Abstract
Increasing evidence suggests that postnatal neovascularization involves the recruitment of circulating endothelial progenitor cells (EPCs). Hematopoietic and endothelial cell lineages share common progenitors. Cytokines formerly thought to be specific for the hematopoietic system have only recently been shown to affect several functions in endothelial cells. Accordingly, we investigated the stimulatory potential of erythropoietin (Epo) on EPC mobilization and neovascularization. The bone marrow of Epo-treated mice showed a significant increase in number and proliferation of stem and progenitor cells as well as in colony-forming units. The number of isolated EPCs and CD34+/flk-1+ precursor cells was significantly increased in spleen and peripheral blood of Epo-treated mice compared with phosphate-buffered saline–treated mice. In in vivo models of postnatal neovascularization, Epo significantly increased inflammation- and ischemia-induced neovascularization. The physiologic relevance of these findings was investigated in patients with coronary heart disease. In a multivariate regression model, serum levels of Epo and vascular endothelial growth factor were significantly associated with the number of stem and progenitor cells in the bone marrow as well as with the number and function of circulating EPCs. In conclusion, the present study suggests that Epo stimulates postnatal neovascularization at least in part by enhancing EPC mobilization from the bone marrow. Epo appears to physiologically regulate EPC mobilization in patients with ischemic heart disease. Thus, Epo serum levels may help in identifying patients with impaired EPC recruitment capacity.
Introduction
Increasing evidence suggests that several cytokines and interleukins (ILs) formerly thought to be specific for the hematopoietic system, notably granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, IL-4, IL-6, and IL-8, are capable of affecting metabolism and function of endothelial cells.1-9 This responsiveness of both vascular and hematopoietic systems to certain cytokines and interleukins may reflect the common ontogenesis of endothelial and hematopoietic cells. Indeed, differentiation of the vascular endothelium during embryonic development is closely linked to the appearance of primitive hematopoietic cells, suggesting that both cell lineages share a common progenitor, the hemangioblast.10,11
Postnatal neovascularization was initially thought to result exclusively from the migration and proliferation of pre-existing, fully differentiated endothelial cells (a process referred to as angiogenesis).11 Recent studies, however, demonstrated that circulating endothelial progenitor cells (EPCs) home to sites of neovascularization and differentiate into endothelial cells in situ12-16 in a manner consistent with a process termed vasculogenesis.17 It was demonstrated that recruitment of EPCs is essential for tumor vascularization.18 Importantly, heterologous, homologous, or autologous EPCs administered systemically to animals with operatively induced hind-limb ischemia were found to incorporate into foci of neovascularization. Enhanced incorporation of EPCs appears to augment neovascularization of ischemic tissue.13,15,19-22 Preliminary studies in humans support the hypothesis that progenitor cell administration may represent a useful strategy for the treatment of ischemic heart disease23 and peripheral artery disease.24
Erythropoietin (Epo) exerts its hematopoietic effects by stimulating the proliferation of early erythroid precursors and the differentiation of later precursors of the erythroid lineage.25 Mature endothelial cells also express Epo receptors (EpoRs)26 and Epo induces a proangiogenic response in cultivated mature endothelial cells, as evidenced by stimulation of endothelial cell proliferation, migration, endothelin-1 release, and increase in cytosolic-free calcium concentration.27-30 A physiologic significance for the Epo-EpoR signaling pathway has been demonstrated for the estrogen-dependent cyclical angiogenesis in the uterus.31 In the present study, we demonstrate the ability of Epo to stimulate hematopoietic stem cell proliferation, resulting in a significantly increased number of circulating EPCs in experimental models and in the stimulation of neovascularization in vivo. Consistently, our human studies identify Epo as an independent predictor of EPC number and function in patients with coronary heart disease. These data suggest that Epo is an important physiologic determinant of EPC mobilization.
Materials and methods
Experimental studies
The present study was performed with permission of the State of Hesse, Regierungspräsidium Darmstadt, according to section 8 of the German Law for the Protection of Animals, and conforms to the Guide for the Care and Use of Laboratory Animals measurements.
Flow cytometry analysis. Hematopoietic stem and progenitor cells were identified by their distinct pattern of surface markers. Stem cells were defined by negative staining (lin–) with lineage panel antibodies (CD3, B220, CD11b, Gr-1, and Ter119; BD Pharmingen, San Diego, CA) and positive staining for stem cell antigen 1 (sca-1; BD Pharmingen). EPCs were defined by positive staining for CD34 (BD Pharmingen) and flk-1 (BD Pharmingen). After a one-hour incubation period, cells were washed in phosphate-buffered saline (PBS), fixed in 2% formaldehyde/PBS, and analyzed with the fluorescence-activated cell sorter (FACS) Calibur (Becton Dickinson, Heidelberg, Germany). Data were analyzed using CellQuest software (Becton Dickinson), and all staining was referred to isotype-matched control antibodies purchased from BD Pharmingen.
Colony-forming units (CFUs) assay. Total bone marrow cells and the mononuclear fraction of spleen homogenates (105 cells each) were seeded in methylcellulose plates (Methocult, StemCell, Vancouver, BC, Canada) supplemented with 50 ng/mL murine stem cell factor (SCF), 50 ng/mL human vascular endothelial growth factor (VEGF), 10 ng/mL murine IL-3, and 10 ng/mL human IL-6. For the investigation of erythroid CFUs, cells were cultured in the presence of 3 U/mL human recombinant Epo. Plates were examined under phase-contrast microscopy, and colonies were scored after 14 days of incubation by 2 independent investigators.
EPC culture assay. Mononuclear cells were isolated by density gradient centrifugation with Biocoll (Biochrom, Berlin, Germany) from disrupted spleen cell extracts. Immediately following isolation, 4 × 106 mononuclear cells were plated on culture dishes coated with human fibronectin (Sigma, St Louis, MO) and maintained in endothelial basal medium (EBM; CellSystems, St Katharinen, Germany) supplemented with endothelial growth medium (EGM) SingleQuots + 20% fetal calf serum (FCS) + 100 ng/mL human VEGF. Cultivated EPCs were characterized by expression of endothelial surface markers (VE-cadherin, VEGF receptor-2, and von Willebrand factor).32
Cell cycle analysis. Bone marrow cells were incubated with bromodeoxyuridine (BrdU; 10 μM) for one hour. Then cells were washed in PBS and incubated with anti–BrdU–fluorescein isothiocyanate (FITC; 1:50) for 20 minutes and 7-amino-actinomycin D (7AAD; 1:30) for 15 minutes according to the manufacturer's instructions (BD Pharmingen).
Proliferation assay. To investigate the proliferation of hematopoietic stem cells, we incubated bone marrow cells with BrdU (10 μM) for one hour. After a thorough washing step, cells were incubated with biotinylated lineage panel antibodies (identified by allophycocyanin [APC]–labeled streptavidin [BD Pharmingen]), phycoerythrin (PE)–labeled sca-1 antibodies (BD Pharmingen), and FITC-labeled BrdU antibodies.
Microarray analysis. The expression of EpoR in human umbilical vein endothelial cells (HUVECs; CellSystems), human microvascular endothelial cells (HMVECs), cultured EPCs, and CD34+ bone marrow cells from healthy donors was analyzed with a microarray. The protocol for sample preparation and microarray processing is available from Affymetrix (Santa Clara, CA). Data were analyzed with the software GeneSpring version 3.0 (Silicon Genetics, San Carlos, CA) as previously described.33
Disc neovascularization system. A disc of polyvinyl alcohol sponge (Rippey, El Dorado Hills, CA), covered with nitrocellulose cell-impermeable filters (Millipore, Burlington, MA), allows capillaries to grow only through the rim of the disc.34 Those discs were subcutaneously implanted into the back of 20-week-old C57BL/6N mice (n = 8 per group). Mice were treated with Epo (Janssen-Cilag, Neuss, Germany; 1000 IU/kg body weight) or PBS via subcutaneous injections. Treatment was administered once daily during the first 3 days of each week for the 2-week study period. Then 2 weeks later, mice were anesthetized and space-filling fluorescent microspheres (0.2 μm; Molecular Probes, Eugene, OR) were injected into the left ventricle to deliver them to the systemic microvasculature.35 Both the area of the disc invested by vascular ingrowth and the vessel density were assessed.34
Murine ischemic hind-limb model. The impact of Epo on ischemia-induced neovascularization was investigated in a murine model of hind-limb ischemia,36 by use of 20-week-old C57BL/6N mice fed a 0.15% cholesterol diet for 10 weeks (Harlan Teklad, Madison, WI). The proximal portion of the femoral artery, including the superficial and the deep branch as well as the distal portion of the saphenous artery, was ligated with 6-0 silk suture. All arterial branches between the ligations were obliterated using an electrical coagulator. The overlying skin was closed using 3 surgical staples.
We measured ischemic (right)/normal (left) limb blood flow ratio using a laser Doppler blood flow meter (Laser Doppler Perfusion Imager System, moorLDI-Mark 2; Moor Instruments, Wilmington, DE). Excess hair was removed from the hind limbs using a depilatory cream. Before initiating scanning, mice were placed on a heating pad at 37°C to minimize variations in temperature. After twice recording laser Doppler color images, the average perfusions of the ischemic and nonischemic limb were calculated on the basis of colored histogram pixels. To minimize variables including ambient light and temperature, calculated perfusion was expressed as the ratio of ischemic to nonischemic hind-limb perfusion.
Capillary density was determined in 5-μm frozen sections of the adductor and semimembranous muscles. Endothelial cells were identified using an FITC-labeled rat monoclonal antibody directed against mouse CD31 (BD Pharmingen). Capillary density is expressed as number of capillaries per myocyte. Conductance vessels were identified by size (> 20 μm) and α-actin staining using a Texas Red–labeled rat monoclonal antibody directed against mouse α-actin (Dako, Hamburg, Germany).37
Human studies
Patients. Prospectively studied were 38 patients with angiographically documented coronary artery disease (CAD), including 11 patients with acute coronary syndromes and 9 patients with ischemic cardiomyopathy. Patients with concomitant inflammatory or malignant disease were excluded. Since hydroxymethylglutaryl coenzyme A inhibitors (statins) have been shown to significantly increase the number and function of circulating EPCs, we included only patients without prior statin therapy.32 Informed consent was obtained from all patients and healthy volunteers, and the study protocol was approved by the local Ethics Committee of the University of Frankfurt.
Bone marrow and progenitor cell isolation. Peripheral blood–derived EPCs were isolated from venous blood. Mononuclear cells were isolated by density gradient centrifugation with Biocoll, and 4 × 106 mononuclear cells were plated on 24-well culture dishes coated with human fibronectin (Sigma) in endothelial basal medium (EBM; CellSystems) supplemented with endothelial growth medium SingleQuots and 20% FCS. After 4 days in culture, nonadherent cells were removed by thoroughly washing with PBS. The adherent cell fraction was stained with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine–labeled acetylated low density lipoprotein (DiI-ac-LDL; 2.4 μg/mL at 37°C for one hour), fixed with 2% formaldehyde for 10 minutes, and then stained with FITC-labeled Ulex europaeus agglutinin I (lectin; 10 mg/mL for one hour). Double-staining cells were judged as EPCs32 and counted in 3 randomly selected high-power fields by 2 independent investigators. In other patients, bone marrow was aspirated under local anesthesia from ilium and mononuclear bone marrow cells were isolated by Ficoll density separation.
Flow cytometry analysis. A volume of 100 μL peripheral blood or bone marrow aspirate was incubated for 15 minutes in the dark with FITC-conjugated monoclonal antibodies against human CD34 (BD Pharmingen) in combination with monoclonal antibodies against human kinase domain receptor (KDR; Sigma) followed by PE-conjugated secondary antibody or with the PE-conjugated monoclonal antibody against human CD133 (Miltenyi Biotech, Bergisch-Gladbach, Germany). Isotype-identical antibodies served as controls (BD Pharmingen). After incubation, erythrocytes in peripheral blood were lysed. Then, cells were washed with PBS and fixed in 2% formaldehyde before analysis. Each analysis included 60 000 events.
Migration assay. Isolated EPCs were detached using 1 mM EDTA (ethylenediaminetetraacetic acid) in PBS (pH 7.4), harvested by centrifugation, resuspended in 500 μL EBM, and counted. Then, 2 × 104 EPCs were placed in the upper chamber of a modified Boyden chamber. The chamber was inserted in a 24-well culture dish containing EBM and human recombinant VEGF (50 ng/mL). After 24 hours incubation at 37°C, the lower side of the filter was washed with PBS and fixed with 2% formaldehyde. For quantification, cell nuclei were stained with 4′,6-diamidine-2-phenylidole dihydrochloride (DAPI). Cells migrating into the lower chamber were counted manually in 3 randomly selected microscopic fields.
Biochemical markers. Serum levels of vascular endothelial growth factor (VEGF), tumor necrosis factor-α, granulocyte-macrophage colony-stimulating factor, hepatocyte growth factor, and Epo were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (all from R&D Systems, Wiesbaden, Germany). For interleukin-10 (IL-10) measurements, we used a high-sensitivity ELISA system (R&D Systems). C-reactive protein was determined by nephelometry (Behring BN II Nephelometer; Dade Behring, Deerfield, IL).
Statistical analysis
All results for continuous variables are expressed as means ± standard deviation. Comparisons between groups were analyzed by t test (2-sided) or analysis of variance (ANOVA) for experiments with more than 2 subgroups. Post hoc range tests and pair-wise multiple comparisons were performed with the t test (2-sided) with Bonferroni adjustment. Comparison of categoric variables was generated by the Pearson χ2 test. After blind assessment of biochemical markers and EPC characterization in blood samples from patients, test results were merged with the clinical database. To identify biochemical determinants of circulating EPCs, a multivariate linear regression analysis for EPC number and function was performed. P < .05 was considered statistically significant. All analyses were performed with SPSS 11.0 software (SPSS, Chicago, IL).
Results
Effects of human recombinant erythropoietin in mice
To investigate the effects of Epo on the numbers of EPCs in vivo, mice were subcutaneously treated with human recombinant Epo (100 and 1000 IU/kg body weight) as previously described.38 Epo treatment resulted in a marked rise in human Epo serum levels in mice. Human Epo was undetectable in control mice and increased to 16.1 ± 5.4 IU/L after Epo treatment (100 IU/kg body weight) for 3 days. The higher dose of Epo (1000 IU/kg body weight) resulted in serum levels of 40.6 ± 9.3 IU/L. The white blood cell count significantly increased from 8.7 ± 2.4 cells × 109/L in control mice to 17.4 ± 3.1 cells × 109/L in the Epo group (1000 IU/kg body weight; n = 10; P < .001). Monocyte counts nonsignificantly increased from 0.13 ± 0.07 cells × 109/L to 0.31 ± 0.32 cells × 109/L (P = .23). Hemoglobin level increased from 151 ± 11 to 171 ± 8 g/L (P = .007). Platelet counts were similar between both groups with 337 ± 192 cells × 109/L in the control group compared with 407 ± 162 cells 109/L in the Epo group (P = .55).
Erythropoietin increases the number of stem and progenitor cells in the bone marrow
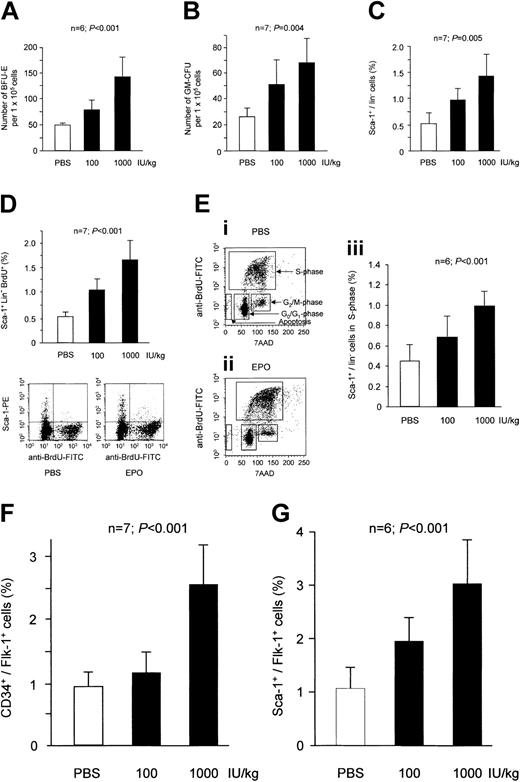
Epo induced a dose-dependent increase in erythroid CFUs (Figure 1A). Interestingly, a similar augmentation was also seen for granulocyte and monocyte colony-forming units (GM-CFUs) in the bone marrow (Figure 1B). Likewise, Epo significantly increased the number of undifferentiated bone marrow–derived stem cells that express the stem cell antigen (sca-1) but lack lineage markers (Figure 1C). Consistently, the proliferative capacity of lin–/sca-1+ cells significantly increased after Epo treatment as assessed by BrdU labeling (Figure 1D). The cell cycle analysis indicated that Epo dose-dependently increased the percentage of bone marrow cells that are in the S-phase (Figure 1E). The number of apoptotic cells was reduced from 9.1 ± 2.3% in PBS-treated mice to 4.8 ± 1.7% in Epo-treated mice (P = .024).
Effect of Epo treatment on bone marrow stem and progenitor cells. Mice were treated with human recombinant Epo for 3 days. Epo dose-dependently increased the number of erythroid CFUs in mice (A). A similar increase in response to treatment with Epo was observed for GM-CFUs (B). FACS analysis showed that Epo significantly enhanced the total number of lin–/sca-1+ stem cells (C). Further analysis using BrdU uptake revealed that Epo treatment caused a significant increase in the percentage of proliferating lin–/sca-1+ stem cells (D). (E) The percentage of total bone marrow cells that are in S-phase was significantly enhanced by Epo (iii). Cells in S-phase are defined as a typical horseshoelike population after staining with BrdU and 7AAD (i-ii). (F) The stimulatory effect of Epo on stem cell proliferation caused a significant increase in the percentage of EPCs defined as CD34+/flk-1+ cells. (G) Murine EPCs can also be identified by positive staining for sca-1 and flk-1. Results for sca-1+/flk-1+ cells showed a similar increase after 3 days of treatment with Epo. Data are shown as means ± SD.
Effect of Epo treatment on bone marrow stem and progenitor cells. Mice were treated with human recombinant Epo for 3 days. Epo dose-dependently increased the number of erythroid CFUs in mice (A). A similar increase in response to treatment with Epo was observed for GM-CFUs (B). FACS analysis showed that Epo significantly enhanced the total number of lin–/sca-1+ stem cells (C). Further analysis using BrdU uptake revealed that Epo treatment caused a significant increase in the percentage of proliferating lin–/sca-1+ stem cells (D). (E) The percentage of total bone marrow cells that are in S-phase was significantly enhanced by Epo (iii). Cells in S-phase are defined as a typical horseshoelike population after staining with BrdU and 7AAD (i-ii). (F) The stimulatory effect of Epo on stem cell proliferation caused a significant increase in the percentage of EPCs defined as CD34+/flk-1+ cells. (G) Murine EPCs can also be identified by positive staining for sca-1 and flk-1. Results for sca-1+/flk-1+ cells showed a similar increase after 3 days of treatment with Epo. Data are shown as means ± SD.
Furthermore, we determined the effect of Epo on EPCs, which are characterized by the expression of the VEGF receptor flk-1.32 Epo also significantly augmented the number of CD34+/flk-1+ cells in the bone marrow (Figure 1F). Double staining for sca-1 and flk-1 has also been considered for the identification of murine EPCs. We observed a similar increase in the number of sca-1+/flk-1+ cells in Epo-treated mice (Figure 1G). These data were confirmed in the EPC culture assay with EPCs characterized by the uptake of DiI-ac-LDL and positive staining for lectin.32 Epo significantly increased the number of DiI-ac-LDL+/lectin+ cells from 91.1 ± 28.6 cells/microscopic field in the control group to 143.0 ± 57.5 (Epo 100 IU/kg) and 202.1 ± 53.2 cells/microscopic field (Epo 1000 IU/kg) (n = 7 each; P = .002).
Erythropoietin increases the number of peripheral progenitor cells
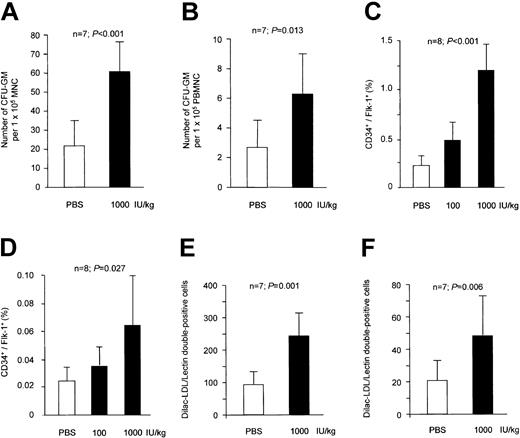
To further determine the effect of Epo on peripheral progenitor cells, we cultivated mononuclear cells from peripheral blood and from spleen homogenates, which serve as a reservoir for peripheral stem/progenitor cells. Epo-treated mice revealed a significant increase in the number of GM-CFUs in spleen and peripheral blood (Figure 2A and B, respectively), which again was similar to the increase that was observed for erythroid CFUs (3.3 ± 1.8-fold increase). Importantly, EPCs defined as CD34+/flk-1+ cells were augmented both in spleen and peripheral blood (Figure 2C and D, respectively). Moreover, the number of DiI-ac-LDL+/lectin+ cultivated EPCs was significantly increased (Figure 2E-F). Of note, the number of late outgrowing colonies after 28 days of cultivation was also altered by Epo treatment. Adherent colonies double-positive for DiI-ac-LDL and lectin were quantified every week. After 4 weeks, DiI-ac-LDL+/lectin+ colonies derived from weekly passaged nonadherent cells were considered as late-outgrowth colonies. Epo (1000 IU/kg) significantly increased the number of late-outgrowth colonies from 13.3 ± 6.2 to 27.4 ± 11.1 cells/microscopic field (n = 6; P = .023).
Effect of Epo treatment on peripheral progenitor cells. Epo treatment for 3 days significantly increased the number of GM-CFUs in spleen (A) and peripheral blood (B). FACS analysis showed that Epo dose-dependently enhanced the number of CD34+/flk-1+ cells in spleen (C) and peripheral blood (D). Consistently, a similar increase in the number of peripheral EPCs was observed in the EPC culture assay for spleen (E) and peripheral blood mononuclear cells (F). Data are shown as means ± SD.
Effect of Epo treatment on peripheral progenitor cells. Epo treatment for 3 days significantly increased the number of GM-CFUs in spleen (A) and peripheral blood (B). FACS analysis showed that Epo dose-dependently enhanced the number of CD34+/flk-1+ cells in spleen (C) and peripheral blood (D). Consistently, a similar increase in the number of peripheral EPCs was observed in the EPC culture assay for spleen (E) and peripheral blood mononuclear cells (F). Data are shown as means ± SD.
Expression of the EpoR on mRNA levels as determined by microarray analysis (fold change of normalized data) was 1.67 ± 0.42 in cultivated human EPCs, suggesting that EPCs are capable of directly responding to Epo. CD34+ cells showed a relative expression level of 1.27 ± 0.62 (n = 4). Similar mRNA expression levels were found in mature endothelial cells (HUVECs: 0.98 ± 0.32; HMVECs: 1.25 ± 0.51; n = 3 each). Consistently, cultivated EPCs showed a consistent, albeit modest, effect after in vitro treatment with Epo. After 4 days of Epo treatment, the number of EPCs was 124 ± 13% compared with control (P = .032).
Erythropoietin increases postnatal neovascularization
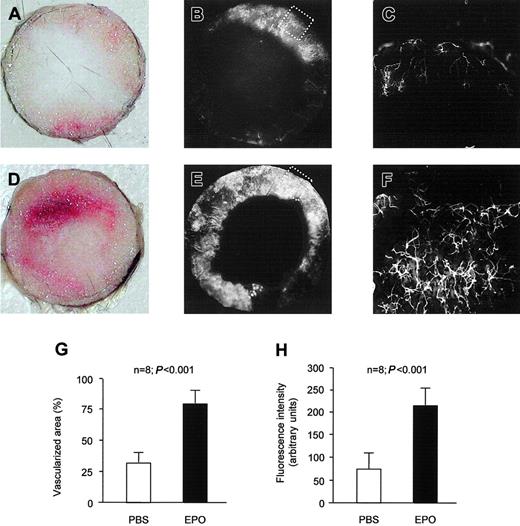
To determine whether above findings may result in enhanced postnatal neovascularization in response to Epo treatment, we used 2 different models of postnatal neovascularization. During the 2-week study period, Epo (1000 IU/kg) or PBS was subcutaneously injected for the first 3 days of each week. In the disc neovascularization system, reflecting both inflammatory angiogenesis and vasculogenesis, the control group showed 33.0 ± 18.4% of the cross-sectional area covered with new vessels during 2 weeks of subcutaneous implantation (Figure 3A-C). Systemic treatment with Epo significantly enhanced neovascularization of the disc (Figure 3D-F). The area of the disc that was covered with vessels increased by more than 2-fold (Figure 3G). Epo increased not only the neovascularized area, but increased also the fluorescence intensity reflecting the density of the vascular network by 3-fold (Figure 3H).
Epo stimulates inflammatory neovascularization in vivo. In the control group, subcutaneous implantation of a polyvinyl sponge for 2 weeks resulted in ingrowth of new vessels. (A) Representative picture of the native disc. (B) Functional neovascularization of the disc was visualized by perfusion with space-filling fluorescent microspheres prior to explantation of the disc. (C) Vessel caliber and density of the network shown by microscopic picture with a × 10 objective (detailed view of tagged area in panel B). Epo increased the vascularized area of the disc (D-E) and the vessel density in the disc (F). Panel F presents a detailed view of tagged area in panel E. Quantification of the vascularized area of the disc (G) as well as the fluorescence intensity of the vascularized area (H) revealed significant differences between PBS- and Epo-treated animals. Data are given as means ± SD.
Epo stimulates inflammatory neovascularization in vivo. In the control group, subcutaneous implantation of a polyvinyl sponge for 2 weeks resulted in ingrowth of new vessels. (A) Representative picture of the native disc. (B) Functional neovascularization of the disc was visualized by perfusion with space-filling fluorescent microspheres prior to explantation of the disc. (C) Vessel caliber and density of the network shown by microscopic picture with a × 10 objective (detailed view of tagged area in panel B). Epo increased the vascularized area of the disc (D-E) and the vessel density in the disc (F). Panel F presents a detailed view of tagged area in panel E. Quantification of the vascularized area of the disc (G) as well as the fluorescence intensity of the vascularized area (H) revealed significant differences between PBS- and Epo-treated animals. Data are given as means ± SD.
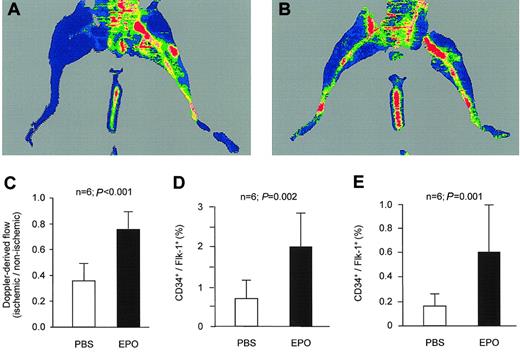
To determine if Epo stimulates neovascularization in the setting of ischemia, we used the murine model of hind-limb ischemia. After unilateral ligation of the superficial and deep femoral artery, we administered Epo (1000 IU/kg) or PBS for 3 weeks. Epo significantly increased hind-limb vascularization as assessed by capillary densitometry (1.61-fold increase relative to PBS-treated mice; P = .011). An increase in capillary density does not necessarily enhance local blood flow in the ischemic hind limb, if not associated with a concomitant increase in collateral vessels. Therefore, we analyzed the number of collaterals defined as vessels with a diameter 0.02 mm or larger coated by an α-actin–positive vascular smooth muscle wall. Compared with control mice, the number of collateral vessels in the ischemic limb of Epo-treated mice nonsignificantly increased from 3.2 ± 0.9 to 4.0 ± 1.4 (P = .26), whereas the mean size of the collateral vessels significantly increased from 0.045 ± 0.019 mm to 0.10 ± 0.042 mm (P = .014). These morphologic differences between control and Epo mice were paralleled by a significantly higher relative hind-limb perfusion as determined by laser Doppler imaging. The ischemic hind limbs in control mice showed 35 ± 13% of the blood flow compared with the nonischemic, contralateral hind limbs (Figure 4A,C). Epo-treated mice, however, showed a much better recovery of blood flow in the ischemic hind limb with 77 ± 16% after 2 weeks (Figure 4B-C).
Epo stimulates ischemia-induced neovascularization in vivo. PBS or Epo was administered by daily subcutaneous injections during the first 3 days of each week. After 2 weeks, perfusion of the ischemic limb was still impaired in PBS-treated mice (A). In contrast, Epo-treated mice showed a marked increase in hind-limb perfusion (B). Quantification of the perfusion of the ischemic hind limb relative to the contralateral, nonischemic hind limb indicated that treatment with Epo caused a significant increase in hind-limb perfusion (C). FACS analysis 7 days after induction of ischemia revealed that Epo treatment further enhanced the ischemia-induced increase in CD34+/flk-1+ cells in spleen (D) and peripheral blood (E). Data are given as means ± SD.
Epo stimulates ischemia-induced neovascularization in vivo. PBS or Epo was administered by daily subcutaneous injections during the first 3 days of each week. After 2 weeks, perfusion of the ischemic limb was still impaired in PBS-treated mice (A). In contrast, Epo-treated mice showed a marked increase in hind-limb perfusion (B). Quantification of the perfusion of the ischemic hind limb relative to the contralateral, nonischemic hind limb indicated that treatment with Epo caused a significant increase in hind-limb perfusion (C). FACS analysis 7 days after induction of ischemia revealed that Epo treatment further enhanced the ischemia-induced increase in CD34+/flk-1+ cells in spleen (D) and peripheral blood (E). Data are given as means ± SD.
As previously described, induction of hind-limb ischemia increases the number of circulating EPCs.13 In the control group, spleen EPCs significantly increased from 0.22 ± 0.11% without ischemia to 0.70 ± 0.45% after 7 days of ischemia (P = .013). The number of spleen EPCs of ischemic animals was further enhanced by Epo treatment to 2.07 ± 0.83% (P = .002 vs control mice after 7 days of ischemia) (Figure 4D). Values for peripheral blood EPCs in control animals were 0.025 ± 0.016% without ischemia and 0.16 ± 0.10% after 7 days of ischemia (P = 0.001). Epo treatment increased peripheral blood EPCs to 0.61 ± 0.18% (P < .001) (Figure 4E).
Erythropoietin serum levels correlate with EPC number and function in patients
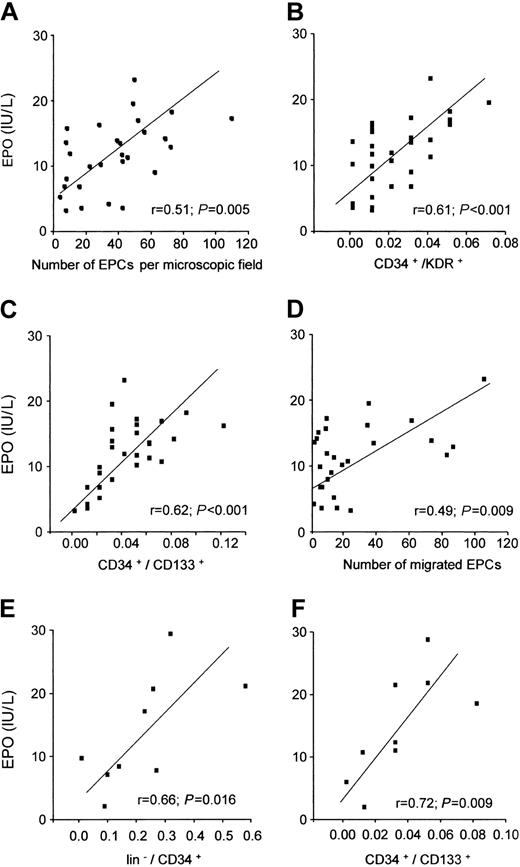
To investigate the (patho-) physiologic relevance of the experimental findings, we analyzed the relation between Epo serum levels and EPC number and function in patients with coronary heart disease. Patients with unstable coronary heart disease had significantly higher Epo serum levels (15.2 ± 4.0 IU/L) compared with patients with stable coronary heart disease (9.7 ± 4.8 IU/L; P = .008) and aged-matched controls (7.9 ± 2.3 IU/L; P = .002). Intriguingly, Epo serum levels significantly correlated with the number of EPCs in the EPC culture assay (Figure 5A), EPCs identified by double-staining for CD34/KDR (Figure 5B), and early hematopoietic progenitor cells identified by double-staining for CD34/CD133 (Figure 5C). In addition, Epo serum levels also correlated with EPC function as assessed in an in vitro migration assay (Figure 5D). In a multivariate linear regression model, serum levels for Epo and VEGF were the only independent predictors of EPC number in the peripheral blood (Table 1).
Epo serum levels in patients with and without coronary heart disease are related to EPC number and function. In patients with coronary heart disease (n = 38), Epo serum levels significantly correlated with the number of EPCs in the EPC culture assay (A), the number of CD34+/KDR+ cells (B), the number of CD34+/CD133+ cells (C), and the migratory capacity of cultivated EPCs (D). In patients with ischemic cardiomyopathy (n = 9), the number of hematopoietic bone marrow stem and progenitor cells (E-F) was significantly associated with Epo serum levels.
Epo serum levels in patients with and without coronary heart disease are related to EPC number and function. In patients with coronary heart disease (n = 38), Epo serum levels significantly correlated with the number of EPCs in the EPC culture assay (A), the number of CD34+/KDR+ cells (B), the number of CD34+/CD133+ cells (C), and the migratory capacity of cultivated EPCs (D). In patients with ischemic cardiomyopathy (n = 9), the number of hematopoietic bone marrow stem and progenitor cells (E-F) was significantly associated with Epo serum levels.
Multivariate linear regression analysis of the association between biochemical markers and number of circulating EPCs (cultured EPCs) in patients with coronary heart disease
. | Multivariate model . | . | |
|---|---|---|---|
| Variable . | R . | P . | |
| Granulocyte-macrophage colony-stimulating factor | -18.6 ± 21.27 | .39 | |
| Vascular endothelial growth factor | 0.12 ± 0.04 | .009 | |
| Hepatocyte growth factor | 0.10 ± 0.09 | .88 | |
| Erythropoietin | 2.22 ± 0.06 | .006 | |
| C-reactive protein | 4.33 ± 5.03 | .40 | |
| Tumor necrosis factor-α | -2.35 ± 7.38 | .75 | |
| Interleukin-10 | 2.77 ± 3.33 | .41 | |
| Troponin T | 1.08 ± 0.53 | .54 | |
. | Multivariate model . | . | |
|---|---|---|---|
| Variable . | R . | P . | |
| Granulocyte-macrophage colony-stimulating factor | -18.6 ± 21.27 | .39 | |
| Vascular endothelial growth factor | 0.12 ± 0.04 | .009 | |
| Hepatocyte growth factor | 0.10 ± 0.09 | .88 | |
| Erythropoietin | 2.22 ± 0.06 | .006 | |
| C-reactive protein | 4.33 ± 5.03 | .40 | |
| Tumor necrosis factor-α | -2.35 ± 7.38 | .75 | |
| Interleukin-10 | 2.77 ± 3.33 | .41 | |
| Troponin T | 1.08 ± 0.53 | .54 | |
In order to investigate whether the correlation between Epo serum levels and number of progenitor cells also extends to the bone marrow, bone marrow aspirates from 9 patients with chronic ischemic cardiomyopathy were analyzed. FACS analysis of the mononuclear fraction of bone marrow aspirate revealed that both lineage-negative hematopoietic stem cells (Figure 5E) and early hematopoietic progenitor cells (Figure 5F) significantly correlated with Epo serum levels.
Discussion
The present data demonstrate that Epo strongly augments neovascularization in vivo in 2 different models of postnatal neovascularization. Epo was previously shown to induce proangiogenic effects in cultivated endothelial cells and in the chick embryo chorioallantoic membrane assay.30,39 By activating the EpoR, Epo stimulates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT)39 and the phosphatidylinositol-3 kinase (PI3K)/Akt pathways,40 which are known to elicit a proliferative, promigratory, and antiapoptotic effect in mature endothelial cells.41 The present study now extends these findings by demonstrating that Epo not only affects mature endothelial cells in the setting of postnatal neovascularization, but also profoundly increases the number of circulating EPCs by mobilizing bone marrow–derived hematopoietic stem cells. The present data suggest that the stimulatory effect of Epo on the formation of new blood vessels is related to the convergence of 2 phenomena, namely (1) stimulation of vasculogenesis by induction of proliferation and differentiation of EPCs and (2) activation of angiogenesis by stimulation of mature endothelial cells.
Epo elicits a similar potency for the improvement of EPC mobilization as VEGF. Administration of VEGF165 (10 μg subcutaneously) in mice induced a 2.1-fold increase in circulating EPC levels in our setting (data not shown), which is comparable with the effect achieved with Epo in the present study (Figure 2). VEGF was the first growth factor described as capable to mobilize EPCs.16 Meanwhile, additional cytokines such as GM-CSF have been shown to increase circulating EPCs.13 However, in contrast to VEGF and Epo, GM-CSF is not directly regulated by hypoxia. Therefore, VEGF and Epo may be considered as endogenous mediators for hypoxia-induced mobilization of EPCs. Indeed, in our studies of patients with coronary heart disease, only VEGF and Epo, but not other cytokines including GM-CSF, were significant and independent predictors for the number and functional activity of circulating EPCs (Table 1). The correlation between Epo serum levels and the number of CD34+ or CD133+ hematopoietic stem cells in the bone marrow in patients with ischemic coronary artery disease further support an important role of endogenous Epo levels. These results also indicate that the effects of Epo are not confined only to circulating EPCs, but extend to the bone marrow. Indeed, our experimental data consistently show that Epo has a profound effect on the number and proliferation of stem and progenitor cells in the bone marrow (Figure 1). To study whether Epo treatment in patients may be associated with an increased number of circulating EPCs, we investigated patients with end-stage renal disease (n = 30). Epo treatment in patients with end-stage renal disease significantly increased Epo serum levels (P = .02 compared with untreated patients), which was paralleled by a significant increase in the number of circulating CD34+/CD133+ cells (P = .008).
This improvement of EPC mobilization and subsequent enhancement of postnatal neovascularization by Epo might be considered unfavorable in the setting of certain forms of cancer, where augmentation of angiogenesis42 and—more importantly—recruitment of EPCs are believed to contribute to tumor growth.18 Thus, supplementary Epo administration for treating anemia might potentially enhance tumor growth via stimulation of tumor neovascularization. However, there is good evidence from multiple clinical trials to recommend use of recombinant human Epo as a treatment option for patients with chemotherapy-associated anemia with a hemoglobin concentration less than 100 g/L (10 g/dL).43 In addition, clinical observations in patients with multiple myeloma receiving recombinant human Epo suggest, in addition to the improved quality of life, a longer survival than expected, considering the poor prognostic features of these patients.44 Experimental studies demonstrated that in tumor-bearing mice Epo treatment induced tumor regression in a large number of animals that was attributed to a T-cell–mediated mechanism.45 Therefore, erythropoietin may act as an antitumor therapeutic agent in addition to its red blood cell–stimulating activity, which together appear to counteract the proangiogenic activities of Epo in cancer patients.
In this respect, our studies in a model of hind-limb ischemia using wild-type mice suggest that Epo might indeed be an important endogenous mediator to stimulate postnatal neovascularization in response to ischemia. Supplementation with Epo leading to activation of the Epo-EpoR signaling pathway induced a marked increase in ischemia-induced neovascularization. This effect may at least in part be mediated by an enhanced mobilization of EPCs from the bone marrow. A potential important role for Epo in the cardiovascular system has recently been demonstrated in Epo and EpoR knock-out mice. Disruption of the Epo-EpoR signaling pathway resulted in a severely disorganized vascular network in the myocardium.46 Moreover, embryonic lethality of Epo and EpoR knock-out mice is partially driven by cardiac failure,46 suggesting an additional role for Epo in cardiac morphogenesis independent of myocardial hypoxia. These results suggest that cardiac failure in mice with genetically inactivated Epo is at least in part related to impaired vessel formation and/or reduced cardiac morphogenesis.
Recently, lines of transgene-rescued EpoR-null mutant mice expressing EpoR exclusively in the hematopoietic lineage have been established.47 Surprisingly, despite the lack of EpoR expression in nonhematopoietic tissues, these mice develop normally and are fertile. These data suggest that nonhematopoietic expression of EpoR is dispensable for normal mouse development. It is important to realize, however, that postnatal neovascularization as investigated in the present study is quite distinct from embryonal vessel development.48 In addition, the redundancy of growth factors expressed by endothelial cells may compensate for the lack of Epo stimulation. These observations suggest that further analyses of the transgene-rescued EpoR-null mutant mice under pathologic conditions (eg, in a model of hind-limb ischemia) are mandatory for a comprehensive understanding of the contribution of the Epo-EpoR signaling system in nonhematopoietic tissue. Indeed, preliminary studies in patients with congestive heart failure indicate that Epo treatment may provide beneficial effects even in the absence of renal insufficiency.49 Therefore, one might speculate that the observed beneficial effects of Epo on cardiac function may be related in part to enhanced mobilization of stem/progenitor cells and subsequently increased tissue revascularization and regeneration.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2003-01-0223.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal