Abstract
Using monoclonal antibodies (mAbs) specific for different natural killer (NK) receptors, we studied the lymphocyte population from 18 patients with NK-type lymphoproliferative disease of granular lymphocytes (LDGL). The analysis of both resting and cultured NK cell populations demonstrated that these patients are frequently characterized by NK cells displaying a homogeneous staining with given anti–killer Ig-like receptor (anti-KIR) mAb (11 of 18 patients). In most patients NK cells were characterized by the CD94/NKG2A+ phenotype, whereas only a minor fraction of the cases expressed CD94/NKG2C. In 7 of these patients we could also assess the function of the various NK receptors. Remarkably those KIR molecules that, in each patient, homogeneously marked the NK cell expansion were found to display an activating function as determined by cross-linking with specific anti-KIR mAb. The KIR genotype analysis performed in 13 of 18 cases revealed that in NK-type LDGL certain activating KIRs, as well as certain infrequent KIR genotypes, were detected with higher frequencies as compared to previously analyzed healthy donors. Moreover, most KIR genotypes included multiple genes coding for activating KIRs. The analysis of non–HLA-specific triggering receptors indicated that the natural cytotoxicity receptors (NKp46, NKp30) were expressed at significantly low levels in freshly drawn NK cells from most patients analyzed. However, in most instances the expression of NKp46 and NKp30 could be up-regulated on culture in interleukin 2. Our data indicate that in NK-LDGL the expanded subset is frequently characterized by the expression of a given activating KIR, suggesting a direct role for these molecules in the pathogenetic mechanisms of this disorder.
Introduction
In healthy individuals, natural killer (NK) cells represent a subset of lymphocytes involved in the immunosurveillance against virus-infected and tumor-transformed cells.1,2 The ability of NK cells to recognize and kill susceptible targets and spare normal autologous cells is commonly determined by the concerted action of different surface receptors (NK receptors [NKRs]), that either trigger or inhibit the NK-mediated cytolytic activity.3-6 The inhibitory NKRs include the killer Ig-like receptors (KIRs; eg, KIR2DL1, KIR2DL2, KIR2DL3, KIR3DL1, and KIR3DL2) each specific for a different group of HLA class I alleles3-5,7 ; the leukocyte Ig-like receptor (LIR-1; also defined as ILT2) that shows a broad HLA class I specificity7-9 ; and the lectin-like heterodimer CD94/NKG2A that recognizes the HLA class Ib, HLA-E molecules.10-12 The triggering receptors are represented by the activating form of the HLA class I–specific KIRs (ie, KIR2DS and KIR3DS),3,13-16 by the CD94/NKG2C heterodimer,17,18 and by a series of non-HLA–specific receptors6 that include the natural cytotoxicity receptors (NCRs; eg, NKp46, NKp30, and NKp44),19-21 the NKG2D receptor,22,23 and certain coreceptors (eg, NKp80 and 2B4).24-26 Whereas the HLA-specific receptors are clonally distributed, the non–HLA-specific triggering receptors are expressed on all NK cells. On the other hand, it has been shown that different NK cells may express different NCR surface density allowing the distinction into NCRbright and NCRdull NK cells. Remarkably, the NCR surface density was shown to correlate with the ability of NK cells to kill a wide range of tumor cell lines.27 Thus, whereas the HLA-specific inhibitory receptors allow NK cells to spare normal autologous HLA class I+ cells, the non–HLA-specific activating receptors are involved in the NK-mediated killing of abnormal target cells that have undergone down-regulation of HLA class I expression.28 A precise role for the activating form of KIRs has not yet been established, although recent data both in mouse and in humans suggest a possible involvement of major histocompatibility (MHC)–specific activating NKRs in the recognition of virally infected cells.29-33
The NK-type lymphoproliferative disease of granular lymphocytes (LDGL) is characterized by the abnormal proliferation of large granular lymphocytes (LGLs) that display a typical NK phenotype (generally CD3–CD56+CD16+) and includes different forms ranging from mild asymptomatic conditions, defined as chronic NK lymphocytosis, to aggressive, usually fatal diseases, often referred to as NK-LGL leukemia.34-38 A role, both direct and indirect, for viral infections in the etiopathogenesis of NK-type LDGL has been proposed pointing to the action of these agents as inciting antigens.34-39 Thus, the assessment of the selective expansion and possibly of the clonality of a discrete NK subset is an important diagnostic and prognostic parameter in NK-LDGL patients. In this context previous studies were focused on the analysis of the NKR distribution by the use of 2 distinct monoclonal antibodies (mAbs) directed to the HLA-C–specific KIRs. By this approach, a homogeneous expansion of a given NK cell subset could be identified in some of the NK-LDGL patients analyzed.40 However, at that time, the limited panel of available KIR-specific mAbs did not allow an exhaustive assessment of these receptors in LDGL.
In the present study, 18 NK-LDGL patients have been analyzed for the expression of a large panel of different NKRs including both HLA-specific and non–HLA-specific receptors. This analysis also included, for the first time, the functional assessment of various NKRs in a series of in vitro–cultured NK cell lines and clones derived from some of the LDGL patients and the analysis of the KIR genotype. We show that in NK-LDGL patients the NK expansions are frequently characterized by a homogeneous expression of one or more KIRs. Strikingly, these receptors displayed an activating function as determined by specific cross-linking with anti-KIR mAbs. These data suggest that LDGL may be induced by a selective expansion of an NK subset expressing a triggering KIR directly involved in the control of inciting (viral?) agents.
Patients, materials, and methods
Patients and control donors
Eighteen patients (9 men and 9 women) were studied. The study was approved by the Università di Genova institutional review board. Informed consent was provided according to the Declaration of Helsinki. Diagnosis was made according to the recently reported criteria.36 In all cases a chronic lymphocytosis, lasting more than 6 months and sustained by at least 2000 granular lymphocytes/mm3, was present in the peripheral blood. At the time of the study none of the patients had received treatment. Analysis of clonality was performed in 3 patients (nos. 1, 15, and 18) using restriction fragment length polymorphism (RFLP) evaluation, as previously reported, showing a polyclonal proliferation.41
All the experiments were performed also on 2 control donors (CTR1 and CTR2) representative of the healthy population. In particular, the NK cell population derived from CTR1 displays a phenotype (70%-90% NKG2A+ NCRbright NK cells) similar to that of approximately 80% of the healthy individuals analyzed in our laboratory (> 100 donors studied), whereas the CTR2 NK cell population displays a less common phenotype (shared by approximately 20% of healthy individuals) characterized by a subset of NCRdull NK cells (40%-50%). These cells are frequently characterized also by the NKG2A– phenotype.6
Purification of PBLs and generation of polyclonal or clonal NK populations from the patients and healthy individuals
Peripheral blood lymphocytes (PBLs) were derived from healthy donors or from patients by Ficoll-Hypaque gradient and depletion of plastic-adherent cells. To obtain enriched NK cells, PBLs were incubated with anti-CD3 (JT3A), anti-CD4 (HP2.6), and anti–HLA-DR (D1.12) mAbs (30 minutes at 4°C) followed by goat antimouse–coated Dynabeads (Dynal, Oslo, Norway; 30 minutes at 4°C) and immunomagnetic depletion.19-21 CD3–CD4–DR– cells were cultured on irradiated feeder cells in the presence of 600 U/mL recombinant interleukin 2 (rIL-2; Proleukin, Chiron, Emeryville, CA) and 1.5 ng/mL phytohemagglutinin (PHA; Gibco, Paisley, United Kingdom) to obtain polyclonal NK cell populations or, after limiting dilution, NK cell clones.
mAbs
The commercially available mAbs used in this study were from Becton Dickinson (Sunnyvale, CA) and included: Leu4 (anti-CD3), Leu11c (anti-CD16), Leu19 (anti-CD56), Leu7 (anti-CD57), and isotype-matched control reagents. The following mAbs were produced in our laboratory: JT3A (IgG2a, anti-CD3), c127 (IgG1, anti-CD16), c218 (IgG1, anti-CD56), EB6b (IgG1, anti-KIR2DL1, and anti-KIR2DS1), GL183 (IgG1, anti-KIR2DL2, anti-KIR2DL3, and anti-KIR2DS2), FES172 (IgG2a, anti-KIR2DS4), Z27 (IgG1, anti-KIR3DL1, and anti-KIR3DS1), Q66 (IgM, anti-KIR3DL2), XA185 (IgG1, anti-CD94), Z199 (IgG2b, anti-NKG2A), F278 (IgG1, anti-LIR1), BAB281 (IgG1, anti-NKp46), Z231 (IgG1, anti-NKp44), AZ20 (IgG1, anti-NKp30), MA152 (IgG1, anti-NKp80), PP35 (IgG1, anti-h2B4), and ECM217 (IgG2b, anti–NKG2-D).
The D1.12 (IgG2a, anti–HLA-DR) mAb was provided by Dr R. S. Accolla (Pavia, Italy). HP2.6 (IgG2a, anti-CD4) mAb was provided by Dr P. Sanchez-Madrid (Madrid, Spain).
Flow cytofluorimetric analysis
The expression of the various receptors was assessed by flow cytofluorimetric analysis using direct or indirect immunofluorescence assays as previously described.14,40 Briefly, cells were stained with the appropriate mAbs either unlabeled or labeled; staining with unlabeled mAb was followed by phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated isotype-specific goat antimouse second reagent (Southern Biotechnology, Birmingham, AL, or Caltag, Burlingame, CA). The results on fresh PBLs were confirmed by the analysis of the CD3–CD16+ gated cells. Samples were analyzed by 1- or 2-color cytofluorimetric analysis (FACScan, Becton Dickinson, Mountain View, CA) and data were processed using CELLQuest Software (Becton Dickinson).
Cytolytic assays
The FcγR+ P815 (murine mastocytoma) target cell line was used for redirected killing experiments.13 NK cells were tested for cytolytic activity in a 4-hour 51Cr-release assay either in the absence or in the presence of various mAbs at 0.5 μg/mL. The effect-to-target (E/T) ratios are indicated in the text.
KIR genotyping
The polymerase chain reaction (PCR)–based sequence-specific primers (PCR-SSP) typing was used to detect the KIR genotype of the LDGL patients. Volume reactions of 15 μL were prepared to include 30 ng test DNA, 1.5 μL10 × PCR buffer, 1.5 mM MgCl2, 0.2 mM deoxyribonucleoside triphosphates (dNTPs), 0.2 U Taq gold (Applied Biosystems, Foster City, CA), 0.4 μM of each primer, and 1% dimethyl sulfoxide (DMSO). PCR conditions were identical for all typing reactions: an initial denaturation of the Taq at 95°C for 8 minutes followed by 35 cycles of 30 seconds at 95°C, 45 seconds at 55°C, and 1 minute 30 seconds at 68°C in a GeneAmp PCR system 9600 thermal cycler (Applied Biosystems). Amplification products were analyzed on ethidium bromide 0.8% agarose gels.
Primer sequences used for KIR genotyping
KIR gene . | Sense primer . | Antisense primer . | Length, bp . | Reference . |
|---|---|---|---|---|
| KIR2DL1 | ACT CAC TCC CCC TAT CAG G | AGG GCC CAG AGG AAA GTC A | 1750 | Uhrberg et al42 |
| KIR2DL1v | ACT CAC TCC CCC TAT CAG G | AGG GCC CAG AGG AAA GTT | 1750 | Uhrberg et al42 |
| ACT CAC TCC CCC TAT CAG G | AGG GCC CAG AGG AAA GTK | 1750 | Uhrberg et al42 | |
| KIR2DL2 | CCA TGA TGG GGT CTC CAA A | GCC CTG CAG AGA ACC TAC A | 1800 | Uhrberg et al43 |
| KIR2DL3 | CCT TCA TCG CTG GTG CTG | CAG GAG ACA ACT TTG GAT CA | 798 | Uhrberg et al43 |
| KIR2DL4 | GTA TCG CCA GAC ACC TGC ATG CTG | GCA GGC AGT GGG GAC CTT AGA CA | 1082 | Hsu et al44 |
| KIR2DL5.1 | CTC CCG TGA TGT GGT CAA CAT GTA AA | GGG GTC ACA GGG CCC ATG AGG AT | 1883 | Hsu et al44 |
| KIR2DL5.2 | GTA CGT CAC CCT CCC ATG ATG TA | GGG GTC ACA GGG CCC ATG AGG AT | 1893 | Hsu et al44 |
| KIR2DS1 | TCT CCA TCA GTC GCA TGA R | AGG GCC CAG AGG AAA GTT | 1800 | Uhrberg et al43 |
| TCT CCA TCA GTC GCA TGA R | AGG GCC CAG AGG AAA GTK | 1800 | Hsu et al44 | |
| KIR2DS2 | TGC ACA GAG AGG GGA AGT A | CAC GCT CTC TCC TGC CAA | 1750 | Uhrberg et al43 |
| KIR2DS3 | TCA CTC CCC CTA TCA GTT T | GCA TCT GTA GGT TCC TCC T | 1800 | Uhrberg et al43 |
| GAC ATG TAC CAT CTA TCC AC | GCA TCT GTA GGT TCC TCC T | 130 | Hsu et al44 | |
| KIR2DS4 | ATC CTG CAA TGT TGG TCG | CTG GAT AGA TGG TAC ATG TC | 1902 | Hsu et al44 |
| KIR1D | ATC CTG CAA TGT TGG TCG | CTG GAT AGA TGG AGC TGC AG | 1885 | Hsu et al44 |
| KIR2DS5 | AGA GAG GGG ACG TTT AAC C | TCC GTG GGT GGC AGG GT | 1950 | Uhrberg et al42 |
| CTG CAC AGA GAG GGG ACG TTT AAC C | TCC AGA GGG TCA CTG GGC | 179 | Hsu et al44 | |
| KIR3DL1 | TAC AAA GAA GAC AGA ATC CAC A | TAG GTC CCT GCA AGG GCA A | 1600 | Uhrberg et al42 |
| TCC CAT CTT CCA TGG CAG AT | TAG GTC CCT GCA AGG GCA A | 1600 | Uhrberg et al42 | |
| KIR3DL2 | CGG TCC CTT GAT GCC TGT | GAC CAC ACG CAG GGC AG | 1900 | Uhrberg et al43 |
| KIR3DL3 | GGA CCT ACA GAT GTT GC | TAG TTG ACC TGG GAA CCC G | 1575 | Rajalingam et al45 |
| KIR3DS1 | GGC AGA ATA TTC CAG GAG G | AGG GGT CCT TAG AGA TCC A | 1750 | Uhrberg et al43 |
| CAG CGC TGT GGT GCC TCG C | CTG TGA CCA TGA TCA CCA T | 249 | Hsu et al44 |
KIR gene . | Sense primer . | Antisense primer . | Length, bp . | Reference . |
|---|---|---|---|---|
| KIR2DL1 | ACT CAC TCC CCC TAT CAG G | AGG GCC CAG AGG AAA GTC A | 1750 | Uhrberg et al42 |
| KIR2DL1v | ACT CAC TCC CCC TAT CAG G | AGG GCC CAG AGG AAA GTT | 1750 | Uhrberg et al42 |
| ACT CAC TCC CCC TAT CAG G | AGG GCC CAG AGG AAA GTK | 1750 | Uhrberg et al42 | |
| KIR2DL2 | CCA TGA TGG GGT CTC CAA A | GCC CTG CAG AGA ACC TAC A | 1800 | Uhrberg et al43 |
| KIR2DL3 | CCT TCA TCG CTG GTG CTG | CAG GAG ACA ACT TTG GAT CA | 798 | Uhrberg et al43 |
| KIR2DL4 | GTA TCG CCA GAC ACC TGC ATG CTG | GCA GGC AGT GGG GAC CTT AGA CA | 1082 | Hsu et al44 |
| KIR2DL5.1 | CTC CCG TGA TGT GGT CAA CAT GTA AA | GGG GTC ACA GGG CCC ATG AGG AT | 1883 | Hsu et al44 |
| KIR2DL5.2 | GTA CGT CAC CCT CCC ATG ATG TA | GGG GTC ACA GGG CCC ATG AGG AT | 1893 | Hsu et al44 |
| KIR2DS1 | TCT CCA TCA GTC GCA TGA R | AGG GCC CAG AGG AAA GTT | 1800 | Uhrberg et al43 |
| TCT CCA TCA GTC GCA TGA R | AGG GCC CAG AGG AAA GTK | 1800 | Hsu et al44 | |
| KIR2DS2 | TGC ACA GAG AGG GGA AGT A | CAC GCT CTC TCC TGC CAA | 1750 | Uhrberg et al43 |
| KIR2DS3 | TCA CTC CCC CTA TCA GTT T | GCA TCT GTA GGT TCC TCC T | 1800 | Uhrberg et al43 |
| GAC ATG TAC CAT CTA TCC AC | GCA TCT GTA GGT TCC TCC T | 130 | Hsu et al44 | |
| KIR2DS4 | ATC CTG CAA TGT TGG TCG | CTG GAT AGA TGG TAC ATG TC | 1902 | Hsu et al44 |
| KIR1D | ATC CTG CAA TGT TGG TCG | CTG GAT AGA TGG AGC TGC AG | 1885 | Hsu et al44 |
| KIR2DS5 | AGA GAG GGG ACG TTT AAC C | TCC GTG GGT GGC AGG GT | 1950 | Uhrberg et al42 |
| CTG CAC AGA GAG GGG ACG TTT AAC C | TCC AGA GGG TCA CTG GGC | 179 | Hsu et al44 | |
| KIR3DL1 | TAC AAA GAA GAC AGA ATC CAC A | TAG GTC CCT GCA AGG GCA A | 1600 | Uhrberg et al42 |
| TCC CAT CTT CCA TGG CAG AT | TAG GTC CCT GCA AGG GCA A | 1600 | Uhrberg et al42 | |
| KIR3DL2 | CGG TCC CTT GAT GCC TGT | GAC CAC ACG CAG GGC AG | 1900 | Uhrberg et al43 |
| KIR3DL3 | GGA CCT ACA GAT GTT GC | TAG TTG ACC TGG GAA CCC G | 1575 | Rajalingam et al45 |
| KIR3DS1 | GGC AGA ATA TTC CAG GAG G | AGG GGT CCT TAG AGA TCC A | 1750 | Uhrberg et al43 |
| CAG CGC TGT GGT GCC TCG C | CTG TGA CCA TGA TCA CCA T | 249 | Hsu et al44 |
Results
Expression and distribution of NKRs in PBLs from LDGL patients
To obtain representative data on the phenotypic profile of NK cell expansions in LDGL, PBLs from 18 patients and from 2 healthy control donors (CTR1 and CTR2; see “Patients, materials, and methods”) were analyzed by cytofluorimetry for the expression of a large panel of NKRs. As shown in Table 2 all the selected patients were characterized by the proliferation of CD3–CD16+ NK cells (range, 34%-85%), whereas CD56 and CD57 were expressed in 11 of 18 and in 12 of 18 cases, respectively (not shown). The expression of each NKR was evaluated on the whole lymphoid population and confirmed using immunologic gate on CD3–CD16+ cells. The analysis of HLA-specific NKRs (Table 2) showed that KIRs were expressed on most patients analyzed.
Phenotypic analysis of PBLs from LDGL patients
Patient no. . | CD16+ CD3- cells, % . | KIR2DL1 KIR2DS1 (EB6b) . | KIR2DL2/3 KIR2DS2 (GL183) . | KIR2DS4 (FES172) . | KIR3DL1 KIR3DS1 (Z27) . | KIR3DL2 (Q66) . | CD94 (XA185) . | NKG2-A (Z199) . | LIR-1 (F278) . |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 82 | 1 | 81† | 0 | 2 | 1 | 85 | 87 | 55 |
| 2* | 51 | 51† | 8 | 13 | 3 | 3 | 77 | 9 | 45 |
| 3* | 64 | 1 | 1 | 65† | 1 | 0 | 67 | 66 | 31 |
| 4* | 75 | 0 | 75† | 0 | 0 | 27 | 76 | 75 | 62 |
| 5 | 71 | 1 | 73† | 0 | 0 | 0 | 79 | 75 | 45 |
| 6 | 48 | 5 | 48† | 0 | 0 | 7 | 62 | 4 | 35 |
| 7 | 40 | 0 | 39† | 1 | 1 | 0 | 45 | 42 | 29 |
| 8 | 35 | 0 | 31† | 1 | 0 | 0 | 39 | 34 | 34 |
| 9* | 34 | 25 | 17 | 0 | 4 | 12 | 35 | 31 | 29 |
| 10* | 62 | 29 | 15 | 0 | 3 | 4 | 33 | 35 | 32 |
| 11 | 50 | 25 | 8 | 6 | 5 | 0 | 38 | 35 | 49 |
| 12 | 46 | 8 | 23 | 0 | 10 | 2 | 45 | 48 | 35 |
| 13 | 70 | 12 | 6 | 21 | 0 | 0 | 74 | 73 | 77 |
| 14 | 59 | 0 | 0 | 25 | 1 | 10 | 42 | 40 | 37 |
| 15* | 73 | 3 | 6 | 0 | 2 | 1 | 75 | 73 | 68 |
| 16 | 41 | 0 | 0 | 1 | 0 | 0 | 48 | 44 | 31 |
| 17 | 71 | 0 | 2 | 9 | 0 | 0 | 78 | 73 | 52 |
| 18 | 67 | 1 | 3 | 1 | 1 | 3 | 69 | 68 | 42 |
| CTR1 | 14 | 6 | 7 | 8 | 7 | 4 | 20 | 12 | 12 |
| CTR2 | 16 | 9 | 9 | 0 | 1 | 4 | 21 | 4 | 4 |
Patient no. . | CD16+ CD3- cells, % . | KIR2DL1 KIR2DS1 (EB6b) . | KIR2DL2/3 KIR2DS2 (GL183) . | KIR2DS4 (FES172) . | KIR3DL1 KIR3DS1 (Z27) . | KIR3DL2 (Q66) . | CD94 (XA185) . | NKG2-A (Z199) . | LIR-1 (F278) . |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 82 | 1 | 81† | 0 | 2 | 1 | 85 | 87 | 55 |
| 2* | 51 | 51† | 8 | 13 | 3 | 3 | 77 | 9 | 45 |
| 3* | 64 | 1 | 1 | 65† | 1 | 0 | 67 | 66 | 31 |
| 4* | 75 | 0 | 75† | 0 | 0 | 27 | 76 | 75 | 62 |
| 5 | 71 | 1 | 73† | 0 | 0 | 0 | 79 | 75 | 45 |
| 6 | 48 | 5 | 48† | 0 | 0 | 7 | 62 | 4 | 35 |
| 7 | 40 | 0 | 39† | 1 | 1 | 0 | 45 | 42 | 29 |
| 8 | 35 | 0 | 31† | 1 | 0 | 0 | 39 | 34 | 34 |
| 9* | 34 | 25 | 17 | 0 | 4 | 12 | 35 | 31 | 29 |
| 10* | 62 | 29 | 15 | 0 | 3 | 4 | 33 | 35 | 32 |
| 11 | 50 | 25 | 8 | 6 | 5 | 0 | 38 | 35 | 49 |
| 12 | 46 | 8 | 23 | 0 | 10 | 2 | 45 | 48 | 35 |
| 13 | 70 | 12 | 6 | 21 | 0 | 0 | 74 | 73 | 77 |
| 14 | 59 | 0 | 0 | 25 | 1 | 10 | 42 | 40 | 37 |
| 15* | 73 | 3 | 6 | 0 | 2 | 1 | 75 | 73 | 68 |
| 16 | 41 | 0 | 0 | 1 | 0 | 0 | 48 | 44 | 31 |
| 17 | 71 | 0 | 2 | 9 | 0 | 0 | 78 | 73 | 52 |
| 18 | 67 | 1 | 3 | 1 | 1 | 3 | 69 | 68 | 42 |
| CTR1 | 14 | 6 | 7 | 8 | 7 | 4 | 20 | 12 | 12 |
| CTR2 | 16 | 9 | 9 | 0 | 1 | 4 | 21 | 4 | 4 |
Surface expression of the various receptors was evaluated by FACS analysis using the mAbs indicated in parentheses. Values indicate percentage of positive cells.
The 7 patients from whom it was possible to derive a polyclonal NKLGL line.
Values in which the percent of NK cells stained by a given anti-KIR mAb approximated that of CD16+CD3- cells.
Importantly, in 8 of 18 cases, NK cells were homogeneously stained by one or another anti-KIR mAb. On the other hand, in 6 of 18 cases the NK-LGL expansion was apparently not characterized by a homogeneous expression of a given KIR. Rather, in these cases only a fraction of the NK cells were stained by one or another anti-KIR mAb. Finally, the NK-LGL expansions from the remaining 4 patients were scored as essentially KIR–. Focusing on the 8 cases that were homogeneously stained by at least one anti-KIR mAb, it is of note that 6 of 8 (patient nos. 1, 4, 5, 6, 7, and 8) were stained by the GL183 mAb, which reacts with KIR2DL2/3 and KIR2DS2, whereas only 1 of 8 cases was stained by the KIR2DL1/S1-specific EB6b mAb (patient no. 2) or by the KIR2DS4-specific FES172 mAbs (patient no. 3). Remarkably, none of the NK-LGL expansions showed a homogeneous reactivity with the KIR3DL1-specific Z27 mAb. This receptor, however, could be detected on minor fractions of NK cells from different NK-LDGL patients. Also, the antibody Q66 specific for KIR3DL2 was found to react with minor NK subsets in a fraction of the patients analyzed.
Table 2 also shows that all patients expressed the CD94 molecule. In 16 of 18 cases this molecule was associated with NKG2A (as assessed by the reactivity with the Z199 mAb), whereas in 2 patients (nos. 2 and 6), NK cells expressed the CD94-NKG2C heterodimer. Indeed, in the latter 2 cases CD94+ cells did not react with Z199 mAb but reacted with P25 mAb (not shown), which recognizes both NKG2A and NKG2C molecules. The LIR-1 (ILT2) was generally expressed in all patients; in particular, the cell fraction stained by the LIR-1–specific mAb ranged between 29% and 77% in the various individuals analyzed.
The assessment of NCR expression (Table 3) indicated that NKp30 was strongly down-regulated in all but one of the cases analyzed (patient no. 6). Similarly, the NKp46 receptor in most instances was detected only in small fractions of NK cells. Thus, with the exception of patients nos. 4 and 15, in whom the percent of NKp46+ cells was comparable to that of the total NK cells, all the other patients were characterized by NK cells expressing little NKp46 receptor. These striking phenotypic results suggested in these patients the occurrence of a defect in NCR expression that was reminiscent of that recently reported in patients with acute myeloid leukemia.46
Reduced expression of NCRs in LDGL patients
Patient no. . | CD16+CD3- cells, % . | NKp46 (BAB281) . | NKp30 (AZ20) . | NKp44 (Z231) . |
|---|---|---|---|---|
| 1 | 82 | 0 | 2 | 0 |
| 2 | 51 | 4 | 2 | 2 |
| 3 | 64 | 26 | 5 | 1 |
| 4 | 75 | 56 | 3 | 0 |
| 5 | 71 | 10 | 1 | 0 |
| 6 | 48 | 10 | 51 | 0 |
| 7 | 40 | 1 | 0 | 0 |
| 8 | 35 | 1 | 0 | 0 |
| 9 | 34 | 11 | 3 | 0 |
| 10 | 62 | 12 | 12 | 5 |
| 11 | 50 | 22 | 2 | 0 |
| 12 | 46 | 3 | 5 | 1 |
| 13 | 70 | 15 | 3 | 0 |
| 14 | 59 | ND | 6 | 5 |
| 15 | 73 | 61 | 2 | 0 |
| 16 | 41 | 1 | 1 | 0 |
| 17 | 71 | 3 | 0 | 0 |
| 18 | 67 | 2 | 1 | 1 |
| CTR1 | 14 | 12 | 12 | 0 |
| CTR2 | 16 | 8 | 6 | 0 |
Patient no. . | CD16+CD3- cells, % . | NKp46 (BAB281) . | NKp30 (AZ20) . | NKp44 (Z231) . |
|---|---|---|---|---|
| 1 | 82 | 0 | 2 | 0 |
| 2 | 51 | 4 | 2 | 2 |
| 3 | 64 | 26 | 5 | 1 |
| 4 | 75 | 56 | 3 | 0 |
| 5 | 71 | 10 | 1 | 0 |
| 6 | 48 | 10 | 51 | 0 |
| 7 | 40 | 1 | 0 | 0 |
| 8 | 35 | 1 | 0 | 0 |
| 9 | 34 | 11 | 3 | 0 |
| 10 | 62 | 12 | 12 | 5 |
| 11 | 50 | 22 | 2 | 0 |
| 12 | 46 | 3 | 5 | 1 |
| 13 | 70 | 15 | 3 | 0 |
| 14 | 59 | ND | 6 | 5 |
| 15 | 73 | 61 | 2 | 0 |
| 16 | 41 | 1 | 1 | 0 |
| 17 | 71 | 3 | 0 | 0 |
| 18 | 67 | 2 | 1 | 1 |
| CTR1 | 14 | 12 | 12 | 0 |
| CTR2 | 16 | 8 | 6 | 0 |
Surface expression of the various receptors was evaluated by FACS analysis using the mAbs indicated in parentheses. Values indicate percentage of positive cells.
ND indicates not done.
Regarding NKp44, which is normally expressed only in activated NK cells,21 this receptor was not expressed at significant levels in the PBLs of the patients analyzed.
A completely different pattern was observed for NKG2D, NKp80, and 2B4 molecules that were homogeneously expressed on NK cells in the majority of the patients (17 of 18, 14 of 16, and 17 of 18 cases, respectively; not shown). Altogether, these data suggest that, in NK-LDGL patients, peripheral blood NK cells are often characterized by a homogeneous expression of a given KIR and by an unusually low surface expression of NCRs.
Characterization of NK cell lines from LDGL patients
Although providing useful information on the phenotype of freshly drawn NK cells from LDGL patients, the data do not allow understanding the actual function of the various NKRs. In this context it is of note that most of the available KIR-reactive mAbs cannot discriminate between activating and inhibitory forms of the receptor. Thus, as mentioned, the EB6b mAb recognizes both KIR2DL1 (inhibitory) and KIR2DS1 (activating) molecules, whereas the GL183 mAb recognizes both KIR2DL2/3 (inhibitory) and KIR2DS2 (activating) molecules. Moreover, the KIR3DL1-specific Z27 mAb47 also reacts with an activating KIR (likely represented by KIR3DS1).48 In the latter case, however, the fluorescence intensity is different in NK cells expressing one or another KIR3D receptor. This allows us to distinguish a Z27dull NK subset (KIR3DS1+) from a Z27bright population (KIR3DL1+). Given its low surface density the detection of this Z27-reactive activating receptor by cytofluorimetric analysis is often difficult especially in unfractionated PBLs. Thus, in the cytofluorimetric analysis described, peripheral blood NK cells expressing this receptor may have been underestimated.
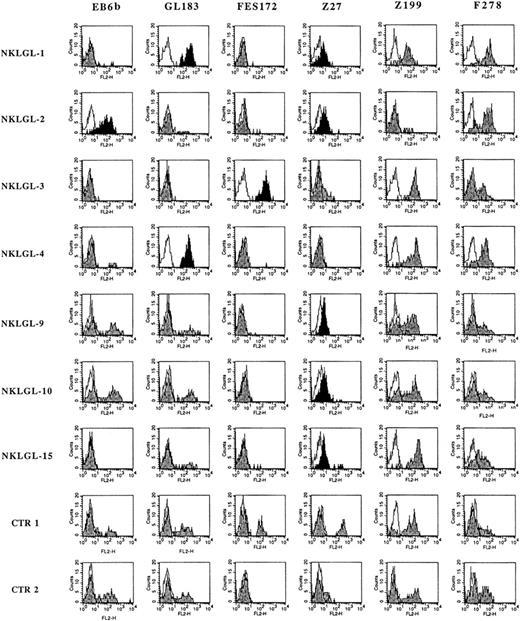
To obtain a more precise functional/phenotypic assessment of the various receptors, NK cells from the patients were also analyzed after culture in the presence of IL-2. A polyclonal NK cell line (thereafter termed NKLGL) could be generated from 7 of 12 patients who were available for these experiments (marked with an asterisk in Table 2) and from 2 healthy controls (CTR1 and CTR2). Importantly, these cultured NK cells showed a KIR distribution pattern similar to that observed in the corresponding PBLs. In particular (Figure 1), NKLGL-1 and NKLGL-4 homogeneously expressed KIR molecules reactive with GL183 mAb, whereas NKLGL-2 was stained by EB6b mAb and NKLGL-3 was stained by FES172 mAb. All these patients were scored as homogeneously positive for the above markers (that are specific for different KIR2D molecules) also in PBLs. On the other hand, patients 9 and 10 were heterogeneous for the expression of KIR2D both in PBLs (Table 2) and in the cultured NKLGL cell lines (Figure 1), whereas patient 15 had little expression of KIR2D in both PBLs and in the NKLGL cell line.
Surface expression of HLA-specific NK receptors in polyclonal NK cell lines from LDGL patients. Polyclonal NK cell lines (NKLGL) derived from 7 LDGL patients and from 2 controls were stained with the indicated mAb specific for different NKRs. In particular, EB6b mAb reacts with KIR2DL1 and S1, GL183 mAb reacts with KIR2DL2/3 and S1, FES172 mAb reacts with KIR2DS4, Z27 mAb reacts with KIR3DL1, Z199 mAb reacts with NKG2A, and F278 mAb reacts with LIR1/ILT2. Open profiles indicate the isotype controls, and gray profiles refer to the specific fluorescence with the above mAbs, whereas black profiles bring into evidence the homogeneous reactivity of given anti-KIR mAb with the whole NK cell population.
Surface expression of HLA-specific NK receptors in polyclonal NK cell lines from LDGL patients. Polyclonal NK cell lines (NKLGL) derived from 7 LDGL patients and from 2 controls were stained with the indicated mAb specific for different NKRs. In particular, EB6b mAb reacts with KIR2DL1 and S1, GL183 mAb reacts with KIR2DL2/3 and S1, FES172 mAb reacts with KIR2DS4, Z27 mAb reacts with KIR3DL1, Z199 mAb reacts with NKG2A, and F278 mAb reacts with LIR1/ILT2. Open profiles indicate the isotype controls, and gray profiles refer to the specific fluorescence with the above mAbs, whereas black profiles bring into evidence the homogeneous reactivity of given anti-KIR mAb with the whole NK cell population.
Remarkably, most NKLGL lines (patients no. 1, 2, 9, 10, and 15) appeared to express the activating KIR recognized by the Z27 mAb (ie, Z27dull phenotype). It is of note that, due to the low surface density of this receptor, the corresponding PBLs were essentially scored as Z27– (Table 2). Thus, the combined analysis of both circulating and in vitro–cultured NK cells allowed the identification of a homogeneous KIR+ NK cell population in 11 of 18 patients analyzed (patient nos. 1-10, and 15 in Table 2 and Figure 1). The expression of the various KIRs in the CTR1 and CTR2 showed the expected absence of homogeneous NK cell populations because all the anti-KIR mAbs reacted with only a fraction of the various NK cell lines. Notably, whereas the CTR1 cell line expressed the Z27bright phenotype in an NK subset, the CTR2 cell line was characterized by a subset expressing the Z27dull phenotype.
NK cells from LDGL patients express activating KIR
We next assessed whether the various KIRs expressed by cultured NK cells from patients with LDGL were able to trigger or to inhibit the NK cell–mediated cytotoxicity. To this end the 7 different patient-derived NKLGL cell lines and the 2 control NK cell lines (CTR1 and CTR2) were analyzed by redirected killing assays against the FcγR+ P815 cell line, either in the absence or in the presence of different anti-KIR mAbs. As shown in Figure 2, in each NKLGL line, those anti-KIR mAbs that homogeneously stained all NK cells were characterized by the ability to induce a strong cytolytic response.
Cytolytic responses to anti-KIR mAbs in polyclonal NK cell lines from LDGL patients. Seven NKLGL cell lines derived from the indicated patients and 2 controls were analyzed for cytolytic responses in a redirected killing assay either in the absence or in the presence of different mAbs: EB6 (a), GL183 (b), FES172 (c), Z27 (d), or anti-CD16 (e). The latter has been indicated with black bars and constitutes a positive control for each experiment. The spontaneous lysis of P815 target cells is represented by the horizontal line in each graph. The bars over the line indicate increase of cytotoxicity, whereas those under the line indicate inhibition. The E/T ratio was 5:1.
Cytolytic responses to anti-KIR mAbs in polyclonal NK cell lines from LDGL patients. Seven NKLGL cell lines derived from the indicated patients and 2 controls were analyzed for cytolytic responses in a redirected killing assay either in the absence or in the presence of different mAbs: EB6 (a), GL183 (b), FES172 (c), Z27 (d), or anti-CD16 (e). The latter has been indicated with black bars and constitutes a positive control for each experiment. The spontaneous lysis of P815 target cells is represented by the horizontal line in each graph. The bars over the line indicate increase of cytotoxicity, whereas those under the line indicate inhibition. The E/T ratio was 5:1.
The anti-KIR mAbs that reacted only with a fraction of NK cells had in most instances either little or no effect. In particular, NKLGL-1 was strongly triggered by GL183 and Z27 mAbs; NKLGL-2 by EB6b and Z27 mAbs; NKLGL-3 by FES172 mAb, and only in part by Z27 mAb; NKLGL-4 by GL183 mAb; NKLGL-9 by Z27 and, in part, EB6b mAbs; NKLGL-10 by Z27 mAb; and NKLGL-15 by Z27 mAb.
These results would suggest that in all NKLGL lines most NK cells are expressing the activating form of a given KIR molecule. In line with these findings, the triggering capability of KIR molecules could be also detected on freshly isolated NK cell populations derived from some of the patients analyzed in this study (not shown). These data indicate that the surface phenotype and the functional properties of the NKLGL lines analyzed are reflecting those of the respective “in vivo” NK cell population. Interestingly, as also indicated by the phenotypic results (Figure 1), the activating KIR recognized by the Z27 mAb was homogeneously expressed in 5 of 7 NKLGL lines including the 3 cases that were KIR2D–. Although not shown, it has to be mentioned that a series of representative NK clones were derived from patients 1, 2, 3, 4, and 15. These NK clones displayed the same phenotypic and functional properties of the corresponding polyclonal NKLGL line. Importantly, none of the NK clones analyzed displayed inhibitory instead of activating function of the KIR that homogeneously marked that particular NK cell expansion.
The various NKLGL lines were also analyzed for cytolytic responses to anti-CD94 or anti–LIR-1 mAbs. Although not shown, anti-CD94 mAb triggered cytotoxicity in 1 of 7 cases analyzed. This LGL expansion (patient no. 2) did not express NKG2A but expressed NKG2C (because it was Z199–P25+). Finally, in no instances was the anti–LIR-1 mAb found to induce triggering effects.
It is of note that in the NK cell lines used as controls (derived from healthy individuals), most anti-KIR mAb did not induce triggering effects. This is likely related to the fact that in healthy individuals NK cells expressing one or another triggering KIR do not represent the majority (as in NKLGL patients) but rather the minority of cells. The only exceptions were represented by the FES172 and Z27 mAbs that were able to induce activation in CTR1 and CTR2 cell lines, respectively (Figure 2). However, even in these cases the magnitude of the triggering response was significantly lower than those magnitudes observed in NKLGL lines characterized by the homogeneous expression of these molecules. Regarding the expression and the function of CD94/NKG2, this receptor was found to trigger cytotoxicity in one of the 2 controls analyzed (CTR2). Indeed, this individual (CTR2) is characterized by the expression of CD94/NKG2C on a major NK subset (see “Patients, materials, and methods”).
Analysis of KIR genotype in LDGL patients
The KIR genes of 13 of 18 LDGL patients were analyzed by PCR-SSP (Figure 3). In line with previous data obtained in the white population, all individuals were scored as KIR2DL4+, KIR3DL2+, KIR3DL3+ and were characterized by the presence of the KIR2DL2 or KIR2DL3 or both genes.42 Notably most patients expressed KIR genotypes characterized by the presence of multiple genes coding for activating KIRs. These should be classified as individuals bearing a group B haplotype as described by Uhrberg et al.43 On the other hand only one of the patients analyzed (no. 11) displayed a KIR genotype containing only one gene coding for an activating KIR. This genotype indicates the presence of the group A haplotype in a homozygous state.43
KIR gene profiles in LDGL patients. Ten distinct KIR gene profiles were detected in NK-LDGL patients based on the presence (shaded box) or the absence (white box) of individual KIR genes.
KIR gene profiles in LDGL patients. Ten distinct KIR gene profiles were detected in NK-LDGL patients based on the presence (shaded box) or the absence (white box) of individual KIR genes.
Seven of 12 patients were scored as KIR3DS1+ and 6 of these were also characterized by the presence of the KIR2DS5 gene. Moreover, among the KIR3DS1+ individuals, 2 pairs (patient nos. 7 and 15 and patient nos. 9 and 10, respectively) were found to share the same KIR genotype. Although the number of patients analyzed is too limited for a precise statistical analysis, it is interesting to note that such genotypes appear to be more frequent in LDGL patients compared with the healthy individuals analyzed in previous studies.42 In line with this observation another genotype that was previously detected at low frequency (3.3%) among healthy individuals could be observed in 3 of 13 patients analyzed (nos. 12, 13, and 17). Finally, the 2 different KIR genotypes determined in patients 1 and 2 appear to be among the most infrequent (< 1%) in the white population.42
Altogether, the above data suggest that the majority of the KIR genotypes detected in LDGL patients are relatively rare compared with the frequencies of KIR genotypes previously reported in the white population. More importantly, the high frequency of group B haplotypes (ie, presence of multiple activating KIRs) may suggest an involvement of activating KIRs in the pathogenesis of this disease.
Expression and function of non–HLA-specific triggering receptors on cultured NKLGL lines
Because freshly drawn NK cells from LDGL patients were generally characterized by low NCR expression, we next analyzed in vitro–cultured NKLGL lines for the expression and the function of these receptors. Controls included the 2 representative polyclonal NK cell lines shown in the above experiments. These controls were characterized either by the expression of a homogeneous NCRbright phenotype (CTR1) or by a bimodal NCR surface distribution that included both NCRbright and NCRdull NK cells (CTR2).
As shown in Figure 4, NCRs were expressed in all NKLGL lines, but their surface density displayed major differences according to the patient analyzed. Thus, in patients 1 and 2 NCRs were expressed at very low surface density, whereas in the remaining patients their mean fluorescence intensity ranged within the values commonly observed in healthy individuals. Comparison between fresh and cultured NK cells indicated that the expression of the NKp46 molecule was substantially up-regulated in patients 3, 9, and 10, whereas the expression of NKp44 and NKp30 was significantly augmented in all NKLGL lines (Figure 4; Table 3).
Surface expression of NCRS in polyclonal NK cell lines from LDGL patients. The same NKLGL lines illustrated in Figure 1 were stained with different anti-NCR mAbs. These were represented by BAB281 mAb specific for NKp46, by Z25 mAb specific for NKp30, and by Z231 mAb specific for NKp44. Isotype controls are represented by open profiles; gray profiles represent the reactivity with the above mAbs. The 2 controls (CTR1 and CTR2) differ for the expression of NCRs because CTR1 was brightly stained by all the mAbs, whereas CTR2 displayed a bimodal distribution (ie, bright versus dull) of both NKp46 and NKp30 molecules.
Surface expression of NCRS in polyclonal NK cell lines from LDGL patients. The same NKLGL lines illustrated in Figure 1 were stained with different anti-NCR mAbs. These were represented by BAB281 mAb specific for NKp46, by Z25 mAb specific for NKp30, and by Z231 mAb specific for NKp44. Isotype controls are represented by open profiles; gray profiles represent the reactivity with the above mAbs. The 2 controls (CTR1 and CTR2) differ for the expression of NCRs because CTR1 was brightly stained by all the mAbs, whereas CTR2 displayed a bimodal distribution (ie, bright versus dull) of both NKp46 and NKp30 molecules.
That certain NKLGL lines were defective in the expression of NCR was further substantiated by the functional analysis of the various receptors in redirected killing assays.
Thus, although not shown, the 2 NKLGL lines that were characterized by very low expression of NCR (patients no. 1 and 2) gave little cytolytic responses to anti-NCR mAbs, whereas the other 5 cases displayed responses comparable to controls.
Discussion
In this study we show that in patients with NK-type LDGL the abnormal LGL expansion can be frequently identified as a population that homogeneously reacts with one or another anti-KIR mAb (11 of 18 cases). Importantly, functional studies showed that in vitro–cultured NK cell lines derived from 7 LDGL patients were characterized by the homogeneous expression of at least one activating KIR. These features make NK cell expansions from NK-LDGL different from normal NK cell populations because in healthy individuals, NK cells usually show a diversified NKR repertoire and prevalently express inhibitory rather than activating KIR. Consistent with this finding, the analysis of the KIR gene repertoire in LDGL patients suggests that the susceptibility to this disease may be influenced at least in part by the KIR genotype. Indeed, in these patients the KIR genotype is frequently characterized by multiple genes coding for activating KIRs. Finally, we show that, in most instances, NK cells freshly drawn from patients display an unusual low NCR expression that, at least in part, can be reverted on in vitro culture in the presence of IL-2.
A previous study indicated that in NK-LDGL patients, the use of EB6 or GL183 mAb or both (reactive with different KIR2D molecules) offered the invaluable opportunity to distinguish pathologic LGL expansions (characterized by the expression “all or nothing” of these receptors) from normal NK cells (characterized by the expression of these molecules only in a fraction of cells).40 In a recent paper, Hoffmann and colleagues, using a wide panel of mAbs against KIR, confirmed our previous data and extended the analysis to T-LDGL.49 In this study, however, patients with NK-type LDGL represented only 2 cases of 9 analyzed and a functional evaluation of these cells was not provided. Our present data allow a more complete phenotypic and functional characterization of the NK cell expansion in a large number of NK-type LDGL and settle the analysis of KIR expression and function as a crucial tool in the diagnosis of this disease. Indeed, only in 7 of 18 patients analyzed (nos. 11-14, 16-18) NK cells were apparently not represented by a homogeneous expansion of cells expressing a given KIR. In patients 16 and 18, however, our study could not be extended to the analysis of polyclonally activated NK cell lines nor to the assessment of the KIR genotype. Therefore, it cannot be ruled out that NK cells from these patients may have been characterized by other undefined triggering KIRs. These receptors might be either expressed at low surface density in freshly drawn NK cells or may not be recognized by the available anti-KIR mAbs. Indeed, in some patients (nos. 1, 2, 9, 10, and 15) the expression of the Z27dull phenotype could be detected only on cultured (but not on fresh) LGLs. Despite the homogeneous expression of a given KIR, the NK cell expansions from most of the patients analyzed were also characterized by a heterogeneous expression of other NKRs. These, which included NKG2A, LIR1, and additional KIRs (Table 2; Figure 1), were confined to different subsets accounting for different percentages of the NK-LGL population. Considering the clonal distribution of the quoted receptors, it appears that the majority of the LGL expansions analyzed in this study, although characterized by the homogeneous expression of a given KIR, are composed of phenotypically distinct NK cell subsets. The finding that the KIRs expressed by NK cells from LDGLs are prevalently of the activating type is in line with our previous identification of KIR-associated KARAP (DAP12) signaling molecule in selected NK cell expansions.16 Altogether, the above data may imply that one of the first steps in the development of NK-type LDGL may be the selection and the expansion of NK cells bearing activating KIRs. The mechanism by which NK cells are selected and induced to proliferate during NK-LDGL pathogenesis is a matter of debate. Accumulating data suggest that exogenous agents, in particular viruses, might play a role in this phenomenon. Indeed, detection of viral infections represents a usual event in NK-type LDGL.39 In a recent study, serologic reactivity to the Ba21 epitope of human T-cell leukemia virus 1 (HTLV-1) env p21 was shown in a cohort of such patients.50 Furthermore, a correlation between the detection of Epstein-Barr virus (EBV) infection and the expression of the EB6 mAb reactive KIRs was recently shown in some patients.51 However, despite a frequent serologic positivity for different viruses (ie, EBV, hepatitis B virus, hepatitis C virus),39 a direct infection of NK cells could be demonstrated only in some cases.52,53 These observations lead to the hypothesis that the chronic NK lymphocytoses may be induced by an initial reactive response to the viral infection. The finding that in such diseases cells are selected that express activating KIR suggests that these receptors may be directly involved in priming the LGL proliferation. In this context previous data indicated that triggering of activating KIR induces in vitro proliferation of NK cells derived from healthy individuals.14 Moreover, activating MHC-specific NKRs have been proposed to recognize viral products both in humans and in mice.29-33 Thus, the recognition of virally infected cells through activating KIRs may be the initial event at the onset of the polyclonal NK lymphocytosis.
Along this line one may ask whether those individuals who are carrying a KIR genotype characterized by the presence of a large number of genes coding for activating KIR may be more susceptible to the occurrence of the disease. Although our number of cases is small, our data show that most of the patients analyzed (12 of 13) were characterized by relatively rare KIR genotypes. Most of these genotypes included multiple genes coding for activating KIRs. More strikingly, some of these genotypes were shared by different patients. Furthermore, the frequencies of certain genes coding for activating KIRs (eg, KIR3DS1 and KIR2DS5) were found to be higher in the patients analyzed compared with those previously reported in the white population. Based on these results it cannot be ruled out that indeed the expression of given KIR genotypes may influence the susceptibility to the disease. Studies are in progress to evaluate the KIR haplotype in a large number of cases to address this issue.
Whatever the mechanisms, NK-LDGL patients display a dramatic increase of NK cells that express activating KIRs. This abnormal NK cell population may be harmful because such cells could drive autoreactive phenomena. However, at variance with T-LDGL, autoimmune manifestations are not frequent in NK-type lymphocytosis. It is possible that the fail-safe mechanism in NK-LGLs could be represented by the expression of the CD94/NKG2A or the LIR-1 inhibitory receptors that have the ability to sense HLA class I expression with broad specificity. In this context it should be noted that although the LIR1+ phenotype is generally poorly represented within normal NK populations, it is expressed in a major fraction of the patients with NK-LGLs analyzed. Interestingly, high expression of LIR-1 molecules was recently shown also in T-LDGL patients who lacked autoimmune manifestation.54
The analysis on the non–HLA-specific triggering receptors revealed that in most patients freshly drawn NK cells expressed the NCR molecules at low density. On culture in IL-2, however, these receptors were up-regulated and were demonstrated to be functional, thus suggesting that the expanded NK cell population may have conserved at least in part the immune competence. In conclusion, our data suggest a pathogenic model for the comprehension of the early events that induce granular lymphocyte proliferation in patients with NK-type LDGL. An exogenous event, likely a virus, would act on the NK cell pool by selecting those cells that better fit in the antiviral response (ie, cells equipped with a given activating KIR). It is likely that the release of cytokines, particularly IL-15,55 might play a crucial role in maintaining the proliferation over time even if a spontaneous resolution of LDGL might occur unless a second, still unknown, event (chromosomal alteration?) could favor the progression of disease.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2002-12-3898.
Supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC), Consiglio Nazionale delle Ricerche (CNR), Istituto Superiore di Sanità (ISS), Ministero della Sanità, Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), and CNR, Progetto Finalizzato Biotecnologie. The financial support of Fondazione Compagnia di San Paolo, Torino, Italy, is also gratefully acknowledged.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Tiziana Baffi for secretarial assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal