Abstract
The identification of a safe, orally active iron chelator is critically important for the prevention of morbidity and early death in patients receiving regular red cell transfusions. Based on our findings in a 1-year multicenter, prospective study of the safety and efficacy of deferiprone in patients with thalassemia major, we have extended the treatment period to 4 years. The mean dose of the chelator was 73 mg/kg per day during 531 patient-years. The rates of agranulocytosis (absolute neutrophil count [ANC] < 500 × 109/L) and milder forms of neutropenia (ANC, 500-1500 × 109/L) were 0.2 and 2.8 per 100 patient-years, respectively. Neutropenia occurred significantly more commonly in patients with intact spleens. Gastrointestinal and joint symptoms decreased significantly after the first year of therapy, and led to discontinuation of deferiprone in only one patient in years 2 to 4. The mean alanine aminotransferase (ALT) value of 71 U/L after 4 years of therapy was significantly higher than the baseline value of 61 U/L. Trend analysis showed no increase in the ALT levels or the percentage of patients with ALT levels greater than twice the upper limit of the reference range. Ferritin levels did not change significantly from the values at the time of change from deferoxamine to deferiprone in either the intention-to-treat analysis or in the 84 patients who completed 4 years of therapy. Because of concerns regarding the effectiveness of the studied dose of deferiprone, 47 patients discontinued therapy, whereas 15 patients interrupted therapy because of concerns regarding low iron levels. The results of this study help to define the safety and effectiveness of long-term therapy with deferiprone.
Introduction
Successful iron-chelation therapy is necessary for the optimal management of thalassemia major and other transfusion-dependent disorders.1-3 Some patients with transfusional iron overload continue to develop iron-related complications and to die despite the availability of deferoxamine.4-7 The ongoing morbidity and mortality are attributable, in large part, to poor compliance due to the need to administer deferoxamine by prolonged subcutaneous infusions at least 5 days per week. These findings underscore the need for a safe and effective orally active chelator.
In a large multicenter study, we have previously characterized the safety profile and the effectiveness of therapy with the orally active iron chelator deferiprone for 1 year in patients with thalassemia major.8,9 The study was continued for an additional 3 years, and we now report the results after 4 years of treatment.
Patients and methods
Patients
Investigators in 3 thalassemia centers in Italy (Cagliari, Ferrara, Torino) and one center in the United States (Philadelphia, PA) enrolled 187 patients with thalassemia major in the study. The inclusion and exclusion enrollment criteria have been described previously.8 The patients were 10 to 41 years old (mean, 18 years) at the time of enrollment. Prior to receiving deferiprone in this trial, 181 of 187 patients were treated with deferoxamine at a mean prescribed dose of 40 mg/kg of body weight and a mean prescribed frequency of 6.5 infusions weekly.
Study design
The trial was designed initially as a prospective 1-year study of deferiprone primarily to determine the incidence of agranulocytosis and other severe adverse events and secondarily to determine efficacy as assessed by serum ferritin level. We subsequently extended the study for an additional 3 years with similar outcome measures.
The preparation and administration of deferiprone and the monitoring of therapy in the first year have been previously described.8 In brief, deferiprone was manufactured by Apotex (Toronto, ON, Canada) as 500-mg scored tablets. The dose of deferiprone was 25 mg/kg body weight, administered 3 times a day for a total daily dose of 75 mg/kg body weight.
A complete blood count (CBC) and white cell differential count were measured weekly (range, 5-10 days) throughout the 4-year study. If the neutrophil count fell to lower than 1.5 × 109/L, therapy with deferiprone was interrupted, and a CBC with a manual differential count was repeated the next day. If the second neutrophil count was higher than 1.5 × 109/L, deferiprone therapy was reinstituted. If the 2 consecutive neutrophil counts were lower than 1.5 × 109/L, the patient was considered to have confirmed neutropenia. A confirmed neutrophil count lower than 0.5 × 109/L was defined as agranulocytosis. In the first year of the study, confirmed neutropenia required discontinuation of therapy with deferiprone. Subsequently, based on analysis of data from the first year by the investigators and the Data and Safety Monitoring Committee, patients with neutropenia but not agranulocytosis were allowed to restart deferiprone after resolution of neutropenia.
Serum alanine aminotransferase (ALT) levels were measured at baseline and every 3 months for 4 years. Immunologic studies and serum zinc levels were not measured routinely after the first year. Serum ferritin levels to assess the degree of iron overload were measured at baseline and every 3 months for as long as 4 years. Although the protocol did not require measurement of liver iron concentration, some patients underwent such assessments during the 4-year study period as part of their clinical management, as part of other studies, or in response to concerns about deferiprone therapy raised by other investigators.10
The study, designed in accordance with the standards of Good Clinical Practice, was approved by the institutional review board of each of the participating centers. Informed consent was obtained from all patients and/or their parents.
The Data and Safety Monitoring Committee reviewed reports of serious adverse events and advised the Steering Committee based on reports of other studies of deferiprone.
Statistical methods
A 2-sample t test or chi-square test, where appropriate, was used to compare baseline characteristics between different subgroups of patients.
Chi-square analysis was used to evaluate the difference in incidence of agranulocytosis or milder neutropenia between splenectomized and nonsplenectomized patients. Cochran-Mantel-Haenszel statistics were used to evaluate potential effects of baseline serum ferritin level on the incidence of gastrointestinal complaints, joint problems, and ALT levels greater than twice the upper limit of the reference range. Kaplan-Meier curves were constructed for the time to the first occurrence of agranulocytosis, neutropenia, gastrointestinal symptoms, and joint problems.
A paired t test was performed to examine the change in ALT level from baseline to 48 months after the initiation of therapy. A chi-square test was conducted to compare the proportion of patients with ALT levels greater than twice the upper limit of the reference range between baseline and 48 months. Trend analysis of the ALT data was performed using the MIXED model approach. The variable time (in months) was modeled as a regression (continuous) variable. The other factor included in the model was the baseline hepatitis C status of the patient. The MIXED procedure in SAS (SAS Institute, Cary, NC) was used for the analysis; data within each patient were considered repeated measures, and the covariance structure used was autoregressive of order 1. In addition, the percentage of patients with ALT values greater than twice the upper limit of the reference range at various times on treatment was examined for trend using the GENMOD procedure in SAS. The factors studied in this model were time and the baseline hepatitis C status of patients.
The change in serum ferritin level between baseline and termination was examined using a paired t test for all patients and for different subgroups.
All statistical analyses were performed using SAS System for PC, Release 6.12. A type I error (α) of 0.05 was used to determine statistical significance in all tests.
Results
Study patients
Clinical characteristics of the 187 patients with thalassemia major enrolled in the trial have been described previously.9 There were 162 patients who completed the first year of study and 84 patients who completed 4 years of therapy with the same dose of deferiprone and no exposure to other chelators. The 84 study completers and 103 noncompleters did not differ significantly in age (mean, 19 years for both groups) or sex (49% female versus 51% male). Study completers were significantly more likely than noncompleters to have undergone splenectomy (50% versus 31%; P = .008) and to have lower serum ferritin levels at study entry (2268 μg/L versus 3044 μg/L; P = .004). The total drug exposure was 531 patient-years. The mean daily dose was 73 ± 2 mg/kg.
Adverse events
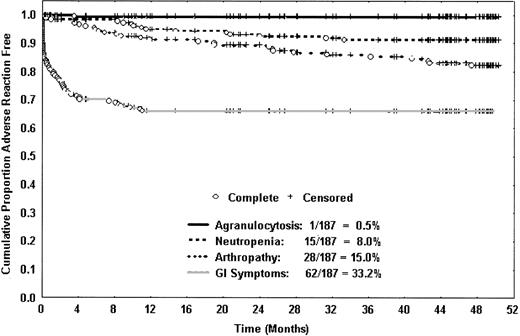
Gastrointestinal symptoms and joint problems. In 62 patients (33%), 65 reported episodes of gastrointestinal symptoms occurred, including abdominal pain, nausea, and vomiting. Of these episodes, 94% occurred in the first year (Figure 1). Because of gastrointestinal symptoms in the first year, 3 patients discontinued therapy; no patient discontinued therapy for this reason in subsequent years.
Kaplan-Meier curves show the time to first occurrence of important adverse events. The only case of agranulocytosis occurred in the first year, and gastrointestinal complaints were very uncommon after the first year. Neutropenia and joint problems occurred throughout the 4-year study period but were more common in the first year than in each of the subsequent years.
Kaplan-Meier curves show the time to first occurrence of important adverse events. The only case of agranulocytosis occurred in the first year, and gastrointestinal complaints were very uncommon after the first year. Neutropenia and joint problems occurred throughout the 4-year study period but were more common in the first year than in each of the subsequent years.
In 28 patients (15%), 30 episodes of joint problems occurred, including pain and/or swelling. Of these episodes, 50% occurred in the first year and 50% in the remaining 3 years (Figure 1). Arthropathy was reported in 13% of patients with baseline ferritin levels lower than 2500 μg/L, 17% of patients with baseline ferritin levels of 2500 to 5000 μg/L, and 21% of patients with baseline ferritin levels higher than 5000 μg/L (P = .291).
Only one patient was withdrawn from the study because of joint symptoms. This 18-year-old patient experienced transient knee pain approximately one month before receiving his first dose of deferiprone. During treatment, the patient experienced recurrent episodes of pain and swelling in both knees and elbows. Tests for rheumatoid factor and antinuclear antibody were negative. Arthrocentesis of the left knee revealed a low C3 concentration (40.7 mg/dL; normal range, 90-180 mg/dL). Deferiprone was stopped after approximately 3.5 years on therapy but the pain and swelling persisted.
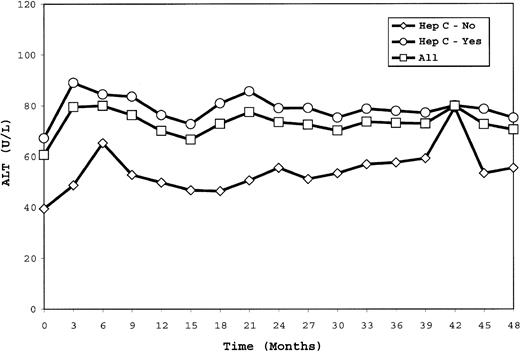
ALT levels. ALT values, previously reported as fluctuating in the first year of therapy, continued to show marked variability in individual patients during the following 3 years (range of change from baseline, –391 to +851 U/L). The mean ALT level was 71 U/L at 48 months in comparison with 61 U/L at baseline (P = .023). Overall, trend analysis showed no significant change in ALT level over time for hepatitis C–positive or –negative patients (Figure 2). The percentage of patients with ALT levels greater than twice the upper limit of the reference range did not differ between baseline (23%) and 48 months (28%) (P = .285). Trend analysis revealed no significant increase over time in the percentage of patients with ALT levels greater than twice the upper limit of the reference range regardless of hepatitis C status. Because of an increase in ALT levels during the first year of therapy, 2 patients withdrew; no patient withdrew for this reason in subsequent years.
ALT values measured every 3 months are shown for all patients as well as for the subgroups of patients who were hepatitis C–seropositive or –seronegative. Trend analysis showed no significant change during the study.
ALT values measured every 3 months are shown for all patients as well as for the subgroups of patients who were hepatitis C–seropositive or –seronegative. Trend analysis showed no significant change during the study.
Agranulocytosis and milder neutropenia. The only case of agranulocytosis during the study occurred in the first year, as previously described.8 Agranulocytosis has not recurred in this patient during 6 years of therapy with deferoxamine. The overall frequency of agranulocytosis was 0.5% and the incidence was 0.2 per 100 patient-years. There were 16 patients (8.5%) who developed milder neutropenia, 9 in the first year and 7 in years 2 to 4 (Figure 1). The overall incidence of milder neutropenia was 2.8 per 100 patient-years. The median duration of neutropenia was one week and no serious complications occurred. Of 113 nonsplenectomized patients, 15 (13%) developed neutropenia, in contrast with 2 (3%) of 74 splenectomized patients (P = .014). One subject restarted therapy with deferiprone upon resolution of neutropenia and after splenectomy; neutropenia did not recur during the next 2 years of therapy.
Ferritin levels and adverse events. The frequencies of gastrointestinal symptoms and joint problems were unrelated to baseline serum ferritin levels (P = .67 and P = .29, respectively). ALT levels greater than twice the upper limit of the reference range were associated with higher serum ferritin levels at study entry (P < .0001) and during treatment (P < .0009).
Withdrawals
Because of adverse events, 29 patients withdrew: 21 in year 1 and 8 in years 2 to 4 (Table 1). In addition, 12 patients voluntarily withdrew, 2 patients withdrew because of protocol violations, 47 withdrew because of questions raised about the effectiveness of the chelator (next section), and 13 withdrew because of exposure to deferoxamine for reasons unrelated to a change in iron stores.
Withdrawals due to adverse events
Adverse event . | Year 1, no. patients (% patients) . | Years 2 to 4, no. patients (% patients) . |
|---|---|---|
| Neutropenia | 9 (4.8) | 6 (3.2) |
| Other | 4 (2.1)* | 1 (0.5)† |
| Nausea/vomiting | 3 (1.6) | 0 |
| Thrombocytopenia | 2 (1.1) | 0 |
| Agranulocytosis | 1 (0.5) | 0 |
| Increased ALT level | 1 (0.5) | 0 |
| Hepatitis | 1 (0.5) | 0 |
| Arthropathy | 0 | 1 (0.5) |
Adverse event . | Year 1, no. patients (% patients) . | Years 2 to 4, no. patients (% patients) . |
|---|---|---|
| Neutropenia | 9 (4.8) | 6 (3.2) |
| Other | 4 (2.1)* | 1 (0.5)† |
| Nausea/vomiting | 3 (1.6) | 0 |
| Thrombocytopenia | 2 (1.1) | 0 |
| Agranulocytosis | 1 (0.5) | 0 |
| Increased ALT level | 1 (0.5) | 0 |
| Hepatitis | 1 (0.5) | 0 |
| Arthropathy | 0 | 1 (0.5) |
Hypersplenism (1), asthenia (1), and decreased neutrophil count (but not meeting criteria for neutropenia) (2).
Depression.
Efficacy
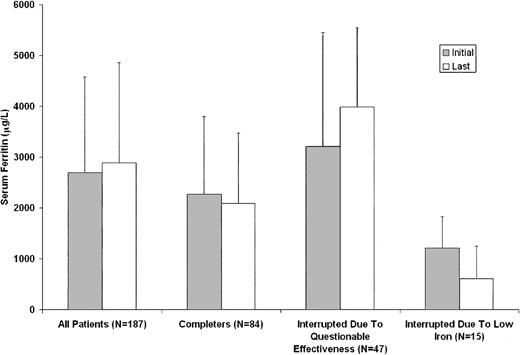
The efficacy of deferiprone was determined by quarterly assessments of serum ferritin levels. The intention-to-treat analysis included all 187 patients enrolled in the study, regardless of time on therapy (range, 7 days to 4 years). For patients who withdrew from the study or were treated at any time with deferoxamine, the last ferritin level on deferiprone was carried forward for the purpose of this statistical analysis. In these patients, the mean serum ferritin levels at baseline and termination were 2696 ± 1877 μg/L and 2889 ± 1972 μg/L, respectively (P = .133) (Figure 3).
Serum ferritin levels before and after treatment with deferiprone. The bars labeled “All Patients” represent the mean initial and last values for all patients enrolled in the study, regardless of time on therapy. “Completers” bars represent comparable mean values for patients who completed 4 years of therapy at a daily dose of 75 mg/kg without exposure to deferoxamine. “Interrupted Due To Questionable Effectiveness” bars represent mean initial and last ferritin levels for patients who discontinued therapy with deferiprone because of concerns about the serum ferritin level and/or liver iron concentration. “Interrupted Due To Low Iron” bars represent comparable mean values for patients who interrupted therapy because of ferritin levels lower than 500 μg/L or decreased liver iron concentration.
Serum ferritin levels before and after treatment with deferiprone. The bars labeled “All Patients” represent the mean initial and last values for all patients enrolled in the study, regardless of time on therapy. “Completers” bars represent comparable mean values for patients who completed 4 years of therapy at a daily dose of 75 mg/kg without exposure to deferoxamine. “Interrupted Due To Questionable Effectiveness” bars represent mean initial and last ferritin levels for patients who discontinued therapy with deferiprone because of concerns about the serum ferritin level and/or liver iron concentration. “Interrupted Due To Low Iron” bars represent comparable mean values for patients who interrupted therapy because of ferritin levels lower than 500 μg/L or decreased liver iron concentration.
For the 84 patients who completed 4 years of study, the mean serum ferritin levels were 2268 ± 1529 μg/L at baseline and 2087 ± 1385 μg/L after 4 years (P = .338) (Figure 3). For patients with initial ferritin levels higher than 2500 μg/L, the ferritin level declined from 3661 ± 1862 μg/L at baseline to 2630 ± 1708 μg/L at the end of 4 years (P = .038). For patients with initial values lower than 2500 μg/L, the mean ferritin level did not change significantly.
Because of questions regarding its effectiveness,10 47 patients discontinued therapy with deferiprone. This decision was based on serum ferritin levels in 28 patients and on liver iron concentration or a combination of these 2 measurements in 19 additional patients. The mean serum ferritin levels were 3212 ± 2228 μg/L at baseline and 3979 ± 1557 μg/L at termination (P = .001; Figure 3). These patients did not differ from study completers in age or sex. However, they were less likely than study completers to have undergone splenectomy (30% versus 50%; P = .025) and they had higher serum ferritin levels at study entry (3212 μg/L versus 2268 μg/L; P = .012).
There were 15 patients who had therapy with deferiprone interrupted due to decreased serum ferritin concentrations, when their ferritin levels fell below 500 μg/L, or for decreased hepatic iron concentration for a total of 41 interruptions. The mean time of interruption was 57 days. For this group, the mean serum ferritin levels were 1207 ± 616 μg/L at baseline and 613 ± 631 μg/L at the time of interruption of therapy (P = .009; Figure 3).
Clinical outcomes
No patient died during the 4-year study. No patient developed clinical findings of congestive heart failure. One patient began treatment for a persistently low ejection fraction diagnosed prior to initiation of deferiprone therapy. The ejection fraction returned to normal during subsequent therapy with enalapril and deferiprone. There were 3 patients who developed insulin-dependent diabetes. No patient developed clinical evidence of end-stage liver disease.
Discussion
This multicenter study was originally designed as a one-year trial to assess the safety and effectiveness of deferiprone. Based on favorable results in the first year, the study was extended for an additional 3 years. Adverse events occurred less frequently after the first year of therapy, and no new drug-related complications were identified. Mean serum ferritin levels declined in those patients with higher baseline levels and remained stable in those with lower baseline levels. Because of adverse events, 29 patients discontinued therapy, and an additional 47 patients discontinued because of concerns regarding the effectiveness of therapy.
Gastrointestinal symptoms, such as nausea, vomiting, and abdominal pain, were the most frequently reported deferiprone-related adverse events. In general, these events were reported early in the first year of therapy and uncommonly thereafter.
Joint problems were associated with the use of deferiprone in 15% of the patients and rarely required discontinuation of therapy. Unlike gastrointestinal problems, joint findings occurred throughout the study period. A large survey of patients with thalassemia receiving deferoxamine found drug-related arthralgia and myalgia in 13% of patients.11 These findings suggest that joint problems may be related to the underlying disease or may be generally associated with iron chelation. Previously, we reported an association between joint symptoms and serum ferritin levels during the first year of therapy with deferiprone.8 That association did not achieve statistical significance in the analysis after 4 years.
The ALT values in individual patients fluctuated throughout the trial. Despite an initial increase in ALT level, trend analyses showed no significant change in ALT level and no significant change in the percentage of patients with ALT levels greater than twice the upper limit of the reference range over 4 years, regardless of hepatitis C status. These findings confirm the transient nature of the changes in ALT levels that has been reported previously12-14 and show no evidence of a progressive increase over time. However, careful monitoring of ALT levels is important for the early detection of individual patients with rising values and may be particularly valuable for monitoring those patients who begin therapy with elevated levels. Until more data are available, interruption of therapy or reduction of the dose of deferiprone should be considered for patients with substantial and progressive increases in the ALT levels.
Agranulocytosis, a serious complication previously reported during deferiprone therapy,12,15,16 occurred in only one patient during the 4-year period of this study. The 0.5% frequency of agranulocytosis found in this prospective study is substantially lower than previous estimates from smaller studies or retrospective analyses.12 Milder neutropenia occurred in 8.5% of patients. All patients with neutropenia subsequently regained normal neutrophil counts. The lower rate of agranulocytosis and the higher rate of milder neutropenia found in this study may be a result of rigid adherence to the protocol's requirements for weekly monitoring of blood counts and the strict guidelines for interrupting or discontinuing therapy.
Data from the first year of study suggested but did not confirm a relationship between the occurrence of neutropenia and the presence or absence of the spleen.8 The analysis after 4 years demonstrates that neutropenia occurs significantly more often in nonsplenectomized patients than in patients who have undergone splenectomy. These findings indicate that milder forms of neutropenia may often be due to hypersplenism rather than chelation therapy with deferiprone.
Overall, iron stores, as assessed by serum ferritin levels, did not change after 4 years of therapy with deferiprone in comparison with baseline values obtained after treatment with deferoxamine. The response to deferiprone was related to the degree of iron overload. Mean ferritin levels declined in patients with baseline levels higher than 2500 μg/L and remained stable in patients with baseline levels lower than 2500 μg/L. Our results show that at a dose of deferiprone of 75 mg/kg per day, iron stores will increase in some patients under these conditions and will decrease in others. In a study of 532 patients who also received 75 mg/kg per day of deferiprone under a special program of the Italian Ministry of Health, Ceci et al analyzed the serum ferritin levels in 151 patients who completed 3 years of therapy with no missing measurements.17 The mean serum ferritin level declined from 2579 μg/L at baseline to 2320 μg/L at 3 years (P = .01). Patients with initial serum ferritin levels higher than 4000 μg/L showed a significant decline in ferritin level consistently over time, and patients with initial serum ferritin levels of 2000 to 4000 μg/L showed a significant decline beginning at 2 years.
Over the course of 4 years, 25% of the patients discontinued therapy because of concerns regarding the effectiveness of this dose of deferiprone. Other investigators have reported higher iron excretion and consistent reduction of ferritin levels with higher doses of deferiprone.18,19 However, long-term safety data for higher doses are not available.
There are 2 factors that may have influenced the attrition rate in this study. First, the protocol did not allow an adjustment in the dose of deferiprone in order to optimize therapy according to the patient's needs, and second, the protocol did not permit the use of deferoxamine. The former may have contributed to the loss of 47 patients who received deferoxamine because of increased iron stores, and the latter was responsible for the loss of 13 patients who used deferoxamine for other reasons, such as prolonged vacations during which monitoring of blood counts was not possible.
This study demonstrates the importance of a large, long-term, and carefully monitored prospective study to evaluate the safety and efficacy of an iron-chelating agent.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2002-10-3280.
Sponsored by Apotex Research, Toronto, ON, Canada. Four of the authors (A.R.C., R.G., A.P., V. De S.) received research support from Apotex Research for the conduct of the study and for meetings related to the study. The same authors have also received research support from Novartis Pharma. One of the authors (F.T.) is employed by Apotex Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Michael Spino, PharmD, Senior Vice-President, Scientific Affairs at Apotex, served with the authors as a member of the Steering Committee. Nancy Olivieri, MD, and Gary Brittenham, MD, served as members of the Steering Committee at the beginning of the study and participated in the organization and initial conduct of the trial but did not take part in the analysis of the data or the preparation of this manuscript. Members of the Data and Safety Monitoring Committee included Elias Schwartz, MD, (Chairman), Sam Charache, MD, Chaim Hershko, MD, Stuart MacLeod, MD, and Giuseppe Masera, MD.
The authors thank the following study coordinators and coinvestigators: Marie Martin, RN, Antonis Kattamis, MD,Debra Friedman, MD (Philadelphia, PA); Anna Lisa Agus, MD, Elisabetta Carta, MD, Maria Teresa Maurichi, Antonella Zappu, PhD, Elisabetta Loi (Cagliari, Italy); Rosa Maria Visconti, Montserrat Collell, PhD, Carmen Gaglioti, MD (Torino, Italy); Maria Rita Gamberini, MD, Anna Lodi, MD, Mauro Gnani, Anna L. Lauriola, MD, Monica Fortini, Rosaria Testa, and Anna Tangerini, MD (Ferrara, Italy). We are indebted to Yu Chung Tsang, PhD, for his biostatistical advice and analyses. We also acknowledge the support of the Ministero Università Ricerca Scientifica (MURST) and Legge Regionale 11 Regione Autonoma Sardegna, Italy. We gratefully acknowledge the participation of the patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal