Abstract
Hemokinin 1 (HK-1) is a new member of the tachykinin peptide family that is expressed in hematopoietic cells. Recent reports studying mouse, rat, and human orthologs of HK-1 demonstrate a broader distribution than originally reported. Our previous studies demonstrated that HK-1, by promoting proliferation, survival, and possibly maturation of B-cell precursors, plays an important role in B lymphopoiesis. Here we present data showing that HK-1 also influences T-cell development at a similar stage of differentiation. This peptide enhanced the proliferation of T-cell precursors and increased the number of thymocytes in fetal thymus organ cultures (FTOCs). Tachykinin antagonists, on the other hand, greatly reduced the cellularity of thymi both in vivo and in vitro. The major reduction occurred in the CD4/CD8 double-positive (DP) cells and the CD44–CD25+ subset of the CD4/CD8 double-negative (DN) cells. Of note, these populations also express HK-1, raising the possibility of autocrine or paracrine pathways influencing T-cell development as we previously reported for B-cell development. Consistent with this, the detrimental effect of tachykinin antagonists could be partially overcome with exogenous HK-1 peptide.
Introduction
Intrathymic T-cell development is a regulated and highly ordered process, in which CD4+CD8+ double-positive (DP) cells are generated from CD4–CD8– double-negative (DN) precursors, and further mature into functional CD4+CD8– and CD4–CD8+ single-positive lymphocytes.1,2 The most immature precursors reside within the DN population. On the basis of the expression of CD25 and CD44, this population can be further divided into 4 subsets.3 DN1, which is identified as being CD44+CD25–, contains cells that are committed to lymphoid lineage but maintain the potential to develop into either T cells, B cells, or natural killer (NK) cells.4 DN1 cells undergo increased CD25 expression to become CD44+CD25+ DN2 cells. Cells at this stage are committed to T-cell lineage and therefore are also called pro-T cells.5 Growth factors, such as interleukin 7 (IL-7) and stem cell factor (SCF), play an important role in this developmental step. Deficiencies in either IL-7– or SCF-signaling pathways resulted in severe defects in early T-cell development, and in combination they led to a complete block.1,2 The next stage of development (DN3 or early pre-T) is characterized by the loss of CD44 expression. At this stage, the T-cell receptor (TCR)–β chain locus is rearranged, and the pre-TCR complex is first assembled and tested (β-selection). Disruption of the complex causes a complete arrest at DN3, as shown in recombination activation gene (Rag)–, TCR-β– and pre–TCR-α–deficient mice.1,2 As further maturation occurs, cells also lose expression of CD25 to become CD44–CD25– DN4 or late pre-T cells. These cells progress to the CD4+CD8+ DP stage via an intermediate, immature CD8 single-positive (ISP) stage with a concomitant burst in cell proliferation.6,7
Tachykinins are a group of neurally derived peptides sharing a common COOH-terminal motif, F-X-G-L-M-NH2. Substance P (SubP) is the prototypic and best-studied member of this family.8,9 Targeted disruption of the genes encoding SubP or its receptor reveals an essential role of SubP in pain transmission and neurogenic inflammatory responses.10-13 In addition, SubP is involved in the regulation of many other biologic processes. In hematopoiesis, SubP stimulates the generation of myeloid cells by inducing various growth factors.14 For T lymphocytes, SubP enhances drendritic cell– or TCR-mediated proliferation,15 inhibits glucocorticoid-induced apoptosis,16 and modulates cytokine secretion of T-helper cells.17 The physiologic role of SubP in these processes, however, is less conclusive because of its neuronal origin and the absence of corresponding defects in knockout mice. More recently, a new tachykinin peptide has been identified and named hemokinin 1 (HK-1) as it was initially thought to be expressed mainly in hematopoietic cells.18 More recent studies, using not only the mouse probe but also rat and human probes, suggest a broader expression pattern of HK-1.19 In addition to the biologic activities common to known tachykinins, HK-1 plays a unique and important role in B-cell development.18
T and B lymphopoiesis share a number of developmental features in common. Progression from earlier to later stages in both T- and B-cell development critically depends on signals provided by antigen receptors and cytokine receptors.20,21 Given the importance of HK-1 in B-cell development, we speculated that similar mechanisms might also be active in T-cell development. Indeed, we found that HK-1 enhanced thymidine incorporation of thymocytes in suspension cultures and increased thymic cellularity in fetal thymus organ cultures (FTOCs). Inhibition of HK-1 function by tachykinin antagonists, on the other hand, blocked T-cell development at specific stages.
Materials and methods
In vivo administration of tachykinin antagonists
C57BL/6 or (C57BL/6XDBA/2)F1 mice were treated with daily intravenous injection of L-732138 (Sigma, St Louis, MO) at 20 mg/kg; L-733060 or L-733061 (Sigma) at 10 mg/kg; or SR140333 (provided by Dr Xavier Emonds-Alt, Sanofi Recherche, Montpellier Cedex, France) at 5 mg/kg for 4 days. Control mice were injected with 70% dimethyl sulfoxide (DMSO), a solvent for the antagonists. We undertook a series of preliminary experiments to determine dose-response parameters and chose a dose in the middle of titration range.
Fetal thymus organ culture
Timed pregnancies were established for C57BL/6, Rag1–/– (The Jackson Laboratory, Bar Harbor, ME) and IL-7–/– (provided by Dr Paulo Vieira, Institut Pasteur, Paris Cedex, France) mice. Fetal thymi were removed at day 15 of gestation. The lobes were placed onto Nucleopore filters (Whatman, Fairfield, NJ) floating on Opti-MEM medium (Gibco BRL, Gaithersburg, MD) supplemented with 1X Nutridoma-SP (Roche Diagnostics, Laval, PQ, Canada). The medium was then changed every 2 days. In preliminary experiments, we tested a range of concentrations of HK-1 (10, 20, 40, 80 μM), SubP (10, 20, 40, 80 μM), and SR140333 (1, 2, 4, 8 μM). The final concentrations of various additives were 40 μM HK-1 (Biotechnology Service Center, Toronto, ON, Canada) or SP (Sigma); 4 μM SR140333; 20 μg/mL anti-CD3ϵ (145-2c11) (Pharmingen, San Diego, CA); and 10 ng/mL IL-7. Typically, cells were harvested after 7 days in culture.
Multiparameter flow cytometric analysis
Thymocytes were stained for the expression of various surface markers with specific antibodies (Pharmingen). For analysis of the whole thymic population, cells were stained with anti-CD4–fluorescein isothiocyanate (FITC) and anti-CD8–phycoerythrin (PE). For analysis of DN subsets, cells were stained with anti-CD4–FITC, anti-CD8–FITC, anti-CD44–PE, and anti-CD25–biotin, followed by streptavidin–quantum red (QR) (Sigma). Stained cells were then analyzed on a FACScan cytometer (Becton Dickinson, San Jose, CA).
Proliferation assay
Adult or day-15 fetal thymocytes were seeded into 96-well plates at 4 × 105 or 4 × 104 per well in serum-free medium containing 0.1 ng/mL IL-7 and 10 ng/mL SCF. The cells were cultured under test conditions as specified in the figure legends for 60 hours, then pulsed with [3H]thymidine (1 μCi [37 kBq] per well), and harvested 12 hours later.
Fractionation of thymocytes
Single-cell suspensions were prepared from C57BL/6 mice. Total thymocytes were stained with anti-CD4–FITC and anti-CD8α–PE, and sorted into CD4+CD8+, CD4–CD8–, CD4+CD8–, and CD4–CD8+ fractions. To fractionate CD4–CD8– cells, total thymocytes were first treated with anti-CD4 (RL 172.4H) and anti-CD8 (3.168) plus guinea pig complement. The resultant cells were then stained with a cocktail of FITC-labeled antibodies (anti-CD4, anti-CD8, anti-CD3, anti-B220, and anti-NK1.1), anti-CD44–PE and anti-CD25–biotin plus streptavidin-QR. FITC-negative cells were subsequently sorted into CD44+CD25–, CD44+CD25+, CD44–CD25+, and CD44–CD25– populations. Sorted cell populations were reanalyzed for their purity and were found to be greater than 95% pure.
Gene expression studies
RNA was prepared from sorted thymocytes with the use of TRIzol reagent (Gibco BRL). Gene expression was determined by reverse transcription (RT)–nested polymerase chain reaction (PCR). Briefly, RNA from 104 to 105 cells was reverse transcribed. The first round of PCR was performed with one tenth of the RT product for 25 cycles. One fiftieth of the first-round product was further amplified with a pair of internal primers for an additional 25 cycles. For β-actin control, a single round of 25 cycles was performed. The final product was gel-fractionated, transferred onto a membrane, and detected by gene-specific probes. The primers used for PCR were as follows: HK-1 first round, 5′-GACAGAGAGGCACCTGCTCA-3′ and 5′-ACATGCAGCGTCTTCTCCAC-3′; HK-1 second round, 5′-AGAGGCAGA GTCCTGGGAGAC-3′ and 5′-CGTTGCTACCAGATGCCAAGAG-3′; SubP first round, 5′-CAAGGAGAGCAAAGAGCGC-3′ and 5′-TGGTAGCTATCACAACACATC-3′; SubP second round, 5′-AGCAAGTGCGCACCTGCGGA-3′ and 5′-TTCACTGCTCACTGACACAGAT-3′; β-actin, 5′-CTGGCACCACACCTTCTACA-3′ and 5′-TCGTACTCCTGCTTGCTGATC-3′.
Results
Reduced thymic cellularity in tachykinin antagonist–treated mice
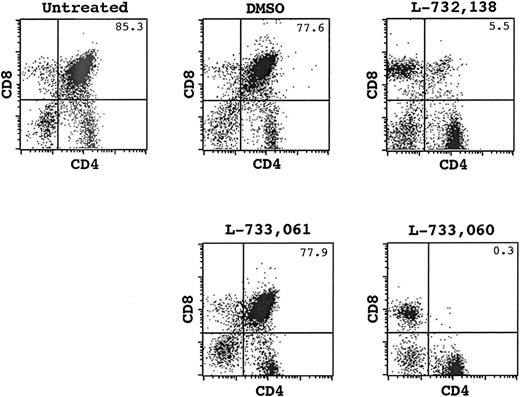
To reveal the potential role of HK-1 in T-cell development, we treated mice with tachykinin antagonists (L-732138 or SR140333)22,23 and subsequently analyzed thymocyte development in these mice. A profound disruption of T-cell development was observed following in vivo administration of the antagonists. The antagonist-treated animals demonstrated remarkably reduced thymic cellularity (Table 1). On average, the total thymocyte number was approximately 40-fold less in these animals than in controls. In addition to the decrease in absolute cell numbers, a distorted picture of CD4/CD8 staining was seen (Figure 1). While CD4+CD8+ cells accounted for approximately 85% of total thymocytes in normal mice, this population dropped to approximately 5% in the antagonist-treated mice. On the other hand, we saw an increased proportion of CD4+CD8– and CD4–CD8+ cells although their absolute numbers were still lower (P < .05). This result, coupled with the fact that no obvious change was observed in the periphery (data not shown), indicates that the antagonist targets mainly cells at early stages of development.
Decreased thymocyte number in tachykinin antagonist-treated mice
Treatment, no. individual mice analyzed . | . | Fractionated thymocytes, × 104 . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Total thymocytes, × 105 . | CD4+CD8+ . | CD4+CD8- . | CD4-CD8+ . | CD4-CD8- . | |||
| No treatment, n = 4 | 410 ± 100 | 3300 ± 600 | 460 ± 290 | 140 ± 70 | 200 ± 80 | |||
| DMSO solvent, n = 4 | 370 ± 150 | 2900 ± 1300 | 380 ± 130 | 140 ± 70 | 190 ± 80 | |||
| Antagonist, n = 6* | 9 ± 16 | 5 ± 9 | 53 ± 92 | 27 ± 50 | 9 ± 10 | |||
Treatment, no. individual mice analyzed . | . | Fractionated thymocytes, × 104 . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Total thymocytes, × 105 . | CD4+CD8+ . | CD4+CD8- . | CD4-CD8+ . | CD4-CD8- . | |||
| No treatment, n = 4 | 410 ± 100 | 3300 ± 600 | 460 ± 290 | 140 ± 70 | 200 ± 80 | |||
| DMSO solvent, n = 4 | 370 ± 150 | 2900 ± 1300 | 380 ± 130 | 140 ± 70 | 190 ± 80 | |||
| Antagonist, n = 6* | 9 ± 16 | 5 ± 9 | 53 ± 92 | 27 ± 50 | 9 ± 10 | |||
All data are given as the mean ± standard deviation (SD) per lobe.
P < .05 with paired, 2-tailed t tests between solvent and antagonist.
FACS analysis for CD4/CD8 expression of thymocytes from an untreated mouse and mice treated with DMSO, L-732138, L-733061 or L-733060. The number at the top-right corner indicates the percentage of CD4+CD8+ cells.
FACS analysis for CD4/CD8 expression of thymocytes from an untreated mouse and mice treated with DMSO, L-732138, L-733061 or L-733060. The number at the top-right corner indicates the percentage of CD4+CD8+ cells.
The DP cells are known to be sensitive to a variety of apoptotic stimuli, and significant reduction of this population is often observed in stress-related conditions.24 To address the concern that the reduced thymic cellularity in our studies may be the result of some nonspecific effects associated with the administration of antagonists, we further tested a stereoselective pair of tachykinin antagonists, L-733060 and its enantiomer L-733061. These 2 molecules demonstrate an approximately 300-fold difference in their affinity for tachykinin receptors, but have similar nonspecific effects.25 In 3 separate experiments, we consistently observed a large (approximately 100-fold) reduction of thymocyte numbers in L-733060–treated mice but no obvious change in mice injected with L-733061. CD4/CD8 staining revealed that DP cells almost disappeared in L-733060–treated mice whereas the same population was intact in L-733061–treated mice (Figure 1).
T-cell developmental blockage following tachykinin antagonist treatment in vitro
Since it was possible that the thymic alterations observed following in vivo application of tachykinin antagonists could be due to systemic mechanisms external to the thymus, we used FTOCs in subsequent studies. Fetal thymi at 15 days of gestation were removed and cultured at the gas-liquid interface in serum-free medium with or without the addition of tachykinin antagonists. Cells were then counted and analyzed by flow cytometry. Table 2 shows a summary of absolute cell numbers for total and CD4/CD8 fractionated thymocytes recovered from 9 independent experiments; the CD4/CD8 staining profile from 1 representative experiment is presented in Figure 2A. Similar to the results from in vivo experiments, tachykinin antagonists had a significant impact on T-cell development in vitro. In addition to a general decrease in absolute cell numbers (P < .01), development of CD4+CD8+ cells was virtually abolished with the addition of antagonists. These experiments also revealed a differential effect of the tachykinin antagonist on the CD4 and CD8 single-positive populations. Whereas CD4+ cells were basically absent from the cultures, substantial numbers of CD8+ cells remained. Preliminary analysis of this CD8+ population revealed considerable heterogenity. While the cells were uniformly CD3low and low/negative for CD25, we found that the population contained both heat stable antigen (HSA)high and HSAlow cells. Approximately 10% to 20% displayed low levels of TCR-β whereas higher numbers of γδ+ cells were present (30% to 70% in different experiments).
Numbers of fractionated thymocytes from FTOCs under various conditions
Condition, no. individual cultures analyzed . | . | Fractionated thymocytes, × 104 . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Total thymocytes, × 104 . | CD4+CD8+ . | CD4+CD8- . | CD4-CD8+ . | CD4-CD8- . | |||
| Medium, n = 10 | 58.9 ± 11.9 | 21.5 ± 9.0 | 4.3 ± 2.9 | 11.9 ± 3.6 | 20.9 ± 7.0 | |||
| HK-1, n = 10*† | 87.6 ± 26.3 | 38.6 ± 17.3 | 6.9 ± 3.2 | 15.0 ± 4.9 | 27.1 ± 11.1 | |||
| SubP, n = 10 | 71.4 ± 23.8*§ | 26.0 ± 11.6*∥ | 6.0 ± 4.4 | 14.4 ± 5.5*§ | 25.1 ± 9.7 | |||
| Antagonist, n = 9*‡ | 19.8 ± 7.5 | 1.0 ± 0.7 | 0.4 ± 0.2 | 6.0 ± 2.3 | 12.1 ± 6.2 | |||
| Antagonist + HK-1, n = 9 | 29.0 ± 11.5*¶ | 3.3 ± 2.1*¶ | 0.8 ± 0.7*¶ | 9.2 ± 4.2 | 16.8 ± 7.6 | |||
| Antagonist + SubP, n = 9 | 22.7 ± 6.6*# | 1.5 ± 1.2*# | 0.4 ± 0.1 | 7.1 ± 2.6 | 13.4 ± 4.4 | |||
Condition, no. individual cultures analyzed . | . | Fractionated thymocytes, × 104 . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Total thymocytes, × 104 . | CD4+CD8+ . | CD4+CD8- . | CD4-CD8+ . | CD4-CD8- . | |||
| Medium, n = 10 | 58.9 ± 11.9 | 21.5 ± 9.0 | 4.3 ± 2.9 | 11.9 ± 3.6 | 20.9 ± 7.0 | |||
| HK-1, n = 10*† | 87.6 ± 26.3 | 38.6 ± 17.3 | 6.9 ± 3.2 | 15.0 ± 4.9 | 27.1 ± 11.1 | |||
| SubP, n = 10 | 71.4 ± 23.8*§ | 26.0 ± 11.6*∥ | 6.0 ± 4.4 | 14.4 ± 5.5*§ | 25.1 ± 9.7 | |||
| Antagonist, n = 9*‡ | 19.8 ± 7.5 | 1.0 ± 0.7 | 0.4 ± 0.2 | 6.0 ± 2.3 | 12.1 ± 6.2 | |||
| Antagonist + HK-1, n = 9 | 29.0 ± 11.5*¶ | 3.3 ± 2.1*¶ | 0.8 ± 0.7*¶ | 9.2 ± 4.2 | 16.8 ± 7.6 | |||
| Antagonist + SubP, n = 9 | 22.7 ± 6.6*# | 1.5 ± 1.2*# | 0.4 ± 0.1 | 7.1 ± 2.6 | 13.4 ± 4.4 | |||
All data are given as the mean ± SD per lobe.
P < .05.
Paired, 2-tailed t tests of medium versus HK-1.
Paired, 2-tailed t tests of medium versus antagonist.
Paired, 2-tailed t tests of medium versus SubP.
Paired, 2-tailed t tests of HK-1 versus SubP.
Paired, 2-tailed t tests of antagonist versus antagonist plus HK-1.
Paired, 2-tailed t tests of antagonist plus HK-1 versus antagonist plus SubP.
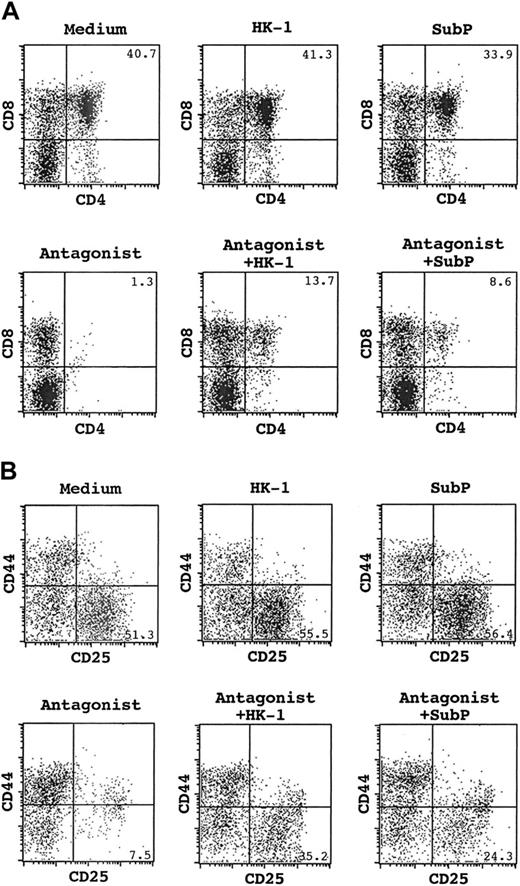
FACS analysis of cells recovered from FTOCs under various conditions. (A) CD4/CD8 staining of total thymocytes. The number at the top-right corner indicates the percentage of CD4+CD8+ cells. (B) CD25/CD44 staining of CD4–CD8– population. The number at the bottom right indicates the percentage of CD25+CD44– DN3 cells.
FACS analysis of cells recovered from FTOCs under various conditions. (A) CD4/CD8 staining of total thymocytes. The number at the top-right corner indicates the percentage of CD4+CD8+ cells. (B) CD25/CD44 staining of CD4–CD8– population. The number at the bottom right indicates the percentage of CD25+CD44– DN3 cells.
To further characterize the developmental abnormalities in antagonist-treated cultures, CD4–CD8– cells were assessed on the basis of CD44 and CD25 expression. As shown in Table 3 and Figure 2B, significant reduction occurred in CD44–CD25+ (DN3) and CD44–CD25– (DN4) subsets (P < .01). DN3 was the most abundant population in normal cultures. Few cells, however, fell into this category in antagonist-treated cultures. In terms of absolute cell numbers, this represented a greater than 4-fold decrease. In contrast to the reduced DN3 population, we saw a slight increase of DN2 cells, both proportionally and in absolute numbers. These results indicate a potential blockage from DN2 to DN3 caused by tachykinin antagonists.
Numbers of fractionated CD4–CD8– cells from FTOCs under various conditions
Condition, no. individual cultures analyzed . | Total CD4-CD8- cells, × 104 . | Fractionated CD4-CD8- cells, × 104 . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | CD44+CD25- . | CD44+CD25+ . | CD44-CD25+ . | CD44-CD25- . | |||
| Medium, n = 10 | 20.9 ± 7.0 | 6.7 ± 2.2 | 1.4 ± 0.5 | 7.8 ± 3.4 | 4.9 ± 1.6 | |||
| HK-1, n = 10 | 27.1 ± 11.1 | 7.5 ± 2.5 | 1.7 ± 0.7 | 10.5 ± 6.3*† | 7.3 ± 3.2*† | |||
| SubP, n = 10 | 25.1 ± 9.7 | 7.8 ± 3.0 | 2.0 ± 0.6 | 9.3 ± 4.6 | 6.0 ± 2.5*‡ | |||
| Antagonist, n = 9 | 12.1 ± 6.2 | 5.8 ± 2.8 | 1.7 ± 1.0 | 1.8 ± 1.9*§ | 2.9 ± 1.1*§ | |||
| Antagonist + HK-1, n = 9 | 16.8 ± 7.6 | 6.8 ± 2.7 | 2.4 ± 1.2 | 3.6 ± 3.2*∥ | 4.0 ± 1.7*∥ | |||
| Antagonist + SubP, n = 9 | 13.4 ± 4.4 | 5.5 ± 1.7 | 1.9 ± 0.8 | 2.5 ± 1.9 | 3.4 ± 1.3 | |||
Condition, no. individual cultures analyzed . | Total CD4-CD8- cells, × 104 . | Fractionated CD4-CD8- cells, × 104 . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | CD44+CD25- . | CD44+CD25+ . | CD44-CD25+ . | CD44-CD25- . | |||
| Medium, n = 10 | 20.9 ± 7.0 | 6.7 ± 2.2 | 1.4 ± 0.5 | 7.8 ± 3.4 | 4.9 ± 1.6 | |||
| HK-1, n = 10 | 27.1 ± 11.1 | 7.5 ± 2.5 | 1.7 ± 0.7 | 10.5 ± 6.3*† | 7.3 ± 3.2*† | |||
| SubP, n = 10 | 25.1 ± 9.7 | 7.8 ± 3.0 | 2.0 ± 0.6 | 9.3 ± 4.6 | 6.0 ± 2.5*‡ | |||
| Antagonist, n = 9 | 12.1 ± 6.2 | 5.8 ± 2.8 | 1.7 ± 1.0 | 1.8 ± 1.9*§ | 2.9 ± 1.1*§ | |||
| Antagonist + HK-1, n = 9 | 16.8 ± 7.6 | 6.8 ± 2.7 | 2.4 ± 1.2 | 3.6 ± 3.2*∥ | 4.0 ± 1.7*∥ | |||
| Antagonist + SubP, n = 9 | 13.4 ± 4.4 | 5.5 ± 1.7 | 1.9 ± 0.8 | 2.5 ± 1.9 | 3.4 ± 1.3 | |||
All data are given as the mean ± SD per lobe.
P < .05.
Paired, 2-tailed t tests of medium versus HK-1
Paired, 2-tailed t tests of medium versus SubP.
Paired, 2-tailed t tests of medium versus antagonist.
Paired, 2-tailed t tests of antagonist versus antagonist plus HK-1.
Restoration of antagonist-induced T developmental defects by HK-1
To test the specificity of the tachykinin antagonists on T-cell development, we attempted to overcome their inhibitory effects using an excess of exogenous peptides. With the inclusion of HK-1 in the culture, a significant number of CD4+CD8+ DP cells were generated even in the presence of the antagonist, and the profile of CD44 and CD25 staining of DN cells was also partially reversed to a normal distribution (Figure 2). With respect to absolute cell number, we saw significant increases in most subsets of thymocytes affected by the antagonist, with CD4+CD8+ DP and CD44–CD25+ DN cells being tripled and doubled, respectively (Tables 2, 3). The results obtained with HK-1 prompted us to further test other known tachykinins for their capacity to correct the T-cell developmental defects associated with tachykinin antagonist treatment. SubP was chosen as a representative since it most closely resembles HK-1 in structure and has been shown to affect many activities of T cells, such as proliferation,15 apoptosis,16 and cytokine secretion.17 In addition, several recent receptor-binding studies using known tachykinin receptors demonstrate that HK-1 and SubP are very similar in their binding properties.26-28 While we did observe partial restoration of T-cell development with SubP in some experiments (Figure 2), we found that this observation was not very reproducible. Over the course of 9 experiments, the addition of SubP was statistically not different from antagonist alone (Tables 2, 3). The significant difference between HK-1 and SubP in their capacity to rescue (P < .05) suggests that the disrupted T-cell development by tachykinin antagonists is most likely the result of direct inhibition of HK-1 function.
The enhanced proliferation of thymocytes by HK-1
Studies with antagonists clearly demonstrate that loss of tachykinin receptor function has a detrimental effect on T-cell development. But a direct effect of HK-1 remains to be shown. In fetal thymus organ cultures, we consistently saw a modest (approximately 50%) but significant increase in the number of cells recovered when HK-1 was added to the cultures (P < .01). Intriguingly, a slight increase (20%) was also observed with SubP (P < .05). This was somewhat surprising since over the course of many experiments SubP failed to overcome the effect of the antagonist. These data may indicate that HK-1 and SubP have different affinities for the receptor that mediates the observed events. Detailed analysis of individual subpopulations in HK-1 cultures indicated increases of cellularity at DN3 and subsequent stages while DN1 and DN2 were not significantly altered (Table 3). This observation is consistent with the T-cell developmental blockage induced by tachykinin antagonists, namely at the transition from DN2 to DN3. Two potential mechanisms for HK-1–induced cellular increase are stimulation of proliferation and maintenance of viability. By propidium iodide and annexin V staining, we failed to see any significant effect of HK-1 on the survival of thymocytes in either suspension cultures or FTOCs (data not shown). To determine if HK-1 could stimulate proliferation of thymocytes, single-cell suspensions were prepared from adult mice and day-15 fetuses and were cultured under various conditions. In the presence of suboptimal concentrations of IL-7 and SCF, addition of HK-1 resulted in a 2-fold increase in thymidine incorporation (Figure 3). No increase, however, was observed with HK-1 alone. A potential explanation is that HK-1 acts on cells of later developmental stages downstream of the IL-7– and SCF-responsive stage. Slightly increased thymidine incorporation was also observed with SubP. Again, its effect was less robust than that of HK-1.
Thymocyte proliferative response to HK-1 and SubP. Single-cell suspensions were made from adult or day-15 fetal thymi and cultured with or without HK-1 or SubP in the presence of suboptimal concentrations of IL-7 and SCF. The concentrations of HK-1 and SubP are indicated in brackets in micromolars. Values of counts per minute (cpm) are the mean of triplicate samples. (A) Proliferation of adult thymocytes. There were 4 × 105 cells plated into each well. (B) Proliferation of day-15 fetal thymocytes. There were 4 × 104 cells plated into each well.
Thymocyte proliferative response to HK-1 and SubP. Single-cell suspensions were made from adult or day-15 fetal thymi and cultured with or without HK-1 or SubP in the presence of suboptimal concentrations of IL-7 and SCF. The concentrations of HK-1 and SubP are indicated in brackets in micromolars. Values of counts per minute (cpm) are the mean of triplicate samples. (A) Proliferation of adult thymocytes. There were 4 × 105 cells plated into each well. (B) Proliferation of day-15 fetal thymocytes. There were 4 × 104 cells plated into each well.
Dissection of the stage specificity of HK-1 action
T-cell precursors at different stages require distinct signals for their developmental progression. The 2 most important signals identified for early T-cell development are those mediated by pre-TCR and IL-7 receptor. Disruption of the transduction of these signals blocks T-cell development at specific stages, DN2 for IL-7 and DN3 for pre-TCR.7,29 Our studies suggest that HK-1 could be another signal critical for T-cell development. We next tested whether HK-1 could substitute for signals from IL-7 or pre-TCR and break the developmental blockage observed in mice lacking IL-7 or Rag. Fetal thymi from IL-7– or Rag-deficient mice were cultured for 7 days in HK-1–containing medium. CD44/CD25 staining of DN cells demonstrated that HK-1 failed to overcome the developmental blockages in both cases (Figure 4), suggesting that HK-1, IL-7, and pre-TCR all provide different signals for T-cell differentiation.
FACS analysis of cells recovered from cultured fetal thymi from Rag1- or IL-7–deficient mice under various conditions. (A) CD25/CD44 staining of CD4–CD8– cells or CD4/CD8 staining of total thymocytes from Rag-deficient FTOCs. The number at the bottom-right corner indicates the percentage of CD25+CD44– DN3 cells, and that at the top left indicates the percentage of CD4–CD8+ cells. (B) CD25/CD44 staining of CD4–CD8– cells from IL-7–deficient FTOCs. The number at the top-right corner indicates the percentage of CD25+CD44+ DN2 cells.
FACS analysis of cells recovered from cultured fetal thymi from Rag1- or IL-7–deficient mice under various conditions. (A) CD25/CD44 staining of CD4–CD8– cells or CD4/CD8 staining of total thymocytes from Rag-deficient FTOCs. The number at the bottom-right corner indicates the percentage of CD25+CD44– DN3 cells, and that at the top left indicates the percentage of CD4–CD8+ cells. (B) CD25/CD44 staining of CD4–CD8– cells from IL-7–deficient FTOCs. The number at the top-right corner indicates the percentage of CD25+CD44+ DN2 cells.
T-cell developmental defects can be corrected completely in IL-7–deficient mice with exogenous IL-7 and, to some extent, in Rag-deficient mice with anti-CD3 antibodies.30,31 FTOCs were thus set up with fetal thymi from IL-7– or Rag1-deficient mice. IL-7 or anti-CD3 was included at the initiation of the cultures, and the addition of tachykinin antagonists was delayed by 24 hours. As shown in Figure 4A, Rag deficiency led to a developmental arrest at the transition from DN3 to DN4. Anti-CD3 antibodies released the blockage and allowed the development of cells of DN4 and subsequent stages. Addition of antagonists resulted in a remarkably diminished DN3 population, as with a normal mouse. In the presence of both anti-CD3 and antagonist, significant or even larger numbers of DN4 cells were still generated, suggesting the differentiation of pre-existing DN3 was not affected. On the other hand, although a large number of DN4 or even CD4–CD8+ cells were produced, no DP or CD4+CD8– cells emerged from the culture, indicating another potential blockage immediately prior to the DP stage. In IL-7–deficient thymi, very few cells were generated beyond the stage of DN2 (Figure 4B). Addition of exogenous IL-7 greatly increased the cellularity and restored the CD44/CD25 staining profile. When the antagonist was also added, the absolute cell number was still comparable to that in IL-7 alone, but there were very few DN3 cells. Instead, a large accumulation of DN2 cells was observed. These results indicate that the antagonist affects the further progression of IL-7–expanded cells rather than the IL-7 expansion itself. Collectively, studies with Rag- and IL-7–deficient fetal thymi suggest 2 checkpoints for tachykinin action in T-cell development: the transition from DN2 to DN3 and the progression from DN4 or CD8 ISP to CD4/CD8 DP.
Expression of HK-1 mRNA in the thymus
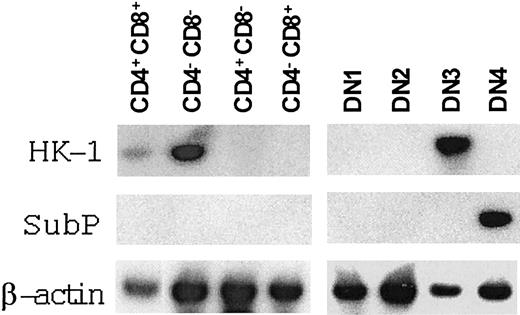
Given the postulated role of HK-1 or other tachykinins in early T-cell development and the fact that HK-1 may work in an autocrine fashion in B-cell development, we investigated the expression of HK-1 and SubP in each thymic subpopulation by RT-PCR (Figure 5). Probably owing to the very low level of expression, significant variations were observed among experiments. Nevertheless, HK-1 mRNA was consistently detected in DN cells (3 of 3), but never in CD4+CD8– or CD4–CD8+ cells (0 of 3). In the DN population, HK-1 mRNA expression was seen mainly in DN3 (3 of 3), but occasionally in other subsets as well. This pattern was particularly interesting in that DN3 cells were the major targets of tachykinin antagonists. While we realize that mRNA expression does not prove that tachykinin precursor proteins and peptides are being expressed, it is reasonable to suggest that autocrine or paracrine mechanisms may be involved in HK-1 signaling pathways. For SubP expression in thymus, previous studies by immuno-histochemical staining demonstrated that its presence was essentially confined to unmyelinated nerve endings.32 It is not known whether there is a nonneuronal source of SubP in the thymus. By RT-PCR, we detected SubP mRNA in DN4 cells, indicating that thymocytes could produce SubP. However, since DN4 is identified by negative staining, it may contain cells other than the T lineage. It remains to be determined whether the T-lineage cells or other accessory cells actually produce SubP.
HK-1 and SubP expression in various subsets of thymocytes. Three CD44/CD25 sortings of DN cells and 2 CD4/CD8 sortings of total thymocytes were performed. RNA was prepared from sorted cells and reverse transcribed. Two rounds of nested PCR were performed, and the products were then detected with corresponding cDNA probes. Results from a representative set of sorted thymocytes are presented.
HK-1 and SubP expression in various subsets of thymocytes. Three CD44/CD25 sortings of DN cells and 2 CD4/CD8 sortings of total thymocytes were performed. RNA was prepared from sorted cells and reverse transcribed. Two rounds of nested PCR were performed, and the products were then detected with corresponding cDNA probes. Results from a representative set of sorted thymocytes are presented.
Discussion
In the early phase of T-cell development prior to β-selection, the small pool of hematopoietic precursors colonizing the thymus undergoes massive expansion. This expansion is driven primarily by cytokines/growth factors. Among them, IL-7 and SCF have attracted the highest attention. IL-7 induced proliferation of DN thymocytes and increased the cell number in FTOCs. Disruption of the IL-7 signaling cascade, on the other hand, resulted in a profound reduction in thymic cellularity and a severe progression blockage beyond the DN2 stage.1,2 SCF is another major factor stimulating the expansion of very immature thymocytes. Analysis of mice bearing mutations in SCF or its receptor c-kit demonstrated a 40-fold reduction in the pro–T-cell compartment.33 Interestingly, there is a synergistic action between IL-7 and SCF. SCF enhanced IL-7–induced proliferation of DN2 cells,3 and the combined deficiency in both IL-7 and SCF signaling pathways demonstrated more profound T-cell developmental defects than single deficiencies in either of the pathways.34 Other factors such as tumor necrosis factor (TNF) and IL-1 also play a role in early T-cell development. In fetal thymus organ cultures, IL-1 and TNF promoted the generation of CD25+ population whereas the antibodies against these 2 factors prevented the development of CD25+ cells.5
In this report, we present evidence showing tachykinins can also be critical factors in the early phase of T-cell development. Blocking tachykinin function by antagonists essentially abolished the development of DP cells both in vitro and in vivo. This inhibitory effect was specific since it occurred only with the active form of a pair of stereoselective antagonists and could be partially overcome with exogenous HK-1 peptides. We further demonstrated that there were 2 potential checkpoints in T-cell development that might be affected by the action of tachykinins. The first checkpoint is at the transition from DN2 to DN3. The antagonist treatment largely eliminated DN3 cells. Meanwhile, a slight increase of DN2 cells was observed. This was more obvious when both exogenous IL-7 and tachykinin antagonist were added to FTOCs derived from IL-7–deficient mice. As expected, T-cell development was restored with the addition of IL-7. In the presence of tachykinin antagonist, however, the IL-7–expanded DN2 cells were not able to proceed to the DN3 stage; this led to the accumulation of DN2 cells but the absence of DN3 cells. The diminished DN3 population was also seen in Rag–/– FTOCs. In these experiments, anti-CD3 was used to drive cells from DN3 to DN4 and beyond. In the presence of antagonist, however, the transition from DN2 to DN3 was blocked while existing DN3 cells were able to move to the DN4 compartment, effectively clearing cultures of DN3 cells. The second checkpoint of tachykinin action is immediately prior to the generation of DP cells. In antagonist-treated FTOCs, some cells bypassed the first blockage at the transition from DN2 to DN3 and developed into the DN4 stage. Nevertheless, no DP or CD4+CD8– cells were generated. Instead, we saw an accumulation of CD4–CD8+ cells. While the identity of the CD4–CD8+ cells remains to be fully defined, it is apparent that this population is quite heterogenous, containing both TCR-β+ cells and γδ+ cells. In early growth response (Egr) transgenic mice35 and mice with double deficiency in the high-mobility group (HMG) box proteins T-cell factor 1 (TCF-1) and lymphoid enhancer factor 1 (LEF-1),36 a similar T-cell developmental arrest at the CD4–CD8+ stage has also been observed. The accumulated cells were postulated to be CD8 ISP cells.
While our studies have demonstrated that tachykinins can play a critical role in T lymphopoiesis, further study is required to determine the specific role of the different members of this family. The antagonists used in our studies are highly specific for the NK-1 receptor, indicating the ligands HK-1 and SubP as likely candidates for mediating the observed functions. Several lines of evidence favor HK-1 as the physiologic mediator. First, the antagonist-induced T-cell developmental defect could be corrected with synthetic HK-1 peptides. This rescue was an HK-1–specific event, and SubP was virtually ineffective. Second, HK-1 had a direct enhancing effect on the proliferation of thymocytes. The same effect elicited by SubP was less robust. Third, the preferential expression of HK-1 in DN3 correlates best with the observed developmental blockage from DN2 to DN3. And last, any major and unique contribution of SubP to T-cell development would predict significant defects in the T-cell compartment of mice carrying a mutated SubP gene. In fact, no such defects have been reported in Ppt-A knockout mice.10,11
The precise mechanisms by which tachykinins like HK-1 regulate T-cell development remain to be defined. The increased thymidine incorporation by HK-1 suggests that it may provide a proliferative signal for thymocytes at a certain developmental stage. Given that the DN3 population was remarkably diminished in the presence of tachykinin antagonists, we speculate that HK-1 may drive the expansion of DN3 cells either by itself or by collaborating with IL-7 similarly to the way in which SCF and IL-7 synergize to expand DN2 cells.3 HK-1 involvement at this stage of T-cell development is reminiscent of its function in B-cell development, where it is found to increase the proliferation of IL-7–expanded B-cell precursors.18 In B lymphopoiesis, HK-1 also enhances cell survival. Although we failed to observe any significant antiapoptotic effect of HK-1 on thymocytes in suspension cultures or FTOCs, the possibility that HK-1 improves thymocyte survival in more relevant situations, such as in positive and negative selection, can't be ruled out. Still another possibility is that HK-1 may act as a differentiation factor, promoting the transitions from DN2 to DN3 and from DN4 or CD8 ISP to DP. Finally, further studies are required to determine whether HK-1 acts through an indirect mechanism, such as influencing cytokine secretion of thymic supporting cells. SubP has been shown to affect myeloid cell development by such a mechanism.14
The majority of the biologic activities observed for tachykinins are mediated by specific G protein–coupled 7 transmembrane domain receptors.37 Signal transduction through these receptors involves several second-message systems, including inositol 1,4,5-triphosphate turnover via phospholipase C, arachidonic acid mobilization via phospholipase A2, and cyclic adenosine monophosphate accumulation via adenylyl cyclase. To date, 3 types of tachykinin receptors have been identified (NK-1, NK-2, and NK-3), each of which shows preference for a specific ligand: NK-1 for SubP, NK-2 for neurokinin A, and NK-3 for neurokinin B. However, the selectivity is generally poor, and a given tachykinin peptide can be a full agonist on all 3 receptors under certain conditions. Recent studies by several groups indicate that HK-1, when applied at different concentrations, can also lead to the activation of all 3 known receptors.26-28 The effect of HK-1 on T-cell development may be mediated by known tachykinin receptors. Since HK-1 demonstrates remarkable selectivity for NK-1 as compared with NK-2 and NK-3,27,28 NK-1 appears to be the most attractive candidate. This hypothesis, however, is not supported by knockout studies. Except for some minor changes in T-cell proliferation response following treatment with anti-CD3 and anti-CD28, no other T-cell defects had been documented in NK-1–deficient mice,12,13,15 arguing against a major role of NK-1–mediated signal in T-cell development.
Previous studies have demonstrated the existence of SubP-binding sites on thymocytes.16,38,39 Given that HK-1 behaves like SubP in receptor binding, these binding sites could be shared by HK-1, and under some circumstances, HK-1 could even be the physiologic ligand. Nevertheless, it remains to be determined what specific tachykinin receptors are involved. We attempted to resolve this question by examining mRNA expression of the known receptors in thymocytes. The results obtained were ambiguous. Extensive PCR amplification (2 rounds of 25 cycles each) revealed mRNA expression of all 3 receptors in various thymic populations (data not shown). But the pattern was inconsistent and the frequency was low, suggesting an extremely low level of expression. It is therefore still not clear what receptor mediates HK-1 action on thymocytes. At this point, it can't be ruled out that HK-1 normally uses a novel, as yet unidentified, receptor. Donaldson and colleagues have reported a fourth tachykinin receptor.40 This new receptor is not only structurally related to NK-3, but also demonstrates binding preference for neurokinin B. It remains possible that additional tachykinin receptors will be identified, one of which would retain sufficient similarity to NK-1 to be inhibited by NK-1 antagonists, but differ sufficiently to distinguish HK-1 from SubP.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-11-3572.
Supported by grants from the Terry Fox Foundation, the National Cancer Institute of Canada, and the Canadian Institute of Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr P. Vieira for IL-7–/– mice, Dr X. Emonds-Alt for SR140333 reagent, and Drs A. Cumano, J. C. Zuniga-Pflucker, and H. Fleming for helpful discussions and critical reading of the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal