Abstract
Based on our previous demonstration that quinine could be used clinically to reverse P-glycoprotein–mediated resistance, we designed a multicenter, randomized trial aiming to determine whether quinine would improve the survival of adult patients (15-60 years old) with de novo acute myelogenous leukemia (AML). These patients randomly received (n = 213) or did not receive (n = 212) a 30 mg/kg/day continuous intravenous infusion of quinine in combination with induction chemotherapy combining idarubicine and cytarabine and, depending on bone marrow examination at day 20, an additional course of cytarabine and mitoxantrone. The mean steady-state quinine concentration was 7.8 mg/L and the mean multidrug resistance reversing activity of serum was 1.96. Complete remission (CR) was obtained in 344 patients (80.9%) without significant influence of quinine. Of the patients in complete remission, 82 were assigned to receive HLA-matched bone marrow transplants, whereas 262 were assigned to 2 courses of intensive consolidation chemotherapy, with or without quinine, depending on initial randomization. The 4-year actuarial overall survival (OS) of the 425 eligible patients was 42.0% ± 2.5%, without significant influence of quinine. Of 160 patients who could be studied, 54 demonstrated rhodamine 123 efflux. In these patients, quinine significantly improved the CR rate from 12 of 25 (48.0%) to 24 of 29 (82.8%) (P = .01). However, there was no significant difference in OS. Neither mdr1 gene nor P-glycoprotein expression influenced the outcome. We conclude that quinine does not improve the survival of adult patients with de novo AML, even though it improves CR rate in a small subgroup of patients defined by rhodamine 123 efflux.
Introduction
The 2 main classes of chemotherapeutic drugs that have demonstrated some efficacy in the treatment of adult de novo acute myelogenous leukemia (AML) are aracytine (Ara-C) and topoisomerase II inhibitors, including anthracyclines, amsacrine, and etoposide. Frontline combinations of these drugs induce complete response rates of 70% to 80% and long-term event-free survival rates of 25% to 40%.1 Current approaches designed to improve outcome for adult patients with de novo AML include the use of new agents directed at various targets, intensive consolidation chemotherapy, stem cell transplantation, differentiation therapy, tumor vaccination, and attempts at reversing leukemic cell resistance.2
The best characterized resistance mechanism in AML is the so-called “multidrug resistance” (MDR).3 This resistance phenotype is the consequence of an increased expression of the mdr1 gene.4 This gene encodes a 170-kDa glycoprotein named P-glycoprotein, an adenosine triphosphate–dependent transmembrane pump that facilitates the cellular efflux of various substances.5 The P-glycoprotein is thought to act as a detoxifying agent by pumping toxins or xenobiotics out of normal cells from various tissues; for example, the gastrointestinal tract, the endothelium of the blood-brain barrier, and bone marrow stromal cells.6,7 When overexpressed in leukemic cells, P-glycoprotein reduces intracellular accumulation of several distinct anticancer drugs including anthracyclines, amsacrine, and epipodophyllotoxins such as etoposide.8 The protein was shown also to specifically interfere with various forms of caspase-dependent cell death.9 In AML, both the expression of mdr1 mRNA and the presence of P-glycoprotein have been correlated with poor response rates to chemotherapy as well as shortened event-free and overall survival.10-14
A number of noncytotoxic drugs have been demonstrated to reverse the P-glycoprotein–mediated resistance in vitro.15-17 The clinical use of most of these compounds is precluded by serum protein binding or clinical toxicity.17,18 We have previously demonstrated that quinine was a good candidate for evaluating the clinical interest of MDR-reversing agents.19 Serum from patients receiving conventional doses of quinine by intravenous infusion reverses the MDR phenotype of various tumor cell lines.19,20 Quinine is safe when combined with mitoxantrone and cytarabine for treating acute leukemia patients.21,22 This compound does not significantly improve the response rate of refractory and relapsed acute myelogenous leukemias22 but increases the response of P-glycoprotein–expressing myelodysplastic syndromes to a mitoxantrone–Ara-C combination.23
Preclinical experiments in which drug-sensitive cancer cells were treated with MDR-related cytotoxic drugs together with P-glycoprotein modulators suggested that these modulators could prevent the emergence of drug resistance.24,25 These observations provided a rationale for considering MDR modulation early in the course of the disease, before the emergence of either P-glycoprotein–mediated or other mechanisms of drug resistance. We therefore undertook a multicenter, randomized trial to determine whether addition of quinine to induction and consolidation chemotherapy regimen could improve the survival of adult patients with de novo AML. Patients were randomly assigned to receive a 30 mg/kg/day continuous intravenous infusion of quinine in combination with 3 courses of chemotherapy including anthracyclines, amsacrine, or etoposide administration. Our results indicate that quinine increases complete remission (CR) rate in a small subgroup of adult patients with de novo AML whose blast cells demonstrate active efflux of rhodamine 123 but does not improve their overall survival.
Patients and methods
Patients
This open phase 3 prospective randomized trial involving 17 medical centers (“Appendix”) was performed from February 1995 to February 1999. The protocol received the assent from institutional ethics committees, and all patients provided informed consent. Patients 15 to 60 years of age with de novo AML were eligible for the study. Each case was classified according to the French-American-British (FAB) system. Patients with AML3, AML7, transformation of myelodysplastic syndromes, or blast crisis of myeloproliferative syndrome were not eligible. Other exclusion criteria included abnormal kidney (creatinine level higher than 250 μmol/L) and hepatic (transaminase levels higher than 4N) functions, clinical or electrocardiolographic signs of heart failure, known hypersensitivity to quinine, and previous history of cancer treated with either chemotherapy or radiotherapy. Cytogenetic abnormalities were classified as favorable [inv16, t(8;21)], unfavorable (abnormalities of chromosomes 5 and/or 7, those involving 11q23 and complexes abnormalities), and intermediate (other changes).
Induction regimen
Induction regimen consisted of a continuous intravenous infusion of Ara-C (200 mg/m2/d) on days 1 through 7 plus intravenous idarubicin (8 mg/m2/d) on days 1 through 5. Patients were randomly assigned to receive or not receive a continuous intravenous infusion of quinine (Quinimax; Labaz Laboratories, Paris, France) at a dosage of 30 mg/kg/day, starting 12 hours before the first dose of idarubicin and ending 12 hours after the end of the last idarubicin infusion. A bone marrow aspiration was performed on day 20. If the marrow contained 20% blasts or more (or fewer than 20% blasts with Auer rods), a second induction course was administered with a combination of Ara-C (3 g/m2 on a 3-hour infusion every 12 hours) on days 1 through 4 (total dose 24 g/m2) and mitoxantrone (12 mg/m2/d) on days 5 and 6. According to the initial randomization, patients also received quinine at the initially defined dose on days 4 through 6.
Consolidation regimen
Patients who, during induction therapy, developed cardiac, renal, or hepatic failure or severe infectious complication (including invasive aspergillosis) were excluded from the trial. Patients in CR after 1 or 2 courses of intensive induction who were aged 45 years or younger with an HLA-identical sibling were proposed allogeneic bone marrow transplantation (BMT) after a standard dose consolidation combining amsacrine (150 mg/m2/d, day 1) and Ara-C (100 mg/m2/d, day 1 to day 5). The conditioning regimen included total body irradiation (12 Gy, 6 fractions from day –6 to day –4), cyclophosphamide (60 mg/kg/d, day –3 to day –2), and granulocyte colony-stimulating factor (G-CSF) (5 μg/kg/d; day –6 to day –1). All other patients in CR after 1 or 2 courses of induction therapy received 2 courses of intensive consolidation chemotherapy (ICC). The first ICC (ICC1) consisted of a combination of Ara-C and mitoxantrone, as in the second induction course. The second ICC (ICC2) consisted of amsacrine (150 mg/m2/d) administered as a 1-hour infusion and etoposide (100 mg/m2/d) administered as a 2-hour infusion from day 1 through day 5. In patients randomly assigned to receive quinine, the MDR-reversing agent (30 mg/kg/day by continuous intravenous infusion) was administered from day 4 to day 6 during ICC1 and from day 1 to day 6 during ICC2. During the first part of the study, patients also were randomly assigned to receive or not receive G-CSF (5 μg/d; Filgrastim, Amgen, and Roche Laboratories) from the day after chemotherapy until granulocyte recovery throughout the 2 courses of consolidation treatment. As reported previously, intermediate analysis demonstrated that G-CSF significantly reduced the duration of neutropenia and hospitalization.26 Subsequently, G-CSF was administered to all the patients who received consolidation therapy.
Monitoring of patients and evaluation of therapy
In all centers, patients were hospitalized for the entire courses of therapy. Toxicity was assessed according to the National Cancer Institute (NCI) grading system. Red blood cell and platelet transfusions as well as prophylaxis and treatment of fever and infections were administered according to the protocols used in each center. CR was defined as a normocellular bone marrow containing fewer than 5% blasts with peripheral granulocyte count higher than 1 G/L and peripheral platelet count higher than 0.1 G/L. Treatment failure was defined as resistant leukemia (partial response or no response) or death (early death during the 7 days of induction treatment or death during bone marrow aplasia). Relapse was defined as reappearance of leukemic cells in the bone marrow or peripheral blood or evidence of extramedullary leukemia.
Assessment of MDR phenotype
Bone marrow samples obtained at diagnosis were shipped by express mail to 1 of 3 reference centers, where blast cells were separated on Fycoll-Hypaque and cryopreserved in liquid nitrogen either as cell suspensions in 50% fetal calf serum and 10% dimethyl sulfoxide or as lysates in RNAzole. To ensure standardization and reproducibility of MDR assessment, each method was performed in a unique expert center that was involved in the definition of consensus recommendations.27 At regular intervals, frozen samples were distributed among these 4 expert centers.
P-glycoprotein expression analysis was studied by L.C. in Saint-Etienne.12,28 Briefly, 5 × 105 thawed cells were incubated with human AB serum (Sigma-Aldrich, St Louis, MO) for 30 minutes at room temperature and washed twice before incubation with 30 μL UIC2 (Immunotech, Marseille-Luminy, France; 200 μg/mL) or 4E 3 (Dako, Glostrup, Denmark; 61 μg/mL) or IgG2a isotopic control (Immunotech; Dako) antibodies for 1 hour at room temperature. After 2 washes in phosphate-buffered saline, the cells were incubated with 10 μL fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse antibodies (Silenus Pty, Victoria, Australia; dilution 1:50) for 15 minutes at 4°C. Propidium iodide (PI) (Sigma-Aldrich) was added immediately before flow cytometry analysis. The human leukemic cell line K562, its adriamycin-resistant subclone K562/ADM, and the KG1a cell line were used as controls. Cells were analyzed using an Epics XL flow cytometer (Beckman-Coulter, Miami, FL). Viable blast cells were gated using side scatter and PI parameters. Results were expressed as mean fluorescence index (MFI = arithmetic mean of the specific antibody fluorescence/arithmetic mean of the respective control) and percentage of stained cells. The threshold for P-glycoprotein positivity was set to MFI higher than 1.5.
mdr1 gene expression was studied in Paris by P.d.C.29 Total RNA was extracted from 2 × 106 cells using the Trizol Reagent (Invitrogen, Carlsbad, CA) and stored in 50 μL RNAse-free distilled water at –80°C. RNA quality was ensured by agarose gel electrophoresis, and 1 μg total RNA was reverse-transcribed. mdr1 transcripts were quantified using real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR) assays with β2 microglobulin transcripts used as endogenous control. Primers and probes were chosen with the assistance of the computer program Primer Express (Applied Biosystems, Foster City, CA). The nucleotide sequences were 5′ tggacaagcactgaaagataagaaag (forward primer), 5′aaagaaacaacggttaagtt (reverse primer), and aggtgctgggaagatcgctactgaag (probe) for mdr1 and have already been published for β2-microglobulin.29 PCR reactions were performed using an ABI Prism 7700 Sequence Detection System and Core Reagent Kit (Applied Biosystems) as described elsewhere.29 Included in every amplification run were 2 nontemplate controls. The human leukemic cell line CEM and its multidrug-resistant subline CEM/VBL10030 were used to generate a calibration curve. Interassay variability was lower than 5%. Quantitative values of transcripts were obtained from the threshold cycle (Ct) number at which the increase in fluorescent signal associated with an exponential increase of PCR products detected, and ΔCt values of each sample were determined as described elsewhere.29 Samples were considered as positive when mdr1 gene expression was at least 2-fold higher than that measured in the sensitive CEM cell line.
P-glycoprotein function was assessed by B.D. in Rennes, who monitored rhodamine 123 uptake and efflux using a previously described flow cytometry method.31,32 Briefly, uptake was measured after a 30-minute incubation with 0.5 μg/mL rhodamine 123 (Sigma) at 37°C, whereas efflux was measured after 90 minutes at 37°C or at 4°C in the absence or in the presence of an MDR-reversing agent (verapamil, 10 μmoL/L). Flow cytometry analysis (Cytoron; Ortho Diagnostic Systems, Raritan, NJ) was performed after 2 further washes. To ensure that efflux was related to leukemic blast cells, leukemic cells were gated according to scatter properties and antigen profile, thus avoiding confusion with other bone marrow cells that potentially express P-glycoprotein, such as CD8-positive T cells and natural killer cells. Control studies were performed in K562-sensitive and -resistant cell lines. This functional assay was scored as positive when more than 10% of leukemic cells demonstrated a verapamil-sensitive efflux.
MDR-reversing activity of serum samples
Serum samples were collected before and 2 days after initiation of quinine intravenous infusion and frozen at –20°C, then collected in each center and tested in a unique center (Dijon). Quinine serum concentration was measured using a previously described high-performance liquid chromatography method.20-22 MDR-reversing activity was measured by incubating MDR-positive K562 cells for 4 hours at 37°C with a mixture of 3% [14C]-doxorubin and 97% nonradioactive drug diluted in either serum-free culture medium or serum samples. Serum-free medium containing various concentrations of quinine was used for positive controls. After 3 washes, cells were disrupted in 1N NaOH, and the radioactivity representing intracellular doxorubicin accumulation (IDA) was measured on a β scintillation counter (LKB 1241 Rackbeta, Stockholm, Sweden). Each determination was performed in duplicate with 2 wells per point. The serum-reversing activity at time Tn was calculated according to the following formula: [(IDA at Tn–IDA at T0)/IDA at T0] × 100, where IDA is expressed as cpm.
Statistical analysis
Central randomization was stratified by center. The data were collected centrally and subsequently reviewed by 2 clinicians. A least 128 patients randomized in each group were required to detect an increase of 4-year survival from 40% to 55% (unilateral test; α, 5%; β, 20%) under quinine exposure. The homogeneity of treatment groups was tested with the chi-square test for binary variables and the Kruskal-Wallis test for continuous variables. Disease-free survival (DFS) was calculated from the date of first remission to the date of first relapse or the date of death from any cause. Overall survival (OS) was defined as the time from first randomization to the time of death. Patients who did not receive the induction treatment as indicated by the protocol were excluded from the calculation of DFS, but data were collected on these patients for OS analysis. Actuarial curves were calculated according to the Kaplan-Meier technique, and the differences between the curves were analyzed with the 2-tailed log-rank test. Multivariate analysis was made by a logistic regression analysis for the CR rate and by the Cox proportional hazard model for DFS and OS data. Comparison between the control and quinine arms was performed according to the intention-to-treat principle. A comparison was made between the patients for whom, according to the protocol, the intention was to perform a BMT and those who should have received the first course of ICC. The median follow-up duration is 49 months (range, 4 to 78 months). Investigators received a follow-up questionnaire every 6 months. The final analysis was performed in August 2002.
Results
Quinine did not improve overall CR rate in adult de novo AML patients
Between February 1995 and January 1999, 435 patients were registered in the study by 17 institutions (“Appendix”). Because of an inadequate diagnosis (n = 5) or a previous history of malignancy (n = 5), 10 patients were ruled ineligible. The characteristics of the 425 eligible patients are indicated on Table 1. There was no significant difference between the control and quinine arms according to age, sex, performance status, FAB classification, initial blood cell counts, and hemoglobin level. Cytogenetic analysis indicated an imbalance between groups with significantly more poor-risk cytogenetic abnormalities in the control (n = 39) than in the quinine-treated (n = 20) group of patients (P = .01). Other cytogenetic subgroups were balanced between control and quinine-treated patients (Table 1).
Characteristics of the 425 eligible patients
. | Without quinine, N = 212 . | With quinine, N = 213 . | P . |
|---|---|---|---|
| Mean age, y | 41 | 41 | NS |
| Sex, n, M/F | 102/110 | 108/105 | NS |
| PS greater than 1, n | 45 | 52 | NS |
| Hemoglobin level, mean g/L | 91 | 90 | NS |
| Leukocyte count, mean G/L | 70.0 | 59.5 | NS |
| Platelet count, mean G/L | 14.4 | 12.0 | NS |
| FAB, n, 0/1/2/4/5/6 | 8/53/67/43/34/7 | 12/53/77/38/28/11 | NS |
| Cytogenetic analysis, n | |||
| Good | 32 | 32 | .01 |
| Intermediate | 112 | 128 | .01 |
| Poor | 39 | 20 | .01 |
| Normal | 80 | 100 | .01 |
| ND/failure | 30 | 37 | .01 |
| MDR phenotype, n | |||
| mdrl gene+ (%) | 36 of 132 (27.3) | 40 of 132 (30.3) | NS |
| 4 E3+ (%) | 30 of 122 (24.6) | 32 of 119 (26.9) | NS |
| UIC2+ (%) | 38 of 126 (30.2) | 39 of 111 (35.1) | NS |
| Rh123 efflux+ (%) | 25 of 78 (32) | 29 of 82 (35.4) | NS |
. | Without quinine, N = 212 . | With quinine, N = 213 . | P . |
|---|---|---|---|
| Mean age, y | 41 | 41 | NS |
| Sex, n, M/F | 102/110 | 108/105 | NS |
| PS greater than 1, n | 45 | 52 | NS |
| Hemoglobin level, mean g/L | 91 | 90 | NS |
| Leukocyte count, mean G/L | 70.0 | 59.5 | NS |
| Platelet count, mean G/L | 14.4 | 12.0 | NS |
| FAB, n, 0/1/2/4/5/6 | 8/53/67/43/34/7 | 12/53/77/38/28/11 | NS |
| Cytogenetic analysis, n | |||
| Good | 32 | 32 | .01 |
| Intermediate | 112 | 128 | .01 |
| Poor | 39 | 20 | .01 |
| Normal | 80 | 100 | .01 |
| ND/failure | 30 | 37 | .01 |
| MDR phenotype, n | |||
| mdrl gene+ (%) | 36 of 132 (27.3) | 40 of 132 (30.3) | NS |
| 4 E3+ (%) | 30 of 122 (24.6) | 32 of 119 (26.9) | NS |
| UIC2+ (%) | 38 of 126 (30.2) | 39 of 111 (35.1) | NS |
| Rh123 efflux+ (%) | 25 of 78 (32) | 29 of 82 (35.4) | NS |
PS indicates performance status; Rh123, rhodamine 123; NS, not significant; and ND, not done.
All eligible patients were evaluated for induction therapy
Of patients evaluated for induction therapy, 213 received quinine and 212 did not. Bone marrow examination at day 20 after the beginning of induction therapy identified more than 19% blasts or blasts with Auer rods in 48 patients of the control group (23%) and in 42 patients (20%) of the quinine group (NS). These patients received a second induction course as described in the therapeutic protocol (Table 2). Mean quinine concentration, measured at day 2 of drug infusion in 161 patients receiving quinine, was 7.8 mg/L (median, 7.7 mg/L; range, 1.1-15.3 mg/L). The mean MDR-reversing activity of serum, determined at day 2 in these patients, was 1.96 (median, 1.89; range, 1.10-4.00) and correlated with quinine serum level (r = 0.48, P < .0001). When tested in the same conditions, serum samples from 69 control patients did not demonstrate any significant MDR-reversing activity (mean ratio, 1.03). Adverse effects due to quinine infusion, including tinnitus, vertigo, and tachycardia, were registered in 97 patients (46%). These effects disappeared after a 20% dose decrease in 79 patients and required quinine treatment cessation in 18 patients. The CR rate in the quinine arm was not significantly influenced by these dose modifications.
Number of patients at each step of the protocol
. | Without quinine, n . | With quinine, n . | Total . |
|---|---|---|---|
| Included | 218 | 217 | 435 |
| Eligible | 212 | 213 | 415 |
| 2 courses of induction | 48 | 42 | 90 |
| ICC eligible | 134 | 128 | 262 |
| ICC1 done | 117 | 114 | 231 |
| ICC2 done | 103 | 91 | 194 |
| HLA identical | 37 | 45 | 82 |
| BMT | 32 | 41 | 73 |
. | Without quinine, n . | With quinine, n . | Total . |
|---|---|---|---|
| Included | 218 | 217 | 435 |
| Eligible | 212 | 213 | 415 |
| 2 courses of induction | 48 | 42 | 90 |
| ICC eligible | 134 | 128 | 262 |
| ICC1 done | 117 | 114 | 231 |
| ICC2 done | 103 | 91 | 194 |
| HLA identical | 37 | 45 | 82 |
| BMT | 32 | 41 | 73 |
ICC indicates intensive consolidation course; BMT, allogeneic bone marrow transplantation.
The incidence of mucositis provoked by induction therapy was significantly higher in the quinine group than in the control group (P < .05). Other induction regimen–related toxicities were similar in both groups, for example, quinine did not increase the rate of early deaths and deaths in aplasia (Table 3). CR was achieved in 171 of 212 patients (80.7%) in the control group and in 173 of 213 patients (81.2%) in the quinine group (Table 3). In patients who achieved CR, quinine did not influence the duration of cytopenia, the rate of red cell and platelet transfusions, the duration of antibiotic and antifungal drug administration, and the time to discharge from the hospital (Table 3). Univariate analysis identified 5 parameters of significant value according to their impact on CR achievement: initial white blood cell count (cutoff, 30 × 109/L), karyotype (4 groups), performance status, FAB classification (M2/M4 versus others), and rhodamine 123 efflux (Table 4). Using these 5 parameters for logistic regression analysis, karyotype (P = .0004), white blood cell count (P = .002), and performance status (P = .002) remained significant.
Induction regimen: results and toxicity
. | Without quinine, n = 212 . | With quinine, n = 213 . | P . |
|---|---|---|---|
| Number of patients in CR (%) | 171 (80.6) | 173 (81.2) | NS |
| CR after 1 course of induction, n (%) | 139 (65.6) | 145 (68.1) | NS |
| CR after 2 courses of induction, n (%) | 32 (15.1) | 28 (13.1) | NS |
| Time to discharge from hospital, d (range) | 31 (19-100) | 30 (16-81) | NS |
| Duration of neutropenia of fewer than 0.5 G/L, d (range) | 26 (11-102) | 25 (9-67) | NS |
| Duration of thrombopenia of fewer than 100 G/L, d (range) | 22 (8-134) | 23 (9-96) | NS |
| Red cell transfusion, units (range) | 11 (3-50) | 12 (2-38) | NS |
| Platelet transfusion, units (range) | 8 (0-40) | 9 (1-40) | NS |
| Duration of intravenous antibiotics, d (range) | 25 (0-100) | 25 (0-66) | NS |
| Duration of intravenous antifungal agents, d (range) | 9 (0-60) | 12 (0-60) | NS |
| Early death, n (%) | 3 (1.4) | 1 (0.5) | NS |
| Death in aplasia, n (%) | 6 (2.8) | 12 (5.7) | NS |
| Failure, n (%) | 32 (15.1) | 27 (12.7) | NS |
| Toxicity—NCI grade above 2, % | |||
| Mucositis | 10.2 | 17.2 | .05 |
| Nausea/vomiting | 3.9 | 4.4 | NS |
| GI tract | 6.3 | 8.1 | NS |
| Kidney | 1.5 | 2.5 | NS |
| Liver | 16.4 | 23.1 | NS |
| Central nervous system | 3.4 | 4.4 | NS |
. | Without quinine, n = 212 . | With quinine, n = 213 . | P . |
|---|---|---|---|
| Number of patients in CR (%) | 171 (80.6) | 173 (81.2) | NS |
| CR after 1 course of induction, n (%) | 139 (65.6) | 145 (68.1) | NS |
| CR after 2 courses of induction, n (%) | 32 (15.1) | 28 (13.1) | NS |
| Time to discharge from hospital, d (range) | 31 (19-100) | 30 (16-81) | NS |
| Duration of neutropenia of fewer than 0.5 G/L, d (range) | 26 (11-102) | 25 (9-67) | NS |
| Duration of thrombopenia of fewer than 100 G/L, d (range) | 22 (8-134) | 23 (9-96) | NS |
| Red cell transfusion, units (range) | 11 (3-50) | 12 (2-38) | NS |
| Platelet transfusion, units (range) | 8 (0-40) | 9 (1-40) | NS |
| Duration of intravenous antibiotics, d (range) | 25 (0-100) | 25 (0-66) | NS |
| Duration of intravenous antifungal agents, d (range) | 9 (0-60) | 12 (0-60) | NS |
| Early death, n (%) | 3 (1.4) | 1 (0.5) | NS |
| Death in aplasia, n (%) | 6 (2.8) | 12 (5.7) | NS |
| Failure, n (%) | 32 (15.1) | 27 (12.7) | NS |
| Toxicity—NCI grade above 2, % | |||
| Mucositis | 10.2 | 17.2 | .05 |
| Nausea/vomiting | 3.9 | 4.4 | NS |
| GI tract | 6.3 | 8.1 | NS |
| Kidney | 1.5 | 2.5 | NS |
| Liver | 16.4 | 23.1 | NS |
| Central nervous system | 3.4 | 4.4 | NS |
CR indicates complete remission; GI, gastrointestinal; and NS, not significant.
Prognostic factors for complete remission
. | N . | CR, % . | P . |
|---|---|---|---|
| Age younger than 46 y | 247 | 83.8 | .09 |
| Age older than 45 y | 178 | 77 | |
| FAB 2 and 4 | 225 | 84.9 | |
| FAB other | 200 | 76.5 | .04 |
| Performance status less than 3 | 327 | 84.4 | .001 |
| Performance status higher than 2 | 97 | 69.0 | |
| WBC count lower than 30 × 109/L | 281 | 86.5 | < .0001 |
| WBC count higher than 30 × 109/L | 144 | 75.4 | |
| Karyotype | < .0001 | ||
| Normal | 180 | 82.2 | |
| Good | 64 | 96.9 | |
| Intermediate | 61 | 67.2 | |
| Poor | 59 | 69.5 | |
| Rh123 efflux positive | 54 | 65.4 | .04 |
| Rh123 efflux negative | 106 | 81.3 | |
| mdr gene positive | 76 | 82.9 | NS |
| mdr gene negative | 188 | 83.8 | |
| UIC2 positive | 77 | 80.6 | NS |
| UIC2 negative | 160 | 78.8 | |
| 4E3 positive | 62 | 83.7 | NS |
| 4E3 negative | 169 | 76.3 | |
| Quinine dose reduction | 79 | 80.2 | NS |
| No quinine dose reduction | 134 | 81.8 |
. | N . | CR, % . | P . |
|---|---|---|---|
| Age younger than 46 y | 247 | 83.8 | .09 |
| Age older than 45 y | 178 | 77 | |
| FAB 2 and 4 | 225 | 84.9 | |
| FAB other | 200 | 76.5 | .04 |
| Performance status less than 3 | 327 | 84.4 | .001 |
| Performance status higher than 2 | 97 | 69.0 | |
| WBC count lower than 30 × 109/L | 281 | 86.5 | < .0001 |
| WBC count higher than 30 × 109/L | 144 | 75.4 | |
| Karyotype | < .0001 | ||
| Normal | 180 | 82.2 | |
| Good | 64 | 96.9 | |
| Intermediate | 61 | 67.2 | |
| Poor | 59 | 69.5 | |
| Rh123 efflux positive | 54 | 65.4 | .04 |
| Rh123 efflux negative | 106 | 81.3 | |
| mdr gene positive | 76 | 82.9 | NS |
| mdr gene negative | 188 | 83.8 | |
| UIC2 positive | 77 | 80.6 | NS |
| UIC2 negative | 160 | 78.8 | |
| 4E3 positive | 62 | 83.7 | NS |
| 4E3 negative | 169 | 76.3 | |
| Quinine dose reduction | 79 | 80.2 | NS |
| No quinine dose reduction | 134 | 81.8 |
NS indicates not significant.
Quinine increased the CR rate in adult de novo AMLs with rhodamine 123 efflux
The centralized blast cell collection procedure allowed the analysis of mdr1 gene expression, P-glycoprotein expression, and rhodamine 123 efflux in 264, 231, and 160 patients, respectively (Table 5). At least one of these resistance-associated markers was studied in 341 patients. In other patients, blast cells were either not collected (n = 46) or unusable for any analysis after storage (n = 38). MDR markers were balanced between quinine-treated and control patients. The disease variables were similar in untested and tested patients, with the exception of the white blood cell (WBC) count that was higher in the rhodamine 123–tested group (median WBC count, 20.0 × 109/L in tested; 9.9 × 109/L in nontested patients, P = .03). Each marker was scored positive in about 30% of the patients studied. However, due to the limited correlation between the various MDR markers tested, the influence of MDR phenotype on CR rate was examined mainly by considering each parameter individually.
Multidrug resistance phenotype analysis at diagnosis
. | Without quinine . | . | With quinine . | . | Total . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | N . | CR, % . | N . | CR, % . | N . | P . | |||
| mdr1 gene+ | 36 | 80.0 | 40 | 87.5 | 76 | NS | |||
| mdr1 gene- | 96 | 80.4 | 92 | 86.2 | 188 | NS | |||
| 4 E3+ | 30 | 83.3 | 32 | 87.5 | 62 | NS | |||
| 4 E3- | 82 | 76.8 | 87 | 75.9 | 169 | NS | |||
| UIC2+ | 38 | 81.6 | 39 | 79.5 | 77 | NS | |||
| UIC2- | 88 | 75.0 | 72 | 80.6 | 160 | NS | |||
| Rh123 efflux+ | 25 | 48.0 | 29 | 82.8 | 54 | .01 | |||
| Rh 123 efflux- | 53 | 86.8 | 53 | 77.4 | 106 | NS | |||
. | Without quinine . | . | With quinine . | . | Total . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | N . | CR, % . | N . | CR, % . | N . | P . | |||
| mdr1 gene+ | 36 | 80.0 | 40 | 87.5 | 76 | NS | |||
| mdr1 gene- | 96 | 80.4 | 92 | 86.2 | 188 | NS | |||
| 4 E3+ | 30 | 83.3 | 32 | 87.5 | 62 | NS | |||
| 4 E3- | 82 | 76.8 | 87 | 75.9 | 169 | NS | |||
| UIC2+ | 38 | 81.6 | 39 | 79.5 | 77 | NS | |||
| UIC2- | 88 | 75.0 | 72 | 80.6 | 160 | NS | |||
| Rh123 efflux+ | 25 | 48.0 | 29 | 82.8 | 54 | .01 | |||
| Rh 123 efflux- | 53 | 86.8 | 53 | 77.4 | 106 | NS | |||
Criteria for positivity are indicated in “Patients and methods.” NS indicates not significant.
In the group of patients whose blast cells demonstrated rhodamine 123 efflux ex vivo (functional assay), quinine significantly improved the CR rate from 12 of 25 (48.0%; control arm) to 24 of 29 (82.8%, quinine arm) (P = .01). In contrast, in patients whose blast cells did not demonstrate any significant rhodamine 123 efflux, quinine had no influence on the CR rate (46 of 53 in the control arm, 41 of 53 in the quinine arm) (Table 5). Neither mdr1 gene nor P-glycoprotein expression assessed by 2 distinct antibodies demonstrated any significant influence on the response to induction therapy in the absence and presence of quinine (Table 5). Similarly, no significant difference was observed when mdr1 gene or P-glycoprotein expression were analyzed as continuous variables in patients in CR compared to those in failure (not shown). The CR rate was 76% in the 33 patients with all studied MDR markers positive, whereas it was 83% in the 131 patients with 2 or 3 concordant negative markers (NS). Finally, in the quinine-treated group, no correlation was identified between MDR-reversing activity in day-2 serum and the response rate (not shown).
Quinine did not improve the outcome of adult de novo AML patients
An HLA-matched sibling was identified in 82 of 207 patients aged 45 years or younger who entered CR. Of those patients, 73 received a standard dose consolidation chemotherapy, then an allogeneic BMT; 9 patients did not receive BMT due to disease relapse (n = 4), toxicity (n = 3), and donor-related exclusion criteria (n = 2). The median interval between the first day of induction therapy and BMT was 59.5 days in the control arm and 62 days in the quinine arm (P = .33).
Of the 262 patients who were assigned to ICC, 31 were considered ineligible for the first course (toxicity, n = 9; relapse, n = 5; protocol violation, n = 5; death, n = 4). The median interval between CR achievement and the first ICC was 16 days in the control group and 19 days in the quinine group (NS). Of the 231 patients who received the first ICC, 37 were considered ineligible for the second course (toxicity, n = 20; relapse, n = 9; protocol violation, n = 1; death, n = 7). The median interval between the first day of ICC1 and the first day of ICC2 was 56 days in the control arm and 58 days in the quinine arm (P = .98).
Quinine-related adverse effects led to a 20% dose decrease in 31 of 128 patients (24%) during ICC1 and 40 of 91 patients (44%) during ICC2, whereas quinine infusion was stopped in 12% of patients during both ICC1 and ICC2. Addition of quinine to ICC1 significantly increased the duration of neutropenia (P = .046) and thrombocytopenia (P = .014) as well as the requirement for platelet transfusion (P = .01). Other toxicities were similar in both the control and the quinine arm. In patients who received ICC2, quinine-induced increase in the duration of cytopenia did not reach significance, and the regimen toxicity remained very low.
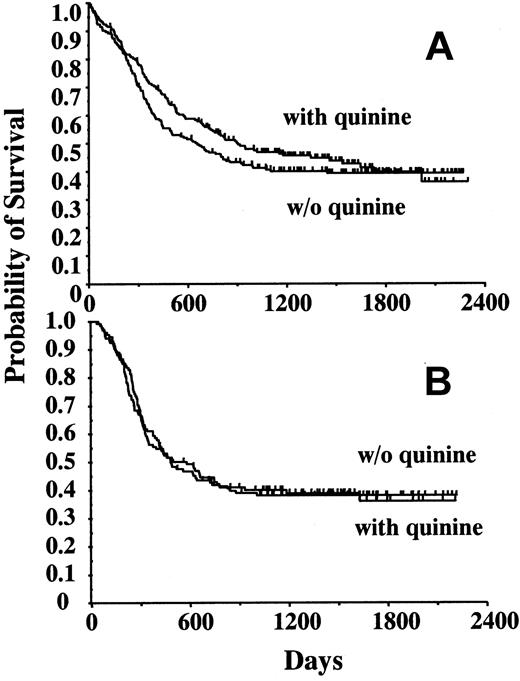
Among the 344 patients who entered CR, the probability of remaining alive and free of disease at 4 years was 43.1% ± 2.7%. Quinine did not influence the 4-year DFS (without quinine, 41.1% ± 3.8% [n = 171]; with quinine, 45.3% ± 3.8%, [n = 173] P = .29) (Figure 1A) nor overall survival (Table 6), even when considering patients who did not undergo BMT (DFS without quinine, 38.1% ± 4.2% [n = 134]; DFS with quinine, 38.8% ± 4.8% [n = 128] NS) (Figure 1B). In patients whose blast cells demonstrated rhodamine 123 efflux ex vivo, quinine improved both the event-free survival (Figure 2) and OS, that is, quinine increased the median survival from 12 to 24 months and the 4-year survival rate from 32% to 47%, respectively (not shown). However, the number of patients in each group remained low, and these results did not reach statistical significance. Neither mdr1 gene expression nor P-glycoprotein expression demonstrated any significant influence on DFS and survival, both in the absence and presence of quinine (not shown).
Disease-free survival according to treatment arms. (A) DFS of the 344 patients in whom complete remission was obtained, depending on initial randomization (without quinine, n = 171; with quinine, n = 173). (B) DFS of the 262 patients who were assigned to intensive consolidation therapy, without or with quinine according to initial randomization (without quinine, n = 134; with quinine, n = 128).
Disease-free survival according to treatment arms. (A) DFS of the 344 patients in whom complete remission was obtained, depending on initial randomization (without quinine, n = 171; with quinine, n = 173). (B) DFS of the 262 patients who were assigned to intensive consolidation therapy, without or with quinine according to initial randomization (without quinine, n = 134; with quinine, n = 128).
Analysis of factors influencing survival
. | N . | Median survival, mo . | 4-year survival, % . | P . |
|---|---|---|---|---|
| Without quinine | 212 | 22 | 39.3 ± 3.4 | .3 |
| With quinine | 213 | 30 | 43.7 ± 3.6 | |
| BMT, intent to treat | 82 | NR | 63.0 ± 5.4 | .07 |
| ICC, intent to treat | 262 | 33 | 46.1 ± 3.2 | |
| Age younger than 46 y | 247 | 31 | 46.7 ± 3.3 | .006 |
| Age older than 45 y | 178 | 18 | 34.5 ± 3.7 | |
| FAB 2 and 4 | 225 | 36 | 47.4 ± 3.4 | .003 |
| FAB other | 200 | 20 | 34.8 ± 3.5 | |
| Performance status less than 3 | 327 | 30 | 45.4 ± 2.8 | .001 |
| Performance status higher than 2 | 97 | 15 | 29.4 ± 4.8 | |
| WBC count lower than 30 G/L | 281 | 36 | 46.9 ± 3.0 | < .0001 |
| WBC count higher than 30 G/L | 144 | 15 | 31.3 ± 4.0 | |
| Karyotype | .003 | |||
| Normal | 180 | 27 | 40.8 ± 3.8 | |
| Good | 64 | NR | 58.7 ± 6.5 | |
| Intermediate | 61 | 22 | 39.5 ± 6.4 | |
| Poor | 59 | 12 | 26.8 ± 5.8 | |
| Rh123 efflux positive | 54 | 18 | 39.1 ± 6.6 | .33 |
| Rh123 efflux negative | 106 | 34 | 46.2 ± 5.0 |
. | N . | Median survival, mo . | 4-year survival, % . | P . |
|---|---|---|---|---|
| Without quinine | 212 | 22 | 39.3 ± 3.4 | .3 |
| With quinine | 213 | 30 | 43.7 ± 3.6 | |
| BMT, intent to treat | 82 | NR | 63.0 ± 5.4 | .07 |
| ICC, intent to treat | 262 | 33 | 46.1 ± 3.2 | |
| Age younger than 46 y | 247 | 31 | 46.7 ± 3.3 | .006 |
| Age older than 45 y | 178 | 18 | 34.5 ± 3.7 | |
| FAB 2 and 4 | 225 | 36 | 47.4 ± 3.4 | .003 |
| FAB other | 200 | 20 | 34.8 ± 3.5 | |
| Performance status less than 3 | 327 | 30 | 45.4 ± 2.8 | .001 |
| Performance status higher than 2 | 97 | 15 | 29.4 ± 4.8 | |
| WBC count lower than 30 G/L | 281 | 36 | 46.9 ± 3.0 | < .0001 |
| WBC count higher than 30 G/L | 144 | 15 | 31.3 ± 4.0 | |
| Karyotype | .003 | |||
| Normal | 180 | 27 | 40.8 ± 3.8 | |
| Good | 64 | NR | 58.7 ± 6.5 | |
| Intermediate | 61 | 22 | 39.5 ± 6.4 | |
| Poor | 59 | 12 | 26.8 ± 5.8 | |
| Rh123 efflux positive | 54 | 18 | 39.1 ± 6.6 | .33 |
| Rh123 efflux negative | 106 | 34 | 46.2 ± 5.0 |
NR indicates not reached.
Event-free survival according to rhodamine 123 (Rh123) efflux. Of 160 studied patients, 54 demonstrated Rh123 efflux, of whom 29 were randomized in the quinine arm, and 106 were scored Rh123 negative, of whom 53 received quinine. The differences in EFS observed in the absence of quinine (without quinine: Rh123+, n = 25; Rh123–,n = 53) do not reach statistical significance.
Event-free survival according to rhodamine 123 (Rh123) efflux. Of 160 studied patients, 54 demonstrated Rh123 efflux, of whom 29 were randomized in the quinine arm, and 106 were scored Rh123 negative, of whom 53 received quinine. The differences in EFS observed in the absence of quinine (without quinine: Rh123+, n = 25; Rh123–,n = 53) do not reach statistical significance.
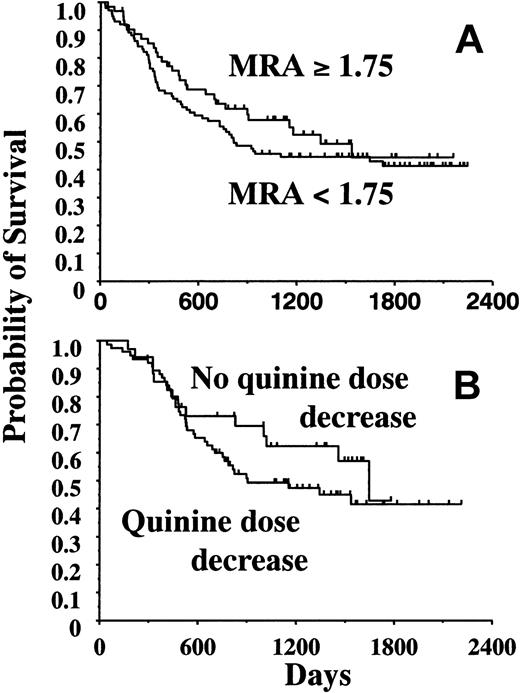
We also studied the influence of MDR-reversing activity in day-2 serum on survival. We did not identify any threshold over which survival was significantly improved (Figure 3A). Similarly, quinine dose reduction did not influence EFS, DFS, and survival, either in the whole population of quinine-treated patients or in subgroups, for example, in the 114 patients who received ICC1 with quinine (Figure 3B).
Influence of MDR-reversing activity and quinine dose decrease on survival. (A) Analysis of the prognostic value of the MDR-reversing activity measured at day 2 of quinine infusion in 163 patients. Overall survival was not significantly improved in the 101 patients with an MDR-reversing activity higher than 1.75 compared with 62 patients with an MDR-reversing activity lower than 1.75 (P = .32). Other tested cutoff values were 1.20, 1.50, and 2.00 (not shown). (B) Influence of quinine dose decrease on overall survival in 114 patients who received ICC1. Quinine dose was decreased during either ICC1 or ICC2 in 76 patients (P = .27).
Influence of MDR-reversing activity and quinine dose decrease on survival. (A) Analysis of the prognostic value of the MDR-reversing activity measured at day 2 of quinine infusion in 163 patients. Overall survival was not significantly improved in the 101 patients with an MDR-reversing activity higher than 1.75 compared with 62 patients with an MDR-reversing activity lower than 1.75 (P = .32). Other tested cutoff values were 1.20, 1.50, and 2.00 (not shown). (B) Influence of quinine dose decrease on overall survival in 114 patients who received ICC1. Quinine dose was decreased during either ICC1 or ICC2 in 76 patients (P = .27).
HLA-matched BMT was the best intensification therapy. The 4-year actuarial OS of the 425 eligible patients was 42.0% ± 2.5% (Figure 4A). In patients aged younger than 46 years and compared with the intensification arm, BMT intention significantly improved 4-year DFS (58.1% ± 5.5% in the BMT group [n = 82] versus 41.8% ± 4.5% in the ICC group [n = 125]; P = .018), whereas its favorable effect on survival did not reach significance (Table 6). In this same group of patients, BMT favorably influenced DFS (Figure 4B) and OS (not shown) when compared with ICC in patients who received treatments. Univariate analysis identified the following prognostic factors for survival: karyotype, age, white blood cell count, FAB classification, and performance status (Table 6; Figure 5). In multivariate analysis, karyotype appeared as the main prognostic parameter that influenced both DFS (P = .0004) and survival (P = .0006). This multivariate analysis also demonstrated that BMT favorably and independently influenced DFS (P = .005) and OS (P = .009) in younger patients who received the assigned therapy. When patients assigned to BMT were excluded, karyotype remained the only significant prognostic parameter (not shown).
Influence of treatment on survival. (A) Overall survival of the 425 eligible patients, including the confidence interval. The 4-year survival rate was 42.0% ± 2.5%. (B) DFS of patients who received allogeneic bone marrow transplantation (BMT) was significantly better that that of patients who received at least 1 intensive consolidation course (ICC1) and those who received 2 intensive consolidation courses (ICC2).
Influence of treatment on survival. (A) Overall survival of the 425 eligible patients, including the confidence interval. The 4-year survival rate was 42.0% ± 2.5%. (B) DFS of patients who received allogeneic bone marrow transplantation (BMT) was significantly better that that of patients who received at least 1 intensive consolidation course (ICC1) and those who received 2 intensive consolidation courses (ICC2).
Parameters influencing overall survival. Age (A), karyotype (B), FAB subtype (C), white blood cell (WBC) count (D), and performance status (not shown) were the 5 main prognostic parameters influencing survival in univariate analysis (Table 6). Both karyotype and WBC remained significant in multivariate analysis of the whole population of patients. Only karyotype was a significant prognostic parameter when excluding transplantation (not shown).
Parameters influencing overall survival. Age (A), karyotype (B), FAB subtype (C), white blood cell (WBC) count (D), and performance status (not shown) were the 5 main prognostic parameters influencing survival in univariate analysis (Table 6). Both karyotype and WBC remained significant in multivariate analysis of the whole population of patients. Only karyotype was a significant prognostic parameter when excluding transplantation (not shown).
Discussion
Several observations argued in favor of using P-glycoprotein modifier agents early in the treatment of AML. The MDR1 phenotype is an independent variable for predicting in vivo drug resistance.10-14 In addition, preclinical studies suggested that MDR-reversing agents could prevent both selection of blast cells expressing a functional P-glycoprotein and induction of the resistance phenotype in residual tumor cells.24,25 Although recently challenged by studies of the allelic expression of mdr1 in AML,33 this latter observation led to the hypothesis that the systematic combination of an MDR-reversing agent with induction and consolidation regimen might improve eradication of the residual leukemic disease. The present study demonstrates that using quinine as an MDR-reversing agent does not improve survival in adult patients between 15 and 60 years old with de novo AML. However, a small group of patients defined as having blast cells with quinine-sensitive rhodamine 123 efflux could benefit from this reversion strategy, at least when considering CR induction.
Several randomized trials of P-glycoprotein modulation in AML have now been reported.23,34,35 The greatest success was obtained with cyclosporin A combined with induction and consolidation therapy in patients with relapsed/refractory AML.34 Conversely, cyclosporin A in combination with ADE (cytarabine, daunorubicin, etoposide) was found to decrease survival of patients older than 60 years with refractory/relapsed AML.35 A similar observation was made in testing the cyclosporin A–related PSC833 (Valspodar) in combination with ADE regimen in untreated AML patients aged 60 years and older.36 At variance with cyclosporin A and its derivative PSC833 (Valspodar),37,38 quinine and derivatives have limited influence on the pharmacokinetics of cytotoxic drugs39,40 including idarubicin (J.-L.H., unpublished data, November 2002). We have shown previously that quinine could improve the survival of patients with P-glycoprotein–positive high risk myelodysplastic syndrome treated with mitoxantrone and high-dose cytarabine.23 Results from the present study suggest that de novo adult AML patients are less suited for MDR reversion than relapsed, refractory AML patients and high-risk myelodysplastic syndrome patients.
We have shown previously that the MDR-reversing activity of quinine was retained in the serum of patients despite protein binding,20-22 and results obtained with quinine in P-glycoprotein–positive myelodysplastic syndromes suggest that this drug behaves as an efficient MDR modulator when administered intravenously.23 We identified a significant MDR-reversing activity in most serum samples from quinine-treated patients, and this activity was linearly related to quinine serum level.22 Thus, the failure to demonstrate a positive effect on clinical outcome may not be related to a lack of genuine inhibition of P-glycoprotein function in vivo, although we cannot rule out that a more potent inhibitor or a molecule with a larger spectrum of action would have done better. The decrease of quinine doses in about one third of patients did not significantly decrease the efficacy of the MDR-reversing agent. However, a careful monitoring of quinine serum level during treatment may be more efficient than side-effect–based dose adaptation to accurately determine the dose of quinine to be infused.
P-glycoprotein expression was initially found to be of prognostic significance for CR achievement and survival duration in de novo AML patients treated with daunorubicin-containing induction therapy.11,41,42 Cellular pharmacological studies demonstrated P-glycoprotein–mediated efflux of several cytotoxic drugs included in the present study, such as mitoxantrone, etoposide, and amsacrine,43-45 but suggested that the plasma membrane pump had less effect on idarubicin cellular uptake.46,47 Compared with other anthracyclines, idarubicin demonstrated a modest but significant benefit for induction therapy in AML,48-51 which was attributed to a high level of active metabolite46,47 and decreased sensitivity to P-glycoprotein–mediated efflux.48-51 The present study demonstrates that the response to induction regimen including idarubicin is decreased in patients whose blast cells demonstrate rhodamine 123 efflux, a negative effect that is corrected by quinine administration.
Difficulties in defining which methods are most appropriate for determining the MDR status of a patient's blast cells have complicated the identification of those patients who could benefit from MDR-reversing strategies.52,53 Several consensus recommendations have been proposed in an attempt to decrease the variability in measurements of MDR factors, including mdr1 gene, P-glycoprotein, and cellular efflux.27,54 The present study was performed according to these recommendations and biologic assays were centralized to prevent variability between centers. In these conditions, only rhodamine 123 efflux in blast cells appeared as a significant prognostic factor, which, interestingly, could be reversed by quinine. Various parameters have been proposed to account for the lack of concordance between drug efflux activity and either mdr1 gene expression level or P-glycoprotein detection at the blast cell surface,55 for example, posttranslational modifications or intracellular location of the P-glycoprotein, the choice of antibodies used for detection, and the way of gating the leukemic cell population in flow cytometry analyses.47,56-59 Our study and a recently published trial36 strongly argue for using functional assays to identify patients who could benefit from MDR reversion.
The influence of allogeneic BMT on AML patient outcome has been a controversial issue.59-62 In the present study, patients with an HLA-identical sibling donor demonstrated a better disease-free survival than patients without a donor, and their survival was not influenced by addition of quinine to induction therapy. Our study confirms the prognostic value, in terms of both initial response and survival, of such clinical and biologic variables as cytogenetic abnormalities and advanced age.63,64 A poor prognostic karyotype has been associated with P-glycoprotein–mediated resistance.65 In the present study, rhodamine 123–positive (n = 54) and –negative (n = 107) AML were not significantly different according to cytogenetic subgroups, mean age, FAB subtypes, and performance status.
In conclusion, quinine infusion induces an efficient MDR-reversing activity in patient serum and prevents the decrease in CR rate observed in adults with de novo AML whose blast cells efflux rhodamine 123. The lack of significant effect of the drug on survival could be due to the limited number of patients who may benefit from this strategy (about one third of these patients). Quinine also could sensitize rhodamine 123–positive leukemic cells to induction regimen without preventing selection of cells that resist through other mechanisms and are responsible for relapse. In addition to P-glycoprotein, several mechanisms contribute to the resistance of leukemic cells to cytotoxic drugs. These include transporters that, like P-glycoprotein, increase efflux and/or modify the intracellular distribution of drugs and dyes.66 MDR correlates with a deregulated expression of such critical regulators of apoptosis as Bcl-2–related proteins and heat shock proteins,67 and P-glycoprotein itself has been proposed to negatively interfere with various caspase-dependent cell death pathways.68 Although these multiple resistance mechanisms could account for disappointing results of most trials translating the concept of MDR reversion into clinical application, results from the present study suggest that a small group of adult patients with de novo AML, who could be identified by dye efflux assays, may benefit from MDR reversion.
Appendix
The following French centers and investigators from the GOELAMS group participated in this study: Amiens (B. Desablens, J. Fernandes), Angers (N. Ifrah, M. Hunault-Berger, M. Gardembas), Besançon (J. Y. Cahn, E. Deconninck, F. Garnache), Bobigny (P. Casassus), Brest (C. Berthou), Colmar (B. Audhuy), Clermont-Ferrand (P. Travade), Dijon (E. Solary, D. Caillot, F. Mugneret, F. Kara-Slimane), Metz (V. Dorvaux), Nancy (F. Witz, B. Witz, M. C. Bene), Nantes (J. L. Harousseau, P. Moreau, N. Milpied), Orléans (S. Le Tortotrrec), Poitiers (F. Guilhot, A. Sadoun), Reims (B. Pignon), Rennes (T. Lamy, M. Bernard, B. Drenou), Saint-Etienne (D. Guyotat, L. Campos, C. Mounier), Strasbourg (B. Lioure,A. Falkenrodt), Tours (P. Colombat, M. Delain).
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-11-3419.
Supported by a grant from the Programme Hospitalier de Recherche Clinique (J.-L.H.), the Ligue Nationale Contre le Cancer, and the Centre Hospitalier Universitaire (CHU) Dijon (E.S.).
A list of the French centers and investigators from the GOELAMS group that participated in this study appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Caroline Even and Monique Grandjean for helpful monitoring of the study, Karine Tran-Perennou and David Masson for technical assistance, and Marie-Christine Bene and Philippe Solal-Seligny for careful reading of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal