Abstract
In a patient with lethal factor VII (FVII) deficiency, 2 homozygous nucleotide substitutions were identified in the F7 gene: a IVS7+2T>G transversion involving the IVS7 donor splice site, followed by a mutation at nucleotide 10588 that would result in a missense variation (Arg224Gln). The mutated splice site, located within the first repeat of a minisatellite, is followed by a variable number of pseudo-sites, normally silent. To investigate the consequences of this mutation on F7 splicing, we designed normal and mutant minigenes, spanning exons 5 to 8. In cells transfected with the mutant construct, no normal splicing occurred. Only spliced transcripts including the first minisatellite repeat were observed, resulting from the activation of the most proximal wild-type pseudo-site, which would generate a truncated protein (stop codon upstream of nucleotide 10588). These findings, which suggest the existence of a mechanism selecting one single splice site among multiple cryptic sites, explain the patient's phenotype.
Introduction
Inherited factor VII (FVII) deficiency is a rare autosomal recessive disorder. Its clinical presentation correlates poorly with FVII plasma levels. However, severe bleeding diathesis is observed when FVII activity is less than 1%.1 The gene encoding FVII (GenBank accession no. NM_000131),2 spanning 12.8 kb of the q34 region on chromosome 13, is expressed only in hepatocytes. IVS7 of this gene contains a minisatellite characterized by a 4 to 8 repeated 37-bp element.3 The first repeat includes the last 4 bp of exon 7 and the first 33 bp of IVS7. Thus, the IVS7 repeated element provides multiple identical 5′ splice junctions, normally silent.3 Previous studies showed that other gene abnormalities (IVS7+5G>A, IVS7del3-7) weakened the IVS7 consensus sequence, leading to activation of the first downstream pseudo-site.4,5 We recently characterized 2 other mutations (IVS7+2T>G, Arg224Gln) in the homozygous state in a child presenting with a lethal FVII deficiency.6 We investigated here the effects on the splicing of F7 transcripts of the IVS7+2T>G transversion, which abolishes the invariant GT dinucleotide. Because the proband had died, transcripts were characterized through in vitro studies.
Study design
A F7 genomic region of 3.5 kb, spanning intron 4 to exon 8 (nucloetides 7688-11188), was amplified from a healthy individual and from the patient (forward primer Pa 5′AGAACACCACTGCTGACCCA3′, reverse primer Pm 5′ACAGTTCGACGCAGCCTTGG3′; Figure 1A) using the Expand high-fidelity polymerase chain reaction (PCR) system (Boehringer-Mannheim, Mannheim, Germany). Both wild-type and mutant F7 alleles carried 6 repeats of the 37-bp element in the homozygous state. Wild-type and mutant PCR fragments were cloned into the cytomegalovirus promoter-based expression vector pTracer using the T4-DNA ligase (Invitrogen, Carlsbad, CA). All coding sequences and splice junctions were sequenced. Chinese hamster ovary (CHO) cells were grown in Iscove medium (Invitrogen) supplemented with 10% fetal calf serum. Transfections of wild-type and IVS7 mutant F7 minigenes were performed with lipofectamin (Invitrogen). Total RNA was extracted using RNAplus (Bioprobe Systems, Montreuil, France). Following precipitation, RNA was treated with DNaseI to remove contaminating plasmid DNA. First-strand cDNA synthesis was performed with 5 μg total RNA as a template, with random hexamers as primers (Pharmacia, Uppsala, Sweden) and Superscript II (Invitrogen). One tenth of each of the first-strand synthesis reaction products was used in each of the 3 subsequent standard PCRs with oligonucleotides Pb (5′TCTGTGTGAACGAGAACGGC3′) and Pm; Pe (5′AAGAAATGCCAGCAAACCCC3′) and Pm; Ph (5′GGAGCTCAGTTGTGTGGGGG3′) and Pm (Figure 1A). Nonpurified PCR products were cloned into the vector pTOPO-4 (Invitrogen). To characterize the aberrant transcripts, both strands of inserts were sequenced with the forward and reverse primers used for PCR. Informed consent was obtained from the patient's parents.
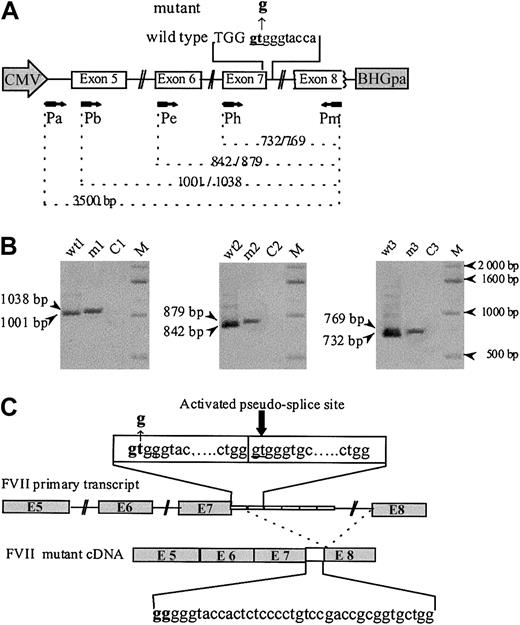
Analysis of in vitro expression of F7 mRNA splicing products. (A) The human F7 gene region cloned into the EcoRI site of the vector pTracer-CMV contains complete coding sequence from exon 5 to 8, part of intron 4, and complete introns 5 to 7. Both wild-type and mutant inserts carried 6 repeats of the 37-bp monomer. The mutated region is indicated in bold letters, the exonic sequences by capital letters, and the intronic sequences by lowercase letters. A schematic representation of the primers (arrows) used for PCR and their corresponding location in the sequence are given. (B) RT-PCR amplification of RNA from CHO cells transfected with wild-type (wt) and mutant (m) constructs, detected on 1% agarose gel stained with ethidium bromide. Lanes were loaded with wt and mutant cDNA amplified with primers Pb and Pm (lanes wt1 and m1), Pe and Pm (lanes wt2 and m2), and Ph and Pm (lanes wt3 and m3). Lanes C1, C2, and C3 are controls without cDNA template. Fragments of different lengths are observed for the wild-type and the mutant. (C) Schematic drawing of the F7 cDNA structure spanning exon 5 to 8 in the mutant. The IVS7+2 T>G donor site mutation leads to the insertion of the first 37-bp element between exons 7 and 8, due to the use of the IVS7 5′ donor pseudo-site, located at IVS7+38 in the second 37-bp repeated element of the IVS7 minisatellite.
Analysis of in vitro expression of F7 mRNA splicing products. (A) The human F7 gene region cloned into the EcoRI site of the vector pTracer-CMV contains complete coding sequence from exon 5 to 8, part of intron 4, and complete introns 5 to 7. Both wild-type and mutant inserts carried 6 repeats of the 37-bp monomer. The mutated region is indicated in bold letters, the exonic sequences by capital letters, and the intronic sequences by lowercase letters. A schematic representation of the primers (arrows) used for PCR and their corresponding location in the sequence are given. (B) RT-PCR amplification of RNA from CHO cells transfected with wild-type (wt) and mutant (m) constructs, detected on 1% agarose gel stained with ethidium bromide. Lanes were loaded with wt and mutant cDNA amplified with primers Pb and Pm (lanes wt1 and m1), Pe and Pm (lanes wt2 and m2), and Ph and Pm (lanes wt3 and m3). Lanes C1, C2, and C3 are controls without cDNA template. Fragments of different lengths are observed for the wild-type and the mutant. (C) Schematic drawing of the F7 cDNA structure spanning exon 5 to 8 in the mutant. The IVS7+2 T>G donor site mutation leads to the insertion of the first 37-bp element between exons 7 and 8, due to the use of the IVS7 5′ donor pseudo-site, located at IVS7+38 in the second 37-bp repeated element of the IVS7 minisatellite.
Results and discussion
The patient's phenotype (undetectable FVII plasma levels and lethal bleeding diathesis), combined with the IVS7 mutation, led us to investigate its effect on the F7 transcripts. Figure 1B shows the electrophoretic pattern of the reverse transcriptase–PCR (RT-PCR) products obtained from RNA extracted from cells transfected with the various constructs. In experiments carried out with the wild-type construct, all 3 PCRs yielded single bands of the expected size. Identical experiments, performed with the mutant construct, generated in all cases a single slightly larger band. Nonpurified RT-PCR products were subsequently subcloned and sequenced. Analysis of 5 clones showed correct splicing of the transcripts from cells transfected with the wild-type construct. In contrast, splice products generated from cells transfected with the mutant construct revealed the inclusion of the first intronic 37-bp repeat, between exons 7 and 8, demonstrating activation of the IVS7 5′ pseudo-site located in the second repeat. The possible existence of residual normal splicing or, less frequently, of abnormal splice products was investigated by the analysis of 50 additional clones; all of them contained the same aberrant splicing product, allowing us to estimate the level of all other possible splice events below 2%.
Several lines of evidence suggest that the IVS7+2T>G transversion is the disease-causing mutation. (1) It involves the GT invariant dinucleotide, the alteration of which is known to impair normal splicing.7-10 (2) This mutation that, according to our experiments, indeed impairs normal splicing, activates a cryptic site at IVS7+38 (Figure 1C). This would lead to a 37-bp insertion between exons 7 and 8, resulting in a frameshift, with a premature termination codon at position 231. If synthesized, such a truncated protein would lack the catalytic domain. By contrast, the 10588G>A (Arg224Gln) is likely to be a non–disease-causing substitution; the Arg224 surface residue is located in an evolutionary nonconserved region, and, moreover, its conversion by alanine scanning did not lead to activity or tissue factor–binding decrease.11 This mutation was found in the heterozygous state in an asymptomatic subject, in whom FVII levels (FVII:c 78%; FVII:Ag 94%) are consistent with a normal function.6 Finally, this nucleotide substitution is located 12 nucleotides downstream to the premature termination codon introduced by the 37-bp insertion.
Different phenotypes were observed among patients carrying the other homozygous IVS7 mutations,4,5 although aberrantly spliced products were identical to those found in our study. This situation illustrates that mutations located in less-conserved nucleotides, unlike those involving invariant GT dinucleotide, allow various amounts of normal splicing to occur,9 sufficient to prevent lethal bleeding diathesis.
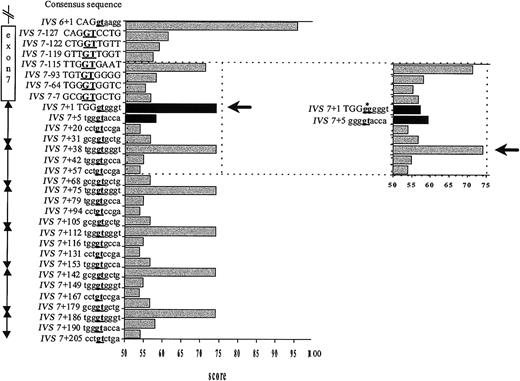
To gain insight into the mechanism of splice site selection, the consensus values (CVs), which reflect the base-pairing stability between the U1 snRNA and a given splice sequence,7 of the GT-containing sequences in the surrounding region were calculated (Figure 2). As expected, the authentic IVS7 5′ splice site and each IVS7 pseudo-site have an identical CV. The IVS7+2T>G mutation results in a dramatic decrease of the CV of the IVS7 donor splice site to a value that is below that of several neighboring potential splice sites (Figure 2). The CV of the GT-containing sequence at IVS7+5 is slightly increased by the mutation (Figure 2).
Strength of potential splice sites as measured by their CVs. The statistical rules of Shapiro and Senopathy were applied to assign a CV expressed in percentage of homology to the consensus sequence.7 A score of 100 represents the best match; 0, the worst match. The CV of the IVS6 donor site and the authentic and mutated IVS7 donor splice site were rated according to this procedure, and potential cryptic sites along exon 7 and in each 37-bp element of the IVS7 minisatellite were researched alike. Because the CV of the GT-containing sequence in the close vicinity of the mutated splice site ranged from 53.9 to 74.7, we chose the value 53.9 as a threshold to define a potential alternative splice site. On the vertical axis are given the location and the sequence of the potential cryptic sites. Position IVS7+1 corresponds to the first nucleotide of IVS7, and position IVS7-1 to the last nucleotide of exon 7. Capital letters denote exonic sequences and lowercase letters intronic sequences. Underlined boldface letters indicate the position of the splicing site. The horizontal axis shows the CV assigned for each position. The black arrow indicates the activated site. In the insert figure, the CV changes caused by the IVS7+2 T>G mutation (black columns) and the cryptic site subsequently activated (black arrow and asterisk pointing to the mutated nucleotide) are shown.
Strength of potential splice sites as measured by their CVs. The statistical rules of Shapiro and Senopathy were applied to assign a CV expressed in percentage of homology to the consensus sequence.7 A score of 100 represents the best match; 0, the worst match. The CV of the IVS6 donor site and the authentic and mutated IVS7 donor splice site were rated according to this procedure, and potential cryptic sites along exon 7 and in each 37-bp element of the IVS7 minisatellite were researched alike. Because the CV of the GT-containing sequence in the close vicinity of the mutated splice site ranged from 53.9 to 74.7, we chose the value 53.9 as a threshold to define a potential alternative splice site. On the vertical axis are given the location and the sequence of the potential cryptic sites. Position IVS7+1 corresponds to the first nucleotide of IVS7, and position IVS7-1 to the last nucleotide of exon 7. Capital letters denote exonic sequences and lowercase letters intronic sequences. Underlined boldface letters indicate the position of the splicing site. The horizontal axis shows the CV assigned for each position. The black arrow indicates the activated site. In the insert figure, the CV changes caused by the IVS7+2 T>G mutation (black columns) and the cryptic site subsequently activated (black arrow and asterisk pointing to the mutated nucleotide) are shown.
Several studies, reporting simultaneous activation of multiple cryptic sites on a single 5′ splice site mutation,12-15 postulate that cryptic sites compete at different efficiencies for activation, according to their ability to bind U1snRNA.12-14 This is especially observed when a mutation within the authentic site decreases its affinity for U1snRNA to the same approximate level as that of putative cryptic sites.16 In one case (LPL gene), the use, at low efficiency, of a highly homologous pseudo-site was even demonstrated in the normal allele.12 In the present case, the mutant F7 pre-mRNA contains multiple potential cryptic sites of strengths higher than that of the mutated authentic site. According to these studies, these sites could be competitively activated at different efficiencies, with a higher degree of activation for each IVS7 5′ pseudo-site.16 Strikingly, only the most upstream of the IVS7 5′ pseudo-sites, located in the second IVS7 minisatellite repeat, is activated. No exon skipping was observed despite the high strength of the donor site of the preceding intron (IVS6; Figure 2).
Taken together, these data strongly suggest the existence, in the IVS7-F7 gene, of a specific physiologic mechanism that selects the most upstream 5′ splice site, like that discussed for G triplets.17,18 In our case, the possible repression of the 3′ following pseudo-sites is another hypothesis that could account for this situation; however, this possibility was rejected because the mutation of the authentic 5′ splice site was shown to result in the activation of the following 5′ pseudo-site. This model also accounts for the absence of the alternatively spliced products resulting from the activation of other pseudo-sites, even at very low efficiency, both from the healthy control and from the mutated gene.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-09-2951.
O.C. is the recipient of a grant from INSERM, France, and the Direction de la Recherche Scientifique, Algeria.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Henri Wajcman for useful discussions throughout the course of this study and critical reading of the manuscript. We thank Philippe Coudol for assistance with the sequencing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal