Abstract
Human hepcidin, a 25–amino acid peptide made by hepatocytes, may be a new mediator of innate immunity and the long-sought iron-regulatory hormone. The synthesis of hepcidin is greatly stimulated by inflammation or by iron overload. Evidence from transgenic mouse models indicates that hepcidin is the predominant negative regulator of iron absorption in the small intestine, iron transport across the placenta, and iron release from macrophages. The key role of hepcidin is confirmed by the presence of nonsense mutations in the hepcidin gene, homozygous in the affected members, in 2 families with severe juvenile hemochromatosis. Recent evidence shows that deficient hepcidin response to iron loading may contribute to iron overload even in the much milder common form of hemochromatosis, from mutations in the HFE gene. In anemia of inflammation, hepcidin production is increased up to 100-fold and this may account for the defining feature of this condition, sequestration of iron in macrophages. The discovery of hepcidin and its role in iron metabolism could lead to new therapies for hemochromatosis and anemia of inflammation.
Introduction
The pathogenesis of anemia of inflammation and the regulation of iron absorption and distribution rank among the major unsolved problems in classical hematology. In the last 2 years, rapid progress has been made on both of these problems by elucidation of the central role of hepcidin, an iron-regulatory hormone and a mediator of innate immunity. To communicate the excitement of this evolving work, I will provide the essential background information on iron metabolism and its relationship to host defense, and then review the studies that led to our current understanding of the role of hepcidin in iron metabolism and host defense. Finally, I will discuss those areas where our insight is sparse and where more research is needed.
Background
Iron is an essential element for nearly all living organisms.1 It is a key functional component of oxygen transporting and storage molecules (eg, hemoglobin and myoglobin) and of many enzymes that catalyze the redox reactions required for the generation of energy (eg, cytochromes), the production of various metabolic intermediates, and for host defense (eg, nicotinamide adenine dinucleotide phosphate [NADPH] oxidase). Humans and other vertebrates strictly conserve iron by recycling it from senescent erythrocytes and from other sources. The loss of iron in a typical adult male is so small that it can be met by absorbing only 1 to 2 mg of iron per day.2 In comparison, the total body iron in an adult male is 3000 to 4000 mg and the daily iron requirement for erythropoiesis is about 20 mg. Such conservation of iron is essential because many human diets contain just enough iron to replace the small losses. However, when dietary iron is more abundant, absorption is appropriately attenuated.
Important homeostatic mechanisms prevent excessive iron absorption in the proximal small intestine and regulate the rate of iron release from macrophages involved in recycling.2 Why is this necessary? Cellular iron that is not used by other ferroproteins accumulates in ferritin whose capacity for iron is limited, and may be exceeded after a prolonged period of iron excess. As illustrated by patients with severe forms of hemochromatosis or iron overload, whose total body iron is more than 5 to 10 times normal, excessive tissue iron causes widespread organ damage. It is also possible that rapid release of iron from macrophages could create local iron overload and cause localized tissue injury. The toxic effects of free iron are ascribed to its ability to catalyze the generation of reactive free radicals.1
Another reason why iron must be tightly controlled is that resistance to infection is in part dependent on the outcome of a tug-of-war over iron between the host and the invading bacteria. Bacteria have evolved multiple sophisticated mechanisms3 for acquiring iron in environments where very little free iron is available. These devices include the secretion of iron-binding organic molecules, siderophores, and their reuptake by bacteria (the avidly iron-binding desferroxamine is a siderophore). The most highly adapted pathogens even evolved the ability to wrest iron from host iron-binding proteins such as hemoglobin, transferrin, and lactoferrin. Bacteria incur metabolic costs to obtain iron in iron-poor environments, and these may limit growth. Iron deprivation may also inhibit the formation of resistant bacterial biofilms4 and favor the mobile but vulnerable unicellular forms that are better equipped to reach alternative iron sources. Conversely, bacteria grow faster and form biofilms more readily when iron is abundant. For these or possibly other reasons, patients and mice with iron overload are more susceptible to a number of intracellular and blood pathogens,5,6 and even relatively modest increase in iron intake may diminish host resistance to infection.7
The connection between iron and innate immunity is also evidenced by at least 2 key proteins of iron metabolism that have homologs in innate immunity. Transferrin, the main iron transport protein in plasma, is closely related to the lactoferrin, a highly abundant iron-binding protein of neutrophils and epithelial secretions. The antimicrobial effect of lactoferrin is due in part to its ability to chelate iron.8 In intestinal epithelial cells, iron is absorbed through the apical transporter DMT1 (divalent metal transporter 1) also called Nramp2 (natural-resistance-associated macrophage protein 2)9,10 because of its close relationship to Nramp1, a divalent cation transporter in phagocytic vacuoles of macrophages. Nramp1 mutations diminish the microbicidal activity of macrophages and cause increased susceptibility to infections with intracellular pathogens in mice and humans.11
Analysis of iron metabolism in human diseases and animal models suggests several major influences2 that determine how much iron is absorbed from food and how much recycled iron is released from macrophages. Iron requirements of bone marrow erythropoiesis stimulate iron absorption in the small intestine, while excessive iron stores (accumulated predominantly in the liver) provide an inhibitory signal. Both of these responses are homeostatic under most conditions. In diseases characterized by increased destruction of erythrocyte precursors (eg, in thalassemias), the dominance of the stimulus of erythropoietic demand over inhibition by iron stores can cause iron overload, even in the absence of transfusions. Infections and inflammatory diseases induce iron sequestration in macrophages, the hallmark of anemia of inflammation (formerly known as anemia of chronic disease), and also decrease iron absorption in the small intestine. It is generally assumed that the iron sequestration response may increase the resistance to infections by restricting the availability of iron to microbes5 but the details remain speculative.
Since the sites of iron absorption, recycling, storage, and utilization are distant from each other, it was reasonable to expect that iron-regulatory hormones must exist to account for the observed interactions between these compartments, and that inflammatory substances may also be involved in iron regulation. However, the molecular basis of these signals was elusive for many years, until a series of converging and often serendipitous discoveries provided the long-sought opening.
Hepcidin is a new mediator of innate immunity
During studies of antimicrobial properties of various human body fluids, Park et al12 isolated a new peptide from human urine and named it hepcidin, based on its site of synthesis (the liver, hep-) and antibacterial properties in vitro (-cidin). Independently, Krause et al13 isolated the same peptide from plasma ultrafiltrate and named it LEAP-1 (liver-expressed antimicrobial peptide). The major hepcidin form was a cationic peptide with 25 amino acid residues and 4 disulfide bridges. Surprisingly, and unlike other antimicrobial peptides, hepcidin sequences were remarkably similar among various mammalian species, and searches of expressed sequence tag (EST) databases also identified several clearly related fish hepcidins. In humans, the peptide is derived from the C-terminus of an 84–amino acid prepropeptide, encoded by a 0.4–kilobase (kb) mRNA generated from 3 exons of a 2.5-kb gene on chromosome 19. Compared with other antimicrobial peptides whose sequences have evolved rapidly and vary significantly even between closely related mammalian species, the evolution of hepcidin was constrained and hinted at the possibility that this peptide might specifically interact with other conserved macromolecules. Hepcidin composition and its site of synthesis was reminiscent of drosomycin, a 4-disulfide insect defensin synthesized in the fat body of drosophila (the equivalent of the liver) in response to infections. In the course of these initial studies, one of the urine donors developed a systemic infection, and the concentration of hepcidin was increased about 100-fold in the acute urine samples. This observation reinforced the notion that hepcidin was a mediator of innate immunity.
Hepcidin structure
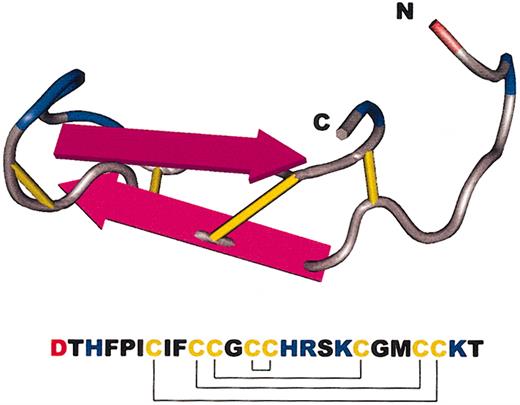
Although the predominant form of hepcidin in human urine contained 25 amino acids (hepcidin-25), two peptides shorter at the amino terminus were also found, hepcidin-22 and hepcidin-20. Synthetic versions of hepcidin-20 and -25 were prepared and could be refolded at moderate yield after reduction under denaturing conditions and reoxidation12 (see also Kluver et al14 for an alternative procedure). As judged by mass spectrometry, electrophoretic migration in acid-urea polyacrylamide gel electrophoresis, retention on C18 reverse-phase high-performance liquid chromatography (HPLC) columns, and reactivity with antihepcidin antibody, the natural and synthetic forms were identical. Hunter et al15 analyzed the synthetic forms by nuclear magnetic resonance spectrometry and established their connectivity and solution structure (Figure 1). The molecule is a simple hairpin whose 2 arms are linked across by disulfide bridges in a ladderlike configuration. One highly unusual feature of the molecule is the presence of disulfide linkage between 2 adjacent cysteines near the turn of the hairpin. Compared with most disulfide bonds, disulfide bonds formed between adjacent cysteines are stressed and could have a greater chemical reactivity. Like other antimicrobial peptides, hepcidin displays spatial separation of its positively charged hydrophilic side chains from the hydrophobic ones, a characteristic of peptides that disrupt bacterial membranes.
Amino acid sequence and a model of the major form of human hepcidin. The amino and carboxy termini are labeled as N and C, respectively. Disulfide bridges are in yellow, basic amino acids in blue, and acidic in red. The pattern of disulfide linkages between the 8 cysteines is also shown in the amino acid sequence.
Amino acid sequence and a model of the major form of human hepcidin. The amino and carboxy termini are labeled as N and C, respectively. Disulfide bridges are in yellow, basic amino acids in blue, and acidic in red. The pattern of disulfide linkages between the 8 cysteines is also shown in the amino acid sequence.
Iron loading induces hepcidin
The connection between hepcidin and iron metabolism was first made by Pigeon et al16 during studies of the hepatic responses to iron overload. They found the mRNA for a murine hepcidin by subtractive hybridization of iron-overloaded versus normal livers and showed that the mRNA was predominantly expressed by hepatocytes. The hepcidin mRNA was induced not only by dietary or parenteral iron overload but also by treatment of the mice with lipopolysaccharide. Moreover, β2-microglobulin knock-out mice, a murine model of hemochromatosis, showed increased levels of hepcidin mRNA on a normal diet but not a low-iron diet. These results clearly indicated that hepcidin was regulated by iron as well as by immune stimuli. The authors also mapped and sequenced the human and murine hepcidin genes and noted that the mouse had 2 closely related hepcidin genes and transcripts (hepcidin-1 and -2), whereas there was only 1 in humans. Importantly, the authors also placed the hepcidin gene into the close vicinity of the upstream stimulatory factor-2 (USF2) gene.
Hepcidin is the dominant regulator of the absorption of dietary iron and the release of iron from macrophages
The USF2 gene was under investigation as a hepatic transcription factor involved in the regulation of glucose and lipid metabolism.17 Unexpectedly, Nicolas et al18 found that mice with USF2 gene knock-out developed spontaneous hemochromatosis. The pattern of organ involvement was similar to early human hemochromatosis, in that the liver and the pancreas had increased iron content but the spleen was iron-deficient, even relative to spleens of control C57BL6 mice. After they observed that the livers of the USF2 knock-out mice completely lacked both hepcidin mRNAs, the authors concluded that hepcidin was a negative regulator of iron uptake in the small intestine and of iron release from macrophages. In this model, the absence of hepcidin would account for unregulated hyperabsorption of iron leading to iron overload, as well as unregulated discharge of macrophage iron, leading to iron-depleted macrophages in the spleen. In an accompanying editorial,19 Fleming and Sly proposed that hepcidin was the long-anticipated iron regulatory hormone and raised the possibility that the overproduction of hepcidin during infections and inflammation may be responsible for anemia of inflammation.
At this point, the potential role of USF2 was still confounding. However, in a follow-up study,20 Nicolas et al demonstrated that another line of USF2 knock-out mice prepared with a different knock-out construct had normal hepcidin mRNA levels and no detectable iron abnormalities. Thus, the original USF2 knock-out exerted a “cis” effect, interfering with the transcription of hepcidin genes located near the site of disruption. Moreover, transgenic mice constructed to overexpress hepcidin in their livers20 died shortly after birth of severe iron deficiency, indicating that hepcidin was also a negative regulator of placental transport of iron to the fetus. Some mosaic founders, presumably exposed to less hepcidin, survived but suffered from severe iron deficiency, which could apparently not be fully reversed by parenteral iron. These observations indicate that hepcidin may exert its blocking effect on iron transport at multiple sites, including the intestinal epithelium, the placenta, macrophages, and perhaps other cell types as well.
In a study that mirrored the mouse model of hepcidin overexpression, Weinstein et al21 analyzed resected tumors from 2 patients with large hepatic adenomas and severe iron-refractory microcytic anemia. In this clinical syndrome, resection of the tumor fully reverses the hematologic abnormalities. They demonstrated that the tumors autonomously overexpressed hepcidin mRNA and proposed that overproduction of hepcidin in these patients was the cause of their anemia, and by extension, a likely mediator of anemia of inflammation.
Induction of hepcidin by infection and inflammation
In the meantime, the connection between hepcidin and infection/inflammation was also becoming clearer. Shike et al22 showed that in white bass liver, infection with the fish pathogen Streptococcus iniae increased hepcidin mRNA expression 4500-fold. In another study by Nicolas et al,23 injections of turpentine, a standard inflammatory stimulus, into mice induced hepcidin mRNA 4-fold and led to a 2-fold decrease in serum iron. The hypoferremic response to turpentine-induced inflammation was absent in the USF2/hepcidin-deficient mice, indicating that this response is fully dependent on hepcidin. Nemeth et al24 assayed urinary hepcidin peptide in patients with anemia of inflammation due to chronic infections or severe inflammatory diseases, and observed as much as a 100-fold increase in hepcidin excretion (adjusted for urinary creatinine), with smaller increases in patients with less severe inflammatory disorders. Urinary hepcidin was also increased about 100-fold in patients with iron overload from transfusions for sickle cell anemia or myelodysplasia. Hepcidin excretion correlated well with serum ferritin, which is also increased by both iron loading and inflammation. Studies of the effect of iron or cytokines on isolated primary human hepatocytes24 revealed that hepcidin mRNA was induced by lipopolysaccharide and strongly induced by monokines from monocytes exposed to lipopolysaccharide. Among the cytokines, interleukin-6 (IL-6), but not IL-1α or tumor necrosis factor α (TNF-α), strongly induced hepcidin mRNA. Surprisingly, exposure of hepatocytes to iron-saturated transferrin or to ferric ammonium citrate not only failed to induce hepcidin mRNA but suppressed it. These findings indicated that hepcidin production by hepatocytes is indirectly regulated by both infection and iron. Infections, and in particular pathogen-specific macromolecules such as lipopolysaccharide, probably act on macrophages, including hepatic Kupffer cells, to induce the production of IL-6, and the cytokine in turn induces the production of hepcidin mRNA in hepatocytes.24 In the aggregate, the increase of hepcidin production by inflammation and the ability of transgenic or tumor-derived hepcidin to suppress erythropoiesis by iron starvation strongly suggest that hepcidin is the key mediator of anemia of inflammation. However, it still remains to be shown that hepcidin peptide administration to mice or humans will cause iron sequestration and iron-limited erythropoiesis.
Suppression of hepcidin by anemia or hypoxia
In addition to iron stores and inflammation, anemia and hypoxia also affect iron metabolism. These stimuli would be expected to decrease hepcidin production and remove the inhibitory effect on iron absorption and iron release from macrophages so that more iron is available for compensatory erythropoiesis. Weinstein et al21 and Nicolas et al23 confirmed that these effects indeed take place. Anemia induced by mouse mutations that restrict the uptake of iron in the small intestine (sla and mk mice) showed markedly decreased hepatic hepcidin mRNA. Anemia induced by phlebotomy, or by hemolysis from phenylhydrazine, also suppressed hepatic hepcidin mRNA. Importantly, the suppressive effect of hemolytic anemia was seen even in iron-overloaded mice, suggesting that the suppression of hepcidin by anemia is a stronger effect than the stimulation of hepcidin by iron overload. This hierarchy of effects could explain why iron overload commonly develops with certain hemolytic disorders. However, these observations do not explain why iron overload occurs much more commonly in intramedullary hemolysis than in peripheral hemolysis or nonhemolytic anemias.
The role of hepcidin in hemochromatosis
Since hepcidin deficiency caused severe iron overload in mice, the search was on for human mutations that would cause or contribute to hemochromatosis.25-27 A polymorphism resulting in a substitution of methionine for threonine in the proregion of the hepcidin precursor25,26 had no detectable effect on iron metabolism, even in a homozygous state. Finally, 2 families with severe juvenile hemochromatosis were identified by Roetto et al28 : One family had a homozygous frameshift, the other a homozygous premature stop mutation, both in the coding region of hepcidin. This is a very severe form of hemochromatosis in which organ damage and even death can occur before the age of 30 years, with equal sex distribution.29 The severe phenotype of hepcidin deficiency confirms the central role of hepcidin in iron homeostasis. However, most juvenile hemochromatosis is not due to hepcidin deficiency but has been linked to an as yet unknown gene on chromosome 1q. This gene may encode an essential component of the hepcidin receptor or its signaling pathway. Since direct purification of the hepcidin receptor may be difficult, the pursuit of this gene by genetic techniques should be very informative. It is now likely that hepcidin is also involved in the pathogenesis of the much less severe but much more common form of hemochromatosis caused by mutations in the HFE gene, a transmembrane protein of the major histocompatibility complex (MHC) class I family. Despite intensive efforts by a number of excellent investigators, the cellular function of HFE and the mechanism by which HFE defects cause hemochromatosis have remained a mystery. Recent studies by Ahmad et al30 and Bridle et al31 indicate that in HFE knock-out mice, unlike in normal mice, hepatic hepcidin mRNA levels do not increase after iron-loading. Moreover, patients with hemochromatosis due to HFE mutations have lower than normal hepcidin mRNA in liver biopsies despite hepatic iron overload.31 Early studies of hepcidin excretion in a small number of patients with compensated hemochromatosis also indicate that their production of hepcidin is inappropriately low relative to plasma ferritin levels.24 However, apparently contradictory data emerged from the studies of hepcidin mRNA responses to iron-loading in β2-microglobulin knock-out mice.16 Since β2-microglobulin is required for the subcellular targeting of HFE molecules to the plasma membrane, these mice are thought to mimic the HFE defect. On normal iron diets, the β2-microglobulin knock-out mice had a 2-fold higher level of hepatic hepcidin mRNA than did normal mice. However, because normal and knock-out mice were not matched for hepatic iron content, transferrin saturation, or ferritin levels, this study would not have detected the possibly blunted hepcidin response of β2-microglobulin knock-out mice to iron loading. Since the predominant site of HFE expression in the liver is in macrophages and sinusoidal cells, iron sensing may take place in these cells and could be communicated to the hepatocytes to regulate their production of hepcidin. In the absence of functioning HFE, either the sensing mechanism or the communication to hepatocytes could be impaired, resulting in inadequate hepcidin response to iron. Without this relayed stimulus from Kupffer cells or another cell type involved in iron sensing, the direct effect of iron on hepatocytes may be to suppress hepcidin production.24
How does hepcidin regulate iron transport?
The molecules that mediate the transport of nonheme iron in enterocytes (most recently reviewed by Andrews32 and Roy and Enns33 ) have been elucidated through studies of mutations that cause hypochromic microcytic anemias in mice, rats, and zebrafish or by expression cloning in Xenopus oocytes. Ferric (Fe3+) iron from the digestate is taken up at the apical surfaces of enterocytes after reduction by a ferric oxidoreductase “duodenal cytochrome B” (DcytB) that converts it to ferrous (Fe2+) iron, which then moves inside the cell via the transporter DMT1 (Nramp2). An alternative and important absorption pathway takes up iron in heme but it has not yet been fully characterized.34 In enterocytes, iron can either be stored in ferritin or move to the basolateral surface of the cell where it is transported out by ferroportin (Ireg1), reoxidized by hephaestin, then collected by transferrin for distribution to tissues. A similar set of transporters is probably mediating the transplacental movement of iron.35
The situation in macrophages may be more complex. Macrophages contain multiple iron transporters, including Nramp1 and 2 (DMT1) and ferroportin (Ireg1). During recycling of iron from senescent red cells, macrophages phagocytize the erythrocytes and break them down in the phagosome. Although is not known with certainty how red cell iron enters the macrophage cytoplasm there is evidence that it exits the macrophage via ferroportin, assisted by the ferroxidase ceruloplasmin. In particular, patients who are heterozygotes for certain ferroportin mutations develop a form of hemochromatosis characterized by early iron overload in Kupffer cells and splenic macrophages rather than hepatocytes.36-40 A similar picture is also seen in patients and mice who lack ceruloplasmin, a plasma ferroxidase that facilitates the release of iron from hepatocytes and macrophages,41 fulfilling a function homologous to that of hephaestin in the duodenal iron absorption. Macrophages also share with other cell types the ability to take up iron from transferrin, transport it across the endosomal membrane via Nramp2 (DMT1), and incorporate it in ferroproteins, including the storage protein ferritin.
Which of these molecules could mediate the effects of hepcidin? Frazer et al42 studied in rats the responses of hepcidin and various components of the absorption pathway to a switch from an iron-replete to an iron-deficient diet, or to Freund complete adjuvant, a potent inducer of innate immunity. Within 2 to 6 days after the switch to an iron-deficient diet, the fractional absorption of a test dose of iron increased and DcytB, DMT1 (Nramp2), and ferroportin (Ireg1) were induced but hephaestin was not changed. The increases correlated with the drop in hepatic hepcidin mRNA: at times when hepcidin mRNA levels were high, transporter mRNAs were always low, and vice versa. After Freund adjuvant, hepcidin mRNA was maximally induced by 8 hours, and DcytB and Nramp2 (DMT1) were suppressed by 16 hours, but ferroportin (Ireg1) and hephaestin were not changed. Unfortunately, these experiments, while important, do not differentiate between primary responses to hepcidin and secondary responses due to changes in cellular iron concentrations. The synthesis of many proteins involved in iron metabolism is regulated directly by iron-regulatory proteins (most recently reviewed by Cairo and Pietrangelo43 ), and one form of Nramp2 (DMT1) and ferroportin (Ireg1) mRNAs both contain the appropriate iron-response motifs. Moreover, the regulation of transport need not occur by changes in the number of transport molecules since modulation of their subcellular location or the transport rates of individual transporters (gating) would lead to the same outcome. Furthermore, transporters are not the only potential regulators of iron release since, at least in principle, the release of iron from duodenal cells or macrophages could also be regulated by the avidity and perhaps the size of the ferritin compartment.
Frazer et al42 noted that the timing of hepcidin mRNA suppression was simultaneous (within 1 day) with the increase in duodenal transporter mRNA expression. This observation raises doubts about one current model of duodenal transport, which postulates that the rate of duodenal transport is set during the differentiation of crypt cells to epithelial cells.33 In this model, the iron sensing is done by the crypt cells exposed to plasma transferrin but not to luminal iron. Depending on the degree of iron saturation of transferrin, the iron absorption rate is “programmed” (presumably by synthesizing a high or low number of transport molecules), and this absorption rate persists for the remaining 2 days of the life of the epithelial cell, as it ascends on the villus, until the cell is shed from the villous tip. The model was developed before the role of hepcidin was appreciated and may need to be reconsidered since the key iron sensors need not be located in the intestine but could be in the liver and the bone marrow or perhaps in other tissues as well.
How is hepcidin production regulated by iron?
We do not yet understand the mechanisms by which iron overload in mice induces an increase of hepcidin mRNA in the liver,16 and iron overload in humans leads to greatly increased urinary hepcidin excretion.24 In the simplest model for iron-mediated regulation of hepcidin production, the hepatocyte would serve as the iron-sensor cell as well as the producer of hepcidin. However, direct exposure of murine16 or human24 hepatocytes to ferric iron, with or without serum, or of human hepatocytes to iron-saturated transferrin24 did not induce hepcidin mRNA, and at higher concentrations of iron suppressed it. This suggests that iron-sensing may take place in other cell types. Based on the likely involvement of macrophage-derived IL-6 in hepcidin induction during infections, Kupffer cells or perhaps the sinusoidal cells30 could sense iron and communicate to hepatocytes to regulate hepcidin production. The nature of this signal remains to be determined.
The cellular concentrations of ferritin, transferrin receptor-1, and probably other iron-regulated proteins43 are posttranscriptionally controlled by iron-regulatory proteins that bind to iron-regulatory elements in the respective mRNAs. Curiously, hepcidin mRNA does not contain recognizable iron regulatory elements. However, inspection of the hepcidin promoter reveals several CCAAT/enhancer-binding protein (C/EBP) consensus sequences as well as signal transducer and activator of transcription (STAT) and hepatocyte nuclear factor 4 (HNF-4) motifs.44 Courselaud et al44 showed that hepcidin mRNA levels were regulated in part by the transcription factor C/EBPα and that iron loading induced C/EBPα protein in mouse livers about 2.1-fold. Moreover, C/EBPα-deficient mice showed decreased hepcidin mRNA and periportal hepatic iron overload. Although C/EBPα clearly affects hepcidin expression and is in turn regulated by iron stores, it is not clear whether this transcription factor is a part of the major physiologic regulatory pathway for hepcidin production.
Therapeutic implications of hepcidin
Complete hepcidin deficiency resulting in juvenile hemochromatosis appears to be very uncommon.28 However, hepcidin mRNA measurements in HFE knock-out mice30 and in patients with HFE-related hemochromatosis30,31 suggest that partial hepcidin deficiency may contribute to iron overload in the most common form of hemochromatosis. If this hypothesis is correct, it would be possible to treat hemochromatosis by hepcidin replacement, either using optimized forms of the peptide or small molecular agonists. If hepcidin follows the pattern of other peptide hormones or cytokines, its actions will be mediated by cell surface receptors. Elucidation of the receptor and its transduction pathways should lead to the development of hepcidin antagonists, some of which could be useful in treatment of anemia of inflammation, a condition often resistant to erythropoietin therapy.45 Improved understanding of the host defense role of iron sequestration would facilitate such applications.
Conclusions
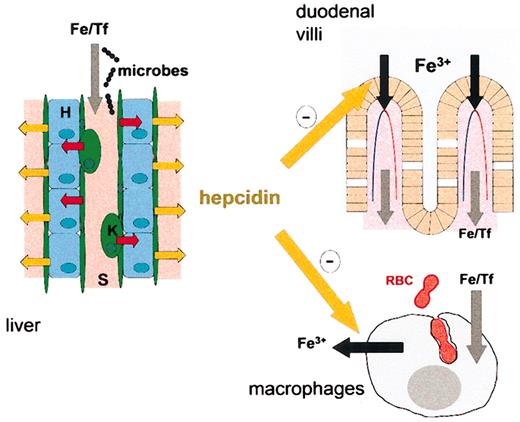
Hepcidin may be the principal iron-regulatory hormone, the key mediator of anemia of inflammation, and a bridge between innate immunity and iron metabolism (Figure 2). Studies of the molecular mechanisms of hepcidin activity could transform our understanding of the regulation of iron transport and should lead to new therapies for hemochromatosis and anemia of inflammation.
Hepcidin synthesis in the liver and its effects on iron metabolism. Hepatic sinusoids (S, pink) are lined by endothelial cells (green) and Kupffer cells (K, green). Exposure of these cells to microbes or highly iron-saturated transferrin (Fe/Tf) causes the release of IL-6 and possibly other signals (red arrows) that act on hepatocytes (H, light blue cells) to induce the synthesis and secretion of hepcidin (yellow arrows). Plasma hepcidin (large yellow arrows) inhibits iron uptake in the duodenum and iron release from macrophages in the spleen and elsewhere.
Hepcidin synthesis in the liver and its effects on iron metabolism. Hepatic sinusoids (S, pink) are lined by endothelial cells (green) and Kupffer cells (K, green). Exposure of these cells to microbes or highly iron-saturated transferrin (Fe/Tf) causes the release of IL-6 and possibly other signals (red arrows) that act on hepatocytes (H, light blue cells) to induce the synthesis and secretion of hepcidin (yellow arrows). Plasma hepcidin (large yellow arrows) inhibits iron uptake in the duodenum and iron release from macrophages in the spleen and elsewhere.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2003-03-0672.
Supported by National Institutes of Health HL 46809 and the Will Rogers Fund.
I am grateful to Elizabeta Nemeth for many illuminating discussions and the careful reading and editing of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal