecules are extrachromosomal circular DNA generated during V(D)J recombination that do not replicate themselves during mitosis and are diluted-out upon cellular proliferation. As a consequence of this behavior, peripheral blood signal-joint TREC (sjTREC) frequencies are considered surrogate markers for thymic function. Furthermore, peripheral blood quantification of the TREC molecules generated during T-cell receptor beta-chain variable region chain rearrangements (ie, distinct Dβ-Jβ or Vβ-DβJβ TRECs13 ) illustrates the assortment of the different gene rearrangement events that occurred in the thymus, thereby providing a direct assessment of the diversity of recent thymic emigrants (RTEs).
Graft-versus-host disease (GVHD) remains the most common complication of AHSCT. Patients are often left with severe lymphoid atrophy and inadequate immune responses to both recall and neoantigens.14-17 One of the important hallmarks of this disease is the profound defect in the peripheral T-cell repertoire, which was shown to be strongly skewed following AHSCT and particularly during chronic GVHD (cGVHD) episodes.18,19 Thymic output has been extensively studied following allogeneic/syngeneic bone marrow transplantation through sjTREC peripheral blood frequency quantification, particularly during the initial period of immune reconstitution.20-23 However, the diversity of recent thymic emigrants in patients who underwent transplantation, as well as its influence in the long-lasting perturbations of the peripheral T-cell compartment, remain to be assessed.
Naive T-cell frequencies are usually reduced for long periods of time following AHSCT. Such a reduction can be the consequence of an impaired thymic function but can also reflect modifications of the peripheral naive T-cell homeostasis. Previous studies demonstrated reduced levels of thymic activity in the early period following AHSCT as well as during acute and cGVHD episodes. In this study, we analyzed thymic function and naive T-cell homeostasis in a cohort of patients who underwent transplantation more than a year before being sampled. Determination of peripheral blood TREC frequencies indicates that the adult thymus remains active long after HLA-matched AHSCT, while cGVHD episodes decrease the magnitude of thymocyte emigration. The peripheral RTE TCR repertoire, defined by peripheral blood Dβ1Jβ1.1 to Dβ1Jβ1.6 TRECs representation, was found to be similar among all study groups (even during cGVHD episodes, when thymic output is reduced). We also demonstrated that patients who underwent transplantation present a permanent defect in postthymic naive T-cell survival despite functional thymopoiesis and increased peripheral naive T-cell proliferation. Interestingly, cGVHD- patients show, for several years after transplantation, a significant reduction in the expression of both interleukin-7 receptor α (IL-7Rα) chain and Bcl-2 on the surface of naive T cells. These findings document for the first time a direct mechanism that may account for the long-lasting reduction of naive T-cell counts observed following AHSCT.
Patients and methods
Patients and control subjects
Blood samples were obtained from 38 healthy volunteers and 33 AHSCT recipients. AHSCT recipients had undergone transplantation 1 to 10 years before study for the following conditions: SAA (n = 1), ALL (n = 3), AML (n = 10), CML (n = 14), myelodysplastic syndrome (n = 1), non-Hodgkin lymphoma (n = 2), or MM (n = 2). The AHSCT myeloablative regimen for patients with hematologic malignancies consisted of cyclophosphamide (120 mg/kg) and total body irradiation (12 Gy), or busulfan (16 mg/kg) and cyclophosphamide (200 mg/kg).24,25 The patient with SAA received 200 mg/kg cyclophosphamide. Transplanted cells were obtained from the bone marrow or the peripheral blood of an HLA genotypically identical sibling. No specific procedure was carried out to enrich or deplete for a specific cell population. Diagnosis of acute and cGVHD was based on standard criteria, and treatment consisted of corticosteroids with cyclosporine and/or mycophenolate mofetil and/or tacrolimus.26,27 Among the patient group, 10 were experiencing cGVHD at sampling time (cGVHD+). The 23 remaining patients were not suffering from cGVHD at sampling time (GVHD-); they either never suffered from cGVHD or were devoid of cGVHD clinical symptoms for at least 8 months. No significant age difference was noted between the 3 groups of individuals (mean age for control group: 46.9 years [range, 23-78 years], cGVHD-: 42.5 years [range, 25-60 years], cGVHD+: 42.7 years [range, 21-57 years]; P ≥ .17 for all 3 comparisons). Clinical protocols were approved by the Human Subjects Protection Committee of the Maisonneuve-Rosemont Hospital. Samples were obtained with the informed consent of the patients.
Quantitative and qualitative analysis of thymic function: sjTREC and DβJβ TREC quantification
Primers specific for each of the sjTREC (δRec-ψJα), the 6 Dβ1-Jβ1 deletion circles (DβJβ TRECs), and a fragment of the human CD3γ chain gene were defined on the human germ-line sequence (GenBank accession numbers AE00061, U66061, and X06026, respectively) (Table 1). The amplicons for the DβJβ TRECs and the sjTREC, defined by the 5′/3′ out primers, amplified from human thymocytes, were individually cloned into Bluescript vector (Stratagene, La Jolla, CA) together with the CD3γ amplification product (amplified from human chromosomal DNA using CD3γ out 3′/5′ primer pair). These plasmids, amplified in the same run as the experimental samples, were used to generate standard curves for real-time quantitative polymerase chain reaction (PCR)-based assay. Parallel quantification of each deletion circle together with the CD3γ amplicon was performed for each sample using LightCycler technology (Roche Diagnostics, Basel, Switzerland). This protocol allows us to precisely measure the input DNA for each quantification, thus providing an absolute number of TRECs per 105 cells. Cells (approximately 2 × 105 peripheral blood mononuclear cells [PBMCs]) were lysed in Tween-20 (0.05%), nonidet P-40 (NP-40, 0.05%), and Proteinase K (100 μg/mL) for 30 minutes at 56°C and then 15 minutes at 98°C. Multiplex PCR amplification was performed for sjTREC or each of the 6 DβJβ TRECs, together with the CD3γ chain, in 100 μL (10 minutes initial denaturation at 95°C, 30 seconds at 95°C, 30 seconds at 60°C, 2 minutes at 72°C for 22 cycles) using the “outer” 3′/5′ primer pairs (Table 1). The linearity of this first-round multiplex assay was demonstrated in triplicate experiments up to 22 cycles when using a maximum of 2 × 105 PBMCs (data not shown). These PCR conditions were used for all subsequent experiments. Following the first round of amplification, the PCR products were diluted 10-fold prior to online, real-time amplification using LightCycler technology. PCR conditions in the LightCycler experiments were as follows: 1 minute initial denaturation at 95°C, 1 second at 95°C, 10 seconds at 60°C, 15 seconds at 72°C for 40 cycles; fluorescence measurements were performed at the end of the elongation steps. For each PCR product, the TREC and CD3γ second-round PCR quantifications were performed in separate capillaries and in independent LightCycler experiments but quantified on the same first-round serially diluted standard curve. This highly sensitive nested quantitative PCR assay allows the detection of one copy per PCR reaction for each DNA circle. The results are expressed as absolute number of TRECs per 105 cells. Since only T cells contribute to the peripheral blood sjTREC content, the sjTREC frequencies were normalized to the relative CD3+ T-cell frequency as measured by fluorescence-activated cell sorter (FACS) analysis for each sample. Quantification of sjTREC frequencies was performed in duplicate. Interexperimental variation was always lower than 20% (n = 71).
Oligonucleotides and probes for TREC quantification
DNA circles quantification | ||||
| sjTREC | 5′ -In: | GCTCTGAAAGGCAGAAAGAGG | ||
| 5′ -Out: | CTCTCCTATCTCTGCTCTGAA | |||
| 3′ -In: | ACATTTGCTCCGTGGTCTGTG | |||
| 3′ -Out: | ACTCACTTTTCCGAGGCTGA | |||
| Dβ1 | 3′ -In: | TGTGACCCAGGAGGAAAGAAG | ||
| 3′ -Out: | CTCATCTGGGCCTGTCCTTGT | |||
| Jβ1S1 | 5′ -In: | CCCTCTCTATGCCTTCAATGT | ||
| 5′ -Out: | GAACCTAGGACCCTGTGGA | |||
| Jβ1S2 | 5′ -In: | CAGATCCGTCACAGGGAAAAGT | ||
| 5′ -Out: | ACAAGGCACCAGACTCACAGTT | |||
| Jβ1S3 | 5′ -In: | TGTCCCTGTGAGGGAAGAGTT | ||
| 5′ -Out: | AAGGGAACACAGAGTACTGGAA | |||
| Jβ1S4 | 5′ -In: | TGGACTTGGGGAGGCAGGA | ||
| 5′ -Out: | GGATCACACGGGGCCTAATT | |||
| Jβ1S5 | 5′ -In: | CTCATAAAATGTGGGTCAGTGGA | ||
| 5′ -Out: | GAAACTGAGAACACAGCCAAGAA | |||
| Jβ1S6 | 5′ -In: | TGAATCCAGGCAGAGAAAGG | ||
| 5′ -Out: | ATCCTCCCTCTTATGTGCATGG | |||
| CD3γ | 5′ -In: | TGGCTGTCCTCATCCTGG | ||
| 5′ -Out: | ACTGACATGGAACAGGGGAAG | |||
| 3′ -In: | CTTGGCCTATGCCCTTTTGG | |||
| 3′ -Out: | CCAGCTCTGAAGTAGGGAACATAT | |||
| LightCycler probes | ||||
| Sj | P1: | AATAAGTTCAGCCCTCCATGTCACACTf | ||
| P2: | (Red! 640) TGTTTTCCATCCTGGGGAGTGTTTCAp | |||
| Dβ1 | P1: | CTGGGAGTTGGGACCGCCAGAGAGGTf | ||
| P2: | (Red! 640) TTTGTAAAGGTTTCCCGTAGAGTTGAATCATTGTp | |||
| CD3γ | P1: | GGCTGAAGGTTAGGGATACCAATATTCCTGTCTCf | ||
| P2: | (Red! 705) CTAGTGATGGGCTCTTCCCTTGAGCCCTTCp | |||
DNA circles quantification | ||||
| sjTREC | 5′ -In: | GCTCTGAAAGGCAGAAAGAGG | ||
| 5′ -Out: | CTCTCCTATCTCTGCTCTGAA | |||
| 3′ -In: | ACATTTGCTCCGTGGTCTGTG | |||
| 3′ -Out: | ACTCACTTTTCCGAGGCTGA | |||
| Dβ1 | 3′ -In: | TGTGACCCAGGAGGAAAGAAG | ||
| 3′ -Out: | CTCATCTGGGCCTGTCCTTGT | |||
| Jβ1S1 | 5′ -In: | CCCTCTCTATGCCTTCAATGT | ||
| 5′ -Out: | GAACCTAGGACCCTGTGGA | |||
| Jβ1S2 | 5′ -In: | CAGATCCGTCACAGGGAAAAGT | ||
| 5′ -Out: | ACAAGGCACCAGACTCACAGTT | |||
| Jβ1S3 | 5′ -In: | TGTCCCTGTGAGGGAAGAGTT | ||
| 5′ -Out: | AAGGGAACACAGAGTACTGGAA | |||
| Jβ1S4 | 5′ -In: | TGGACTTGGGGAGGCAGGA | ||
| 5′ -Out: | GGATCACACGGGGCCTAATT | |||
| Jβ1S5 | 5′ -In: | CTCATAAAATGTGGGTCAGTGGA | ||
| 5′ -Out: | GAAACTGAGAACACAGCCAAGAA | |||
| Jβ1S6 | 5′ -In: | TGAATCCAGGCAGAGAAAGG | ||
| 5′ -Out: | ATCCTCCCTCTTATGTGCATGG | |||
| CD3γ | 5′ -In: | TGGCTGTCCTCATCCTGG | ||
| 5′ -Out: | ACTGACATGGAACAGGGGAAG | |||
| 3′ -In: | CTTGGCCTATGCCCTTTTGG | |||
| 3′ -Out: | CCAGCTCTGAAGTAGGGAACATAT | |||
| LightCycler probes | ||||
| Sj | P1: | AATAAGTTCAGCCCTCCATGTCACACTf | ||
| P2: | (Red! 640) TGTTTTCCATCCTGGGGAGTGTTTCAp | |||
| Dβ1 | P1: | CTGGGAGTTGGGACCGCCAGAGAGGTf | ||
| P2: | (Red! 640) TTTGTAAAGGTTTCCCGTAGAGTTGAATCATTGTp | |||
| CD3γ | P1: | GGCTGAAGGTTAGGGATACCAATATTCCTGTCTCf | ||
| P2: | (Red! 705) CTAGTGATGGGCTCTTCCCTTGAGCCCTTCp | |||
Flow-cytometric analysis
PBMCs cryopreserved in liquid nitrogen were thawed for staining and analysis. Cell viability, as determined by trypan blue exclusion assay, was more than 95%. The following mouse antihuman antibodies were used in different combinations for cell surface staining: anti-CD27 (immunoglobulin G1 [IgG1], L128) coupled to R-Phycoerythrin (R-PE; BD Biosciences, San Jose, CA); anti-CD45RA CyChrome (IgG2a, HI100; BD PharMingen, San Diego, CA); and anti-CD3 (IgG2a, HIT3a; BD PharMingen). For CD127 expression analysis, the following combination of antibodies was used: anti-CD3 allophycocyanin (APC; BD PharMingen), anti-CD45RA CyChrome (BD PharMingen), anti-CD27 fluorescein isothiocyanate (FITC; BD PharMingen), and anti-CD127 PE (Immunotech, Marseille, France). For the Bcl-2 expression analysis, anti-CD3 APC (BD PharMingen), anti-CD45RA CyChrome (BD PharMingen), anti-CD127 PE (Immunotech Marseilles, France), and anti-Bcl-2 FITC (BD PharMingen) were used. Stainings were performed by incubating cells with the appropriate pool of antibodies for 30 minutes at 4°C followed by a series of washes with phosphate-buffered saline solution (PBS) supplemented with 2% fetal calf serum (FCS). For intracellular Ki-67 and Bcl-2 analyses, cells were fixed with FACS lysing solution (BD Biosciences) after surface marker labeling and then permeabilized with FACS Permeabilizing solution (BD Biosciences) as per the manufacturer's instructions prior to intracellular staining with anti-Ki-67 FITC (IgG1, Ki-67; DAKO, Glostrup, Denmark) or anti-Bcl-2 FITC (BD PharMingen). Isotype-matched controls were used for intracellular staining and specificity was ascertained by performing competition assays of the FITC-labeled antibody with the purified Ki-67-specific antibody. A minimum of 3 × 105 events in the live cell gate, as defined by forward and side scatter, was accumulated for each sample. Data were acquired on a FACSCalibur system and analyzed on a 4-log scale using CellQuest Pro software (Becton Dickinson Systems, Heidelberg, Germany).
Statistical analysis
Statistical analysis (2-tailed Student t test, Pearson correlation test, R, and P values) was performed using Microsoft Excel spreadsheets (Seattle, WA). An R value of 0.3 or more, -0.3 or less, and a P value of .05 or less were considered significant. In order to perform 2-tailed Student t test for checkerboard representations, we scored a Gaussian distribution (Figure 2A) and a detected DβJβ TREC family (Figure 3B) with a value of 1. Biased distributions and absence of a DβJβ TREC family were attributed the value of 0.
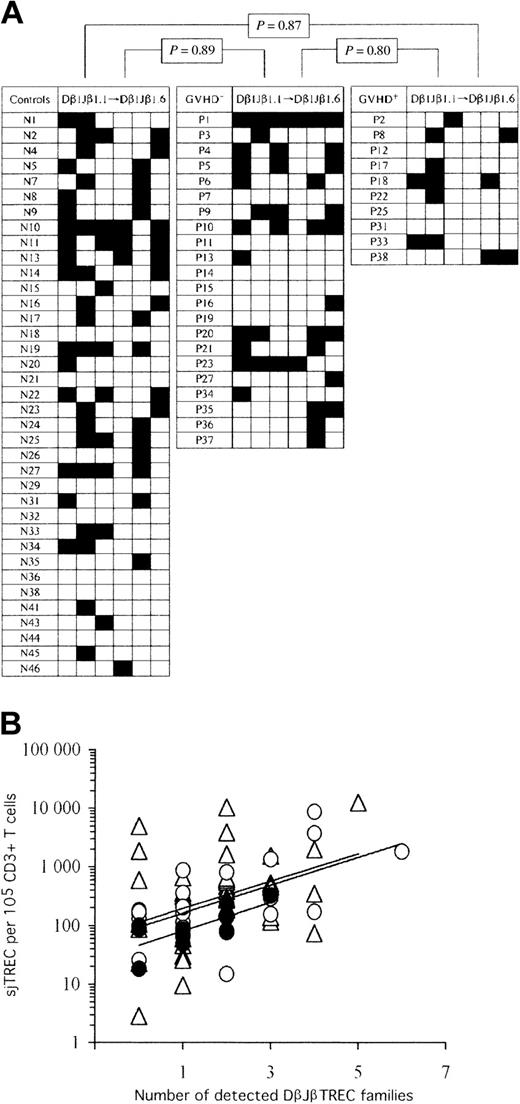
Thymic function remains diversified following AHSCT, despite the occurrence of cGVHD. (A) Checkerboard representation of peripheral blood RTE repertoire diversity in controls (left) and AHSCT patients (GVHD-: middle; cGVHD+: right). TCR Dβ1Jβ1.1 to Jβ1.6 TREC frequencies were enumerated by real-time quantitative PCR on 105 peripheral blood mononuclear cells. Black squares represent detected (≥ 1 per 105 cells) DβJβ TREC families, whereas empty squares correspond to frequencies of less than 1 DβJβ TREC per 105 cells. In order to perform statistical analysis, a binary code was applied to the data set; “1” and “0” represent “detected” and “undetected” DβJβ TRECs, respectively. Statistical significance in the representation of TCR DβJβ TREC families among the 3 experimental groups is shown on top. Similar results were obtained in a duplicate experiment (not shown). (B) Positive association between peripheral sjTREC frequencies and the number of detectable Dβ1Jβ1.1 to Jβ1.6 TREC families. Empty triangles (▵) correspond to healthy control adults, whereas empty (▴) and filled (•) circles represent cGVHD- and cGVHD+ AHSCT patients, respectively. Solid lines (—) indicate linear regression curves for each study group (top: controls; middle: GVHD-; bottom: GVHD+).
Thymic function remains diversified following AHSCT, despite the occurrence of cGVHD. (A) Checkerboard representation of peripheral blood RTE repertoire diversity in controls (left) and AHSCT patients (GVHD-: middle; cGVHD+: right). TCR Dβ1Jβ1.1 to Jβ1.6 TREC frequencies were enumerated by real-time quantitative PCR on 105 peripheral blood mononuclear cells. Black squares represent detected (≥ 1 per 105 cells) DβJβ TREC families, whereas empty squares correspond to frequencies of less than 1 DβJβ TREC per 105 cells. In order to perform statistical analysis, a binary code was applied to the data set; “1” and “0” represent “detected” and “undetected” DβJβ TRECs, respectively. Statistical significance in the representation of TCR DβJβ TREC families among the 3 experimental groups is shown on top. Similar results were obtained in a duplicate experiment (not shown). (B) Positive association between peripheral sjTREC frequencies and the number of detectable Dβ1Jβ1.1 to Jβ1.6 TREC families. Empty triangles (▵) correspond to healthy control adults, whereas empty (▴) and filled (•) circles represent cGVHD- and cGVHD+ AHSCT patients, respectively. Solid lines (—) indicate linear regression curves for each study group (top: controls; middle: GVHD-; bottom: GVHD+).
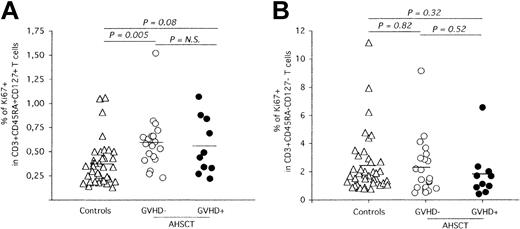
AHSCT induces long-term perturbation of naive T-cell homeostasis. Phenotypic analysis of naive and memory peripheral blood T cells was performed by multiparameter flow cytometry using conjugated antibodies against CD3, CD45RA, and CD27. The frequency of proliferating naive (A) and memory (B) T cells was determined by quantification of Ki-67 nuclear expression. The solid horizontal lines represent the average Ki-67+ frequencies for each group. Statistical significance was calculated by the 2-tailed Student t test and is shown on top of graphics. N.S. indicates not significant.
AHSCT induces long-term perturbation of naive T-cell homeostasis. Phenotypic analysis of naive and memory peripheral blood T cells was performed by multiparameter flow cytometry using conjugated antibodies against CD3, CD45RA, and CD27. The frequency of proliferating naive (A) and memory (B) T cells was determined by quantification of Ki-67 nuclear expression. The solid horizontal lines represent the average Ki-67+ frequencies for each group. Statistical significance was calculated by the 2-tailed Student t test and is shown on top of graphics. N.S. indicates not significant.
Results
The reduction of naive T-cell counts following AHSCT is not a consequence of impaired thymic function
FACS analysis for CD3, CD45RA, and CD27 cell surface expression was performed on all subjects under study (Figure 1A). Naive T-cell frequencies are reduced in cGVHD- AHSCT recipients (% of CD45RA+CD27+ gated on CD3+ = 24.56%, n = 21) compared with healthy controls (32.76%, P = .03, n = 38). Naive T-cell frequencies are also reduced during cGVHD episodes (20.71%, P = .02, n = 10). Reduced naive T-cell frequencies were not associated with time after AHSCT (R = -0.21, P = .27, data not shown).
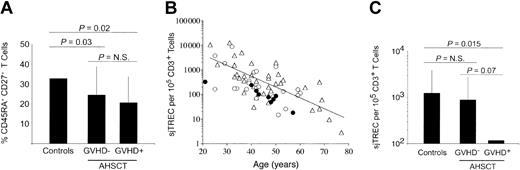
Influence of AHSCT and cGVHD episodes on peripheral blood naive T-cell frequencies and de novo T-cell production. (A) Naive T-cell frequencies, as defined by CD45RA and CD27 dual expression on CD3+ cells, were quantified in PBMCs by multiparameter flow cytometry. A minimum of 3 × 105 events in the live cell gate, as defined by forward and side scatter, was accumulated for each sample. (B) Age-related decrease of sjTREC content normalized for CD3+ T-cell frequencies. Peripheral blood sjTREC frequencies were obtained using real-time quantitative PCR and normalized to CD3+ T-cell frequencies as estimated by FACS analysis. Healthy adult controls (n = 37) are represented with empty triangles (▵), while the solid line (—) indicates the negative linear regression trend from control individuals' data (y = 31 581e-0,1012x). AHSCT patients exempt of cGVHD (n = 22) at sampling time are shown as empty circles (▴), whereas patients who underwent transplantation currently undergoing cGVHD (n = 10) are illustrated with filled circles (•). (C) Histogram representation of average peripheral sjTREC frequencies in these cohorts. Statistical significance (P ≤ .05) was calculated by the 2-tailed Student t test and is shown on top of graphics. The vertical lines indicate the group's standard deviation. N.S. indicates not significant.
Influence of AHSCT and cGVHD episodes on peripheral blood naive T-cell frequencies and de novo T-cell production. (A) Naive T-cell frequencies, as defined by CD45RA and CD27 dual expression on CD3+ cells, were quantified in PBMCs by multiparameter flow cytometry. A minimum of 3 × 105 events in the live cell gate, as defined by forward and side scatter, was accumulated for each sample. (B) Age-related decrease of sjTREC content normalized for CD3+ T-cell frequencies. Peripheral blood sjTREC frequencies were obtained using real-time quantitative PCR and normalized to CD3+ T-cell frequencies as estimated by FACS analysis. Healthy adult controls (n = 37) are represented with empty triangles (▵), while the solid line (—) indicates the negative linear regression trend from control individuals' data (y = 31 581e-0,1012x). AHSCT patients exempt of cGVHD (n = 22) at sampling time are shown as empty circles (▴), whereas patients who underwent transplantation currently undergoing cGVHD (n = 10) are illustrated with filled circles (•). (C) Histogram representation of average peripheral sjTREC frequencies in these cohorts. Statistical significance (P ≤ .05) was calculated by the 2-tailed Student t test and is shown on top of graphics. The vertical lines indicate the group's standard deviation. N.S. indicates not significant.
Quantification of sjTREC was performed by real-time, online PCR on PBMCs of patients following AHSCT (GVHD-: n = 22 and cGVHD+: n = 10) and compared with control individuals (n = 37). For each individual, the sjTREC content was normalized to CD3+ T-cell counts (Figure 1B-C). Peripheral sjTREC frequencies per 105 CD3+ T cells average 1255 [range, 3-12 521], 897 [range, 15-8738], and 119 [range, 18-328] in controls, cGVHD-, and cGVHD+ patients, respectively. While cGVHD- patients exhibit only a 1.4-fold decrease in sjTREC frequencies (P > .56), a highly significant decrease (10.5-fold, P = .015) is observed in patients experiencing cGVHD, demonstrating the quantitative impact of cGVHD on thymic function.
A positive correlation was observed for both cGVHD- and the control groups when comparing sjTREC frequencies with the relative frequency of CD45RA+ CD27+ circulating T cells (Pearson R = 0.42, P = .002 for control group and R = 0.43, P = .011 in the patient group). This correlation was not seen in the cGVHD+ group.
Within the cGVHD- group, 15 patients experienced past episodes of cGVHD (more than 8 months before sampling; “Patients and methods”), while the remaining 8 were exempt of clinical symptoms since AHSCT. No difference in the peripheral sjTREC content was observed between these 2 groups (GVHD-: 526/105 CD3+ T cells, past cGVHD: 1109/105 CD3+ T cells, P > .40), demonstrating the reversibility of the impact cGVHD has on thymopoiesis. The strong reduction of sjTREC frequency observed in peripheral blood during cGVHD episodes indicates that this immunopathology can lead to a temporary dysfunction of the thymus in sustaining T-cell maturation.
The thymus of AHSCT patients produces a diversified population of RTEs
The thymus is one of the principal targets for autoimmune reactions during GVHD.28 One can expect some of its normal physiologic processes (ie, positive and negative selection) to be modified by cGVHD, leading to biased thymocyte exportation. Moreover, as most patients who underwent transplantation develop subclinical or clinical cGVHD, thymopoiesis could be affected even in the absence of clinical cGVHD. Having demonstrated that RTE concentration in peripheral blood is significantly reduced only during cGVHD episodes, we assessed the diversity of the exported RTEs in both groups of patients compared with control individuals (Table 2; Figure 2). DβJβ TRECs are generated during TCRβ chain rearrangement; thus, their peripheral blood frequencies reflect the extent of diversity in TCR DβJβ gene segment use among RTEs. Accordingly, we quantified Dβ1Jβ1.1 to Dβ1Jβ1.6 TRECs in AHSCT patients' peripheral blood lymphocytes. Representative examples of these quantifications are shown in Table 2. A biased thymic output would lead to either an over- and/or an underrepresentation of certain DβJβ TREC families in periphery, which in turn would alter the expressed peripheral repertoire. Accordingly, 2 different analyses of the DβJβ TREC results were performed on the 3 groups of individuals in order to evaluate the relative abundance of DβJβ TREC families in the patients' peripheral blood mononuclear cells.
Representative sjTRECs and DβJβ TRECs frequencies in adults who did not undergo transplantation and AHSCT patients
Patients and identification nos. . | . | Months after AHSCT . | sjTRECs per 105 CD3* . | Dβ1Jβ1.1 to Dβ1Jβ1.6 TRECs/105 CD3+ cells† . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Age, y . | . | . | 1.1 . | 1.2 . | 1.3 . | 1.4 . | 1.5 . | 1.6 . | |||||
| Controls who did not undergo transplantation | ||||||||||||||
| 2 | 33 | NA | 1547‡ | — | 4 | 2 | — | — | 3 | |||||
| 4 | 26 | NA | 1631 | — | 4 | — | — | — | 5 | |||||
| 7 | 33 | NA | 3841 | — | 6 | — | — | 5 | — | |||||
| 11 | 42 | NA | 346 | 2 | — | 1 | 3 | — | 9 | |||||
| 13 | 37 | NA | 116 | 4 | — | — | 4 | — | 2 | |||||
| 18 | 65 | NA | 23 | — | — | — | — | — | — | |||||
| 24 | 40 | NA | 596 | — | 16 | — | — | 6 | — | |||||
| cGVHD− AHSCT patients§ | ||||||||||||||
| 3 | 39 | 16 | 356 | — | 7 | — | — | — | — | |||||
| 5 | 50 | 32 | 1348 | 11 | — | 9 | — | — | 28 | |||||
| 6 | 41 | 108 | 814 | 3 | — | — | — | 1 | — | |||||
| 13 | 42 | 48 | 869 | 4 | — | — | — | — | — | |||||
| 20 | 25 | 30 | 3746 | 7 | 10 | — | — | 2 | 30 | |||||
| 35 | 51 | 36 | 15 | — | — | — | — | 7 | 6 | |||||
| 37 | 59 | 36 | 92 | — | — | — | — | 6 | — | |||||
| cGVHD+ AHSCT patients§ | ||||||||||||||
| 8 | 40 | 44 | 240 | — | 2 | — | — | — | 2 | |||||
| 17 | 49 | 20 | 64 | — | 5 | — | — | — | — | |||||
| 18 | 21 | 36 | 328 | 6 | 5 | — | — | 2 | — | |||||
| 22 | 50 | 19 | 85 | — | 3 | — | — | — | — | |||||
| 25 | 57 | 25 | 77 | — | — | — | — | — | — | |||||
| 33 | 42 | 12 | 141 | 2 | 3 | — | — | — | — | |||||
| 38 | 47 | 48 | 87 | — | — | — | — | 1 | 1 | |||||
Patients and identification nos. . | . | Months after AHSCT . | sjTRECs per 105 CD3* . | Dβ1Jβ1.1 to Dβ1Jβ1.6 TRECs/105 CD3+ cells† . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Age, y . | . | . | 1.1 . | 1.2 . | 1.3 . | 1.4 . | 1.5 . | 1.6 . | |||||
| Controls who did not undergo transplantation | ||||||||||||||
| 2 | 33 | NA | 1547‡ | — | 4 | 2 | — | — | 3 | |||||
| 4 | 26 | NA | 1631 | — | 4 | — | — | — | 5 | |||||
| 7 | 33 | NA | 3841 | — | 6 | — | — | 5 | — | |||||
| 11 | 42 | NA | 346 | 2 | — | 1 | 3 | — | 9 | |||||
| 13 | 37 | NA | 116 | 4 | — | — | 4 | — | 2 | |||||
| 18 | 65 | NA | 23 | — | — | — | — | — | — | |||||
| 24 | 40 | NA | 596 | — | 16 | — | — | 6 | — | |||||
| cGVHD− AHSCT patients§ | ||||||||||||||
| 3 | 39 | 16 | 356 | — | 7 | — | — | — | — | |||||
| 5 | 50 | 32 | 1348 | 11 | — | 9 | — | — | 28 | |||||
| 6 | 41 | 108 | 814 | 3 | — | — | — | 1 | — | |||||
| 13 | 42 | 48 | 869 | 4 | — | — | — | — | — | |||||
| 20 | 25 | 30 | 3746 | 7 | 10 | — | — | 2 | 30 | |||||
| 35 | 51 | 36 | 15 | — | — | — | — | 7 | 6 | |||||
| 37 | 59 | 36 | 92 | — | — | — | — | 6 | — | |||||
| cGVHD+ AHSCT patients§ | ||||||||||||||
| 8 | 40 | 44 | 240 | — | 2 | — | — | — | 2 | |||||
| 17 | 49 | 20 | 64 | — | 5 | — | — | — | — | |||||
| 18 | 21 | 36 | 328 | 6 | 5 | — | — | 2 | — | |||||
| 22 | 50 | 19 | 85 | — | 3 | — | — | — | — | |||||
| 25 | 57 | 25 | 77 | — | — | — | — | — | — | |||||
| 33 | 42 | 12 | 141 | 2 | 3 | — | — | — | — | |||||
| 38 | 47 | 48 | 87 | — | — | — | — | 1 | 1 | |||||
NA indicates not applicable; —, less than 1.
sjTREC frequencies were normalized according to CD3+ T-lymphocyte proportions, as determined by flow cytometry.
TREC frequencies were normalized according to CD3+ T-lymphocyte proportions, as determined by flow cytometry. Similar results were obtained in a duplicate experiment.
sjTREC content is shown as the mean of 2 independent quantifications.
AHSCT patients who underwent transplantation were classified according to their cGVHD history/status. cGVHD+ patients (n = 10) were symptomatic at sampling time, whereas cGVHD− patients were exempt of symptoms since engraftment (n = 8) or for at least 8 months prior sampling (n = 14).
In the first analysis, we investigated whether one or more of the DβJβ TREC families were underrepresented in peripheral blood of patients who underwent transplantation as a consequence of a biased thymic output. For each individual, the number of undetected DβJβ TREC families (< 1 copy per 105 cells) among the 6 tested was evaluated, and statistical analysis was performed between the study groups (Figure 2A). The frequency of undetectable DβJβ TREC families was not significantly different among the 3 groups (controls: mean = 4.22, [range, 1-6]; cGVHD-: mean = 4.18 [range, 0-6], and cGVHD+: mean = 4.8 [range, 3-6]; P ≥ .15). In a duplicate experiment, similar means, ranges, and statistics were obtained (data not shown). As the number of detected DβJβ TREC families correlates with sjTREC frequencies (R ≥ 0.31, P < .014 for all 3 groups, Figure 2B), the previous analysis was repeated after normalization for the sjTREC content (DβJβ TREC frequency divided by sjTREC frequency). Again, no significant difference was observed among groups (P ≥ .80 for all comparisons).
The second analysis aimed at evaluating a possible overrepresentation of DβJβ TREC families in peripheral blood that could also result from a biased thymic function in patients who underwent transplantation. The mean frequency and standard deviation (SD) for each DβJβ TREC family, normalized to sjTREC content (to normalize the magnitude of thymic function), were calculated for the healthy control group. Dβ1Jβ1.1 to Dβ1Jβ1.6 TREC frequencies, for the 71 individuals of the study, were compared with these means. A DβJβ TREC frequency was considered overrepresented when its value was 3 SD above the control mean for this particular DβJβ TREC. The frequency of overrepresented DβJβ TRECs was 4.05%, 6.82%, and 0% in controls, cGVHD-, and cGVHD+, respectively, showing that neither AHSCT nor cGVHD episodes lead to overrepresentation of particular DβJβ TRECs.
Thus, the absence of clear significant differences among the 3 groups in both analyses demonstrates that the population of cells produced by the thymus of patients who underwent transplantation is diversified, even during cGVHD episodes, when the overall thymic production is reduced.
Increased nuclear Ki-67 expression within phenotypically defined naive T cells of cGVHD- patients
Apart from thymic export, peripheral naive T-cell counts are regulated through homeostatic proliferation.29-31 Experiments were carried out to define if the observed low levels of naive T cells in patients who underwent transplantation (Figure 1A) could be the result of an impaired capacity of this T-cell compartment to sustain homeostatic proliferation. Accordingly, Ki-67 expression, a nuclear antigen expressed throughout all phases of the cell cycle except G0,32 was quantified in CD3+CD45RA+CD27+ T cells (Figure 3A). Strikingly, naive T cells from AHSCT recipients free of cGVHD clinical symptoms at sampling time demonstrated increased proliferation levels compared with healthy individuals (controls: 0.37%, cGVHD-: 0.59%, P = .005). Moreover, no significant difference was observed in the frequency of Ki-67-expressing naive T cells between both groups of patients who underwent transplantation (GVHD+: 0.56%, P = .75). In contrast, similar proliferation rates were observed in the memory compartment of all groups (controls: 2.42%, cGVHD-: 2.29%, cGVHD+: 1.83%, P ≥ .38), suggesting that the increased naive T-cell proliferation is a response to specifically restore the naive compartment and does not reflect increased levels of general T-cell proliferation, possibly observed during T-cell lymphopenia (Figure 3B).
Thus, the observed reduction of naive T-cell counts in patients who underwent transplantation does not originate from the inability of these cells to sustain homeostatic proliferation. On the contrary, naive T-cell lymphopenia seems to result in a feedback mechanism leading to enhanced peripheral proliferation of these cells.
Reduced expression of IL-7Rα (CD127) and Bcl-2 in naive T cells of GVHD- patients
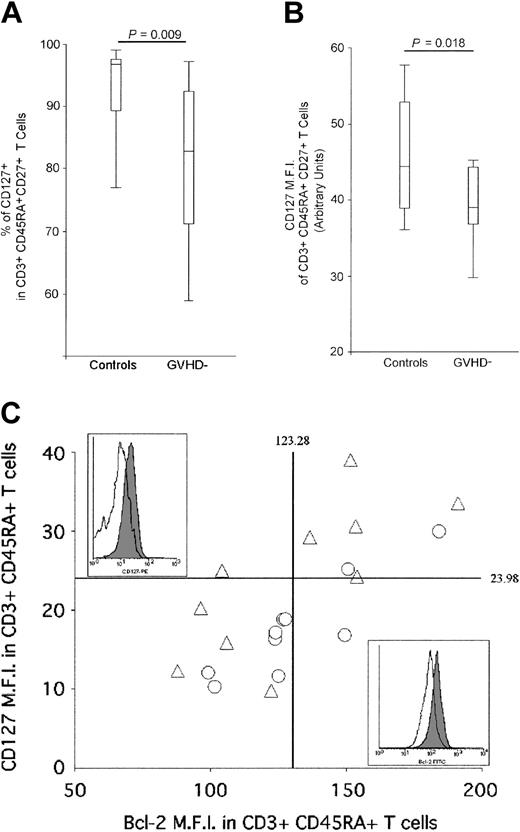
Having demonstrated that neither a reduced thymic function nor an impaired proliferation can account for the reduction of the naive T-cell population, we investigated the third arm of homeostatic regulation: death and survival signals. Naive T-cell survival is regulated through the interaction between interleukin 7 (IL-7) and its receptor (CD127: IL-7Rα), which modulates Bcl-2 expression levels.29,33,34 The proportion of IL-7Rα+ naive T cells (CD45RA+, CD27+) was evaluated by FACS analysis in cGVHD- patients and compared with healthy individuals in order to investigate whether the observed long-lasting naive T-cell deficiency is attributable to a defect in naive T-cell survival. In healthy adults, most CD3+, CD45RA+, CD27+ naive T cells express CD127 (92.61%, [range, 73.28%-99.62%]) (Figure 4A). In cGVHD- patients, the proportion of CD3+, CD45RA+, CD27+ cells expressing CD127 is significantly reduced (80.42%, [range, 51.67%-99.15%], P = .009). Moreover, CD127 mean fluorescence intensity (MFI) within CD127+ naive T cells is significantly lower in the patient group (mean MFI control: 45.44, cGVHD-: 38.98, P = .01) (Figure 4B). In parallel, we measured Bcl-2 expression on CD3+, CD45RA+ naive T cells. In both groups, a strong positive correlation was observed between the level of expression of CD127 and Bcl-2 (R = 0.72, P = .004 and R = 0.89, P = .0001 for control and cGVHD- patient groups, respectively), demonstrating that low CD127 expression leads to the down-regulation of Bcl-2 on CD3+, CD45RA+ naive T cells (Figure 4C). While healthy donors' naive T cells harbor a large range of expression of both Bcl-2 and CD127, 7 of 10 of the AHSCT patients demonstrate a reduced expression of these 2 molecules, which should impair naive T-cell survival (Figure 4C).29,33-35 It is important to point out that cells expressing very low levels of Bcl-2 cannot be found in vivo as they are likely to die by apoptosis. Despite this phenomenon that leads to the underestimation of the frequency of cells expressing low levels of CD127 and Bcl-2 in AHSCT patients, statistically significant differences were observed between study groups.
Reduction of IL-7Rα (CD127) expression on naive T cells from cGVHD- patients. (A) FACS analysis of IL-7Rα+ naive T-cell frequencies of AHSCT patients and control group. CD127+ lymphocytes were quantified in CD3+, CD45RA+, CD27+ naive T cells by multiparameter flow cytometry. A minimum of 1 × 105 events in the live cell gate, as defined by forward and side scatter, was accumulated for each sample. A “percentile” analysis was performed for the control and AHSCT GVHD- patient groups in order to illustrate the data. The top and bottom horizontal lines represent the 90% and 10% percentile values, respectively, whereas the top and bottom edges of the rectangle represent the 75% and 25% percentile, respectively. The horizontal line located within the rectangle defines the 50% percentile. (B) Mean fluorescence intensity (MFI) of CD127-expressing CD3+, CD45RA+, CD27+ naive T cells of AHSCT patients and control groups. Again, “percentile” analysis, identical to panel A, was performed to represent the data for the control and AHSCT GVHD- patient groups. (C) Representation of the mean fluorescence intensity (MFI) of CD127- and Bcl-2 expression on naive T cells. The vertical and horizontal solid lines represent the average IL-7Rα and Bcl-2 MFI of the control group. Empty triangles (▵) represent healthy adults, whereas empty circles (▴) correspond to cGVHD- patients. Representative example of Bcl-2 (top left panel) and CD127 (bottom right panel) are shown for control who did not undergo transplantation (filled area) and AHSCT patient (empty area).
Reduction of IL-7Rα (CD127) expression on naive T cells from cGVHD- patients. (A) FACS analysis of IL-7Rα+ naive T-cell frequencies of AHSCT patients and control group. CD127+ lymphocytes were quantified in CD3+, CD45RA+, CD27+ naive T cells by multiparameter flow cytometry. A minimum of 1 × 105 events in the live cell gate, as defined by forward and side scatter, was accumulated for each sample. A “percentile” analysis was performed for the control and AHSCT GVHD- patient groups in order to illustrate the data. The top and bottom horizontal lines represent the 90% and 10% percentile values, respectively, whereas the top and bottom edges of the rectangle represent the 75% and 25% percentile, respectively. The horizontal line located within the rectangle defines the 50% percentile. (B) Mean fluorescence intensity (MFI) of CD127-expressing CD3+, CD45RA+, CD27+ naive T cells of AHSCT patients and control groups. Again, “percentile” analysis, identical to panel A, was performed to represent the data for the control and AHSCT GVHD- patient groups. (C) Representation of the mean fluorescence intensity (MFI) of CD127- and Bcl-2 expression on naive T cells. The vertical and horizontal solid lines represent the average IL-7Rα and Bcl-2 MFI of the control group. Empty triangles (▵) represent healthy adults, whereas empty circles (▴) correspond to cGVHD- patients. Representative example of Bcl-2 (top left panel) and CD127 (bottom right panel) are shown for control who did not undergo transplantation (filled area) and AHSCT patient (empty area).
These results highlight for the first time that the long-lasting reduction in naive T-cell counts associated with HLA-matched AHSCT, even in the absence of clinical cGVHD, involves a postthymic defect that hampers naive T-cell survival. Homeostatic regulation of this peripheral compartment is, in the absence of clinical GVHD, maintained through normal thymic export and enhanced homeostatic proliferation. During cGVHD episodes, this homeostatic equilibrium is disrupted as a consequence of the impairment of thymic function.
Discussion
AHSCT recipients often exhibit an immunodeficiency of varying severity and duration. This period is characterized by a long-lasting (> 48 months) reduction of peripheral blood naive T-cell frequencies. This is demonstrated looking at naive T-cell frequencies (Figure 1A) and reflects itself in absolute naive T-cell counts (cGVHD-: 221/μL; cGVHD+: 155/μL) compared with normal value of ± 600/μL as defined in historical controls.36 This cohort of patients was sampled after the initial reconstitution phase, when general lymphopenia is resolved and patients who underwent transplantation have quasinormal levels of T cells (total T-cell count = 1189 ± 749 and 988 ± 558/mL in cGVHD- and cGVHD+ patients, respectively). In cGVHD- patients, CD45RA+ CD27+ T-lymphocyte frequency remained low despite normal thymic function (Figure 1B) and a higher proportion of naive T cells expressing Ki-67 (P = .005; Figure 3A).
GVHD leads to a skewed peripheral T-cell repertoire.37-39 Given the critical role of thymic architecture in positive and negative selection during thymopoiesis, autoimmune manifestations of GVHD, known to affect the thymus, could bias thymocyte ontogeny and/or exportation in a way that would impair diversification of the RTE TCR repertoire. In order to investigate whether thymic output is biased following AHSCT, and particularly during cGVHD episodes, we developed an original approach aimed at precisely quantifying Dβ1Jβ1.1 to Dβ1Jβ1.6 TRECs using a panel of primers/probes that covers 46% of the RTE repertoire. In both control subjects and patients who underwent transplantation, average peripheral blood DβJβ TREC frequencies are in accordance with known TCR DβJβ gene segment use in single positive thymocytes.40 Dβ1Jβ1.3 (range, 0-9) is the less frequent DβJβ TREC, while Dβ1Jβ1.1 (range, 0-17) and Dβ1Jβ1.6 TRECs (range, 0-29) are well represented, demonstrating that such an approach is suitable to analyze RTE diversity. Using this tool, we provide direct evidence supporting the notion that the thymus maintains its ability to export diversified T cells after AHSCT, even during a cGVHD episode (Figure 2). These data are in agreement with the polyclonal TCR repertoire expressed by human thymocytes,41 as well as naive T cells from T-cell-depleted CD34-selected hematopoietic progenitor cells in patients who underwent transplantation.42 Therefore, the induction of peripheral T-cell repertoire perturbations characterizing patients who underwent transplantation37,43-45 is not consecutive of qualitative alterations of thymopoiesis.
Peripheral sjTREC frequencies, reflective of thymic output, are severely reduced (> 1 log) during cGVHD episodes following AHSCT, compared with cGVHD- patients or healthy controls (Figure 1B-C). Although fluctuations in peripheral T-cell proliferation levels can influence sjTREC frequencies,46 their effect, if any, in this cohort of patients is very limited: (1) the proportion of Ki-67+ naive T cells remained comparable between cGVHD- and cGVHD+ groups (P = .75) and (2) no inverse correlation was observed between Ki-67 expression in naive T lymphocytes and sjTREC frequencies. Therefore, the reduced peripheral blood RTE concentration seen during cGVHD episodes is most likely the consequence of aberrant thymocyte development/ontogeny47 induced by autoimmune responses directed at thymocytes or thymic stromal cells during cGVHD episodes.48 It is also important to point out that thymic function and peripheral naive T-cell frequencies were comparable between patients who never had cGVHD (8/23) and those who did have GVHD episodes but were cGVHD-free for at least 8 months (15/23) prior to the initiation of study (P = .40 and .54, respectively), indicating that the quantitative defect in thymic output is reversible. Furthermore, the effect of the prednisone-based regimen for the treatment of GVHD episodes was not determined and could also lead to reduced thymic activity as a result of acceleration of thymocyte apoptosis.49
The absence of a statistically significant correlation between the evolution of TREC frequencies and naive T-cell proliferation can also reflect the existence of a physiologic dichotomy between naive T cells and RTEs. As suggested by Berzins et al,50 the RTE population represents an independent compartment composed of short-lived T cells. When receiving the adequate signals, probably provided by peripheral niches, these cells further mature to naive T cells. It seems logical that, despite important modifications of peripheral naive T-cell proliferation and survival observed in cGVHD- patients, the TREC quantification, which represents a quantitative assessment of the RTE compartment, is barely influenced by peripheral events. In agreement with this hypothesis, the slight decrease in sjTREC frequencies is most probably the consequence of the observed increased proliferation of CD3+ CD45RA+ CD27+ naive T cells in cGVHD- patients.
Naive T-cell homeostasis is the consequence of a delicate balance between cell maturation, proliferation, survival, and death. In order to maintain viability, T cells require continuous signals from their in vivo environment. IL-7 is a key element of T-cell homeostasis29 and promotes peripheral naive T-cell survival.33,35,51-54 Signal transduction, induced through IL-7 receptor complex, involves the association of the IL-7Rγ chain with Jak1 and Jak3,55 followed by the induction of members of the signal transducer and activator of transcription (STAT) family, reportedly STAT1, STAT5, and possibly STAT3,56 ultimately leading to Bcl-2 expression. As Bcl-2 expression also requires signaling through Jak3/STAT,57 the observed reduction of both the frequency of CD127-expressing naive T cells and the level of expression of this molecule on CD127+ cells, associated with the reduction of Bcl-2 expression, will most likely impair naive T-cell survival.35,58
The findings reported in this paper are consistent with a “steady-state” model in which naive T-cell homeostasis is regulated through 3 different parameters: thymic output, peripheral proliferation, and cell death. In AHSCT patients, the low frequency of peripheral naive T cells could lead to feedback mechanisms, such as proliferation of these cells46,59 and/or increased thymic output, aiming at restoring this population. Accordingly, increased proliferation levels of naive T cells were observed following AHSCT (Figure 3). According to the steady-state hypothesis, increased proliferation and thymic output should be compensated by increased cell death (in order to keep the influx and outflux of T cells constant). This is consistent with the observed reduction of IL-7Rα and Bcl-2 expression levels (Figure 4). This naive T-cell equilibrium in cGVHD- patients, characterized by stable but reduced peripheral naive T-cell counts, was observed for up to 10 years after transplantation, indicating that a new peripheral naive T-cell frequency “set-point,” significantly lower than in controls, has been established. It remains to be demonstrated whether the defect in naive T-cell survival following AHSCT is a consequence of a transplantation-induced modification of the peripheral environment or is intrinsic to thymic educational processes.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1428.
Supported by grants to C.P. from Canadian Institute of Health Research (CIHR) and to R.-P.S. from National Institutes of Health (NIH), CIHR, and CANVAC. R.-P.S. is a CRC chair in human immunology. J.-F.P., M.-L.D., and A.D. are/were doctoral research award recipients of the CIHR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work was carried out as a partial fulfillment of J.-F. Poulin's thesis at McGill University, Montréal. J.-F. Poulin is now a postdoctoral fellow at the Gladstone Institute of Virology and Immunology (GIVI, San Francisco, CA) in the laboratory of Joseph M. “Mike” McCune. The authors would like to thank Chantal Baron and Sophie Ouellet for help with flow cytometry and technical assistance. We also thank Zvi Grossman, Martin Meier-Schellersheim, Sophie Gratton, and Gaël Dulude for critical reading of this manuscript and insightful thinking. R. Cheynier is an invited/senior visiting scientist from Institut Pasteur (Paris, France).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal