About 75% of patients with newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML) treated initially with imatinib achieve complete cytogenetic remission (CCyR),1 and imatinib also induces Ph negativity, though less often, in patients treated in advanced phases of CML. Patients in CCyR may however still have as many as 1010 leukemia cells in their body,2 and in CML the quantity of such “residual disease” can be monitored by quantitating BCR-ABL transcripts.3 We have studied serially BCR-ABL transcript numbers in a series of CML patients treated with imatinib who achieved CCyR. The results confirm that they constitute a heterogeneous group whose individual prognoses may be very different. The failure to detect BCR-ABL transcripts on repeated testing was rare.
We identified 42 CML patients (37 originally in CP, 5 in accelerated phase [AP]) treated with imatinib at 8 collaborating United Kingdom centers who were classified as having achieved CCyR on the basis of 100% Ph negativity in at least 20 marrow metaphases. At the time of starting imatinib the patients were newly diagnosed (n = 15), chronic phase refractory to interferon-α (n = 24), or in relapse after stem cell transplantation (n = 2). The median age was 52.3 years (range, 24.8-69.9 years), and median time to achieve CCyR was 5.5 months (range, 1.8-16.4 months). The daily dose of imatinib was 400 mg (CP) or 600 mg (AP). Serial quantitative polymerase chain reaction (Q-PCR) assays were performed at regular intervals at a single center using Taq-man technology (Applied Biosystems, Foster City, CA).4 The median number of samples per patient was 5 (range, 3-15 samples). Results were expressed as a percentage ratio using ABL transcripts as the internal control. The failure to detect BCR-ABL by Taq-man assay was confirmed with a 2-step nested technique as previously described.5
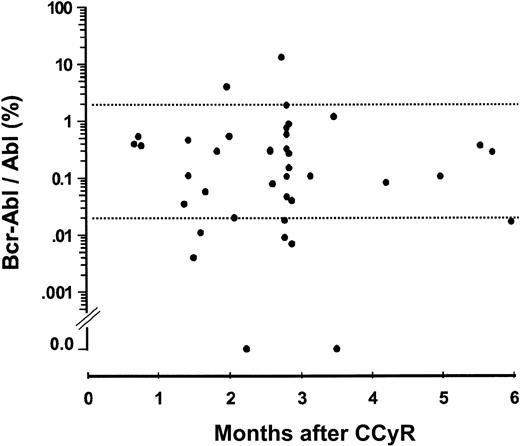
The median BCR-ABL/ABL ratio was 0.19% (range, 0.0%-13.1%) for the first sample after achieving CCyR; only 2 patients had ratios above 2.0% (Figure 1). During serial monitoring thereafter 17 (40%) patients (15 originally in CP; 2 originally in AP) achieved a BCR-ABL/ABL ratio less than 0.02% on at least 2 occasions 4 or more weeks apart. Only 1 of the 17 patients who achieved a ratio less than 0.02% later lost the CCyR and progressed to hematologic relapse. Thirty-three patients always had detectable BCR-ABL transcripts. In 7 cases transcripts were undetectable on at least one occasion but positive on others. Two patients had no detectable transcripts on serial monitoring for at least 6 months; in one case both Taq-man and nested PCR studies were negative on 3 occasions over 11 months, and in the other, 3 sequential specimens were negative over 6 months.
BCR-ABL/ABL ratios in first available sample from 42 patients reported to be in complete cytogenetic remission. Two patients had values above 2.0%, and 7 patients had values below 0.02%. There was no obvious relationship between the interval from first classification as CCyR and the level of BCR-ABL transcripts.
BCR-ABL/ABL ratios in first available sample from 42 patients reported to be in complete cytogenetic remission. Two patients had values above 2.0%, and 7 patients had values below 0.02%. There was no obvious relationship between the interval from first classification as CCyR and the level of BCR-ABL transcripts.
In summary we have shown that Q-PCR values in CML patients in CCyR are very variable. The failure to detect any transcripts, as described by Barbany et al6 in one patient, was a rare occurrence and frequently was not sustained. We suggest that the term these authors employed, “molecular remission,” is imprecise since it depends very much on the sensitivity of the assay employed. It would be preferable to refer to responding patients as having transcripts undetectable for a specified period. Our data support the recommendation that quantification of BCR-ABL transcripts is essential for optimum management of CML patients who achieve CCyR on imatinib.7

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal