Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is a proven curative therapy for many hematologic malignancies. HSCT from HLA-identical sibling donors (ISDs) is still the golden standard. For the remaining 70% of the patients lacking an ISD, alternative (partially) HLA-matched family donors (MFDs) and HLA-matched unrelated donors (MUDs) are now widely accepted. However, it is presently unclear whether outcome after HSCT from an MFD or an MUD is superior. Thus, the classical clinical end points after HSCT from an ISD (n = 138), MFD (n = 86), and MUD (n = 101) were compared by means of univariate and multivariate statistical analyses. MFD transplantations with HLA class II (DRB1 ± DQB1) mismatches in graft-versus-host (GVH) direction showed an increased risk of grades II to IV graft-versus-host disease, and MFD transplantations with more than a single HLA class I (A ± B ± C) mismatch in host-versus-graft (HVG) direction were associated with a higher risk of graft failure. However, no significant difference in overall survival was detectable among the 3 study groups after adjustment for the main predictors of transplantation outcome. Thus, for patients lacking an ISD, an already identified MFD with an HLA-DRB1 ± DQB1 mismatch in GVH or a combined HLA-A ± B ± C mismatch in HVG direction should be accepted only in clinically urgent settings that leave no time to identify an MUD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an established curative therapy for a variety of hematologic malignancies.1 Genotypically HLA-identical sibling donors (ISDs)—which are available for about 30% of white patients2 —are still regarded as the best donors for HSCT.3 However, for the remaining 70% of patients, alternative donors, that is, (partially) HLA-matched family donors (MFDs) other than HLA-identical siblings and HLA-A–, B-, C-, DRB1-, and DQB1-matched unrelated donors (MUDs), are meanwhile routinely accepted.4-7
The fact is that the clinical outcome after HSCT from an MFD4,8,9 as well as an MUD10-13 has meanwhile clearly improved, probably because of progress made in the domains of HLA-typing techniques14-16 and supportive therapy.17,18
However, it is presently unclear whether HSCT from an MFD or from an MUD has a superior outcome since the clinical studies cited in the previous two paragraphs do not directly compare these 2 allogeneic approaches. Thus, the question is still open on how to proceed if the donor search among the patient's siblings—which is always run first—has identified only an MFD, but no ISD. Should any or at least a subgroup of MFDs (which remains to be defined) be accepted immediately without further effort to identify an MUD? Or should an unrelated donor search, even if expensive and time consuming, be run for all of these patients?
The present clinical study directly compares the classical clinical end points of transplantation outcome from MFDs and MUDs, with ISD transplantations serving as controls, and identifies 2 subgroups of MFDs that should not be accepted routinely for HSCT.
Patients and methods
Entry criteria
All patients who underwent transplantation at our institution during the period from January 1, 1994, to July 31, 2000, and fulfilled the following entry criteria were enrolled: (1) 16 years or older; (2) allotransplantation for chronic myeloid leukemia (CML), acute myeloid leukemia (AML), or acute lymphatic (ALL) leukemia; (3) first transplantation (ie, no preceding allotransplantation or autotransplantation); (4) no graft manipulation (eg, no T-cell depletion); (5) myeloablative conditioning regimen used (containing 4 × 2.5 Gy fractionated total body irradiation and cyclophosphamide); (6) cyclosporine plus short-course methotrexate protocol used for graft-versus-host disease (GVHD) prophylaxis; and (7) transplantations performed under strict regimens for gut decontamination of anaerobic bacteria and reverse isolation (laminar air flow conditions). At the time of analysis all enrolled patients (N = 325) who survived had a follow-up of 18 months or longer.
Strategy of donor search and selection of graft source
The strategy of donor search was in accordance with the German Consensus on Immunogenetic Donor Search.19 Thus, for patients lacking an ISD, an MFD was accepted (without a further effort to identify an MUD) if matched with the patient in graft-versus-host (GVH) direction for at least 5 of the 6 HLA-A, B, and DRB1 antigens (regardless of the number of additional HLA mismatches in host-versus-graft [HVG] direction). If more than one MFD was available we tried to avoid selection of a female donor for a male patient and of a cytomegalovirus (CMV)–negative donor for a CMV-positive recipient. Since October 1994, we preferred peripheral blood over marrow as graft source in patients with an increased risk of graft failure and/or with a recent serious infectious disease as outlined previously.9
Histocompatibility studies
HLA class I (A, B, and C) typing of patients and donors relied on conventional serology (supplemented by one-dimensional iso-electric focusing [1D-IEF]) until 1996 and on low-resolution DNA-based typing (polymerase chain reaction and sequence-specific primer [PCR-SSP]) thereafter. For the purposes of the present study more than 90% of donors and recipients belonging to the MFD group were retyped for HLA-A, B, and C by low-resolution PCR-SSP. For identification and selection of HLA class II (DRB1, DQB1)–matched donors, the mixed lymphocyte culture (MLC) test was routinely used until 1994, supplemented by HLA-DR and DQ serology. After 1994, the DRB1 and DQB1 antigens of all donors and recipients were identified according to the German consensus on immunogenetic donor search.19 Thus, for patients and donors belonging to the ISD and MFD group, low-resolution PCR-SSP (generic level, 2-digit code) was used, supplemented by a high-resolution typing technique in case of ambiguous results (eg, homozygosity). In the MFD setting, German experts did not feel a need for routine high-resolution HLA-DRB1 and DQB1 typing if low-resolution typing had already disclosed a donor/recipient DRB1 and/or DQB1 mismatch (eg, DRB1 *01 vs *04) and the test results were supported by a pedigree plot segregation analysis. In contrast high-resolution PCR-SSP (allelic level, 4-digit code) was routinely used in the unrelated donor-recipient setting.
Baseline characteristics of study transplantations
The following variables were used for initial (ie, at presentation) characterization: type of donor (MUD, yes = 1 vs other = 0; and in parallel MFD, yes = 1 vs other = 0), patient sex, donor sex, sex mismatch (female donor for male recipient = 1, other = 0), patient age, donor age, ABO blood group major incompatibility, underlying disease (CML, AML, ALL), disease stage (early [CML first chronic phase, and AML/ALL first complete remission] = 0 vs advanced [all other stages] = 1), and graft source (peripheral blood [PB] = 0 vs bone marrow [BM] = 1). For all MFD transplantations the following initial parameters were documented additionally: donor/recipient HLA match/mismatch as detailed by HLA locus (A, B, C, DRB1, and DQB1) and involved immunologic vector (GVH, HVG direction), sum of HLA class I, as well as sum of HLA class II mismatches in GVH and in HVG direction (Figure 1).
HLA characteristics of transplantations from HLA-matched family donors other than genotypically HLA-identical siblings (MFD study group). HLA matches (white boxes) and HLA mismatches (black boxes) are detailed by the HLA loci (A, B, C, DRB1, and DQB1) and the immunologic vector (GVH indicates graft-versus-host; HVG, host-versus-graft). N indicates number of transplantations for a given HLA constellation. More than 95% of the given HLA mismatches were full antigen mismatches (eg, A*02 vs A*03, DRB1*01 vs DRB1*04), whereas the remaining mismatches were HLA antigen split mismatches (eg, B*15 [62] vs B*15 [63], B*40 [60] vs B*40 [61]).
HLA characteristics of transplantations from HLA-matched family donors other than genotypically HLA-identical siblings (MFD study group). HLA matches (white boxes) and HLA mismatches (black boxes) are detailed by the HLA loci (A, B, C, DRB1, and DQB1) and the immunologic vector (GVH indicates graft-versus-host; HVG, host-versus-graft). N indicates number of transplantations for a given HLA constellation. More than 95% of the given HLA mismatches were full antigen mismatches (eg, A*02 vs A*03, DRB1*01 vs DRB1*04), whereas the remaining mismatches were HLA antigen split mismatches (eg, B*15 [62] vs B*15 [63], B*40 [60] vs B*40 [61]).
Outcome characteristics of study transplantations
In order to document transplantation outcome, the following variables were selected: overall survival (OS), treatment-related mortality (TRM), engraftment, grades II to IV acute graft-versus-host disease (aGVHD), and relapse (REL). For all outcome variables, time after transplantation to event was documented together with the corresponding status variable (event/censored). Engraftment was assumed if self-sustaining blood neutrophil counts higher than 1 × 109/L (1000/μL) together with untransfused platelet counts higher than 20 × 109/L (20 000/μL) were reached by day 28 after transplantation. The grades of aGVHD were assessed according to the published standard criteria.20 The diagnosis of relapse was established by cytomorphology and/or cytogenetics.
Univariate statistical analysis
For direct comparison of initial parameters of the 3 study groups, the Kruskal-Wallis test was used for skewed continuous variables (such as time to therapy), a one-way analysis of variance (ANOVA) for symmetrically distributed continuous variables (such as age), and the chi-square test for independence for categoric variables. To illustrate the dependence of time to event of the 3 transplantation groups (ISD, MFD, and MUD) Kaplan-Meier diagrams were used. If “stage of disease” was the dominating influence parameter the corresponding Kaplan-Meier diagram is shown after stratification for disease stage. Since all Kaplan-Meier diagrams are shown for illustrative rather than inferential analysis, P values have been deferred to the multivariate analysis.
Multivariate statistical analysis
The influence of all baseline variables listed in Table 1 on the times to achieve the analytic end points of transplantation outcome was evaluated by Cox proportional hazard regression with backward elimination of parameters with a P value more than .2. For the analysis of interactions, the variables of main interest “MFD: yes/no” and “MUD: yes/no” were forced to remain in the Cox model. Interactions between the variables “MFD: yes/no” and “MUD: yes/no” and the identified risk factors in the final Cox model were evaluated by including interaction terms (eg, the interaction between patient age and MFD, and the interaction between patient age and MUD) one at a time and testing whether the explained deviation of the model exceeded the 0.95 quantile of the chi-square distribution with one degree of freedom.
Initial characteristics of transplantation study groups
. | ISD . | MFD . | MUD . | P . |
|---|---|---|---|---|
| N, total | 138 | 86 | 101 | |
| Patient age | ||||
| Median, y (range) | 40.5 (16-59) | 35.5 (16-57) | 40.0 (16-57) | .008* |
| Older than 37 y (%) | 84 (61) | 32 (37) | 58 (57) | .002† |
| Patient sex, n, M:F (ratio) | 79:59 (1.3:1) | 49:37 (1.3:1) | 58:43 (1.3:1) | NS† |
| Diagnosis (%) | NS† | |||
| CML | 107 (77) | 56 (65) | 76 (75) | |
| AML | 27 (20) | 25 (29) | 17 (17) | |
| ALL | 4 (3) | 5 (6) | 8 (8) | |
| Disease stage | ||||
| Early vs advanced (%) | 110 vs 28 (80 vs 20) | 48 vs 38 (56 vs 44) | 66 vs 35 (65 vs 35) | .001† |
| Interval: diagnosis → TX, d, median (range) | ||||
| CML | 490 (38-5.162) | 573 (111-4.057) | 678 (102-5.182) | <.0001‡ |
| AML/ALL | 190 (72-1.293) | 280 (120-1.589) | 601 (104-2.003) | .001‡ |
| Stem cell source | ||||
| BM vs PB (%) | 80 vs 58 (58 vs 42) | 40 vs 46 (46 vs 54) | 76 vs 25 (75 vs 25) | <.0001† |
| Donor age, y | ||||
| Median (range) | 41 (12-54) | 43 (9-71) | 36 (20-59) | .006* |
| Older than 38 y (%) | 78/138 (56) | 50/86 (58) | 35/101 (35) | .001† |
| Donor sex, n, M:F (ratio) | 63:75 (0.8:1) | 38:48 (0.8:1) | 65:36 (1.8:1) | .006† |
| Sex mismatch, | ||||
| yes, donor F, patient M (%) | 43/138 (31) | 27/86 (31) | 13/101 (13) | .001† |
. | ISD . | MFD . | MUD . | P . |
|---|---|---|---|---|
| N, total | 138 | 86 | 101 | |
| Patient age | ||||
| Median, y (range) | 40.5 (16-59) | 35.5 (16-57) | 40.0 (16-57) | .008* |
| Older than 37 y (%) | 84 (61) | 32 (37) | 58 (57) | .002† |
| Patient sex, n, M:F (ratio) | 79:59 (1.3:1) | 49:37 (1.3:1) | 58:43 (1.3:1) | NS† |
| Diagnosis (%) | NS† | |||
| CML | 107 (77) | 56 (65) | 76 (75) | |
| AML | 27 (20) | 25 (29) | 17 (17) | |
| ALL | 4 (3) | 5 (6) | 8 (8) | |
| Disease stage | ||||
| Early vs advanced (%) | 110 vs 28 (80 vs 20) | 48 vs 38 (56 vs 44) | 66 vs 35 (65 vs 35) | .001† |
| Interval: diagnosis → TX, d, median (range) | ||||
| CML | 490 (38-5.162) | 573 (111-4.057) | 678 (102-5.182) | <.0001‡ |
| AML/ALL | 190 (72-1.293) | 280 (120-1.589) | 601 (104-2.003) | .001‡ |
| Stem cell source | ||||
| BM vs PB (%) | 80 vs 58 (58 vs 42) | 40 vs 46 (46 vs 54) | 76 vs 25 (75 vs 25) | <.0001† |
| Donor age, y | ||||
| Median (range) | 41 (12-54) | 43 (9-71) | 36 (20-59) | .006* |
| Older than 38 y (%) | 78/138 (56) | 50/86 (58) | 35/101 (35) | .001† |
| Donor sex, n, M:F (ratio) | 63:75 (0.8:1) | 38:48 (0.8:1) | 65:36 (1.8:1) | .006† |
| Sex mismatch, | ||||
| yes, donor F, patient M (%) | 43/138 (31) | 27/86 (31) | 13/101 (13) | .001† |
The P values refer to differences among the 3 study groups with regard to the indicated baseline characteristics of transplantations. M indicates male; F, female; NS, not significant; TX, transplantation; BM, bone marrow; and PB, peripheral blood
One-way ANOVA
Chi-square test
Kruskal-Wallis test
For graft failure as an event, the scarcity of events did not allow a higher dimensional analysis without severe risk of biased estimation. Therefore, an exploratory analysis of the biologically plausible variables was performed in order to test whether the obtained results are within the range of results reported by previously studies.
Results
Overall survival (OS)
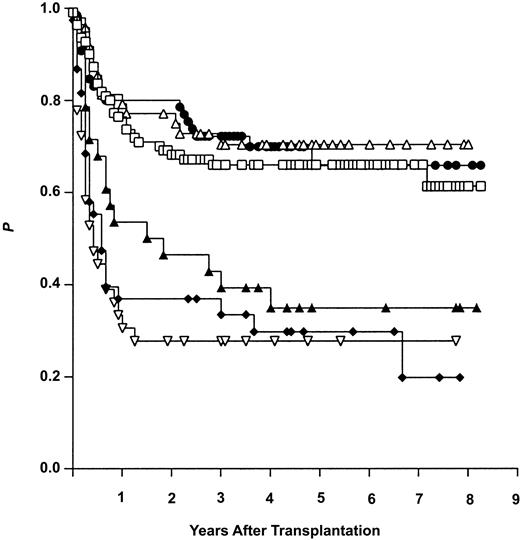
Univariate statistical analysis showed the stage of disease and the age of the patient to have a marked influence on OS after HSCT. The 5-year OS rate, for example, reached 67% for patients with early disease, but declined to 29% for patients with advanced disease. The corresponding data for OS were 65% for patients aged 37 years or younger, but dropped down to 47% for patients older than 37 years. In contrast, univariate analysis could not demonstrate any impact on OS of all other initial characteristics listed in Table 1, including the type of donor used. In order to illustrate the results of univariate analysis, the OS curves for the 3 study transplantation groups (ISD, MFD, and MUD) are shown in Figure 2 after stratification for the dominating variable “stage of disease.”
Overall survival (Kaplan-Meier estimates) after allogeneic hematopoietic stem cell transplantation from genotypically HLA-identical siblings (ISDs), alternative (partially) HLA-matched family donors (MFDs), and HLA-matched unrelated donors (MUD). Results are given after stratification for early (ear) and advanced (adv) disease stage. ▵ indicates MFD ear (n = 48); •, MUD ear (n = 66); □, ISD ear (n = 110); ▴, ISD adv (n = 28); ▿, MUD adv (n = 35); and ⋄, MFD adv (n = 38).
Overall survival (Kaplan-Meier estimates) after allogeneic hematopoietic stem cell transplantation from genotypically HLA-identical siblings (ISDs), alternative (partially) HLA-matched family donors (MFDs), and HLA-matched unrelated donors (MUD). Results are given after stratification for early (ear) and advanced (adv) disease stage. ▵ indicates MFD ear (n = 48); •, MUD ear (n = 66); □, ISD ear (n = 110); ▴, ISD adv (n = 28); ▿, MUD adv (n = 35); and ⋄, MFD adv (n = 38).
Multivariate statistical analysis revealed the parameters “disease stage,” “patient age,” “time interval between diagnosis and transplantation,” as well as “donor age” to be independent risk factors for OS (cf, Table 2). In contrast, the type of donor was excluded from the Cox model for OS, irrespective of whether tested as “MUD: yes/no” (P = .6) or as “MFD: yes/no” (P = .4). For comparison with the explanatory parameters, listed in Table 2, the hazard ratios (95% confidence intervals) for the excluded parameters “MUD” and “MFD” were 1.17 (0.7-1.7) and 1.15 (0.7-1.7), respectively.
Influence of baseline variables on transplantation outcome: clinical end points and corresponding Cox models (backward exclusion, P > .02)
Clinical end point . | Baseline variable . | Hazard ratio . | 95% Confidence interval . | P . |
|---|---|---|---|---|
| Overall survival | Disease stage | 3.31 | 2.3-4.6 | <.0001 |
| Patient age | 1.03* | 1.01-1.05 | .002 | |
| Interval Dg → TX | 1.09* | 1.00-1.2 | .07 | |
| Donor age | 1.01* | 0.99-1.02 | .15 | |
| Treatment-related mortality | Disease stage | 3.45 | 2.3-5.0 | <.0001 |
| Patient age | 1.04* | 1.02-1.06 | <.001 | |
| Donor age | 1.02* | 1.00-1.03 | .04 | |
| Acute graft-vs-host disease | Donor MFD | 2.18 | 1.3-3.5 | <.0001 |
| Donor MUD | 1.72 | 1.05-2.79 | .03 | |
| Patient age | 1.02* | 1.00-1.05 | .02 | |
| Donor age | 1.01* | 0.99-1.03 | .15 | |
| Disease stage | 1.31 | 0.98-1.99 | .14 | |
| Relapse | Disease stage | 3.15 | 1.7-5.6 | <.0001 |
| Donor MUD | 0.42 | 0.2-0.8 | .01 | |
| Donor MFD | 0.51 | 0.2-1.0 | .05 | |
| Graft source | 1.91 | 1.0-3.5 | .03 | |
| Sex mismatch | 0.42 | 0.2-0.9 | .12 |
Clinical end point . | Baseline variable . | Hazard ratio . | 95% Confidence interval . | P . |
|---|---|---|---|---|
| Overall survival | Disease stage | 3.31 | 2.3-4.6 | <.0001 |
| Patient age | 1.03* | 1.01-1.05 | .002 | |
| Interval Dg → TX | 1.09* | 1.00-1.2 | .07 | |
| Donor age | 1.01* | 0.99-1.02 | .15 | |
| Treatment-related mortality | Disease stage | 3.45 | 2.3-5.0 | <.0001 |
| Patient age | 1.04* | 1.02-1.06 | <.001 | |
| Donor age | 1.02* | 1.00-1.03 | .04 | |
| Acute graft-vs-host disease | Donor MFD | 2.18 | 1.3-3.5 | <.0001 |
| Donor MUD | 1.72 | 1.05-2.79 | .03 | |
| Patient age | 1.02* | 1.00-1.05 | .02 | |
| Donor age | 1.01* | 0.99-1.03 | .15 | |
| Disease stage | 1.31 | 0.98-1.99 | .14 | |
| Relapse | Disease stage | 3.15 | 1.7-5.6 | <.0001 |
| Donor MUD | 0.42 | 0.2-0.8 | .01 | |
| Donor MFD | 0.51 | 0.2-1.0 | .05 | |
| Graft source | 1.91 | 1.0-3.5 | .03 | |
| Sex mismatch | 0.42 | 0.2-0.9 | .12 |
Disease stage indicates early = 0, advanced = 1; patient age, the patient age at transplantation (years); interval Dg → TX, time interval between diagnosis and transplantation (years); donor age, donor age at transplantation (years); donor MFD, use of a matched family donor = 1 versus other type of donor = 0; donor MUD, use of a matched unrelated donor = 1 versus other type of donor = 0; graft source, bone marrow = 1, peripheral blood = 0; and sex mismatch, female donor for male patient = 1 versus other constellation = 0
Note that the given hazard ratios are calculated on a per-year basis
Thus, in contrast to advanced disease and advanced patient age, the type of donor (ISD, MFD, or MUD) had no significant impact on OS after HSCT in our study.
Treatment-related/non–relapse-related mortality
Univariate statistical analysis. Treatment-related mortality (TRM) was found to be clearly influenced by disease stage and patient age. The 5-year TRM, for example, was 38% for patients with early disease but 60% for patients with advanced disease, and 25% for patients 37 years or younger compared with 48% for patients older than 37 years. In contrast, the other initial variables listed in Table 1 including the type of donor had no significant influence on TRM. To illustrate the latter finding, we calculated the TRM for ISD, MFD, and MUD group patients after stratification for the dominating risk factor (ie, disease stage). For patients with early disease we documented a 5-year TRM of 27%, 29%, and once again 29% in the ISD, MFD, and MUD groups, respectively; the corresponding percentages for patients with advanced disease were 55%, 58%, and 65%, respectively.
Multivariate statistical analysis. Cox model building suggested 3 of the initial transplantation characteristics listed in Table 1 to be independent risk factors for TRM, namely “disease stage,” “patient age,” and “donor age” (cf, Table 2). In contrast, the type of donor was eliminated from the Cox model irrespective of whether tested as “MUD: yes/no” (P = .5) or “MFD: yes/no” (P = .2). The calculated hazard ratios (95% confidence intervals) for the excluded parameters “MUD” and “MFD” were 1.2 (0.7-1.9) and 1.3 (0.8-2.0), respectively.
Graft failure (GF)
Only patients surviving day 28 after transplantation (n = 318) were included into the analysis. Notably, no case of GF was observed in the 136 patients who underwent an ISD transplantation, whereas GF occurred in 8 of 84 patients who underwent an MFD transplantation and in 5 of 98 patients grafted with an MUD.
The Kaplan-Meier procedure was used to evaluate the impact of the type of donor on the risk of primary GF on day 28 after transplantation. The calculated risk of GF differed significantly between the 3 groups (ISD: 0%, MFD: 8.5%, and MUD: 5.2%; P < .004, log-rank test). In contrast, the difference between the MFD and MUD group was not significant.
To study the influence of the other baseline variables listed in Table 1 on GF, cross-table calculations including all evaluable MFD and MUD group patients (n = 182) were performed. This type of analysis revealed the variable “graft source (BM vs PB)” to have an impact on the risk of GF. In the MUD group, BM was used in 74 cases and PB in 24 cases, and all observed 5 cases of GF were within the BM subgroup. In the MFD group, BM was used in 38 cases and PB in 46 cases. Of the 8 cases with graft failure, 7 were within the BM and only 1 in the PB subgroup. For MFD patients the observed difference in the graft failure rate between BM and PB was significant (2-sided Fisher exact test, P = .02).
Thus, the use of an MFD or an MUD instead of an ISD is clearly associated with a higher risk of GF.
Acute graft-versus-host disease (aGVHD)
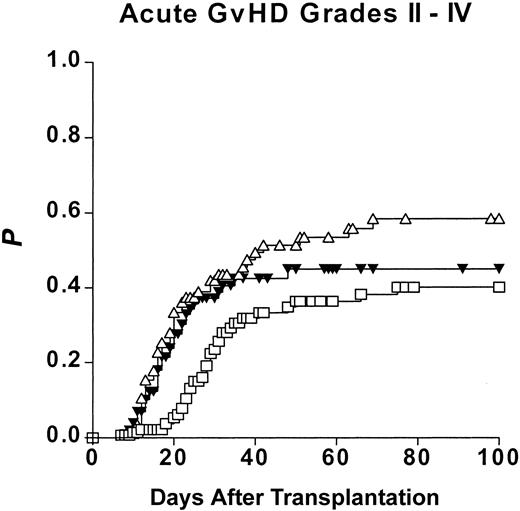
Univariate statistical analysis disclosed the risk of grades II to IV aGVHD to be influenced by 3 parameters, namely the type of donor (as depicted in Figure 3), patient age (≤ 37 years: 37%; 37 years: 60%), and donor age (≤ 38 years: 39%; > 38 years: 57%).
Risk of acute graft-versus-host disease (Kaplan-Meier estimates) after hematopoietic stem cell transplantation from genotypically HLA-identical siblings (ISDs), alternative (partially) HLA-matched family donors (MFDs), and HLA-matched unrelated donors (MUDs). □ represents ISDs; ▵, MFDs; and ▾, MUDs.
Risk of acute graft-versus-host disease (Kaplan-Meier estimates) after hematopoietic stem cell transplantation from genotypically HLA-identical siblings (ISDs), alternative (partially) HLA-matched family donors (MFDs), and HLA-matched unrelated donors (MUDs). □ represents ISDs; ▵, MFDs; and ▾, MUDs.
Multivariate statistical analysis confirmed the above results since it suggested (P < .2) the following initial variables as independent risk factors for grades II to IV aGVHD: type of donor “MFD,” type of donor “MUD,” “patient age,” “donor age,” and “disease stage” (cf, Table 2). The analysis of interactions between the parameters of main interest “MFD” and “MUD” and the other risk factors of the final Cox model revealed that the introduction of the interaction variable “MFD * Patient age” improved the so-far presented Cox model significantly (increase of Chi-square > 3.841).
Thus, the risk of aGVHD is clearly increased after HSCT from an MFD as well as from an MUD compared with an ISD and appears to be especially high in case of patients with advanced age who underwent an MFD transplantation.
Relapse (REL)
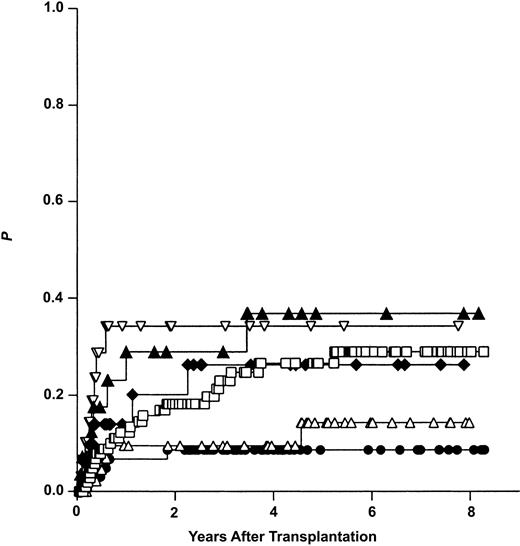
Univariate statistical analysis identified several variables to influence the risk of REL. The stage of disease had the greatest impact (early: 19%, advanced: 33%), followed by the type of donor (ISD: 29%, MFD: 19%, MUD: 16%), and the graft source (PB: 15%, BM: 27%). Figure 4 illustrates the calculated risk of relapse for ISD, MFD, and MUD group patients after stratification for the dominating influence variable “stage of disease.” Of major interest was the high risk of REL in ISD group patients with early disease compared with MFD and MUD group patients with early disease.
Risk of relapse (Kaplan-Meier estimates) after allogeneic hematopoietic stem cell transplantation from genotypically HLA-identical siblings (ISDs), alternative (partially) HLA-matched family donors (MFDs), and HLA-matched unrelated volunteers (MUDs). Results are given after stratification for early (ear) and advanced (adv) disease stage. ▴ indicates ISD adv (n = 28); ▿, MUD adv (n = 35); □, ISD ear (n = 110); ⋄, MFD adv (n = 38); ▵, MFD ear (n = 48); and •, MUD ear (n = 66).
Risk of relapse (Kaplan-Meier estimates) after allogeneic hematopoietic stem cell transplantation from genotypically HLA-identical siblings (ISDs), alternative (partially) HLA-matched family donors (MFDs), and HLA-matched unrelated volunteers (MUDs). Results are given after stratification for early (ear) and advanced (adv) disease stage. ▴ indicates ISD adv (n = 28); ▿, MUD adv (n = 35); □, ISD ear (n = 110); ⋄, MFD adv (n = 38); ▵, MFD ear (n = 48); and •, MUD ear (n = 66).
Multivariate statistical analysis confirmed the results given above since it suggested (with P < .2) the following independent risk factors for relapse: “disease stage,” type of donor “MUD,” type of donor “MFD,” “graft source,” and “sex mismatch” (cf, Table 2).
Thus, the use of an MFD or an MUD instead of an ISD seems to be protective (hazard ratio < 1) against relapse at least in patients with early disease.
Impact of HLA mismatches
The data presented above demonstrated MFD and MUD group patients to be at a higher risk of aGVHD and of graft failure compared with ISD group patients. However, the MFD group was very heterogeneous with regard to the pattern of donor/recipient HLA mismatches, as detailed in Table 1. Thus, we decided to evaluate whether the documented increased risk of aGVHD and of GF of the MFD group can be attributed to transplantations with special patterns of donor/recipient HLA mismatches.
Impact of HLA mismatches on aGVHD
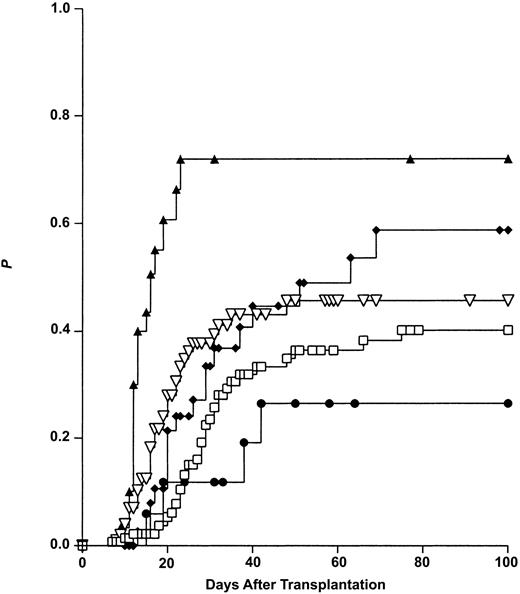
The Kaplan-Meier procedure was used to calculate the risk of aGVHD for the following subgroups of transplantations: “MFD class I” = MFD transplantations with one or more HLA class I mismatches but no HLA class II mismatch in GVH direction (n = 41), “MFD class II” = MFD transplantations with no class I but 1 or 2 class II mismatches in GVH direction (n = 21), “MFD MM” = MFD transplantations with no class I and no class II mismatch in GVH direction (n = 16), and MUD transplantations. Outcome data of ISD transplantations served as controls. As shown in Figure 5 the aGVHD risk was found to be highest in the “MFD class II” group (75%), intermediate in the “MFD class I” (61%) as well as MUD (46%) groups, and lowest in the ISD (40%) and the “MFD no MM” (28%) groups.
Impact of mismatched HLA loci on the risk of acute graft-versus-host disease (Kaplan-Meier estimates). Class I MM: 1A or 1B or 1C or 1B + 1C mismatch, but no class II mismatch in GVH direction. Class II MM: 1 DRB1 and/or 1 DQB1 mismatch, but no class I mismatch in GVH direction. For comparison, the outcomes after transplantation from HLA-identical siblings (ISDs) and HLA-matched unrelated donors (MUDs) are also shown. ▴ indicated MFD-MM II (n = 21); ⋄, MFD-MM I (n = 41); ▿, MUD (n = 101); □, ISD (n = 138); and •, MFD-no MM (n = 16).
Impact of mismatched HLA loci on the risk of acute graft-versus-host disease (Kaplan-Meier estimates). Class I MM: 1A or 1B or 1C or 1B + 1C mismatch, but no class II mismatch in GVH direction. Class II MM: 1 DRB1 and/or 1 DQB1 mismatch, but no class I mismatch in GVH direction. For comparison, the outcomes after transplantation from HLA-identical siblings (ISDs) and HLA-matched unrelated donors (MUDs) are also shown. ▴ indicated MFD-MM II (n = 21); ⋄, MFD-MM I (n = 41); ▿, MUD (n = 101); □, ISD (n = 138); and •, MFD-no MM (n = 16).
Multivariate statistical analysis was confined to the enrolled MFD and MUD transplantations (n = 187). The type of donor was tested as “MUD: yes/no,” “sum of HLA class I mismatches in GVH direction (A + B + C),” and “sum of HLA class II mismatches in GVH direction (DRB1 + DQB1).” Multivariate analysis suggested the “sum of HLA class II mismatches” (P < .001) and “patient age” (P = .08) as independent risk factors for aGVHD after HSCT from an MFD or an MUD with hazard ratios (95% confidence interval) of 2.1 (1.5-3.1) and 1.02 (0.99-1.04), respectively. In contrast, the variables “MUD” and “sum of HLA class I mismatches” were eliminated from the Cox model with P values of .5 and .6 and hazard ratios (95% confidence interval) of 1.2 (0.6-2.5) and 1.1 (0.7-1.7), respectively.
Thus, MFD transplantations mismatched for HLA class II (DRB1 ± DQB1) antigens in GVH direction imply a higher risk of grades II to IV aGVHD compared with MFD transplantations mismatched for HLA class I (A, B, and C) antigens in GVH direction or MUD transplantations. Due to the high linkage disequilibrium between HLA-DRB1 and DQB1 isolated DRB1 and DQB1 donor/recipient disparities were too rare to allow for an analysis of the impact of single locus HLA class II mismatches on aGVHD.
Impact of HLA mismatches on primary GF
All MFD (n = 83) and MUD (n = 98) group patients surviving day 28 after transplantation were included in the analysis. First, cross-table calculations for the occurrence of GF were performed in patients with 0, 1, 2, and 3 HLA class I (A + B + C) mismatches in HVG direction, irrespective of the presence or absence of additional HLA class II mismatches in HVG direction. Consequently, all evaluable MUD group patients figured with 0 mismatches. The rate of GF was shown to increase significantly with the number of HLA class I disparities from 6 (4.8%) of 125 and 1 (3.6%) of 28 for patients with nil and 1 mismatch, respectively to 3 (13.6%) of 22 and 3 (50%) of 6 for patients exhibiting 2 and 3 mismatches, respectively (P = .003, 2-sided Fisher exact test). In contrast, HLA class II disparities had no influence on primary GF after adjustment for class I disparities and class II disparities.
Finally, we tested the impact of the sum of HLA class I (A + B + C) and the sum of HLA class II (DRB1 + DQB1) mismatches in HVG direction on GF by Cox model building. The sum of HLA class II mismatches was not found to have an impact on GF (P = .7, hazard ratio 1.1 [95% confidence interval, 0.5-2.3]), whereas the variable “sum of HLA class I mismatches in HVG direction” appeared as a prominent risk factor for GF with a P value of less than .001 and a hazard ratio (95% confidence interval) of 2.6 (1.6-4.2). Thus, MFD transplantations with more than one HLA class I (A ± B ± C) mismatch in host-versus-graft (HVG) direction are associated with a higher risk of graft failure compared with other subgroups of MFD transplantations or with MUD transplantations.
Discussion
The present retrospective single-center study compares 3 different approaches of HSCT as practiced at our institution, namely HSCT from genotypically HLA-identical siblings (ISDs), related donors other than ISDs (MFD), and HLA-matched unrelated volunteers (MUDs). The main aim of the study was to answer the question of whether HSCTs from MFDs or from defined subgroups of MFDs have an inferior clinical outcome compared with MUDs.
Most differences in initial characteristics between the 3 study cohorts documented in Table 1 were an integral part of the evaluated procedures, for example, the higher mean donor age in the MFD group (since the patients' parents served as MFDs in multiple cases) or the lower frequency of sex-mismatched transplantations in the MUD group (since exclusion of sex-mismatched donors was feasible only when several donors were available for one patient, which is a rare event in the ISD and MFD settings). Nevertheless, all differences in initial characteristics were regarded as possibly confounding variables. Thus, results suggesting an impact of the parameter “type of donor used” on clinical outcome were accepted only if confirmed by multivariate analysis.
The sample size of the presented single-center study appears to be sufficient with regard to the study objective, especially because the scientific question has not been adequately considered by other studies. However, even if the quality of the presented data allows for making valid statistical predictions, we are aware that the number of cases enrolled is comparatively low in view of the clinical impact of our main conclusions. This applies especially to our finding that overall survival appears to be similar between the 3 study groups (cf, Figure 2 and Table 2).
Our finding of a similar long-term overall survival among the 3 study groups is surprising at least at first glance, since at the same time the present study demonstrated an increased risk of primary GF (cf, “Results,” section 3) as well as acute GVHD grades II to IV (cf, “Results,” section 4) in the MFD and MUD compared with the ISD group, and GF as well as clinically relevant aGVHD are well-known life-threatening complications after HSCT.
However, our findings are in accordance with those of a recent large multicenter study, comparing the outcome after ISD and MUD transplantations for CML,21 and may be explained as follows. In a statistical analysis focusing on long-term OS after HSCT the impact of GF on OS may become “invisible” for the following reasons: (1) primary GF is a rare event even in the MFD (8.5%) and MUD (5.2%) groups and occurs early after transplantation. Thus, more frequent causes of death may “dilute” the impact of GF on OS at the long term, and (2) patients successfully undergoing retransplantation after GF are notably not censured in the Kaplan-Meier analysis for OS. Furthermore, the adverse effect of acute GVHD on OS after transplantation from MFDs or MUDs seem to be compensated at the long-term by the documented reduced risk of relapse at least in patients who underwent transplantation for early disease (cf, Figure 4). At any rate, our finding of a similar OS after transplantation from an ISD, MFD, and MUD allows for 2 conclusions: (1) MFDs and MUDs, as defined by this study, are both acceptable in principal if an ISD is not available and (2) the worldwide efforts in building up “unrelated marrow donor registries” were worthwhile.6,22-24
Another important result of this study is that 2 subgroups of MFD transplantations had an inferior outcome compared with the ISD and MUD groups: (1) donor/recipient HLA class II (DRB1 ± DQB1) antigen mismatches in GVH direction were associated with an increased risk of clinically relevant aGVHD (cf, Figure 5), and (2) HLA class I (A ± B ± C) mismatches in HVG direction (especially if combined) resulted in an elevated risk of graft failure. Thus, for patients lacking an HLA-identical sibling donor, an available related donor mismatched for HLA-DRB1 ± DQB1 in GVH or for HLA-A ± B ± C in HVG direction should be selected only in clinically urgent settings that leave no time to identify a possibly available MUD.
Additionally, our study gave a detailed insight into the biology of HLA in the context of allogeneic HSCT from related donors. Obviously, HLA class II mismatches in GVH direction play a key role for the development of aGVHD, whereas HLA class I mismatches in HVG direction have a major impact on the risk of graft failure. Of note, our findings on HLA biology in the related setting are identical to those of a recent study from Seattle analyzing a large cohort of unrelated transplantations.25 Another point of interest is the relatively high relapse rate for the early disease group (cf, Figure 4). A straightforward explanation for this phenomenon is that the use of an MFD or an MUD is protective against relapse only in case of early disease, but not in case of (generally more aggressive) advanced disease stages.
Finally, the data of this study indirectly contribute to the recent discussion of whether donor/recipient HLA-A, B, and C sequence-based typing (SBT)26-28 will improve the outcome after unrelated HSCT without compromising donor availability.29,30 Our present data clearly argue against the routine use of SBT. We did not use this time-consuming typing technique and could nevertheless achieve the same long-term OS results after transplantation from an MUD and a “golden-standard” ISD. Of interest is that a recent editorial published in the New England Journal of Medicine31 endorses our present view. Nevertheless, our data on the value of SBT are still preliminary, since our outcome results were reached under special supportive therapy modalities32 (strict reverse isolation and consequent gut decontamination of anaerobic bacteria).
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-09-2866.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This paper is dedicated to Prof Dr Ulrich W. Schaefer, Director of the Department of Bone Marrow Transplantation, University Hospital of Essen, who died on the 18th of August, 2002.
We thank Dr Shraga Goldmann, University Hospital of Ulm, and our local unrelated donor search coordinator, Mrs Sabine Riebschläger, for fruitful discussions.

![Figure 1. HLA characteristics of transplantations from HLA-matched family donors other than genotypically HLA-identical siblings (MFD study group). HLA matches (white boxes) and HLA mismatches (black boxes) are detailed by the HLA loci (A, B, C, DRB1, and DQB1) and the immunologic vector (GVH indicates graft-versus-host; HVG, host-versus-graft). N indicates number of transplantations for a given HLA constellation. More than 95% of the given HLA mismatches were full antigen mismatches (eg, A*02 vs A*03, DRB1*01 vs DRB1*04), whereas the remaining mismatches were HLA antigen split mismatches (eg, B*15 [62] vs B*15 [63], B*40 [60] vs B*40 [61]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/3/10.1182_blood-2002-09-2866/6/m_h81534714001.jpeg?Expires=1769206838&Signature=YpIUZOo6rY7lf81arHKXV7Q3yU-kprS0T1eWuSpAQieJQYLI7-LyDoVrVodNIawl5x0eNTP~bmr7kIlpn7476RmkzSLKYWuv9vUGnTIx62LXGTprF6QJ9TJvKDZfQa3x-67ARX8gmezE9z2BAndy30lA-DMLdlq9WIgawRk0Cq9aY4CNkQQ2L3uDq~VOMfj9NmnJuWofb26KkabdJojEWS5QZn2h93IVONfUoDQ8m0tcM0yBlCWhKjGLWdSRomkPUeWGNbojDban4HP-G6lhinB2h4nReaS~xe-QoKFMBAPFro-WTqeXoXOKXYPHWqXafEjRdKnPaPXpM6OJhROB3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal