Abstract
A regimen of busulfan and cyclophosphamide is standard therapy before transplantation of allogeneic hematopoietic stem cells in patients with chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS). The clinical trial reported here was undertaken to test the hypothesis that fludarabine can replace cyclophosphamide in this regimen and facilitate donor engraftment with reduced toxicity. The conditioning regimen consisted of 30 mg/m2 intravenous fludarabine daily from day -9 to day -6, and oral busulfan given at 1 mg/kg 4 times a day every 6 hours from day -5 to day -2, with doses adjusted to target plasma levels of 900 ± 100 ng/mL at steady state. Cyclosporine and methotrexate were used for prophylaxis for graft-versus-host disease. Enrolled were 42 patients with high-risk CML (n = 4) or MDS (n = 38). The median patient age was 52 years (range, 12-65 years). Mobilized blood stem cells were obtained from HLA-compatible siblings (n = 16) or unrelated donors (n = 26). Engraftment was achieved in all patients, and the day-100 regimen-related mortality was 7%. With a median follow-up of 18 months (range, 13-27 months), the probabilities of overall survival, disease-free survival, and nonrelapse mortality were 42.4%, 34.9%, and 24%, respectively. These data indicate that the combination of fludarabine and targeted busulfan is sufficiently immunosuppressive to facilitate engraftment of blood stem cells from HLA-matched siblings and unrelated donors. Based on these encouraging results, further studies of fludarabine and targeted busulfan are warranted in standard-risk patients.
Introduction
Myeloablative transplantation protocols for patients with chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS) have used conditioning regimens consisting of cyclophosphamide and whole-body irradiation or busulfan. Cyclophosphamide has been included in allogeneic protocols predominantly for its potent immunosuppressive activity, while irradiation or busulfan has been used primarily for its antileukemia activity. A serious limitation of cyclophosphamide-based regimens has been the associated toxicity that requires patient hospitalization and intense supportive care and predisposes the patient to mortality from infections and graft-versus-host disease (GVHD).1,2
Decreasing the dose of total-body irradiation, or substituting busulfan for irradiation, particularly when combined with plasma level targeting, has resulted in decreased regimen-related toxicity (RRT) and, possibly, less graft-versus-host disease (GVHD).3,4 In patients with early CML, the incidence of relapse has not increased with this approach.5
High exposure to a specific metabolite of cyclophosphamide was recently shown to be associated with major toxicities and increased nonrelapse mortality after conditioning with cyclophosphamide and total-body irradiation.2 This observation provides the rationale for replacing cyclophosphamide with other less toxic immunosuppressive agents capable of achieving sustained engraftment of allogeneic hematopoietic stem cells not only from matched related but also from unrelated donors.
Fludarabine is a purine analog that inhibits lymphocyte proliferation, promotes lymphocyte apoptosis, and is effective in the treatment of chronic lymphocytic leukemia and indolent lymphomas.6-8 The compound has shown minimal extramedullary toxicity at doses between 90 and 125 mg/m2 per course, although central nervous system toxicity may occur at higher doses. The immunosuppressive properties of fludarabine have been well documented in previous clinical trials, demonstrating engraftment of hematopoietic stem cells from HLA-identical siblings and unrelated donors, when used in combination with reduced doses of alkylating agents or whole-body irradiation.9-12 In addition, fludarabine has been incorporated into conditioning regimens promoting engraftment of highly purified blood stem cells from related donors mismatched for one HLA-haplotype.13 Recently, the combination of 250 mg/m2 fludarabine with standard doses of intravenous busulfan and thymoglobulin has been associated with a low rate of transplant-related mortality.14
The hypothesis of this study was that a combination of 120 mg/m2 fludarabine and 16 mg/kg busulfan over 4 days with dose adjustments to plasma levels of 900 ± 100 ng/mL would lead to sustained engraftment of peripheral blood stem cells (PBSCs) from HLA-compatible related or unrelated donors. The steady-state plasma level of 900 ng/mL was chosen because previous studies had shown that maintaining the levels at or above this threshold could reduce the risk of relapse in patients with CML.5 The goal of the current trial was to reduce day-100 nonrelapse mortality in a patient cohort with advanced disease to less than 20%. Both hypotheses were supported by the results of this study.
Patients and methods
Patient eligibility and accrual
Between October 2000 and November 2001, 42 patients, 12 to 65 (median, 52) years of age, were enrolled in identical prospective protocols at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA (n = 30), and at the University of Dresden, Germany (n = 12). Patient, disease, and transplantation characteristics are summarized in Table 1. Diagnosis included CML in blast phase (n = 2), CML in blast phase/remission (n = 2), refractory anemia (RA) with high-risk cytogenetics (n = 6), RA with excess of blasts (RAEB, n = 11), RAEB in transformation (RAEB-t, n = 4), untreated (n = 2) or treated acute myeloid leukemia (AML) developing from MDS (n = 10; 5 in complete remission [CR]; 5 resistant), and chronic myelomonocytic leukemia (CMML, n = 5). Patients with MDS were grouped according to the International Prognostic Scoring System (IPSS) into intermediate-1 (n = 7), intermediate-2 (n = 10), and high (n = 4) risk.15 Cytogenetic risk categories and blast counts before conditioning therapy and a number of prior therapies for all MDS patients are provided in Table 1. Of the patients, 5 with MDS and 10 who had progressed to AML had received 1 to 6 regimens of chemotherapy; 3 of the MDS patients had also been treated with thalidomide. Treated with growth factors, including granulocyte colony-stimulating factor (G-CSF), erythropoietin, and interleukin-11, were 7 patients, whereas 1 patient received immunosuppressive therapy (antithymocyte globulin [ATG] and a chimeric tumor necrosis factor α [TNF-α] receptor).
Patient and transplantation characteristics
Characteristic . | Number . |
|---|---|
| No. of patients | 42 |
| Age, y, range (median) | 12-65 (52) |
| Sex, M/F | 27/15 |
| CMV status | |
| R+/D+ | 12 |
| R+/D- | 10 |
| R-/D+ | 6 |
| R-/D- | 14 |
| Time to transplantation, mo, range (median) | 3-112 (9) |
| No. of prior therapies, n = 22, range (median) | 1-6 (2) |
| Disease | |
| CML | 4 |
| BP | 2 |
| BP in remission | 2 |
| MDS | 38 |
| FAB | |
| RA high-risk | 6 |
| RAEB | 11 |
| RAEB-t | 4 |
| Cytogenetic risk* | |
| Good | 6 |
| Intermediate | 4 |
| Poor | 11 |
| IPSS* | |
| Low | 0 |
| Intermediate-1 | 7 |
| Intermediate-2 | 10 |
| High | 4 |
| AML after MDS or CMML | 12 |
| Untreated | 2 |
| CR | 5 |
| Resistant | 5 |
| BM blasts,† %, range (median) | 0-82 (6.5) |
| PB blasts,† %, range (median) | 0-26 (0) |
| CMML | 5 |
| PBSC donor | |
| HLA-matched related | 16 |
| Unrelated | |
| HLA-A, B, C, DRB1, and DQB1 matched | 15 |
| A, B, or C mismatch | 7 |
| DRB1 mismatch | 4 |
| PBSC dose | |
| CD34+ cells, × 106/kg, median (range) | 7.48 (1.3-25.4) |
| CD3+ cells, × 108/kg, median (range) | 3.2 (1.3-6.2) |
Characteristic . | Number . |
|---|---|
| No. of patients | 42 |
| Age, y, range (median) | 12-65 (52) |
| Sex, M/F | 27/15 |
| CMV status | |
| R+/D+ | 12 |
| R+/D- | 10 |
| R-/D+ | 6 |
| R-/D- | 14 |
| Time to transplantation, mo, range (median) | 3-112 (9) |
| No. of prior therapies, n = 22, range (median) | 1-6 (2) |
| Disease | |
| CML | 4 |
| BP | 2 |
| BP in remission | 2 |
| MDS | 38 |
| FAB | |
| RA high-risk | 6 |
| RAEB | 11 |
| RAEB-t | 4 |
| Cytogenetic risk* | |
| Good | 6 |
| Intermediate | 4 |
| Poor | 11 |
| IPSS* | |
| Low | 0 |
| Intermediate-1 | 7 |
| Intermediate-2 | 10 |
| High | 4 |
| AML after MDS or CMML | 12 |
| Untreated | 2 |
| CR | 5 |
| Resistant | 5 |
| BM blasts,† %, range (median) | 0-82 (6.5) |
| PB blasts,† %, range (median) | 0-26 (0) |
| CMML | 5 |
| PBSC donor | |
| HLA-matched related | 16 |
| Unrelated | |
| HLA-A, B, C, DRB1, and DQB1 matched | 15 |
| A, B, or C mismatch | 7 |
| DRB1 mismatch | 4 |
| PBSC dose | |
| CD34+ cells, × 106/kg, median (range) | 7.48 (1.3-25.4) |
| CD3+ cells, × 108/kg, median (range) | 3.2 (1.3-6.2) |
CMV indicates cytomegalovirus; R, recipient; +, seropositive; D, donor; -, seronegative; CML, chronic myelogenous leukemia; BP, blast phase; MDS, myelodysplastic syndrome; FAB, French-American-British classification; RA, refractory anemia; RAEB, refractory anemia with excess of blasts; AML, acute myelogenous leukemia; CMML, chronic myelomonocytic leukemia; CR, complete remission; BM, bone marrow; PB, peripheral blood; and PBSC, peripheral blood stem cell
Relates to MDS only and was scored according to International Prognostic Scoring System15
Relates to AML only
There were 5 patients who met the criteria of therapy-related MDS/AML. Risks associated with this treatment protocol were fully explained to all patients and donors. Informed consent was obtained in all cases and was documented using forms approved by the institutional review boards at both participating institutions.
Treatment regimen
All patients were conditioned with fludarabine, 30 mg/m2 infused over 30 minutes once a day on 4 consecutive days (total dose, 120 mg/m2), followed by busulfan at a prescribed dose of 1.0 mg/kg administered orally every 6 hours on each of 4 successive days (total dose, 16 mg/kg). The dose was calculated based on the ideal body weight. Busulfan was administered as 2-mg tablets or lyophilized drug in gelatin capsules. Busulfan dose adjustments were made as described below. For prevention of seizures, patients at the FHCRC received oral phenytoin, with a loading dose of 15 mg/kg divided into 3 doses before busulfan administration, and a maintenance dose of 300 mg daily by mouth continued until 24 hours after the last dose of busulfan. Patients at the University of Dresden received oral diazepam, 10 mg per day during busulfan administration. All patients received transplants of G-CSF–mobilized peripheral blood stem cells.
Prophylaxis for acute GVHD consisted of methotrexate (MTX) and cyclosporine (CSP). All patients were scheduled to receive intravenous MTX on day 1 and 10 mg/m2 on days 3, 6, and 11. CSP was either administered intravenously at a dose of 3 to 5 mg/kg/d or given at an bioequivalent amount of the oral formulation in 2 divided doses starting on the day before blood stem cell infusion (day -1). Starting on day 50, oral CSP administration was tapered by 5% weekly if GVHD was inactive. The dose of CSP was adjusted to maintain blood levels between 150 and 450 ng/mL. The dose of MTX was decreased for severe mucositis, extravascular fluid accumulation, or renal dysfunction. Acute and chronic GVHD were diagnosed and graded using established criteria.16,17 Acute and chronic GVHD were treated with prednisone, CSP, or tacrolimus. One patient received mycophenolate mofetil for treatment of chronic GVHD.
Donor selection and blood stem cell harvest
Related or unrelated donors were selected based on compatibility for HLA-A, B, C, DRB1, and DQB1 by intermediate- or high-resolution DNA typing. Mismatch for a single HLA-A, B, C, DRB1, and DQB1 allele was allowed within the same broad serotype (ie, A*0101 vs 0102) or within a cross-reactive group (ie, A*0101 vs 0301).18 Data are summarized in Table 1. Donors and patients were sex mismatched in 18 cases, with a female donor for a male recipient in 8 cases.
Injection of the donor with G-CSF and apheresis were performed according to the policy of the collection center. Peripheral blood stem cells (PBSCs) were stored overnight at 4°C when necessary. A target dose of more than 5.0 × 106 CD34+ cells/kg was requested. The dose of infused CD34+ and CD3+ cells is shown in Table 1. Blood stem cells were infused on day 0 without further manipulation.
Pharmacokinetic studies of fludarabine and busulfan targeting
During the 4 days of fludarabine administration, blood samples were collected at 0, 0.5, 1, 4, 8, 12, and 24 hours after each dose. 2-Fluoroadenine (F-Ara-A) plasma levels were determined using liquid chromatography with mass spectrometry detection (LC/MSC): 250 μL plasma was combined on ice with 20 μLof 10 ng/μL sulfamerizine (internal standard) and 100 μL of 10% perchloric acid. The mixture was vortexed and centrifuged. Subsequently, 200 μL of the supernatant was added to 75 μL of cold 20% KHCO3, vortexed, and centrifuged. The supernatant was then transferred to spin filter tubes and centrifuged. The filtrate was injected into an LC/MSD running an acetate/methanol gradient mobile phase through a Synergi Hydro-reverse phase column (Phenomenex, Torrance, CA) in aminoisophthalic acid—dimethyl ester, monitoring m/z 265 (internal standard) and 286 (F-Ara-A).
Details of the chemical and pharmacokinetic analyses for busulfan have been described.19,20 To provide accurate assessments of busulfan exposure during conditioning, blood samples were collected at 0, 1, 2, 4, and 6 hours after each morning dose on days 1, 2, and 3 of busulfan administration. The busulfan concentration at steady state (CSS) is the ratio of busulfan area under the curve (AUC) over the dosing interval between doses (6 hours). If the CSS achieved was lower than 800 or more than 1000 ng/mL, subsequent doses were linearly adjusted to achieve the target.
Regimen-related toxicity
Regimen-related toxicity was scored using the Common Toxicity Criteria version 2 of the National Cancer Institute of the United States (http://ctep.cancer.gov/reporting/ctc.html). During the first 100 days after transplantation we evaluated mucositis and neurologic, gastrointestinal, hepatic, pulmonary, and hematologic toxicity. Any positive blood culture for pathogenic bacteria or Candida species was documented as bacteremia or candidemia, respectively. Bacterial and yeast organ site infection as well as invasive mold infection required positive culture results from a biopsy taken at a sterile site or histologic evidence. The duration of hospitalization was recorded.
Engraftment and rejection
On day 75 and after one year, peripheral blood mononuclear cells were obtained, and CD33+ myeloid cells and CD3+ T cells were isolated by fluorescence-activated cell sorting for chimerism testing. When a sex difference existed between donor and patient, in situ Y chromosome hybridization was performed. When patient and donor were of the same sex, cellular DNA was amplified for at least 3 variable number tandem repeat or short-tandem repeat loci. The amplified fragments were examined to identify informative host or donor markers.21,22
Supportive care
Tests for cytomegalovirus (CMV) pp65 antigen or polymerase chain reaction (PCR) for CMV DNA were performed weekly. In case of a positive test result, preemptive therapy with 5 mg/kg ganciclovir every 12 to 24 hours was initiated and administered until 100 days from transplantation or until PCR results became negative, whichever was last. Infectious disease prophylaxis strategies were used during this study in both centers including the use of systemic antibacterial antibiotics and fluconazole. All CMV-seronegative patients received either screened or filtered blood products. Acyclovir was given for herpes simplex virus (HSV) and varicella zoster virus (VZV) prophylaxis to seropositive patients for one month and one year, respectively.
Causes of death
Deaths after relapse were categorized as due to malignancy irrespective of the proximate cause. Deaths without relapse were categorized as nonrelapse deaths. Infection was listed as the cause of death when a bacterial, viral, or fungal infection was the proximate cause of death in patients who had not relapsed. GVHD was indicated as a contributor to death if active GVHD was present at the time of death from any cause in the absence of leukemia relapse.
Statistical analysis
The primary end point of the study was to determine the incidence of nonrelapse mortality by 100 days after transplantation. Secondary end points included the incidence of donor stem cell engraftment, the incidence and severity of acute GVHD, and the risk of persistent or recurrent disease. Acute GVHD was categorized as grade II or higher, or grade III or IV. Estimates of survival and disease-free survival (DFS) were obtained by the method of Kaplan and Meier where patients were censored at last follow-up if still alive.23 The incidences of relapse, nonrelapse mortality, and acute GVHD were calculated using cumulative incidence estimates.24 Univariate analyses of the influence of different variables on survival and incidence were performed with the proportional hazards regression model, where nonrelapse mortality and relapse were treated as competing risks. The data set was locked on January 23, 2003.
Results
Fludarabine plasma levels
The pharmacokinetic parameters of 2-fluoroadenine (F-Ara-A) are provided in Table 2. No accumulation of F-Ara-A was observed over the 4-day administration period. Mean maximum concentration (Cmax) values were positively correlated with the corresponding mean AUC. There was no significant association of mean F-Ara-A AUC and time to neutrophil and platelet engraftment or level of T-cell chimerism on day 75. The patient with the highest exposure to F-Ara-A (45.2 μM/h) experienced no extraordinary toxicity during the first 100 days.
Pharmacokinetics of 2-fluoroadenine
. | t1/2 terminal, h . | AUC, μM/h . | Cmax, μM . | Clearance, L/h/m2 . | Trough blood level, μM . |
|---|---|---|---|---|---|
| Mean | 11.2 | 19.1 | 4.4 | 6.3 | 0.29 |
| Standard deviation | 3.9 | 7.0 | 4.8 | 2.4 | 0.15 |
| Range | 4.5-21.3 | 8.0-45.2 | 1.3-7.8 | 2.2-13.3 | 0.08-0.66 |
. | t1/2 terminal, h . | AUC, μM/h . | Cmax, μM . | Clearance, L/h/m2 . | Trough blood level, μM . |
|---|---|---|---|---|---|
| Mean | 11.2 | 19.1 | 4.4 | 6.3 | 0.29 |
| Standard deviation | 3.9 | 7.0 | 4.8 | 2.4 | 0.15 |
| Range | 4.5-21.3 | 8.0-45.2 | 1.3-7.8 | 2.2-13.3 | 0.08-0.66 |
t1/2 indicates half-life time; and Cmax, maximum concentration
Busulfan levels and targeting
Busulfan Css levels were measured for all but 2 patients in the study. On days 1 to 3, the median BU Css level was 908 ng/mL (range, 774-1188 ng/mL), with 35 of 40 patients in the prescribed target range of 800 to 1000 ng/mL. While 8 patients achieved the target level with the prescribed doses, 9 patients required busulfan dose reductions and 18 required increments. The median total dose of busulfan administered was 14.4 mg/kg (range, 9.8-18.7 mg/kg) actual body weight and 15.9 mg/kg (range, 11-21.8 mg/kg) ideal body weight. The median, 25% and 75% percentiles, and ranges for doses of busulfan administered on day 1, and the doses on days 2 and 3 modified according to prior AUC are depicted in Figure 1A. The target levels achieved each day are shown in Figure 1B.
Busulfan dose and steady-state plasma levels. (A) Busulfan dose. (B) Steady-state plasma levels. The boxes are divided at their median with the edges of the boxes at 25% and 75% of the samples. The ends of the whiskers correspond to the minimum and maximum.
Busulfan dose and steady-state plasma levels. (A) Busulfan dose. (B) Steady-state plasma levels. The boxes are divided at their median with the edges of the boxes at 25% and 75% of the samples. The ends of the whiskers correspond to the minimum and maximum.
Engraftment and chimerism
All 41 evaluable patients achieved sustained engraftment. One patient was not evaluated for engraftment because of early death on day 9. Neutrophil counts of 0.5 × 109/L or higher were reached after a median of 16 days (range, 12-25 days). Platelet counts of 20 × 109/L or higher for 7 days without transfusion were reached after a median of 13 days (range, 11-75 days). Chimerism tests showed donor hematopoiesis in all patients assayed (Figure 2). On day 75, chimerism was tested in 31 patients: all showed donor type in 26% to 100% (median, 100%) of the granulocytes (CD33+) and 35% to 95% (median, 75%) of the T (CD3+) cells. The patient with 26% donor myeloid cells experienced hematologic relapse of CMML shortly thereafter. At one year, chimerism was tested in 12 patients: donor type was between 91% and 100% (median, 99.5%) for granulocytes and between 28% and 100% (median, 98%) for T cells; 2 patients with related donors had persistent mixed T-cell chimerism with 28% and 50% donor type cells at one year.
Chimerism. The scatters represent the percentage of donor chimerism in sorted CD3+ and CD33+ cells on day 75 (n = 31) and after one year (day 365; n = 12). A solid line represents the median.
Chimerism. The scatters represent the percentage of donor chimerism in sorted CD3+ and CD33+ cells on day 75 (n = 31) and after one year (day 365; n = 12). A solid line represents the median.
Regimen-related toxicity (RRT)
Toxicities associated with therapy are specified and graded in Table 3. Nonfatal toxicities included mucositis, idiopathic pneumonitis, and sinusoidal obstruction syndrome/veno-occlusive disease of the liver (VOD). No infection with HSV or VZV occurred during the first 100 days after transplantation. The duration of hospitalization during the first 100 days ranged between 8 and 86 (median, 35) days.
Toxicity and causes of death
. | Number . |
|---|---|
| Toxicity, day 0 to 20, median | |
| Maximum serum aspartate aminotransferase, μM/s (range) | 56 (21-516) |
| Maximum serum creatinine, μM (range) | 97 (53-362) |
| Maximum total serum bilirubin, μM (range) | 42 (13-188) |
| Toxicity, nonfatal, day 0 to 100, no. of patients | |
| Mucositis, grade 3 | 31 |
| Veno-occlusive disease, grade 4 | 1 |
| Pneumonitis, grade 4 | 1 |
| Bacteremia | 2 |
| Invasive fungal infection | 2 |
| CMV reactivation, 22 seropositive pairs | 12 |
| Fatal toxicity and causes of death, no. of patients | |
| Relapse or progression of malignancy | 12 |
| Idiopathic pneumonia syndrome | 5 |
| Acute GVHD/bacterial sepsis | 1 |
| Cardiac failure due to suspected endocardial fibrosis | 1 |
| Encephalopathy | 1 |
| Chronic GVHD and aspergillosis | 2 |
. | Number . |
|---|---|
| Toxicity, day 0 to 20, median | |
| Maximum serum aspartate aminotransferase, μM/s (range) | 56 (21-516) |
| Maximum serum creatinine, μM (range) | 97 (53-362) |
| Maximum total serum bilirubin, μM (range) | 42 (13-188) |
| Toxicity, nonfatal, day 0 to 100, no. of patients | |
| Mucositis, grade 3 | 31 |
| Veno-occlusive disease, grade 4 | 1 |
| Pneumonitis, grade 4 | 1 |
| Bacteremia | 2 |
| Invasive fungal infection | 2 |
| CMV reactivation, 22 seropositive pairs | 12 |
| Fatal toxicity and causes of death, no. of patients | |
| Relapse or progression of malignancy | 12 |
| Idiopathic pneumonia syndrome | 5 |
| Acute GVHD/bacterial sepsis | 1 |
| Cardiac failure due to suspected endocardial fibrosis | 1 |
| Encephalopathy | 1 |
| Chronic GVHD and aspergillosis | 2 |
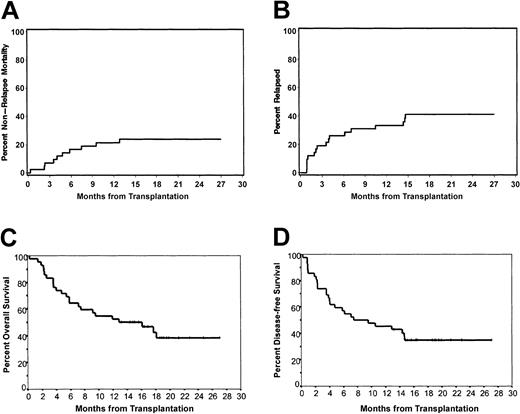
There were 10 deaths in patients who had not relapsed. One patient died on day 9 from encephalopathy (grade 5 toxicity) presumed secondary to conditioning chemotherapy. While exposure to F-Ara-A in this patient was within the therapeutic range (mean AUC, 15.3 μM/h), which does not preclude the possibility that fludarabine contributed to this unexpected toxicity, the mean busulfan Css plasma level was slightly above the targeted range (1018 ng/mL). Death due to idiopathic pneumonia syndrome (grade 5 toxicity) occurred in 5 patients on days 71, 108, 145, 177, and 227. Further causes of death were bacterial sepsis associated with GVHD (day 65), cardiac failure due to suspected pre-existing endocardial fibrosis (day 123) in 1 patient, or cerebral aspergillosis (day 291 and day 391) in 2 patients. The cumulative incidence of death without relapse was 7% at day 100 and 24% at 18 months (Figure 3A).
Nonrelapse mortality, incidence of relapse, and survival. The estimates of the cumulative incidence of death from causes other than relapse or progression (A) and the incidence of relapsing disease (B). With a median follow-up of 18 months (range, 13-27 months), the Kaplan-Meier estimates of overall survival (C) and relapse-free survival (D) are depicted.
Nonrelapse mortality, incidence of relapse, and survival. The estimates of the cumulative incidence of death from causes other than relapse or progression (A) and the incidence of relapsing disease (B). With a median follow-up of 18 months (range, 13-27 months), the Kaplan-Meier estimates of overall survival (C) and relapse-free survival (D) are depicted.
Relapse
Malignancy persisted or recurred in 3 of 4 patients with advanced CML and 13 of 37 with advanced MDS, AML, or CMML. After posttransplantation relapse 4 patients are alive and 12 died. One patient with relapse achieved remission after second transplantation from the same donor. The cumulative incidence of relapse was 41% (Figure 3B).
GVHD
Grades II to IV acute GVHD occurred in 22 (54%) of 41 evaluable patients with onset between 20 and 62 (median, 32) days. Grade III acute GVHD was observed in 9 patients (22%), and 1 patient (2%) had grade IV acute GVHD. There was no detectable difference in the incidence and severity of acute GVHD between patients receiving a graft from related compared with unrelated donors. Beyond day 100, 39 patients (93%) survived and 19 (49%) developed chronic GVHD that was extensive in 9 (23%) and limited in 10 patients. So far, 5 patients have been able to discontinue all immunosuppressive drugs after 125, 131, 387, 510, and 620 days.
Survival
The probabilities of overall survival (OS) and disease-free survival (DFS) at 18 months were 42.4% and 34.9%, respectively (Figure 3C and D, respectively). After a median follow-up of 18 months (range, 13 to 27 months) for surviving patients, 12 (36%) of 33 patients with MDS or secondary AML are in continuous remission, and 4 (12%) patients survive after relapse. Of 5 patients with CMML, 3 survive disease-free, whereas all 4 patients with advanced CML have died. The Karnofsky performance score of the 19 surviving patients ranged between 60% and 100% (median, 90%) by the time of last follow-up. With a Karnofsky score less than 80%, 4 patients are living with relapse (n = 3) or are suffering from chronic GVHD (n = 1).
Statistical analyses
The univariate analysis of individual risk factors is shown in Table 4. Age, disease, type of donor, or HLA-match in case of an unrelated donor did not significantly impact on disease-free survival, incidence of relapse, and nonrelapse mortality. There was a trend toward an improved disease-free survival and a decrease in the incidence of relapse in CMV-negative recipients. A separate analysis of the 33 patients with MDS and AML revealed a significantly better disease-free survival for patients with an IPSS risk score of 0.5 to 1.0 (n = 7, 71.4%) compared with those with a higher score and those progressed to AML (n = 26, 26.0%, P = .02). This difference was mainly explained by an increased incidence of relapse for patients with a risk score higher than 1.0 (43.3%) compared with low-risk patients (14.3%, P = .07). Additional analyses showed no significant influence of age, karyotype, number of prior chemotherapies, and time from diagnosis to transplantation on disease-free survival, incidence of relapse, and nonrelapse mortality in MDS and AML patients.
Univariate analysis
. | N . | DFS at 18 mo (%) . | P . | Relapse at 18 mo (%) . | P . | NRM at 18 mo (%) . | P . |
|---|---|---|---|---|---|---|---|
| Patient age, y | * | — | — | ||||
| 60 or older | 10 | 30.0 | 30.0 | 40.0 | |||
| 50 to 59 | 15 | 40.0 | 40.0 | 20.0 | |||
| Younger than 50 | 17 | 35.3 | 47.1 | 17.6 | |||
| MDS | 21 | 42.9 | 28.6 | 28.6 | |||
| AML | 12 | 22.2 | .20 | 52.8 | .18 | 25.0 | — |
| CMML | 5 | 60.0 | 40.0 | 0.0 | |||
| CML | 4 | 0.0 | 75.0 | 25.0 | |||
| IPSS | .02 | .07 | .17 | ||||
| 0.5 to 1.0 | 7 | 71.4 | 14.3 | 14.3 | |||
| more than 1 and AML† | 26 | 26.0 | 43.3 | 30.8 | |||
| Patient | .08 | .05 | — | ||||
| CMV negative | 20 | 48.9 | 26.1 | 25.0 | |||
| CMV positive | 22 | 22.7 | 54.5 | 22.7 | |||
| Donor | — | .12 | — | ||||
| Related | 16 | 25.0 | 56.2 | 18.8 | |||
| Unrelated | 26 | 41.5 | 31.5 | 26.9 | |||
| Unrelated matched | 15 | 40.0 | — | 26.7 | — | 33.3 | — |
| Unrelated mismatched | 11 | 43.6 | — | 38.2 | — | 18.2 | — |
. | N . | DFS at 18 mo (%) . | P . | Relapse at 18 mo (%) . | P . | NRM at 18 mo (%) . | P . |
|---|---|---|---|---|---|---|---|
| Patient age, y | * | — | — | ||||
| 60 or older | 10 | 30.0 | 30.0 | 40.0 | |||
| 50 to 59 | 15 | 40.0 | 40.0 | 20.0 | |||
| Younger than 50 | 17 | 35.3 | 47.1 | 17.6 | |||
| MDS | 21 | 42.9 | 28.6 | 28.6 | |||
| AML | 12 | 22.2 | .20 | 52.8 | .18 | 25.0 | — |
| CMML | 5 | 60.0 | 40.0 | 0.0 | |||
| CML | 4 | 0.0 | 75.0 | 25.0 | |||
| IPSS | .02 | .07 | .17 | ||||
| 0.5 to 1.0 | 7 | 71.4 | 14.3 | 14.3 | |||
| more than 1 and AML† | 26 | 26.0 | 43.3 | 30.8 | |||
| Patient | .08 | .05 | — | ||||
| CMV negative | 20 | 48.9 | 26.1 | 25.0 | |||
| CMV positive | 22 | 22.7 | 54.5 | 22.7 | |||
| Donor | — | .12 | — | ||||
| Related | 16 | 25.0 | 56.2 | 18.8 | |||
| Unrelated | 26 | 41.5 | 31.5 | 26.9 | |||
| Unrelated matched | 15 | 40.0 | — | 26.7 | — | 33.3 | — |
| Unrelated mismatched | 11 | 43.6 | — | 38.2 | — | 18.2 | — |
IPSS indicates International Prognostic System Score for MDS; CMV, cytomegalovirus; and —, not significant (P > .2)
Only P values of .2 or less are given
Only MDS and AML patients
Discussion
We have shown here that a combination of fludarabine and busulfan targeted to a steady-state plasma level of 900 ± 100 ng/mL can be used successfully to condition patients with advanced MDS and CML for allogeneic transplantation from related and unrelated donors. All patients achieved sustained engraftment and the day-100 regimen-related mortality was low (7%). This result is comparable with that of a retrospective analysis of 109 patients with MDS who had received targeted busulfan (800-900 ng/mL) and 120 mg/kg cyclophosphamide and who showed a day-100 nonrelapse mortality of 13%.25 Since the latter cohort contained more patients with RA/refractory anemia with ring sideroblasts (RARS) (63%), low-risk cytogenetics (58%), and marrow graft recipients (74%), a direct comparison between the two series is not appropriate. The incidence of relapse with fludarabine and targeted busulfan seemed no higher than published for standard conditioning therapies in similar patients.26-29 The results of the current study support the concept that fludarabine is well tolerated and sufficiently immunosuppressive to achieve sustained engraftment of PBSC transplants from related or unrelated donors.
Fludarabine and other purine analogs inhibit the mechanisms of repair from alkylator-induced DNA damage, thus providing a rationale to combine purine analogs and alkylating agents to promote allogeneic engraftment with limited extramedullary toxicity.30 Conditioning regimens combining fludarabine at a dose of 125 mg/m2 and melphalan at a dose of 140 to 180 mg/m2 allowed for engraftment of hematopoietic cell transplants from related and unrelated donors.10-12,31
Slavin et al first showed the efficacy of fludarabine (180 mg/m2) combined with low-dose (8 mg/kg) busulfan and ATG Fresenius (Bad Homburg, Germany), 40 mg/kg, in facilitating engraftment of PBSC transplants from matched related donors.9 One report showed that ATG was not essential to achieve stable engraftment of PBSCs from matched related donors after conditioning with 150 mg/m2 fludarabine and 8 mg/kg busulfan.32 However, graft failure was observed in 9 (21%) of 42 patients (7 with CML or MDS) treated with 150 mg/m2 fludarabine, 8 mg/kg busulfan, 10 mg/kg ATG Merieux (Lyon, France), and hematopoietic cells from unrelated donors.33 A recent study combining a higher dose of fludarabine (250 mg/m2) with 12.8 mg/kg intravenous busulfan and 4.5 mg/kg ATG Merieux proved the regimen to be well tolerated, with only 2 cases of graft failure among 28 patients receiving marrow grafts from unrelated donors.14 In the current study, the combination of fludarabine and targeted busulfan led to sustained engraftment in all recipients of PBSCs from related (n = 16) or unrelated donors (n = 25). Since fludarabine was used at doses of only 120 mg/m2 in this study, we surmise that busulfan mediated additional immunosuppressive effects.
The observation of complete and sustained engraftment of PBSCs from unrelated donors with the present regimen is encouraging particularly when compared with results with so called “nonmyeloablative” regimens, which have been associated with considerable rejection rates in patients with MDS and CML.34 Chimerism analyses showed prompt engraftment of granulocytes (CD33+), whereas mixed T-cell chimerism was observed in most patients up to day 365. More rapid increase of T-cell chimerism has been observed in other studies of reduced intensity regimens in patients who had been previously treated with chemotherapy for diseases other than CML or MDS.35,36
Although the study including mainly patients with advanced MDS and AML evolving from MDS had not been designed to detect stage-specific differences in outcome, disease-free survival was associated with the IPSS risk category as has been shown in a recent report using targeted busulfan and cyclophosphamide as conditioning therapy.25
The analysis of fludarabine pharmacokinetics provides reliable information on the plasma levels and the elimination kinetics of 2-fluoroadenine in patients receiving fludarabine as part of conditioning therapy. We found no accumulation of F-Ara-A and no specific toxicity associated with an increased exposure to the drug in any patient. Given a linear correlation between dose and exposure, the maximum AUC and Cmax values measured were significantly less than those presumably achieved in early studies with 125 mg/m2 of fludarabine for 5 days, a regimen that had been associated with an excess of neurotoxicity.37 Based on these data, we believe that routine determination of F-Ara-A plasma concentration may not be necessary at the dose of fludarabine used in this study and can be restricted to trough blood or Cmax measurements in high-risk patients with impaired renal function.
In this prospective study, data from both centers confirmed the feasibility of daily busulfan dose adjustments based on “real time” determination of drug plasma levels. The mean target level of 900 ± 100 ng/mL was achieved with dose adjustments in the majority of patients during the administration period. Targeted doses of busulfan may be especially important in older patients by sparing the extramedullary toxicity associated with standard protocols. Previous data show that Css busulfan plasma levels of 800 to 900 ng/mL were associated with a favorable outcome for patients 55 to 65 years of age with MDS.26 Lower levels of busulfan exposure were shown to be associated with an increased risk of relapse in CML patients.5 There is also an association between low busulfan AUC and graft failure after mismatched or unrelated donor transplantations.38 This might be another rationale to target the busulfan exposure in patients at risk for these complications. With the advent of intravenous busulfan formulations, more precise targeting of busulfan exposure may be possible in future trials.32,39,40
The combination of fludarabine with busulfan doses adjusted to targeted levels may be advantageous in minimizing early regimen-related toxicity compared with the standard regimen of busulfan and cyclophosphamide. These preliminary results indicate that relapse does not occur more frequently with this reduced intensity regimen. The substitution of fludarabine for cyclophosphamide eliminates from the conditioning regimen a drug with highly variable metabolism that is known to cause toxic injury to hepatic sinusoidal cells and leads to increased nonrelapse mortality.2,41 Pneumonia was the reason of death in 5 patients (12%) in this study. Despite the advanced disease status of the patients included, the incidence of this complication was comparable with published cohorts receiving conditioning with busulfan and cyclophosphamide.4,42 Future controlled studies in standard-risk patients with myeloid malignancies will be needed to assess the relative safety and efficacy of this regimen of fludarabine/busulfan and to determine if disease-free survival is improved compared with standard busulfan/cyclophosphamide.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-11-3567.
Supported in part by grants CA18029, CA36444, CA87948, and the Deutsche Krebshilfe grant 70-2755 (M.B. and C.T.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the medical and nursing staff of both institutions for their important contribution. We are indebted to Lori Hubbard for data management. Many thanks to Silke Soucek for the statistical analyses.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal