The usefulness of the WT1 quantitative assessment by real-time quantitative polymerase chain reaction (RQ-PCR) as a marker for minimal residual disease (MRD) detection after allogeneic bone marrow transplantation (BMT) has been reported in a recent issue of Blood by Ogawa et al1 in a series of 72 patients affected by different types of leukemias. According to their data, the quantitative assessment of WT1 transcript amount acquires a highly significant value in terms of the possibility of being predictive of imminent relapse in this particular setting, as the WT1 transcript amount after BMT is lower than in the normal bone marrow (BM), allowing the transcript to reach a high degree of sensitivity.
These data prompted us to analyze by RQ-PCR the WT1 transcript amount in 18 patients (7 acute myeloid leukemia, 2 acute lymphoblastic leukemia, and 9 chronic myelogenous leukemia) before and after allogeneic BMT. The procedure used has been previously described in detail, and ABL was used as a reference gene.2 In agreement with the data of Ogawa et al,1 we are able to confirm that the determination of the WT1 transcript amount can represent a useful marker to monitor the persistence or the reappearance of leukemic cells after allogeneic BMT and that increasing amounts of WT1 transcript are predictive of relapse. Indeed, as already demonstrated by Ogawa et al,1 an increase of WT1 expression above the upper threshold found in controls (see Figure 1) was detected in 5 of 18 patients, and all 5 of the patients relapsed after a period ranging from 1 to 5 months. By contrast, none of the patients who remain within the normal range of WT1 expression relapsed.
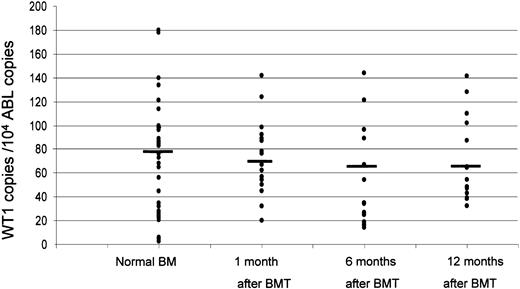
Amount of WT1 transcripts found in the BM samples obtained from healthy controls with respect to those obtained in patients at 1, 6, and 12 months after BMT. The values are expressed as WT1 copies every 104 copies of ABL. The bars indicate the median values for each group. No statistically significant differences were observed.
Amount of WT1 transcripts found in the BM samples obtained from healthy controls with respect to those obtained in patients at 1, 6, and 12 months after BMT. The values are expressed as WT1 copies every 104 copies of ABL. The bars indicate the median values for each group. No statistically significant differences were observed.
However, in contrast to the data of Ogawa et al,1 we were not able to find differences in the WT1 transcript amount expressed by normal control BM samples with respect to the 13 patients in remission during a period of follow-up ranging from 14 to 38 months after BMT. As shown in Figure 1, the median values of WT1 within one month after transplantation are 76 copies/104 ABL copies (range, 20-142), while normal controls (obtained from 42 healthy subjects) expressed a median value of WT1 copies of 78 (range, 3-180) (P = .9 by Student t test). In patients who persisted in complete remission (CR), no significant changes in WT1 expression were noted during follow-up (P = .84 after 6 months, and P = .9 after one year). In addition, no differences were observed between the values obtained in these patients with respect to those obtained in leukemia patients who obtained remission after chemotherapy (P = .86).
As previously reported,2 in our hands WT1 represents a good marker for MRD detection even in patients treated with intensive chemotherapy or autologous bone marrow transplantation and may also represent a good marker to establish disease progression in myelodysplastic syndromes.3
The discrepancy observed between our data and those of Ogawa et al is probably due to differences in the procedures used. Thus, although new quantitative real-time procedures promise to simplify the protocols that are currently in use, standardization and the introduction of rigorous, internationally accepted controls are required to enable RQ-PCR for WT1 transcript quantitative assessment to become a robust and routine basis for therapeutic decisions.
The background level of WT1 expression following allogeneic stem cell transplantation is significantly decreased
In agreement with our data,1 Cilloni et al2 reported that the determination of the WT1 transcript amount could represent a useful marker for monitoring the persistence or the reappearance of leukemic cells after bone marrow transplantation (BMT). Although we observed that the background expression of WT1 was significantly decreased after allogeneic stem cell transplantation, Cilloni et al insist that there were no significant changes in WT1 expression between patients who persisted in complete remission (CR) and healthy controls. However, for the comparison with healthy controls regarding WT1 expression, we did not use samples of patients who persisted in CR, but minimal residual disease (MRD)–negative samples. To define the background level of WT1 gene expression in bone marrow (BM) samples following BMT, we used the expression of chimeric genes. When the expression of chimeric genes was less than 10-5 or undetectable (the expression of major bcr-abl in K562 cells, minor bcr-abl in L2 cells, and AML1-MTG8 in Kasumi-1 cells was defined as 1.0), MRD was defined as negative. WT1 gene expression levels in MRD-negative samples that were obtained up to day 400 were found to be significantly lower (median, 1.0 × 10-4; range, 8.6 × 10-6 to 4.0 × 10-4) than those of healthy volunteer donors (median, 3.8 × 10-4; range, 4.2 × 10-5 to 3.6 × 10-3) (P < .0001, Mann-Whitney U test), as shown in Figure 2 of our article.1 As shown in Table 1 of our article,1 the WT1 expression levels in patients who persisted in CR were distributed over a wide range of 1 × 10-5 to 1 × 10-2. This result indicates that some patients are in continued CR despite the presence of detectable MRD. Therefore, we consider it likely that Cilloni et al could not find any difference in WT1 expression between samples from patients who persisted in CR and those from healthy controls because some of the patients who persisted in CR had MRD. In Philadelphia chromosome–positive acute lymphoblastic leukemia after marrow transplantation, Radich et al reported that at least a positive test for p210 bcr-abl was not associated with an increased relative risk.3 Taken together, these facts imply that WT1 expression greater than the background level or the presence of MRD does not indicate directly impending relapse. However, as we showed in our article in Blood,1 the risk of relapse within a short period of time (within 40 days) increases step-by-step according to the increase in WT1 expression level.
Thus, we do not consider that the discrepancy observed between our data and those of Cilloni et al is due to differences in the procedures used. However, we agree with Cilloni et al that standardization and the introduction of rigorous, internationally accepted controls are required for real-time quantitative polymerase chain reaction for quantitative WT1 transcript assessment.
Correspondence: Hiroyasu Ogawa, Department of Molecular Medicine, Osaka University Graduate School of Medicine, 2-2, Yamada-Oka, Suita, Osaka, Japan, 565-0871; e-mail: ogachan@ceres.ocn.ne.jp
Supported by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), MURST-COFIN 2002, and AIL (Associazione Italiana contro le Leucemie).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal