Abstract
Gene therapy for hemophilia A requires efficient delivery of the factor VIII gene and sustained protein expression at circulating levels of at least 1% to 2% of normal. Adeno-associated viral type 2 (AAV2) vectors have a number of advantages over other viral vectors, including an excellent safety profile and persistent gene expression. However, a major disadvantage is their small packaging capacity, which has hampered their use in treating diseases such as hemophilia A, cystic fibrosis, and muscular dystrophy, which are caused by mutations in large genes. Here we demonstrate that this can be overcome by using small regulatory elements to drive expression of a B-domain–deleted form of FVIII. The use of this vector for hepatic gene transfer in a canine model of hemophilia A resulted in the sustained (> 14 months) expression of biologically active FVIII. FVIII activity levels of 2% to 4% were achieved. These levels correlated with a partial correction in the whole-blood clotting time and cuticle bleeding time. In addition, immunoprecipitation analysis demonstrated the expression of canine FVIII of the predicted size in the plasma of injected animals. These data support the use of AAV2 vectors in human clinical trials to treat hemophilia A patients.
Introduction
Hemophilia A is a common bleeding disorder caused by the deficiency of blood coagulation factor VIII (FVIII). Severe hemophilia A patients have less than 1% of the normal FVIII levels and suffer from spontaneous bleeding episodes and disabling arthropathy.1,2 The current treatment for hemophilia A is infusion of plasma-derived or recombinant FVIII in response to bleeding. Although this treatment has dramatically improved the course of this disease, patients are still at risk for bleeds when plasma factor FVIII levels fall below 1% to 2% of normal. Gene therapy has the potential to provide persistent levels of FVIII, which minimally could prevent arthropathy, and in theory could cure the disease. FVIII is a secreted protein and circulating levels as low as 1% to 2% of normal have been shown to be efficacious using protein replacement therapy.3 The low levels of FVIII required for efficacy and the wide therapeutic window eliminate the need for complete transduction of the liver and for tight regulation of gene expression, and these features make hemophilia A very amenable to gene therapy. Previous work by us and others has demonstrated the feasibility of gene therapy to treat hemophilia A in the mouse model using a variety of viral vectors including adeno-associated viral (AAV),4,5 adenoviral (Ad),6 gutted adenoviral,7 and retroviral vectors.8,9 However, there have been only 2 reports of gene therapy in the hemophilia A dog model using the homologous transgene.10,11 One report used an Ad vector, and transient FVIII expression was observed due to the generation of anti–canine FVIII antibodies and acute hepatotoxicity.10 A recent paper, using a high-capacity Ad vector, achieved transient (2 weeks) expression of FVIII at 3.5% of normal human FVIII levels (or 0.5% of normal canine FVIII).11 We have been developing a liver-directed gene therapy protocol to treat hemophilia A using an AAV vector with the goal of providing sustained FVIII levels of above 1% of normal. Numerous animal studies have demonstrated that AAV vectors have many advantages over other gene delivery vectors, the most important being sustained gene expression. However, one major disadvantage of AAV vectors is their limited packaging capacity.12 To overcome this, we used minimal transcriptional regulatory elements to construct an AAV vector expressing a B-domain–deleted form of canine FVIII. Intra-hepatic injection of this vector into hemophilic A dogs resulted in sustained and therapeutic levels of FVIII for over 1 year, providing strong support for the feasibility of this approach in humans.
Materials and methods
Vector construction, production, and purification
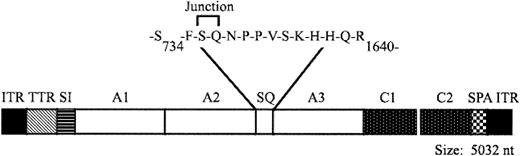
AAV-cFVIII-104 (AAV–canine FVIII–104; Figure 1) was derived from its human counterpart, AAV-hFVIII-104 (H.J., X.Q., S.P.-W. et al, manuscript in preparation, 2003), by replacing the human FVIII-SQ cDNA with the canine FVIII-SQ cDNA. The cDNA encodes the complete A1, A2, A3, C1, and C2 domains of cFVIII and just 14 amino acids of the canine B domain. The B domain is comprised of 3 amino acids from the amino terminus of the B domain fused to 11 amino acids from the carboxy terminus of the B domain. The cDNA is expressed from a 202-bp region of the transthyretin promoter, a 106-bp chimeric intron composed of an EF1α splice donor and an immunoglobulin G (IgG) splice acceptor, and a 45-bp synthetic polyadenylation sequence based on the rabbit β-globin gene.13 Each of these regulatory elements was generated by polymerase chain reaction (PCR) and the expression cassette was assembled in pZERO2 (Invitrogen, Carlsbad, CA). The expression cassette was then cloned between the 2 inverted terminal repeats (ITRs) of pAAV-CMV-FIX (plasmid AAV–cytomegalovirus promoter-FIX).14 AAV vectors were produced using a triple-transfection method15 and were purified by column chromatography.16 Vector genome (vg) titers were determined by quantitative PCR using linearized plasmid DNA as the standard. The concentration of capsid particles (cp) was determined using a commercially available enzyme-linked immunosorbent assay (ELISA; American Research Products, Belmont, MA) and an AAV vector standard developed at Avigen.17
Structure of AAV-cFVIII-SQ vector. AAV vector encoding a B-domain–deleted form of cFVIII. TTR indicates transthyretein promoter; SI, synthetic intron; cFVIII domains A1, A2, A3, C1, and C2; SQ, the deleted B domain; and SPA, synthetic polyadenylation sequence.
Structure of AAV-cFVIII-SQ vector. AAV vector encoding a B-domain–deleted form of cFVIII. TTR indicates transthyretein promoter; SI, synthetic intron; cFVIII domains A1, A2, A3, C1, and C2; SQ, the deleted B domain; and SPA, synthetic polyadenylation sequence.
In vitro transduction assay
Human hepatocellular carcinoma (HepG2) cells were plated in 6-well tissue-culture plates at a density of 1 × 106 cells per well and grown for 24 hours in media containing 20 μM etoposide (Sigma Chemical, St Louis, MO). The cells were infected with AAV-cFVIII-104 at a dose of 104 vg/cell. Twenty-four hours later, the media was replaced with fresh media containing 10% heat-inactivated fetal bovine serum (FBS), and the cells were incubated for an additional 24 hours. Media was analyzed for FVIII activity using the Coatest chromogenic assay (Dipharma, West Chester, OH) or for cFVIII antigen by ELISA (Asserachrom VIIIC:Ag; Diagnostico Stago, Parsippany, NJ).
Animal procedures
The dogs used in this study were from the hemophilia A colony at Queens University and all procedures involving the dogs were reviewed by the Queen's University Animal Care Committee. Prior to surgery, dogs were treated intravenously with 25 mg of diphenhydramine hydrochloride and 100 mg of hydrocortisone. The dogs were then administered cryoprecipitated canine FVIII to raise their plasma FVIII levels to above 50% of normal levels. A midline incision was made in the abdomen of the animal, and AAV-cFVIII-104 vector was administered into the portal vein using a 23-gauge butterfly catheter at a rate of 0.24 to 0.6 mL/kg/minute. One dog (Elisa) received a dose of 6.4 × 1012 vg/kg and the other dog (Junior) received 2.7 × 1013 vg/kg of the AAV-cFVIII vector. After suturing, the dogs were administered 0.24 mg of buprenorphine by intravenous injection, and this dosing was repeated at 7 and 15 hours after treatment. The dogs were also treated with cryoprecipitated FVIII at 7 and 24 hours after surgery. Due to delayed postsurgical bleeding, one dog (Elisa) was given further doses of cryoprecipitated FVIII on days 5 and 6, and one dose of human recombinant FVIIa (NiaStase; Novo Nordisk, Mississauga, ON, Canada) on day 5.
Assays for FVIII activity and inhibitory antibodies
The whole-blood clotting time (WBCT) and cuticle bleeding time (CBT) were measured according to standard protocols. Animal plasma was assayed for biologically active FVIII using the Coatest as described and using normal canine plasma as a standard. The one-stage, activated partial thromboplastin time-based clotting assay (APTT)18 used dilutions of normal canine plasma, in 10% canine FVIII–deficient plasma, as the standards. Canine plasma samples were diluted to 10% in HBS-BSA (Hepes buffered saline–bovine serum albumin) solution. Samples (50 μL) and standards (50 μL) were mixed with an equal volume of human FVIII–deficient plasma (George King Biomedical, Overland Park, KS) and 50 μL of APTT reagent (Organon Tecknika, Durham, NC) followed by incubation for 3 minutes at 37°C. Fifty μL of 25 mM CaCl2 was added and measurement of clotting time was determined using an automated BBL Fibrometer (Becton Dickinson, Franklin Lakes, NJ). FVIII inhibitory antibodies were determined using the Bethesda assay.19
Generation of anti–canine FVIII antibodies
Rabbit and rat anti–canine FVIII heavy-chain (A1-A2 domains) antibodies were generated using canine FVIII-His–tagged recombinant proteins. The canine FVIII A1 fragment (amino acid residues 10-327) and the A2 fragment (residues 341-822) were individually cloned into pET-3 expression vectors (Novagen, Madison, WI), expressed in BL21 cells, and purified using a Ni column. Rabbits and rats were immunized with 500 and 50 μgof purified protein per injection, respectively.
Immunoprecipitation and Western blotting analysis of canine FVIII
Media collected from AAV-cFVIII-104–transduced HepG2 cells, or nontransduced cells, was assayed by ELISA (Asserachrom VIII:C; Diagnostico Stago) to determine the concentration of cFVIII. This ELISA cross-reacts with cFVIII–light chain (LC). Normal canine plasma was used as a standard after diluting to a working range of 0.625 to 20 ng/mL. The maximum sensitivity of the ELISA is 1.5 ng/mL of cFVIII in media and 8 ng/mL in a background of canine hemophilia A (Hem A) plasma. Media containing either 1 or 4 ng of cFVIII was mixed with 500 μL FVIII-deficient canine plasma and was used as a standard for immunoprecipitation, which has a sensitivity of 0.5 ng. Five hundred–microliter samples of canine plasma, collected from AAV-injected dogs, were used per immunoprecipitation. The plasma was precleared by incubation with 50 μL of protein G beads (40 mg/mL; Life-Tech, Stafford, TX) for 2 to 4 hours at 4°C and then centrifuged at 10 000g for 5 minutes. Affinity-purified rabbit anti-cFVIII A1-A2 antibody was cross-linked to protein G beads and 1 μg of the cross-linked antibody was incubated with media or plasma samples overnight at 4°C. Canine FVIII was eluted from the beads and analyzed by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. The membrane was probed with the rat anti-cFVIII heavy-chain antibody, and cFVIII was detected using a horseradish peroxidase (HRP)–conjugated goat antirat IgG (Amersham-Pharmacia Biotech, Piscataway, NJ). The membrane was washed and developed using the chemiluminescent substrate enhanced chemiluminescence (ECL; Amersham-Pharmacia Biotech).
Molecular analysis
A liver biopsy was performed 10 months after injection on the dog injected at a dose of 2.7 × 1013 vg/kg (Junior). Prior to the biopsy, the plasma FVIII levels were raised to 50% of normal by infusion of cryoprecipitated canine FVIII. DNA (10 μg) was extracted from the liver, digested with BamHI, and subjected to Southern blot analysis. The blot was probed with a 974-bp BamHI fragment of the AAV-cFVIII-SQ plasmid, which comprised the entire A3 cFVIII domain. Copy number standards were generated by spiking BamHI-digested naive Hem A dog liver DNA with BamHI-digested AAV-cF8104-SQ plasmid at 5, 1.25, 0.625, 0.313, and 0.163 copies/diploid genome. The hybridized membranes were analyzed using a Storm 860 PhosphorImager (Molecular Devices, Sunnyvale, CA) and the vector copy number was quantified using IMAGEQUANT software (Molecular Devices).
FISH analysis
The tissue obtained from the liver biopsy was also used for RNA fluorescence in situ hybridization (FISH) analysis as described.20 HRP-conjugated canine heavy-chain (HC)– and light-chain (LC)–specific antisense probes (2 of each) were designed: HRP-cFVIII-HC probe cocktail (probe 1: 5′ TTT TCT GGT GCC ACT AAG GCT GAA GGG CAA3′; probe 2: 5′ GAG CTG TCG TCA TCA AAG CTA ACT ACG TCC 3′); and HRP-cFVIII-LC probe cocktail (probe 3: 5′ GAG GCC CTG ATT TTC ATA GTC GCC GTA GAT 3′; probe 4: 5′ ATT GCA GGG AGC TCT ACA GTT CCT TTC CAG 3′; Epoch Biosciences, Bothell, WA).
Sections were hybridized simultaneously with both probe sets, and the signal was detected using a Tyramide signal amplification fluorescein kit (Perkin Elmer, Boston, MA). The sections were counterstained with 4′, 6-diamidino-2-phenlindole, mounted in Prolong antifade (Molecular Probes). For quantitative analysis, sections were examined using a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Thornwood, NY). Images were recorded using a Quips imaging system and software (Applied Imaging, Santa Clara, CA). The transduction efficiency was determined after counting 5 fields of view (400 nuclei) from 5 individual sections representing approximately 10 000 nuclei. For qualitative analysis, images were recorded using the × 40 objective of a Zeiss laser scanning confocal microscope (LSM 510 META; Carl Zeiss).
Results
Characterization of AAV-cFVIII
We constructed an AAV vector, AAV-cFVIII-104 (Figure 1), encoding a B-domain–deleted form of canine FVIII, similar to human FVIII-SQ.21 Due to the limited packaging capacity of AAV,12 the expression cassette contained a minimal promoter derived from the transthyretin gene,22 a synthetic intron, and synthetic poly-adenylation sequence. Recombinant AAV vectors were generated using the triple-transfection system15 and purified by column chromatography. Efficient packaging of the vector was observed despite its large size (5032 nucleotides; 107% of wild-type AAV). The 2 preparations of vector (one for each dog) were evaluated in vitro for biologic activity by transduction of HepG2 cells. Culture media was analyzed by a functional FVIII assay (Coatest) and comparable levels of FVIII activity were observed, demonstrating the functionality of the vectors (Table 1).
In vitro characterization of AAV-cFVIII vectors
Vector lot . | Genome titer, vg/mL . | Capsid titer, cp/mL . | Ratio of total to full capsids . | Transduced HepG2, vg/well . | In vitro activity, mU/mL . | Dog injected . |
|---|---|---|---|---|---|---|
| 17-26 | 2.7 × 1012 | 3.6 × 1013 | 13 | 1 × 106 | 260 | Elisa |
| 510-1 | 4.5 × 1012 | 4.3 × 1013 | 10 | 1 × 106 | 275 | Junior |
Vector lot . | Genome titer, vg/mL . | Capsid titer, cp/mL . | Ratio of total to full capsids . | Transduced HepG2, vg/well . | In vitro activity, mU/mL . | Dog injected . |
|---|---|---|---|---|---|---|
| 17-26 | 2.7 × 1012 | 3.6 × 1013 | 13 | 1 × 106 | 260 | Elisa |
| 510-1 | 4.5 × 1012 | 4.3 × 1013 | 10 | 1 × 106 | 275 | Junior |
Ratio of total to full capsids is equivalent to the ratio of the capsid titer to genome titer.
vg/mL indicates vector genomes/mL; and cp/mL, capsid (empty and fulls) particles/mL.
Correction of hemophilia A in a dog model
The AAV-cFVIII-SQ vector was analyzed both in vitro and in Hem A mice prior to initiating studies in Hem A dogs. Using a dose of 6 × 1012 vg/kg delivered via the portal vein, we observed on average 5.5% of normal canine FVIII levels in the plasma of Hem A mice (H.J., X.Q., S.P.-W. et al, manuscript in preparation, 2003). Having demonstrated the activity of this vector in vitro and in hemophilic mice we proceeded with injections of hemophilia A dogs. The hemophilia A dogs maintained at the colony in Queens University-Kingston Ontario are cross-reacting material–negative (CRM–) and are phenotypically similar to severe hemophilia A patients, having FVIII levels of less than 1% and a propensity for spontaneous musculoskeletal and soft tissue bleeding.23 The FVIII mutation in these dogs was recently characterized and is the result of a rearrangement in intron 22, leading to aberrant splicing and premature termination of the FVIII transcript.24 Two hemophilia A dogs were injected via the portal vein with AAV-cFVIII-104 at doses of 6 × 1012 or 2.7 × 1013 vg/kg (Table 2). No signs of liver toxicity related to AAV vector administration were observed as measured 1 to 2 days after injection by alanine aminotransferase, alkaline phosphatase, and bilirubin levels in the serum. In addition, there were no alterations in hematologic parameters (leukocytes, erythrocytes, hemoglobin, hematocrit, mean corpuscular volume, platelets; data not shown).
Summary of AAV-cFVIII—treated hemophilia A dog results
Dog . | Weight, kg . | Dose, vg/kg . | Ave WBCT, s . | Ave Coatest mU/mL, (%) . | Ave one-stage mU/mL, (%) . | CBT (timepoint, min) . | Inhibitory antibody . |
|---|---|---|---|---|---|---|---|
| Elisa | 10 | 6 × 1012 | 318 ± 38 | 179 ± 31 (2.6) | 124 ± 67 (1.8) | 3-4 (week 16) | — |
| Junior | 12.8 | 2.7 × 1013 | 314 ± 60 | 196 ± 52 (2.8) | 176 ± 53 (2.5) | 4-5 (week 35) | + (weeks 2-9) |
| Normal | NA | NA | 180-300 | 7000 (100) | 7000 (100) | <5 | NA |
| Hem A | NA | NA | >960 | <22.5 (0.3) | <44 (0.6) | >11 | NA |
Dog . | Weight, kg . | Dose, vg/kg . | Ave WBCT, s . | Ave Coatest mU/mL, (%) . | Ave one-stage mU/mL, (%) . | CBT (timepoint, min) . | Inhibitory antibody . |
|---|---|---|---|---|---|---|---|
| Elisa | 10 | 6 × 1012 | 318 ± 38 | 179 ± 31 (2.6) | 124 ± 67 (1.8) | 3-4 (week 16) | — |
| Junior | 12.8 | 2.7 × 1013 | 314 ± 60 | 196 ± 52 (2.8) | 176 ± 53 (2.5) | 4-5 (week 35) | + (weeks 2-9) |
| Normal | NA | NA | 180-300 | 7000 (100) | 7000 (100) | <5 | NA |
| Hem A | NA | NA | >960 | <22.5 (0.3) | <44 (0.6) | >11 | NA |
The Coatest and the one-stage assay are sensitive to 22.5 and 44 mU/mL, respectively. The WBCT, Coatest, and one-stage assay results were averaged over 14 months for Elisa and 7.5 months for Junior. The CBT was performed at a single time point for each injected dog.
A FVIII inhibitory antibody was detected by Bethesda assay in Junior between weeks 2 and 9.
NA indicates not applicable; WBCT, whole-blood clotting time; and CBT, cuticle bleeding time.
The first dog (Elisa) was injected with AAV-cFVIII-104 at a dose of 6 × 1012 vg/kg. Within the first month of vector administration, the whole-blood clotting time (WBCT) decreased from a baseline level of 17 minutes to a near normal value of 6 minutes and has been maintained at 4 to 7 minutes for 14 months (Figure 2A). Normal dogs in this colony have WBCTs of 3 to 5 minutes. Further evidence of FVIII activity was demonstrated by performing 2 functional assays, the chromogenic Coatest, which measures the FVIII-dependent conversion of FX to FXa, and a one-stage activated partial thromboplastin time-based clotting assay, which measures fibrin clot formation. Relative to a human FVIII plasma standard, canine FVIII has 7-fold higher activity in these 2 assays, or 7000 mU/mL, but a similar antigen level (200 ng/mL) when assayed by ELISA. Using the Coatest, FVIII activity levels of 151 mU/mL (2.2% of normal canine activity) were measured 3 weeks after injection. The levels peaked at 265 mU/mL (3.8%) and averaged 179 ± 31 mU/mL (2.6%) over the course of 14 months (Figure 2B). Concordant FVIII values were measured using the one-stage assay (Figure 2C) and these levels would provide a long-term therapeutic effect in patients. A stringent in vivo measure of hemostasis is the cuticle bleeding time, which in a normal dog is shorter than 5 minutes and in a hemophilic dog is longer than 11 minutes. The cuticle bleeding time for Elisa, which was measured at just one time point (5 months after injection) when the FVIII level was 192 mU/mL (2.7% of normal), was 3 to 4 minutes (Table 2), demonstrating therapeutically pertinent in vivo correction of hemostasis in this animal.
Coagulation parameters measured after vector administration. Hemophilia A dogs were injected via the portal vein with 6 × 1012 vg/kg (Elisa, ♦) or 2.7 × 1013 vg/kg (Junior, ▦) of AAV-cFVIII-104. (A) Whole blood was collected at various times after injection and assayed for whole-blood clotting times. Plasma samples from Junior were also assayed by Bethesda assay for FVIII neutralizing antibodies (- - -). (B-C) Plasma was collected and assayed for biologically active FVIII by Coatest and a one-stage assay, respectively. Both dogs received canine cryoprecipitate on days 0 and 1; Elisa received additional injections on days 5 and 6.
Coagulation parameters measured after vector administration. Hemophilia A dogs were injected via the portal vein with 6 × 1012 vg/kg (Elisa, ♦) or 2.7 × 1013 vg/kg (Junior, ▦) of AAV-cFVIII-104. (A) Whole blood was collected at various times after injection and assayed for whole-blood clotting times. Plasma samples from Junior were also assayed by Bethesda assay for FVIII neutralizing antibodies (- - -). (B-C) Plasma was collected and assayed for biologically active FVIII by Coatest and a one-stage assay, respectively. Both dogs received canine cryoprecipitate on days 0 and 1; Elisa received additional injections on days 5 and 6.
A second dog (Junior) was injected with a 4.5-fold higher dose of AAV-cFVIII-104 (2.7 × 1013 vg/kg). Correction of the WBCT from 16 to 6 minutes was observed within one week of injection (Figure 2A). However, 2 weeks after injection, the WBCT began to increase and reverted to the baseline level of 16 minutes by 3 weeks after injection. A correction in the WBCT, to 5 minutes, became apparent 8 weeks following vector administration and it remained at or near this level for at least 34 weeks. FVIII activity levels correlated with the WBCT as low levels were observed for the first 7 weeks when the WBCT was not corrected, but beginning at week 8 FVIII activity levels of 96 mU/mL and 56 mU/mL were seen by Coatest (Figure 2B) and one-stage assay, respectively (Figure 2C). The levels of FVIII activity, as measured by Coatest, continued to increase to as high as 292 mU/mL and averaged 196 ± 52 mU/mL, representing 4.2% and 2.8% of normal canine FVIII activity. Similar increases were observed using the one-stage assay (Figure 2C). A 4- to 5-minute CBT was measured for this dog 35 weeks after injection (Table 2), demonstrating that therapeutic in vivo hemostasis was also achieved with these FVIII levels. We suspected that the slower kinetics of FVIII expression observed in Junior, versus Elisa, was due to a transient immune response against canine FVIII. To investigate this, a Bethesda assay was performed on plasma samples collected at time points when FVIII activity was not observed. Neutralizing antibodies to canine FVIII were detected in plasma from Junior at time points that correlated with a lack of FVIII activity (Figure 2A; Table 2). A peak Bethesda titer of 9 Bethesda units (BUs) was observed 4 weeks after injection and became undetectable by 9 weeks.
The immune response toward canine FVIII could have been elicited toward the canine cryoprecipitate that was infused prior to surgery or to the vector-expressed cFVIII. Approximately 25% of the dogs in this colony have been documented to develop anti–canine FVIII antibodies following treatment with canine cryoprecipitate. To assess these 2 possibilities, a third dog (Sugar) was treated on 3 separate occasions with cryoprecipitate prior to the planned AAV administration. Two weeks following each administration, plasma was evaluated for neutralizing antibodies to cFVIII. Following the third cryoprecipitate administration, this animal developed antibodies to cFVIII and therefore was not injected with AAV. Thus, the antibody generated in Junior could have been elicited either by the cryoprecipitate or by the vector-encoded cFVIII.
Biochemical characterization of cFVIII in plasma
Immunoprecipitation analysis of plasma collected from Elisa at 168 days after injection demonstrated the presence of cFVIII, which migrated at approximately 170 kDa on SDS-PAGE, indicating that the unprocessed form of FVIII was secreted, similar to what was seen in supernatants of transduced HepG2 cells (Figure 3). A contaminating band migrating at 90 kDa prevented us from determining if some of the secreted FVIII was also processed to the heavy chain (data not shown). An estimate of the FVIII protein levels in the plasma was made by comparing a known amount of protein secreted from HepG2 cells (1 and 4 ng) to the amount in 500 μL of canine plasma. Based on this analysis, the concentration of FVIII in the plasma of Elisa was estimated at 6 ng/mL, which correlates with the amount of activity determined by Coatest (5 ng/mL), assuming 7000 mU/200 ng as discussed in “Correction of hemophilia A in a dog model.” Canine FVIII protein was also observed in the plasma of Junior collected 121 days after injection (Figure 3) and was estimated to be at a concentration of 4 ng/mL, slightly lower than what was estimated by Coatest (5.7 ng/mL).
Detection of cFVIII in the plasma of AAV-cFVIII-104–injected dogs. Canine FVIII (1 and 4 ng as measured by ELISA) was immunoprecipitated from media collected from HepG2 cells that were transduced with AAV-cFVIII-104. Media collected from uninfected cells served as a negative control. The media was spiked with 500 μL FVIII-deficient dog plasma prior to immunoprecipitation. Canine FVIII was immunoprecipitated from the plasma (500 μL) of 2 AAV-cFVIII-104–treated dogs, Elisa and Junior. Plasma from an untreated Hem A dog was used as a negative control. SC denotes the position of single-chain cFVIII.
Detection of cFVIII in the plasma of AAV-cFVIII-104–injected dogs. Canine FVIII (1 and 4 ng as measured by ELISA) was immunoprecipitated from media collected from HepG2 cells that were transduced with AAV-cFVIII-104. Media collected from uninfected cells served as a negative control. The media was spiked with 500 μL FVIII-deficient dog plasma prior to immunoprecipitation. Canine FVIII was immunoprecipitated from the plasma (500 μL) of 2 AAV-cFVIII-104–treated dogs, Elisa and Junior. Plasma from an untreated Hem A dog was used as a negative control. SC denotes the position of single-chain cFVIII.
Molecular analysis of transduced liver
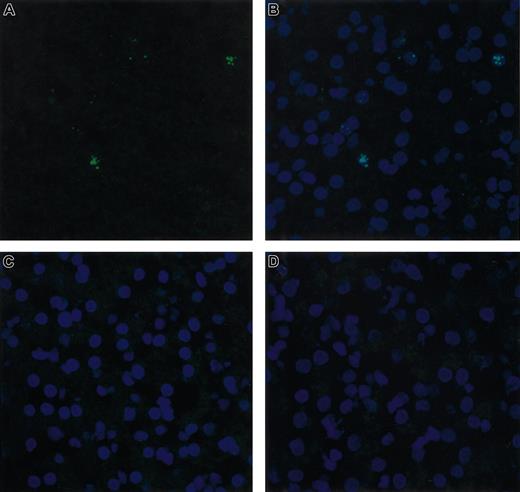
A liver biopsy was performed on Junior 8 months after injection and was used for molecular analysis. DNA was extracted from the right and left lobes of the liver and was subjected to Southern blot analysis. AAV-cFVIII sequences were detected at a level of 1.2 copies and 0.45 copies/diploid genome from the right and left lobes, respectively (Figure 4), indicating good gene transfer (average copy number 0.8 copies/diploid genome) to the dog liver. FISH analysis demonstrated that the cFVIII mRNA was found exclusively in hepatocytes and that approximately 6% of the liver was transduced (Figure 5). These data suggest that the mean AAV content of transduced hepatocytes was 13 copies per cell.
Detection of AAV-cFVIII-SQ transgene in dog liver. DNA was extracted 8 months after injection from the right and left liver lobes of the dog injected with 2.7 × 1013 vg/kg (Junior). Ten μg of DNA was digested with BamHI, Southern blotted, and hybridized with a 32P random-primer–labeled canine FVIII probe. Copy number standards were generated by spiking BamHI-digested naive canine Hem A liver DNA with BamHI-digested cF8104 plasmid DNA at ratios of 5, 1.25, 0.625, 0.313, and 0.16 copies/diploid genome.
Detection of AAV-cFVIII-SQ transgene in dog liver. DNA was extracted 8 months after injection from the right and left liver lobes of the dog injected with 2.7 × 1013 vg/kg (Junior). Ten μg of DNA was digested with BamHI, Southern blotted, and hybridized with a 32P random-primer–labeled canine FVIII probe. Copy number standards were generated by spiking BamHI-digested naive canine Hem A liver DNA with BamHI-digested cF8104 plasmid DNA at ratios of 5, 1.25, 0.625, 0.313, and 0.16 copies/diploid genome.
FISH analysis of cFVIII RNA in dog liver. RNA in situ hybridization was performed on liver sections biopsied from Junior 8 months after injection of AAV-cFVIII-SQ at a dose of 2.7 × 1013 vg/kg. The RNA signal (green staining) was detected using a cFVIII antisense probe cocktail (A). Counter-staining (blue staining) of the same section with 4′, 6-diamidino-2-phenlindole is shown (B). No signal was detected when the sections were pretreated with RNase (data not shown), when a cFVIII sense probe was used (C), or when a naive Hem A liver was analyzed (D). Cells were analyzed under a × 40 objective by confocal microscopy.
FISH analysis of cFVIII RNA in dog liver. RNA in situ hybridization was performed on liver sections biopsied from Junior 8 months after injection of AAV-cFVIII-SQ at a dose of 2.7 × 1013 vg/kg. The RNA signal (green staining) was detected using a cFVIII antisense probe cocktail (A). Counter-staining (blue staining) of the same section with 4′, 6-diamidino-2-phenlindole is shown (B). No signal was detected when the sections were pretreated with RNase (data not shown), when a cFVIII sense probe was used (C), or when a naive Hem A liver was analyzed (D). Cells were analyzed under a × 40 objective by confocal microscopy.
Discussion
In this study, for the first time, we demonstrate sustained expression of therapeutic levels of FVIII in a large animal model of hemophilia A. Previous work in hemophilia A mice, using a number of different vector systems, demonstrated correction of the bleeding disorder. However, attempts to achieve this in the hemophilia A dog model using early generation Ad vectors resulted in only transient expression due to toxicity and to the development of anti–canine FVIII antibodies.10 The use of a high-capacity Ad vector to express cFVIII in Hem A dogs avoided the toxic and immunologic problems associated with earlier generation Ad vectors, but transient expression of the cFVIII (35 mU/mL; 3.5% of normal human FVIII and 0.5% of normal canine FVIII) was still observed.11 It has now been demonstrated in a number of animals species, including rodents, dogs, and nonhuman primates, that AAV vectors do not elicit a cellular immune response or inflammatory response. Indeed, no vector-related toxicities have been observed when AAV vectors purified by cesium chloride centrifugation have been injected into the livers of hemophilia B dogs25 and nonhuman primates.26,27 In the present studies, hemophilia A dogs were administered AAV vectors purified by column chromatography. Vectors purified in this manner have been shown to contain a higher level of empty viral capsids.16 Using these vector preparations no signs of toxicity were seen, demonstrating that AAV vectors purified by either cesium chloride centrifugation or column chromatography can safely be administered to animals. In addition to providing for safety, the lack of toxicity afforded by AAV vectors allows for sustained expression and improved efficacy. It should be noted that in a phase 1 clinical trial involving hepatic artery delivery of an AAV-FIX vector to hemophilia B patients, transient elevations in transaminases were observed in one subject. Therefore, the toxicity of this vector in humans awaits further clinical evaluation. In addition, other potential safety risks using AAV, such as integration28,29 and vector shedding into body fluids,30,31 will need to be addressed more thoroughly. A major disadvantage of AAV is its limited packaging capacity, which has been reported at less than 4.9 kb.12 This, combined with the large size of the B-domain–deleted FVIII cDNA, has hampered the development of efficient AAV-FVIII vectors. Using minimal regulatory elements, we constructed an AAV-cFVIII vector that exceeds the packaging limit, yet can be produced at high titers. We chose a 202-bp region of the transthyretein promoter (TTR) to drive expression of the cDNA. Others have designed AAV-FVIII vectors using other small regulatory regions and have demonstrated plasma FVIII levels as high as 27% of normal in both immunodeficient mice and in mice that became tolerant to the transgene product.5,32,33 We have now introduced an AAV-cFVIII vector with minimal regulatory elements into Hem A dogs. When injected into the portal circulation, FVIII activity levels of 2% to 4% were observed for over 14 months.
Based on prophylactic studies, these levels would convert severe patients to a moderate phenotype and significantly improve their lifestyle. Phenotypic correction was documented in these dogs using 2 parameters. First, WBCTs decreased from a value of 16 to 6 minutes, which is near the normal level (3-5 minutes) in dogs from this colony. Second, upon hemostatic challenge, induced by clipping the animal's cuticle, the dogs showed normal bleeding times. In addition, neither dog required plasma treatment for spontaneous bleeds following vector administration. Untreated dogs are treated on average 4 to 5 times per year to control spontaneous bleeding. Canine FVIII was detected in the plasma of the AAV-injected animals by immunoprecipitation analysis. The single-chain form of B-domain–deleted cFVIII, which migrated at 170 kDa, was detected. The presence of the processed heavy-chain polypeptide of 90 kDa could not be excluded, due to the presence of a contaminating band. However, the absence of a consensus paired basic amino acid–cleaving enzyme (PACE)/furin cleavage site34 at the B-domain/A3-domain junction could account for the observed unprocessed form of cFVIII.
The 2 treated dogs received 4.5-fold different amounts of vector, yet similar FVIII levels were observed. The reason for this lack of a dose response is not clear and could be due to animal variability, technical differences in the infusion, the existence of a nonneutralizing anti-cFVIII antibody in Junior, or saturation of transduction. Column-purified vectors contain a high percentage of empty capsid proteins, which could compete for binding to cells and result in saturation in transduction. Injection of additional dogs at multiple doses with vector preparations that are free of empty capsids will help to elucidate this. We were encouraged by the approximate linear scale-up in FVIII expression observed between mice and dogs and the implications of this for future clinical trials. Hemophilic mice receiving 6 × 1012 vg/kg expressed FVIII levels of on average 5.5% (H.J. et al, in preparation), whereas hemophilic dogs that were injected with the same dose produced levels of 2% to 3%.
One of the biggest risks for the treatment of hemophilia A patients is the development of FVIII neutralizing antibodies. In previously untreated patients (PUPs) this risk is approximately 25% using recombinant and plasma-derived FVIII products. For acceptance in the future treatment of hemophilia A, the risk for inhibitor formation following gene therapy must be no higher than 25% in PUPs and zero in patients that have been previously treated successfully with protein products.35 The transient inhibitor that was generated in Junior could be attributed to either the cryoprecipitate that was administered at the time of vector infusion or to the transgene, since inhibitory antibodies have been elicited following protein replacement in some dogs in this colony. The transient nature of the inhibitor might be the result of tolerance induction to FVIII by gene therapy, which has been observed in a mouse model of hemophilia A.32 Additional studies will be necessary to quantify the risk of inhibitor formation using gene therapy.
In conclusion, we demonstrate that therapeutic levels of FVIII can be achieved and sustained in hemophilia A dogs using an AAV vector of serotype 2. Other AAV serotypes have recently been reported with enhanced tropism for mouse liver.36,37 If these alternate serotypes prove to be superior for dog, and ultimately human liver, curative FVIII levels can be anticipated. The data provided here support the use of AAV2 in human clinical trials to treat hemophilia A patients.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-01-0292.
Several of the authors (C.D.S., H.J., S.L.P.-W., A.E.P., T.L., J.V., D.N., S.K.P., J.F.W., A.M., L.B.C.) are employed by Avigen, Inc. The AAV–Factor VIII vector discussed in this paper is a potential product of Avigen, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal