Abstract
In overhydrated hereditary stomatocytosis (OHSt), Coomassie- and silver-stained polyacrylamide gels show an apparently complete deficit of the 32-kDa membrane protein, stomatin. We have used an antistomatin antibody to examine peripheral blood films, bone marrow, splenic tissue, and hepatic tissue from these patients by immunocytochemistry. This technique revealed that, in fact, some red cells did show positive stomatin immunoreactivity; and consistent with this result, Western blot analysis of the red cell membranes confirmed that about one twentieth to one fiftieth of the normal amount of stomatin was in fact present. Flow cytometry, combining immunoreactive quantitation of stomatin expression with thiazole orange staining for reticulocytes, showed that in OHSt, it was the young cells that had more stomatin. Magnetic-activated cell separation studies, using beads to which an anti–transferrin receptor antibody was conjugated, confirmed that in OHSt there was a correspondence between expression of stomatin and the transferrin receptor. Immunocytochemistry and Western blotting revealed that in OHSt patients, the protein was present in spleen, liver, neutrophils, platelets, monocytes, and about 50% of the peripheral lymphocytes, with the same distribution as in healthy controls. Neither Southern blots, nor direct sequencing of multiple subclones of the cDNA, nor sequencing of amplicons from genomic DNA revealed any significant abnormality in stomatin gene sequence in these patients. The deficiency of stomatin from red cells appears to be due to a loss of stomatin from these red cells on maturation in the bone marrow and in the circulation.
Introduction
The name “stomatocytosis” was coined to describe the morphology in a dominantly inherited hemolytic anemia distinguished from hereditary spherocytosis by the cell shape.1 This original condition is now known as overhydrated hereditary stomatocytosis (OHSt). Aside from the morphology, the anemia shows 2 other features: a catastrophic “leak” across the plasma membrane to the univalent cations Na+ and K+ (Zarkowsky et al2 ), and the deficiency of the 32-kDa integral membrane protein, stomatin, or erythrocyte membrane protein 7.2b.3-5 The condition represents an unusual genetic situation, in which a protein is apparently missing in a dominant, and therefore presumably heterozygous, condition. The gene coding for the missing protein has been cloned and sequenced.5,6 Preliminary reports have indicated that no mutation has been found in American OHSt families, in the protein-coding region at least.7,8
The pedigrees of OHSt are too small for effective genome-wide searches, but other variants of this leaky-cell class of red cell disease have been mapped. An American pedigree with non–stomatin-deficient dehydrated hereditary stomatocytosis (HSt) did not map to the stomatin locus on chromosome 9,9,10 and European pedigrees of this non–stomatin-deficient dehydrated condition map to chromosome 16.11 Other HSt pedigrees with different variants, none of which showed stomatin deficiency, map to yet other loci12 ; there is locus heterogeneity among these leaky membrane diseases.
The stomatin gene and its homologues are widely distributed in both human tissues and in nature; mutations in one homologue, mec-2 (protein product, MEC-2), cause a defect in neuronal mechanosensory function in the nematode, Caenorhabditis elegans. The mutations are genetically associated with the genes mec-4 and -10, themselves homologous to genes encoding subunits of the mammalian epithelial amiloride-sensitive Na+ channel superfamily known as epithelial Na+ channel (ENaC)13,14 : a functional, regulatory, link is suggested. In mammals, stomatin immunoreactivity and ENaC immunoreactivity are both found in lanceolate nerve endings and rapidly adapting mechanoreceptors in the rat vibrissal follicle-sinus complex.15
The true function of stomatin is improperly understood. Stomatin is a raft protein, partly associated with cholesterol + sphingomyelin–rich material, which is insoluble in cold nonionic detergent such as Triton X-100,16,17 as confirmed here (Figure 2F). Some of its homologues are implicated in systems that control the turnover of membrane proteins.18 A murine stomatin knock-out is without overt phenotype.19 Recessive mutations in the human homologue podocin cause a form of nephrotic syndrome.20 One possible unifying hypothesis for these observations is that stomatin is a protein that is concerned with the regulation of the surface expression of membrane proteins via cholesterol + sphingomyelin–rich material in the membrane.18
Western blots of different cell and tissue preparations. (A) Normal and OHSt red cell membranes. Blots 1A, IB, and II were probed with rabbit polyclonal serum, and blot III was probed with mouse monoclonal. Blots IA, IB, and III show comparison of patient A-II-1 with healthy control, whereas blot II shows patient B-II-1. At the shorter ECL exposure time (blot IB), no signal can be seen in the “patient” lane (P), but at the longer exposure time (IA, II), further bands at higher molecular weight are evident in the “healthy” lane (N), which most likely represent oligomers of stomatin itself, since they are not evident in the patient lane. In blot III, the monoclonal antibody identifies further bands at slower migration (*), which probably represent nonspecific binding since the bands are present in both samples. (B) Probing of dilution series of normal red cell membranes with polyclonal antistomatin antibody. Serial dilutions of normal red cell membranes were run on a 12% polyacrylamide gel, blotted to nitrocellulose, and probed with the rabbit polyclonal antibody using a long ECL exposure time. At low dilutions, bands at 55 to 65 kDa are evident, as in blots IA and II seen in panel C. (C) Test of binding of polyclonal antibody to SLP1 and SLP2, in the form of glutathione-s-transferase (GST) fusion proteins expressed in Escherichia coli. Upper panel, Coomassie stain; lower panel, antistomatin antibody Western blot of an identical gel. Lanes 1 to 4 in both panels: E coli lysates containing isopropyl β-D-thiogalactopyranoside (IPTG)–induced fusion proteins (lane 1, GST-SLP1; lane 2, GST-SLP2; lane 3, GST alone; lane 4, GST-stomatin), (aa 144-288; lane 5, molecular weight markers; lane 6, normal red cell membranes; lanes 8-9, 1:50 and 1:100 dilutions of GST-stomatin [aa 144-288] sample used in lane 4). Letters indicate positions of protein bands of interest as follows: a, GST-SLP1; b, GST-SLP2; c, GST alone; d, GST-stomatin (aa 140-288); e, stomatin in normal red cell membranes; f, 1 in 50 dilution of stomatin GST (aa 140-288); and g, 1 in 100 dilution of same. The antibody binds only to GST-stomatin (aa 140-288) (bands d, f, g) and native stomatin in red cells (band e), and not to SLP1 or SLP2. (D) Subcellular fractionation of nonerythrocyte circulating cells from normal blood, probed with polyclonal antibody. Platelets, neutrophils, lymphocytes, and monocytes were isolated as described in “Patients, materials, and methods.” Purities of more than 90% were achieved for each cell type, with less than 0.1% contaminating erythrocytes. Subcellular fractions were isolated by centrifugation on a sucrose step gradient, as described in “Patients, materials, and methods.” In each cell type, all fractions except cytosol show a positive band at 32 kDa. (E) Shown are 4 samples of normal and 1 sample of OHSt liver (patient A-I-2) probed with polyclonal antibody. A predominant single band at 32 kDa is evident. (F) Association of stomatin protein with buoyant, cholesterol + sphingomyelin–rich, Triton-insoluble material purified from normal and OHSt red cell membranes. Red cell membranes were treated with cold Triton X-100 and subjected to sucrose gradient ultracentrifugation.36 Fractions were aspirated and run on 12% SDS polyacrylamide gels and blotted to nitrocellulose. Left-most lanes reflect the lower part of the gradient (45% sucrose wt/vol), whereas the right-most reflect the upper part of the gradient (0% sucrose). The blots were probed with polyclonal antistomatin antibody (upper panels) and antiflotillin antibody (lower panels). The upper panels show that about half of the stomatin was associated with this buoyant raft material, while the remainder was not. The lower panels confirm, first, that flotillin is present in both normal and abnormal red cells, second, that flotillin is exclusively associated with rafts in both cell types, and third, that even in the presence of a deficiency of stomatin, rafts still exist in these abnormal cells.
Western blots of different cell and tissue preparations. (A) Normal and OHSt red cell membranes. Blots 1A, IB, and II were probed with rabbit polyclonal serum, and blot III was probed with mouse monoclonal. Blots IA, IB, and III show comparison of patient A-II-1 with healthy control, whereas blot II shows patient B-II-1. At the shorter ECL exposure time (blot IB), no signal can be seen in the “patient” lane (P), but at the longer exposure time (IA, II), further bands at higher molecular weight are evident in the “healthy” lane (N), which most likely represent oligomers of stomatin itself, since they are not evident in the patient lane. In blot III, the monoclonal antibody identifies further bands at slower migration (*), which probably represent nonspecific binding since the bands are present in both samples. (B) Probing of dilution series of normal red cell membranes with polyclonal antistomatin antibody. Serial dilutions of normal red cell membranes were run on a 12% polyacrylamide gel, blotted to nitrocellulose, and probed with the rabbit polyclonal antibody using a long ECL exposure time. At low dilutions, bands at 55 to 65 kDa are evident, as in blots IA and II seen in panel C. (C) Test of binding of polyclonal antibody to SLP1 and SLP2, in the form of glutathione-s-transferase (GST) fusion proteins expressed in Escherichia coli. Upper panel, Coomassie stain; lower panel, antistomatin antibody Western blot of an identical gel. Lanes 1 to 4 in both panels: E coli lysates containing isopropyl β-D-thiogalactopyranoside (IPTG)–induced fusion proteins (lane 1, GST-SLP1; lane 2, GST-SLP2; lane 3, GST alone; lane 4, GST-stomatin), (aa 144-288; lane 5, molecular weight markers; lane 6, normal red cell membranes; lanes 8-9, 1:50 and 1:100 dilutions of GST-stomatin [aa 144-288] sample used in lane 4). Letters indicate positions of protein bands of interest as follows: a, GST-SLP1; b, GST-SLP2; c, GST alone; d, GST-stomatin (aa 140-288); e, stomatin in normal red cell membranes; f, 1 in 50 dilution of stomatin GST (aa 140-288); and g, 1 in 100 dilution of same. The antibody binds only to GST-stomatin (aa 140-288) (bands d, f, g) and native stomatin in red cells (band e), and not to SLP1 or SLP2. (D) Subcellular fractionation of nonerythrocyte circulating cells from normal blood, probed with polyclonal antibody. Platelets, neutrophils, lymphocytes, and monocytes were isolated as described in “Patients, materials, and methods.” Purities of more than 90% were achieved for each cell type, with less than 0.1% contaminating erythrocytes. Subcellular fractions were isolated by centrifugation on a sucrose step gradient, as described in “Patients, materials, and methods.” In each cell type, all fractions except cytosol show a positive band at 32 kDa. (E) Shown are 4 samples of normal and 1 sample of OHSt liver (patient A-I-2) probed with polyclonal antibody. A predominant single band at 32 kDa is evident. (F) Association of stomatin protein with buoyant, cholesterol + sphingomyelin–rich, Triton-insoluble material purified from normal and OHSt red cell membranes. Red cell membranes were treated with cold Triton X-100 and subjected to sucrose gradient ultracentrifugation.36 Fractions were aspirated and run on 12% SDS polyacrylamide gels and blotted to nitrocellulose. Left-most lanes reflect the lower part of the gradient (45% sucrose wt/vol), whereas the right-most reflect the upper part of the gradient (0% sucrose). The blots were probed with polyclonal antistomatin antibody (upper panels) and antiflotillin antibody (lower panels). The upper panels show that about half of the stomatin was associated with this buoyant raft material, while the remainder was not. The lower panels confirm, first, that flotillin is present in both normal and abnormal red cells, second, that flotillin is exclusively associated with rafts in both cell types, and third, that even in the presence of a deficiency of stomatin, rafts still exist in these abnormal cells.
This paper reports data on the 2 original OHSt pedigrees in the United Kingdom,1,21 both now recognized as stomatin deficient.5 We show 5 main findings: first, that the stomatin protein is not completely missing from all of the red cells in peripheral blood; second, that “young” red cells in peripheral blood show more stomatin than “old”; third, that in the patients stomatin is present in peripheral blood cells (neutrophils, platelets, monocytes, and some lymphocytes) as seen in healthy controls; fourth, sequencing of the complete mRNA, including the 3′ untranslated region, in OHSt patients is normal (although mapping in one small pedigree cannot exclude the stomatin locus); and fifth, that in both healthy controls and patients, the expression of stomatin in lymphocytes is variable. These data strongly suggest that in OHSt red cells the stomatin is lost from the abnormal red cell as an innocent victim rather than because a mutation in the stomatin gene is the primary cause of the disease. The mechanism by which stomatin is lost remains unclear, although some kind of raft-based vesiculation process seems the most likely possibility.
Patients, materials, and methods
Patients
Family trees are shown in Figure 1. Family A was originally described by Lock et al1 and family B, by Meadow.21 Both families show the overtly stomatocytic, high-Na+, low-K+ phenotypes commonly associated with stomatin deficiency.5 In these studies we have focused on individuals A-II-1 and B-II-1. They show hemoglobin (Hb) levels of 90 to 110 g/L (9-11 g/dL) with typical reticulocyte counts (expressed throughout as a proportion of total hemoglobin) of 0.1 to 0.2 (10% to 20%). No patient had been transfused for at least 6 months prior to study. A-II-1 was splenectomized 9 years ago. A tissue sample of his spleen and a bone marrow smear made at the time of his splenectomy were included in our immunocytochemical evaluation. His mother, A-I-2, died some years ago in a parvovirus crisis.22 Individual A-III-1, a female, was born in August 2001. Prolonged neonatal jaundice was present. A sample of cord blood showed the high intracellular [Na+], low-[K+] phenotype (intracellular [Na+], 51 mM cells [normal, 5-11 mM cells]; [K+], 53 mM cells [normal, 85-105 mM cells]) typical of this condition, confirming that the newborn was affected. Approval for these studies was obtained from the University College Hospitals Joint Ethics Committee. Informed consent was provided according to the Declaration of Helsinki.
Family trees. Filled symbols indicate affected individuals; open symbols, not affected. For family A, mapping scores for probes D9S1776, D9S1682, and D9S290 are shown for each individual. Each affected individual has inherited the same haplotype for these markers spanning the stomatin locus.
Family trees. Filled symbols indicate affected individuals; open symbols, not affected. For family A, mapping scores for probes D9S1776, D9S1682, and D9S290 are shown for each individual. Each affected individual has inherited the same haplotype for these markers spanning the stomatin locus.
Methods
Cation flux. Methods for separation of red cells by density in Wintrobe tubes and for evaluation of intracellular [Na+] and [K+] and of isotopic flux rates have been described previously.23
Antibodies. Rabbit polyclonal and mouse monoclonal antibodies were raised against recombinant stomatin fusion proteins (both hexa-histidine tagged and glutathione-S-transferase [GST]) containing amino acids 144 to 288 (end) of the protein. Polyclonal antiserum was affinity-purified using maltose-binding-protein-stomatin fusion protein.23 The clone that initially produced the murine monoclonal antibody ceased to yield antibody after a month and supplies of this antibody then terminated.
Cellular and subcellular fractionation. Cell types from the peripheral blood were purified according to published methods.24 Briefly, citrate phosphate dextrose adenine (CPDa) anticoagulated blood was separated by sedimentation of whole blood in 1% wt/vol dextran followed by layering onto Lymphoprep (Nycomed, Munich, Germany). The interface cells just above the Lymphoprep layer were separated into platelet and mononuclear cell fractions by centrifugation. The mononuclear cells were further separated by adherence (monocytes) or nonadherence (lymphocytes) to tissue-culture plates. The pellet underlying the Lymphoprep layer was subjected to hypotonic lysis and then washed to yield neutrophils free of erythrocytes.25 Subcellular fractions of each cell type were prepared according to Grogan et al.26 Briefly, the cell pellets were resuspended in Lambert break buffer (LBB; in mM: K+, 10; Na+, 3; Mg2+, 4; Cl–, 21; Pipes, 10 [pH 7.4 at room temperature {RT; 25 ± 2°C}]) containing protease inhibitors (phenylmethylsulfonyl fluoride [PMSF], TLCK [L-1-chloro-3-[4-tosyl-amido]-7-amino-2-hepatanone-HCl], and pepstatin, all at 1 μg/mL) and sonicated on ice 4 times in 5-second bursts. This material (start) was loaded onto a 17% to 34% wt/wt sucrose step gradient in LBB and centrifuged at 70 000g for 30 minutes. The upper fraction, above the sucrose, was taken as cytosol; the membranes were taken as the interface of 17% and 34% sucrose steps (to be washed later; below, this paragraph), while the pellet below the 34% sucrose step contained nuclei and granules. The interface material was removed and diluted at least 3-fold in LBB, mixed well, and spun at 100 000g for 30 minutes in a Beckman TLA 100.2 rotor (Beckman Coulter, Hialeah, FL). The supernatant was removed and discarded while the pellet, labeled as “membranes” in Figure 2, was dissolved in 50 μL sodium dodecyl sulfate (SDS) sample buffer. Equal protein amounts of each subcellular fraction were loaded onto SDS polyacrylamide gel electrophoresis gels.
Red cell membranes. Red cell membranes were prepared by hypotonic lysis,27 dissolved in SDS sample buffer, and separated on 12% polyacrylamide gels before blotting to nitrocellulose.28
Stomatin-like proteins (SLPs). Plasmids coding for glutathione-s-transferase (GST) fusion proteins containing SLP1 and SLP2 were kindly gifted by Dr J. S. Morrow (Yale University) and induced as previously described.29
Detergent-insoluble membrane domains. Lipid-rich, cold-detergent–insoluble material was isolated from red cells by sucrose density gradient ultracentrifugation,30 using ice-cold Triton X-100.
Immunocytochemistry. The blood films (peripheral or cord blood) were air-dried at RT for a minimum of 24 hours, then fixed with 100% ethanol for 10 minutes prior to the incubation. For immunocytochemistry, the specimens were washed in phosphate-buffered saline (PBS) and preincubated in a solution containing 20% normal goat serum (NGS), 0.1% Triton X-100, and 0.05% phenylhydrazine in PBS for 30 minutes at room temperature. The primary polyclonal antibody (antistomatin), diluted 1:100 in a solution consisting of 20% NGS, 0.1% Triton X-100, 0.01% Thiomersal, and 0.1% sodium azide in PBS, was applied for 16 hours at room temperature in a humidified chamber. The specimens were then exposed to biotinylated goat anti–rabbit immunoglobulin G (IgG; Vector Laboratories, Burlingame, CA; BA-1000), diluted 1:2000 in PBS-A (2 mg/mL bovine serum albumin in PBS, 0.1% sodium azide wt/vol) for 4 hours at room temperature. For antigen visualization, the blood films were incubated with the ABC reagent (avidin/biotinylated-peroxidase complex; Vector Laboratories) for 2 hours at room temperature, diluted 1:1000 in PBS-A. The substrate reaction was performed with 3,3′ diaminobenzidine. The blood films were then counterstained with hematoxylin, dehydrated in graded ethanol, and mounted in Entellan (Merck, West Point, PA). For negative controls, the primary antibody was omitted.
Smears of bone marrow collected at the time of splenectomy (patient A-II-1) were air-dried and fixed in acetone for 10 minutes at RT. The bone marrow material was stained with murine monoclonal antibody 1H9 raised in mice using the same GST-stomatin fusion protein. For detection in this case, the slides were incubated in the antibody diluted 1:10 in tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) for one hour, after which the bound antibody was detected using the alkaline phosphatase/anti–alkaline phosphatase (APAAP) technique. Alkaline phosphatase activity was detected by the Fast Red technique, which gives a red color. The bone marrow smears were then counterstained with Mayer hematoxylin.31
For tissue sections, the specimens of spleen (patient A-II-1) or liver (patient A-I-2) originally fresh-frozen at the time of splenectomy (spleen, patient A-II-1) or at autopsy (liver, patient A-I-2) and continuously stored at –80°C were directly immersed in fixative (4% paraformaldehyde in PBS) for one day, dehydrated in graded ethanol, embedded in paraffin, and cut at a thickness of 8 μm. For the positive control, paraffin-embedded postmortem material of nonstomatocytosis patients was used. Prior to the antibody incubation, antigen retrieval was performed. The sections were rehydrated, rinsed in deionized water, and incubated in a target retrieval solution (10 mM Na+-citrate buffer, pH 6) for 15 minutes using a microwave oven (800 W). After this treatment the sections were washed in PBS and the immunoincubation was performed as described for blood films using the avidin-biotin-peroxidase method. To distinguish iron deposits from the brown stomatin immunoreaction product, we also performed a Prussian blue reaction after the immunoincubation. In this reaction, the iron-containing deposits give a blue positive ferrocyanide reaction, while cell nuclei were counterstained with nuclear red.
To distinguish B and T cells and stomatin-immunoreactive areas in the spleen, serial sections were cut and incubated using the following primary antibodies: polyclonal antihuman stomatin 1:100 (see “Antibodies”), monoclonal mouse antihuman CD20cy (B-cell marker; DAKO, Glostrup, Denmark; M 0755), and monoclonal mouse antihuman CD3 (T-cell marker; DAKO, Denmark; M 7193). For detection of the monoclonal mouse antibodies, a biotinylated horse anti–mouse IgG antibody (Vector Laboratories; BA 2001) was used as a secondary antibody. For this application, NGS was replaced by 10% horse serum in all incubation steps.
Flow cytometry; magnetic bead cell separation
For combined analysis of red cell maturity and stomatin content, flow cytometry was used. It was believed that the C-terminus of stomatin (containing the polyclonal antibody epitope) was cytoplasmically facing5 and therefore not accessible to the antibody in the whole cell situation. Consistent with this, exposure of whole normal cells to antistomatin antibody followed by fluorescent detection methods gave no signal in the flow cytometer. The cells were therefore fixed and permeabilized to allow the antibody access to the intracellular epitope. Cells were centrifuged at 170g for 10 minutes at RT and the plasma removed. Cells were mixed 1:1 with Alsevier solution (Sigma Chemical, St Louis, MO; no. A3551); 20μL of this suspension was mixed with 1 mL fresh, ice-cold 0.05% vol/vol glutaraldehyde (Sigma Chemical, G5882) and incubated at RT for 10 minutes. Of this mix, 200 μL was mixed with 800 μL ice-cold 0.1% Triton X-100 in PBS and left at RT for 5 minutes, after which 500 μL PBS was added, mixed, and centrifuged at 1600g for 3 minutes in a microcentrifuge. For antistomatin antibody staining, the fixed and permeabilized cells were first exposed to the antistomatin antibody at 1:100 dilution, washed, then exposed to murine anti–rabbit IgG (DAKO; MO737) diluted 1:10. All antibody staining incubations occurred at 0°C and were of 30-minute duration. After further washing, the cells were exposed to a goat anti–mouse IgG antibody labeled with R-phycoerythrin (R-PE; Insight Biotechnology, Wembley, Middlesex, United Kingdom; 115-116-146) diluted 1:25. After washing, the cells were fixed in 1% formaldehyde in PBS. If reticulocyte analysis by thiazole orange was required, immediately prior to flow cytometric analysis the cells were spun and resuspended in Becton Dickinson (BD, San Jose, CA) Retic Reagent (BD 349204) and analyzed on a Beckman Coulter EPICS Elite flow cytometer. Excitation was with the argon laser at 488 nm, and R-PE and thiazole emissions were detected with appropriate bandpass filters at 575 and 525 nm, respectively. At least 104 cells were analyzed in each run.
Magnetic-activated cell sorting (MACS) of erythrocytes
Washed red cells were separated using MACS anti-CD71 (transferrin receptor) microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany; 462-01) using positive selection in media and methods provided with the kit, adapted for 109 cells. Note that an excess of cells was used; thus the flow-through contained some young cells, as shown by subsequent thiazole orange staining (Figure 5D). For flow cytometric analysis after MACS separation, the cells were fixed and permeabilized as described in “Flow cytometry.” For stomatin detection by flow cytometry, the cells were exposed for 30 minutes to a 1:100 dilution of polyclonal antistomatin antibody as described in “Flow cytometry,” then washed and incubated with a fluorescein-5-isothiocynate (FITC)–conjugated swine anti–rabbit IgG antibody (DAKO F0054) at a 1:10 dilution, then washed and fixed in 1% formaldehyde in PBS. The FITC emission was detected at 525 nm.
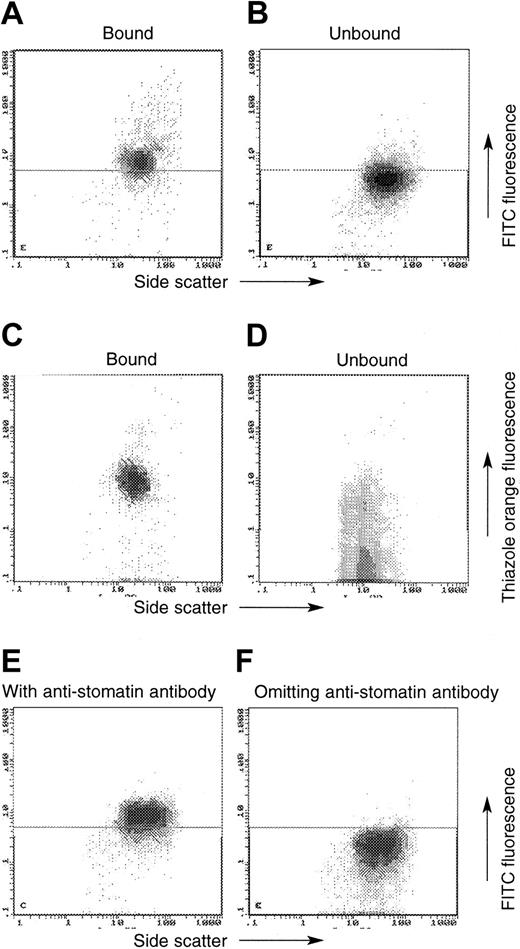
MACS using antitransferrin receptor (TfR) antibody in normal and OHSt cells (patient A-II-1). In these plots, the x-axis always denotes side scatter, whereas the y-axis denotes either the emission of the FITC-labeled swine anti–rabbit IgG (detecting the antistomatin antibody; A-B, E-F) or thiazole orange (C-D). OHSt cells that bound to the anti-TfR beads (A, C) show positive antistomatin antibody immunoreactivity (A) and enhanced thiazole orange staining (C), whereas unbound OHSt cells show less antistomatin antibody immunoreactivity (B) and less thiazole orange staining (D). Control experiments shown in panels E-F show the level of FITC emission from normal cells in the presence (E) and absence (F) of the primary antistomatin antibody. These control experiments define the horizontal demarcating boundary between “stomatin-positive” and “stomatin-negative” cells seen in panels A-B.
MACS using antitransferrin receptor (TfR) antibody in normal and OHSt cells (patient A-II-1). In these plots, the x-axis always denotes side scatter, whereas the y-axis denotes either the emission of the FITC-labeled swine anti–rabbit IgG (detecting the antistomatin antibody; A-B, E-F) or thiazole orange (C-D). OHSt cells that bound to the anti-TfR beads (A, C) show positive antistomatin antibody immunoreactivity (A) and enhanced thiazole orange staining (C), whereas unbound OHSt cells show less antistomatin antibody immunoreactivity (B) and less thiazole orange staining (D). Control experiments shown in panels E-F show the level of FITC emission from normal cells in the presence (E) and absence (F) of the primary antistomatin antibody. These control experiments define the horizontal demarcating boundary between “stomatin-positive” and “stomatin-negative” cells seen in panels A-B.
Gene amplification and sequencing: genetic mapping
For mapping, high-molecular-mass DNA was extracted from peripheral blood leukocytes as described.32 Genotyping at the stomatin locus (9q32-34) was performed using an ABI Prism 377 sequencer and the Linkage Mapping Set Version 2 (Applied Biosystems, Weiterstadt, Germany), comprising 400 fluorescently labeled markers that define a 10-cM resolution human map.33 For cDNA sequencing, total RNA was prepared using guanidinium thiocyanate techniques34 ; mRNA was purified from that by oligo-dT–coupled magnetic beads (Dynal, Oslo, Norway); and cDNA was prepared using Superscript II reverse transcriptase (Gibco, Grand Island, NY). In family A, the 3000 bp cDNA was first amplified in 3 fragments, using Pfu polymerase (Stratagene, La Jolla, CA), using primers designed from the published sequence35 (for primer sequences, go to the Blood website and see the Supplemental Table link at the top of the online article); these gave an informative sequencing interval, between the 3′ ends of the extreme primers of, in total, bases 24-3001. Sequenced were 6 subclones representing each fragment. In a further study on the same patient, the complete gene was then amplified using Elongase (Gibco), and the polymerase chain reaction (PCR) product was sequenced using internal primers (Supplemental Table S1). In family B (B-II-1), the complete cDNA was amplified by Elongase, subcloned by blunt-ended techniques, and 6 subclones were sequenced, using ABI 377 technology, with primers spaced down the gene. Primers for amplification and sequencing of genomic DNA are shown in Table S1. The resulting products were separated from primers using Microcon spin columns (Amicon) filters and sequenced by BigDye v3 Chemistry (ABI) on an Applied Biosystems 3100 sequencer.
Results
Western blots of red cell membrane samples probed with both the rabbit polyclonal antibody and a mouse monoclonal antibody (Figure 2A) show that some protein (about one twentieth to one fiftieth of the normal amount) could be seen at sufficiently high exposures in the patients' membrane samples. At longer enhanced chemiluminescence (ECL) exposures (Figure 2A, blot 1A), both the polyclonal and monoclonal antibodies identified bands at higher molecular weights (MWs) in the control but not in the patient samples. The polyclonal was used to probe a dilution series of normal red cell membranes (Figure 2B). The blot was allowed to develop for a prolonged period, such that these higher MW bands appeared in the undiluted (left-most) lane. At a dilution of 1 in 32, a single relatively faint band was evident at 32 kDa. To ensure that these higher bands were not due to cross-reactivity between this antibody and stomatin-like proteins that have been described,29,37 we tested the reactivity of our antibody against recombinant SLP1 and SLP2; no reaction was seen (Figure 2C). Western blots of purified and fractionated components of other peripheral blood cells (neutrophils, platelets, lymphocytes, and monocytes) each showed a prominent band at 32 kDa in the whole unfractionated cell material, the pellet (unbroken cells, granules, and nuclei), and membrane fractions (plasma membrane, endoplasmic reticulum), but not in cytosol, as expected for this membrane protein (Figure 2D). Western blots of archived liver tissue from 4 healthy controls and patient A-I-2 (Figure 2E) showed a predominant band at 32 kDa.
Stomatin has been shown partly to be associated with cholesterol + sphingomyelin–rich rafts in red cells.16,17 We investigated this aspect in these red cells. Figure 2F shows the results of sucrose gradient studies of normal and OHSt red cells. Blots were probed with antistomatin and antiflotillin antibodies. As expected from the results above (Figure 2A), some stomatin was present in the patient samples. In both the healthy individuals and controls, the stomatin was distributed roughly 50:50 between the buoyant, Triton-insoluble fraction and the soluble fraction. Studies with the antiflotillin antibody confirmed that flotillin was confined to the raft fraction in both cell types, and it was not deficient in these red cells.
Immunocytochemistry of normal and OHSt peripheral blood films is shown in Figure 3 (A-II-1, panel A; B-II-1, panel B). No patient had been transfused for at least 6 months prior to the studies. In each patient case, some red cells (about 10%-15%) were stomatin immunoreactive, while most were nonimmunoreactive. All platelets, all neutrophils, and about half of the lymphocytes were stomatin immunoreactive. In normal blood samples (Figure 3C), all erythrocytes, all platelets, and all neutrophils were immunopositive. As in the OHSt patients, about half of the lymphocytes were positive. In stomatin-immunopositive erythrocytes, whether in normal or OHSt red cells, the distribution pattern of stomatin immunoreactivity was the same: a homogenous brownish staining of the cell cytoplasm. The cellular distribution pattern of stomatin in red cells was dependent on the fixation method. Whereas in acetone- or ethanol-fixed blood smears the immunoreaction product was more homogeneously distributed in red cells, the reaction product in formaldehyde-fixed and paraffin-embedded tissue shows a strong ringlike pattern in contrast to a weak cytoplasmic staining. These results clearly show that formaldehyde fixation is essential to stabilize stomatin in its membrane location. In contrast to erythrocytes, the stomatin immunoreaction product in leukocytes had a granular appearance and showed a homogenous cell distribution pattern.
Immunocytochemistry of peripheral blood films from OHSt and healthy individuals. Blood films were stained with rabbit polyclonal antistomatin antibody (see “Patients, materials, and methods”) and counterstained with hematoxylin. Positive stomatin immunoreactivity is reflected by brown coloring. (A) Patient A-II-1. Some erythrocytes are stomatin immunoreactive (immunoreactive erythrocyte, IRE) but most are nonimmunoreactive (NIRE). All platelets (P) and the neutrophil (N) are immunoreactive. One lymphocyte is immunoreactive (immunoreactive lymphocyte, IRL), but the other is not (NIRL). (B) Patient B-II-1 shows a similar picture to patient A-II-1: mixed population of red cells; all platelets immunoreactive; and nonimmunoreactive lymphocyte. (C) Healthy control blood film. All erythrocytes are stomatin immunoreactive (IRE). All platelets are immunoreactive; there is a mixture of immunoreactive and nonimmunoreactive lymphocytes. (D-F) Negative controls, in which antistomatin antibody was omitted. (D) Patient A-II-1. Negative control and no first antibody. Neutrophil (N) and erythrocytes are all negative. (E) Patient B-II-1. Negative control omitting first antibody. (F) Normal blood film, negative control. Bar represents 10 μm.
Immunocytochemistry of peripheral blood films from OHSt and healthy individuals. Blood films were stained with rabbit polyclonal antistomatin antibody (see “Patients, materials, and methods”) and counterstained with hematoxylin. Positive stomatin immunoreactivity is reflected by brown coloring. (A) Patient A-II-1. Some erythrocytes are stomatin immunoreactive (immunoreactive erythrocyte, IRE) but most are nonimmunoreactive (NIRE). All platelets (P) and the neutrophil (N) are immunoreactive. One lymphocyte is immunoreactive (immunoreactive lymphocyte, IRL), but the other is not (NIRL). (B) Patient B-II-1 shows a similar picture to patient A-II-1: mixed population of red cells; all platelets immunoreactive; and nonimmunoreactive lymphocyte. (C) Healthy control blood film. All erythrocytes are stomatin immunoreactive (IRE). All platelets are immunoreactive; there is a mixture of immunoreactive and nonimmunoreactive lymphocytes. (D-F) Negative controls, in which antistomatin antibody was omitted. (D) Patient A-II-1. Negative control and no first antibody. Neutrophil (N) and erythrocytes are all negative. (E) Patient B-II-1. Negative control omitting first antibody. (F) Normal blood film, negative control. Bar represents 10 μm.
To clarify whether the variable stomatin immunoreactivity in OHSt cells might depend on the maturity of the erythrocytes, we attempted to separate young cells by centrifugation in Wintrobe tubes for immunoincubation. When intact peripheral red cells from both A-II-1 and B-II-1 (with healthy controls) were fractionated by centrifugation (Table 1), the relatively large, high mean red cell volume (MCV) cells with a low mean red cell hemoglobin concentration (MCHC) and higher intracellular Na+ content were found in the uppermost fraction, as in normal blood. In the healthy control blood sample, this upper fraction was enriched in reticulocytes (0.018 [1.8%] in the uppermost fraction compared with 0.002 [0.2%] in the lower), as expected. In both patients, by contrast, there was a degree of relative enrichment of reticulocytes in the lower fractions (eg, in A-II-1, 0.133 [13.3%] in the lower fraction compared with 0.077 [7.7%] in the upper). Immunocytochemistry on these fractions showed no major difference in stomatin expression. We were unable to improve on this reticulocyte separation by any other centrifugal method.
Centrifugation of normal (Ctrl) and OHSt blood in Wintrobe tubes
. | MCV, FI . | . | . | MCHC, g/dL . | . | . | Reticulocyte count, proportion of red blood cells . | . | . | [Na+], mM cells . | . | . | [K+], mM cells . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | ||||||||||
| Whole blood | 136.4 | 138.9 | 83.3 | 27.0 | 25.3 | 34.7 | 0.1019 | 0.233 | 0.008 | 64.3 | 62.3 | 16.7 | 42.0 | 41.4 | 96.5 | ||||||||||
| 1 (upper) | 151.5 | 145.5 | 86.0 | 23.4 | 24.3 | 33.3 | 0.077 | 0.19 | 0.018 | 72.47 | 76.93 | 19.12 | 42.52 | 40.89 | 101.6 | ||||||||||
| 2 | 142.8 | 137.8 | 84.0 | 25.9 | 25.4 | 34.1 | 0.088 | 0.22 | 0.009 | 67.19 | 70.97 | 17.15 | 41.96 | 40.06 | 97.62 | ||||||||||
| 3 | 137.2 | 132.7 | 82.7 | 26.5 | 26.6 | 35.1 | 0.099 | 0.25 | 0.005 | 62.62 | 68.25 | 17.05 | 44.6 | 40.51 | 97.28 | ||||||||||
| 4 (lower) | 126.5 | 121.2 | 78.3 | 29.4 | 29.3 | 37.1 | 0.133 | 0.26 | 0.002 | 55.36 | 72.75 | 18.97 | 52.42 | 38.93 | 91.9 | ||||||||||
. | MCV, FI . | . | . | MCHC, g/dL . | . | . | Reticulocyte count, proportion of red blood cells . | . | . | [Na+], mM cells . | . | . | [K+], mM cells . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | A-II-1 . | B-II-1 . | Ctrl . | ||||||||||
| Whole blood | 136.4 | 138.9 | 83.3 | 27.0 | 25.3 | 34.7 | 0.1019 | 0.233 | 0.008 | 64.3 | 62.3 | 16.7 | 42.0 | 41.4 | 96.5 | ||||||||||
| 1 (upper) | 151.5 | 145.5 | 86.0 | 23.4 | 24.3 | 33.3 | 0.077 | 0.19 | 0.018 | 72.47 | 76.93 | 19.12 | 42.52 | 40.89 | 101.6 | ||||||||||
| 2 | 142.8 | 137.8 | 84.0 | 25.9 | 25.4 | 34.1 | 0.088 | 0.22 | 0.009 | 67.19 | 70.97 | 17.15 | 41.96 | 40.06 | 97.62 | ||||||||||
| 3 | 137.2 | 132.7 | 82.7 | 26.5 | 26.6 | 35.1 | 0.099 | 0.25 | 0.005 | 62.62 | 68.25 | 17.05 | 44.6 | 40.51 | 97.28 | ||||||||||
| 4 (lower) | 126.5 | 121.2 | 78.3 | 29.4 | 29.3 | 37.1 | 0.133 | 0.26 | 0.002 | 55.36 | 72.75 | 18.97 | 52.42 | 38.93 | 91.9 | ||||||||||
Cells were stored overnight in CPDa anticoagulant and centrifuged in Wintrobe tubes at 2000g for 30 minutes at 20°C. The packed cells were then separated by aspiration into 4 fractions (no. 1 uppermost) after which the cells were reconstituted in plasma to original hematocrit. Red cell indices were measured in a Sysmex SE900 counter (Milton Keynes, United Kingdom) and intracellular [Na+] and [K+] were determined on washed cells by flame photometry. Indices on whole blood (ie, prior to centrifugation) are shown for comparison. Effective separation by density was achieved, as evidenced by the lower MCHC in the upper fractions, but while the reticulocytes were enriched in the upper fraction in normal blood (0.018 in the uppermost fraction compared with 0.002 in the lowest), the reticulocytes were enriched in the lower fraction in both patients (0.133 and 0.26 in lower fractions compared with 0.077 and 0.19 in uppermost). The specimens used here were stored overnight. In some leaky red cell conditions, notably cryohydrocytosis, very marked changes in red cell electrolytes can occur on cold storage, but neither pedigree here shows such an effect.
Therefore, flow cytometry was used to correlate stomatin expression with cell age. In the studies shown in Figure 4A-D, we correlated stomatin immunoreactivity (y-axis) with thiazole orange staining (x-axis) on cells fixed and permeabilized as in “Patients, materials, and methods.” The plots are divided into 4 regions, delimiting stomatin-immunoreactive (upper regions, W and X) and thiazole-positive (right-hand-side regions, X and Z) cells. In the healthy control samples (Figure 4C-D), essentially all erythrocytes (> 98%) showed relatively intense stomatin immunoreactivity and the number of reticulocytes was small; most cells were in region W and were stomatin-positive and thiazole-negative. In the patient samples (A-II-1, Figure 4A; B-II-1, Figure 4B), stomatin immunoreactivity (y-axis) was always lower than in healthy controls, but in about 15% to 20% of cells it was significantly greater than baseline, consistent with the immunocytochemistry (Figure 3). In both patients, the majority of the reticulocytes (those cells in regions X or Z) were stomatin-positive, that is, found in region X. In patient A-II-1 (Figure 4A), 24% of the red cells were stomatin-positive (regions X + W) and of these stomatin-positive cells, 23% were thiazole-positive (X). Of the cells, 76% were stomatin-negative (Y + Z), but of these only 6% (Z) were thiazole-positive.
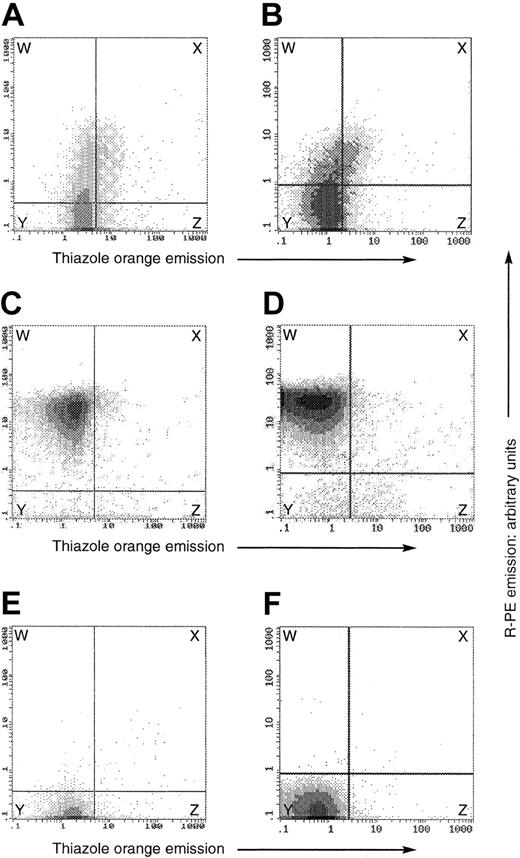
Flow cytometry on fixed and permeabilized, OHSt and normal, red cells. In all plots, the y-axis denotes the emission of the R-PE label conjugated to the secondary antibody in the stomatin detection system, whereas the x-axis denotes thiazole orange fluorescence emission. Both are expressed in arbitrary units. The panels show data from separate experiments on peripheral blood of patient A-II-1 (top row) and patient B-II-1 (lower row). In each experiment, we analyzed a patient (A-B) and a healthy control (C-D) in the presence of the antistomatin antibody and thiazole orange, and then the healthy control in the presence of thiazole only (E-F). The horizontal and vertical demarcation boundaries were set by the control experiments on normal blood shown in panels E-F, in which normal blood, containing about 1% reticulocytes, was subjected to the same analysis in the absence of the antistomatin antibody, defining the threshold fluorescence of the antibody detection method. In the normal samples (C-D), essentially all erythrocytes were stomatin-positive and thiazole-negative, and were present in the upper left box, labeled W. In patients A-II-1 and B-II-1 (panels A and B, respectively), the stomatin expression was always less than that of healthy controls, but nevertheless positive in a minority of the cells (regions W + X), quantitated at 23.6% in patient A-II-1 and 16% in patient B-II-1. In each OHSt case, about 20% to 25% of the stomatin-positive cells were also thiazole-positive (region X, panels A-B), whereas only about 5% of the stomatin-negative cells were thiazole-positive (region Z, panels A-B). Thus stomatin-positive cells tended also to be thiazole-positive.
Flow cytometry on fixed and permeabilized, OHSt and normal, red cells. In all plots, the y-axis denotes the emission of the R-PE label conjugated to the secondary antibody in the stomatin detection system, whereas the x-axis denotes thiazole orange fluorescence emission. Both are expressed in arbitrary units. The panels show data from separate experiments on peripheral blood of patient A-II-1 (top row) and patient B-II-1 (lower row). In each experiment, we analyzed a patient (A-B) and a healthy control (C-D) in the presence of the antistomatin antibody and thiazole orange, and then the healthy control in the presence of thiazole only (E-F). The horizontal and vertical demarcation boundaries were set by the control experiments on normal blood shown in panels E-F, in which normal blood, containing about 1% reticulocytes, was subjected to the same analysis in the absence of the antistomatin antibody, defining the threshold fluorescence of the antibody detection method. In the normal samples (C-D), essentially all erythrocytes were stomatin-positive and thiazole-negative, and were present in the upper left box, labeled W. In patients A-II-1 and B-II-1 (panels A and B, respectively), the stomatin expression was always less than that of healthy controls, but nevertheless positive in a minority of the cells (regions W + X), quantitated at 23.6% in patient A-II-1 and 16% in patient B-II-1. In each OHSt case, about 20% to 25% of the stomatin-positive cells were also thiazole-positive (region X, panels A-B), whereas only about 5% of the stomatin-negative cells were thiazole-positive (region Z, panels A-B). Thus stomatin-positive cells tended also to be thiazole-positive.
To confirm the results seen in Figure 4, we separated red cells from A-II-1 by binding to magnetic beads conjugated with an antihuman transferrin-receptor (CD71) antibody (Figure 5). The transferrin receptor is expressed for 2 to 4 days on immature red cells in the peripheral blood,38,39 and the surface expression of this protein can be used as an affinity ligand for purification of immature erythrocytes.40 All of the bound (ie, immature) red cells were stomatin-immunopositive on subsequent flow cytometry (Figure 5A), while the unbound cells (ie, the relatively more mature) were largely non–stomatin immunoreactive (Figure 5B), confirming that it was the younger cells that were stomatin immunoreactive. Control experiments confirmed that the bound cells were truly thiazole-positive (Figure 5C), whereas the unbound were largely thiazole-negative (Figure 5D).
Thus, using these 2 different techniques for the identification of younger red cells, we were able to show that younger cells were more stomatin immunoreactive.
In smears of a bone marrow sample from patient A-II-1, stomatin immunoreactivity was present in all erythrocyte progenitor cells, as shown for pronormoblasts and normoblasts (Figure 6A-B). The cord blood from the affected newborn A-III-1 (Figure 6F-G) showed marked erythroblastic activity. On this film, all identifiable progenitor red cells were stomatin immunoreactive. In addition to the homogenously distributed stomatin immunoreactivity in the cytoplasm of the normoblasts, a clumpy and condensed immunoreactive material was observed around the nucleus. Normoblasts from normal bone marrow showed the same stomatin-immunoreactive material around their nuclei (Figure 6D-E). Thus, in both normal and OHSt, some stomatin-immunoreactive material was seen to be extruded together with the nucleus.
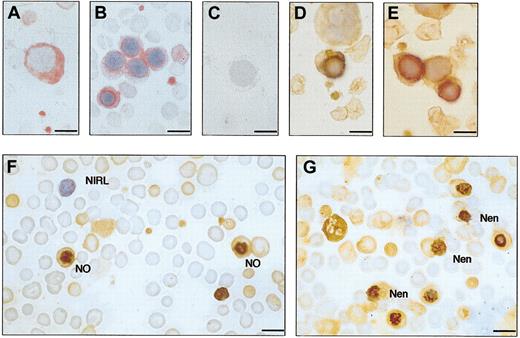
Immunocytochemistry of bone marrow and cord blood. (A-C) Patient A-II-1. Bone marrow, monoclonal antibody; and APAAP detection. (A) Pronormoblast. (B) Normoblasts. (C) Negative control, omitting primary antibody. (D-E) Normal bone marrow, showing normoblasts. Polyclonal antibody, ABC detection. (F-G) Patient A-III-1, cord blood. Polyclonal antibody, ABC detection. Most red cells are nonimmunoreactive. In panel F, 2 normoblasts (NO) are seen with stomatin immunoreactivity at the nucleus. One nonimmunoreactive lymphocyte (NIRL) is seen. In panel G, normoblasts extruding the nucleus can be seen (Nen). Counterstained with hematoxylin. Bar represents 10 μm.
Immunocytochemistry of bone marrow and cord blood. (A-C) Patient A-II-1. Bone marrow, monoclonal antibody; and APAAP detection. (A) Pronormoblast. (B) Normoblasts. (C) Negative control, omitting primary antibody. (D-E) Normal bone marrow, showing normoblasts. Polyclonal antibody, ABC detection. (F-G) Patient A-III-1, cord blood. Polyclonal antibody, ABC detection. Most red cells are nonimmunoreactive. In panel F, 2 normoblasts (NO) are seen with stomatin immunoreactivity at the nucleus. One nonimmunoreactive lymphocyte (NIRL) is seen. In panel G, normoblasts extruding the nucleus can be seen (Nen). Counterstained with hematoxylin. Bar represents 10 μm.
In the study shown in Figure 3, we observed that the stomatin immunoreactivity of lymphocytes could be positive or negative in both patient and control. We examined splenic tissue from patient A-II-1 and controls to see if a pattern of stomatin immunoreactivity could be related to B- or T-cell areas of the white pulp (Figure 7). However, there was no correlation between the stomatin immunoreactivity and T- or B-cell distribution. In the red pulp of the normal spleen, stomatin immunoreactivity was striking due to erythrocytes, platelets, and leukocytes. As expected, the red pulp of OHSt spleen was less stomatin immunoreactive.
Immunocytochemistry of spleen. Serial sections were prepared and stained with anti-CD3 (A-B) and anti-CD20cy (C-D) and antistomatin (E-F) antibodies. Patient (A-II-1) sections are shown in the left column (A,C,E,G) against a normal spleen in the right-hand column (B,D,F,H). T-cell and B-cell areas of the white pulp surrounding a central artery are identified by staining with specific antibodies. In neither normal nor OHSt spleen was stomatin immunoreactivity selectively associated with predominantly T- or B-cell areas of the spleen. In normal spleen (F), the red pulp that is distant from the central artery is strongly stomatin immunoreactive, consistent with the concentrated red cell population, whereas in the patient (E), the equivalent area is less strongly positive. Note the specific stomatin-positive staining of the arterial endothelium in panels E-F (arrows). (G-H) Negative controls for antistomatin antibody (G) and murine monoclonal antibodies (H). Bar represents 10 μm.
Immunocytochemistry of spleen. Serial sections were prepared and stained with anti-CD3 (A-B) and anti-CD20cy (C-D) and antistomatin (E-F) antibodies. Patient (A-II-1) sections are shown in the left column (A,C,E,G) against a normal spleen in the right-hand column (B,D,F,H). T-cell and B-cell areas of the white pulp surrounding a central artery are identified by staining with specific antibodies. In neither normal nor OHSt spleen was stomatin immunoreactivity selectively associated with predominantly T- or B-cell areas of the spleen. In normal spleen (F), the red pulp that is distant from the central artery is strongly stomatin immunoreactive, consistent with the concentrated red cell population, whereas in the patient (E), the equivalent area is less strongly positive. Note the specific stomatin-positive staining of the arterial endothelium in panels E-F (arrows). (G-H) Negative controls for antistomatin antibody (G) and murine monoclonal antibodies (H). Bar represents 10 μm.
A sample of liver was available from patient A-I-2. Immunocytochemistry revealed stomatin-positive hepatocytes with the immunoreaction product closely located to the plasma membrane of the entire circumference of the cell in both patient A-I-2 (Figure 8C) and healthy control (Figure 8A). In the patient, there is additionally in the cytoplasm a large amount of brownish hemosiderin deposits interfering with the immunoreaction product (Figure 8D), but the Prussian blue reaction shown in Figure 8E (in which the iron is stained blue and the nuclei red) shows the distinction between the stomatin immunoreactivity and iron. This can clearly be distinguished from the membrane-associated immunoreaction product (Figure 8E).
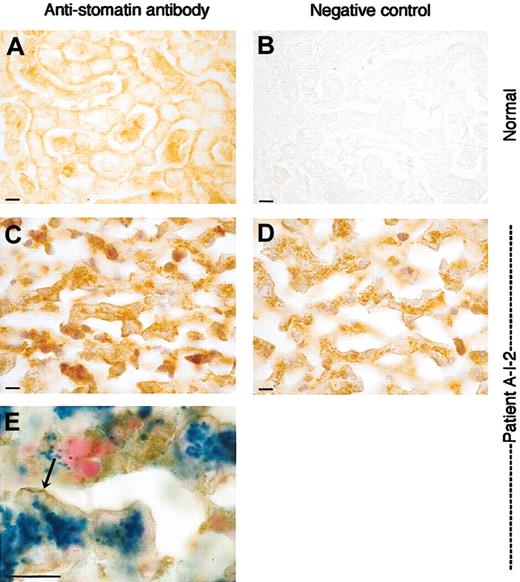
Liver sections stained with antistomatin antibody. Healthy control and OHSt patient. Detail of liver parenchyma showing the trabeculae of hepatocytes and sinusoids. Upper panels (A-B): normal liver; lower panels (C-E): patient A-I-2. (A) Stomatin immunoreactivity in normal liver is associated to the plasma membrane of the hepatocytes. Note the positive staining of red and white blood cells in the sinusoids. (B) Normal liver, negative control, and omitting antistomatin antibody. (C) Patient A-I-2: antistomatin antibody, nuclei counterstained with hematoxylin. As in the control, stomatin immunoreactivity is found associated to the plasma membrane of hepatocytes. Sinusoids are nearly free from red cells due to the fatal hemolytic crisis but show some immunopositive white blood cells. (D) Patient A-I-2: negative control, omitting antistomatin antibody, showing discrete granular brownish deposits of hemosiderin in the cytoplasm. Nuclei were counterstained with hematoxylin. (E) Stomatin immunostaining combined with Prussian blue reaction. Note the blue ferrocyanide-positive deposits in the cytoplasm and the brownish stomatin immunoreaction product that is accentuated at the plasma membrane (arrow). Bar represents 10 μm.
Liver sections stained with antistomatin antibody. Healthy control and OHSt patient. Detail of liver parenchyma showing the trabeculae of hepatocytes and sinusoids. Upper panels (A-B): normal liver; lower panels (C-E): patient A-I-2. (A) Stomatin immunoreactivity in normal liver is associated to the plasma membrane of the hepatocytes. Note the positive staining of red and white blood cells in the sinusoids. (B) Normal liver, negative control, and omitting antistomatin antibody. (C) Patient A-I-2: antistomatin antibody, nuclei counterstained with hematoxylin. As in the control, stomatin immunoreactivity is found associated to the plasma membrane of hepatocytes. Sinusoids are nearly free from red cells due to the fatal hemolytic crisis but show some immunopositive white blood cells. (D) Patient A-I-2: negative control, omitting antistomatin antibody, showing discrete granular brownish deposits of hemosiderin in the cytoplasm. Nuclei were counterstained with hematoxylin. (E) Stomatin immunostaining combined with Prussian blue reaction. Note the blue ferrocyanide-positive deposits in the cytoplasm and the brownish stomatin immunoreaction product that is accentuated at the plasma membrane (arrow). Bar represents 10 μm.
We investigated the stomatin gene in both pedigrees. The pedigree of family A was of just sufficient size perhaps to be able to exclude the stomatin locus (9q32-349 ) by mapping. The intragenic stomatin CA repeat previously described by Gallagher and Forget41 was not informative in this pedigree. Studies using the markers D9S1776, D9S1682, and D9S290, which flank the stomatin locus, showed that the same haplotype (183/150/243, boxed) on chromosome 9 was shared by all 4 affected patients (Figure 1); thus we were not able to exclude the stomatin locus by mapping. Northern blots (previously published5 ) showed normal-sized products. Southern blots, probed with nucleotides 1 to 1357 of the cDNA, showed the expected pattern of restriction fragments (not shown) among the introns involved in protein coding.
The Ensembl stomatin consensus sequence is found at www.ensembl.org/Homo_sapiens/geneview?gene=ENSG00000148175. The sequences determined by direct sequencing of both reverse transcriptase (RT)–PCR products and of at least 6 clones of reticulocyte mRNA were essentially identical to that published by Unfried et al.35 In the coding region, the base immediately preceding the initiator ATG was in all cases a C and not a T as described by Gallagher and Forget.41 At position 388 in the cDNA, all cases showed a C as described by Unfried et al,35 rather than the G described by this group5 and others.41 This is a conservative third base change. On this cDNA sequencing we also found some point changes that were thought to be PCR errors. We amplified the 7 exons from genomic DNA and directly sequenced the amplification products, using primers described in Supplemental Table S1. We found an A-or-G polymorphism at position 2840 on the cDNA (within the 3′ untranslated region [UTR]; 2125 within exon 7), which alters ScaI susceptibility. This segregated with the paternal 175/150/253 haplotype in family A (Figure 1) and was not relevant. No abnormalities were found in the 20 intronic base pairs flanking any exon.
Discussion
The new finding in this report is that stomatin is not simply absent from red cells and their progenitors in OHSt; it appears to be synthesized early in red cell development, but is then lost as the cell matures. Thus, stomatin immunoreactivity is present in some red cells from OHSt peripheral blood. The Western blots (Figure 2) show that the red cells in OHSt display about one twentieth to one fiftieth the normal amount of stomatin as healthy controls. Flow cytometry confirmed that even immature OHSt red cells are never as strongly stomatin immunoreactive as normal cells. These results underline the light microscopical observations that we find in OHSt red cells. The immunoreactivity is weak, just above the detection level, and barely discriminates positive from negative cells, which might reflect the gradual loss of the protein. Flow-cytometric studies, combining quantitative measurements of stomatin expression and cell age, indicate that it is the younger red cells that show the immunoreactivity. These studies quantitating the amount of stomatin in red cells recall previous studies in which the amount of this protein was found to be decreased, on Coomassie blue staining of one-dimensional SDS gels.42,43
The studies of both bone marrow and cord blood indicate that a substantial amount of stomatin is removed from the cell with the extruding nucleus during the maturation of the normoblast in both OHSt and normal bone marrow. Thus, as far as we can tell from these morphologic data, the loss of stomatin in OHSt seems to occur during the maturation of the reticulocyte after extrusion of the nucleus from the normoblast. As the flow cytometry shows that even the immunopositive OHSt cells in the peripheral blood have markedly reduced stomatin immunoreactivity compared with normal red cells, we suggest that the deletion process begins in the bone marrow. According to the magnetic bead separation studies (Figure 5), the loss of stomatin parallels the loss of the transferrin receptor. This may or may not be a coincidence, but it is possible that for some reason the stomatin is being subject to the same degradative processes as other proteins that are removed from the red cell during reticulocyte maturation, for instance in the exosomes in which the transferrin receptor exits from the red cell.44
It remains unclear why stomatin should be lost in this way. The loss of stomatin (for whatever reason) seems unlikely to be a direct cause of the cation leak, given the lack of phenotype in the murine knock-out.19 Nevertheless, the loss of stomatin could be directly associated with the causative lesion, perhaps by virtue of a mutated binding site in a membrane protein that is required for the stability of stomatin in red cells. A primary abnormality in lipid structure of the membrane, examples of which are seen in the non–stomatin-deficient forms of HSt,45,46 could conceivably disrupt the association of the protein with the membrane. Alternatively, the loss of stomatin could be the result of the cation leak. In these cells, 2 secondary effects of the cation leak are evident. Increases in both NaK pump activity and glycolytic activity47 are seen. The stomatin protein has been coimmunoprecipitated with a glucose transporter.48 There may be an inverse relation between Glut 1 glucose transporter activity and stomatin expression49 ; it is conceivable that the up-regulation of glycolytic activity in these cells is somehow associated with the down-regulation of stomatin. It is also possible that the loss of stomatin could represent some kind of regulatory process designed to silence an ion channel. In coexpression studies in oocytes, Goodman et al have recently shown that MEC-2, a homologue of stomatin, increases ion transport via ENaC-type channels.50 Perhaps stomatin is deleted from the red cell in an attempt to down-regulate ion transport in these leaky cells. Such a process could be consistent with the idea of Tavernarakis et al that the stomatin family was generally concerned with control of surface expression of membrane proteins.18 The recent observation that stomatin is contained in exosomes extruded from normal red cells in which the intracellular Ca2+ has been increased by an ionophore may offer a mechanism for the loss of stomatin from these red cells.51 The true molecular answer to these problems will hopefully emerge from mapping studies in related leaky red cell conditions, where large pedigrees are available,11,52 although the locus heterogeneity within these conditions12 has hampered identification of mutant genes.
Like others7,8 we have found no mutation in the stomatin cDNA in these patients. The mapping study that we performed on the small family A pedigree showed that the same haplotype spanning the stomatin locus was present in all 4 affected individuals in this family; this result by no means proves that this locus is involved, but at the same time does not rule it out. A mutation in the promoter is possible, but it is difficult to see how a mutation in only one promoter sequence could influence stomatin transcription from the other allele in this dominantly-inherited and therefore heterozygous condition. Other mapping studies9,11,12,41,53 in the more common non–stomatin-deficient examples of these leaky stomatocytic membrane diseases have never pointed to the stomatin locus on chromosome 9.54 The assertion that the gene is normal, with the corollary that some other gene is responsible for the disease, is consistent with the lack of overt phenotype in the recessive stomatin knock-out mouse.19
The centrifugation studies performed here (Table 1) illustrate how young and old peripheral red cells do not separate by density in the normal way in OHSt. This result recalls the pedigree described by Oski et al55 in which, after centrifugation, the reticulocytes were found in the most dense fraction rather than in the least dense, the normal result. In Oski et al's case it was clear that the cells became more overhydrated as they aged in the circulation, because either the cells became leakier as they matured or because the NaK pump lost activity. Although no studies of stomatin were made in the family studied by Oski et al, it is possible that this condition was a mild version of OHSt, and it is possible that in this pedigree, the stomatin may have been lost more slowly than in our study. Unfortunately, this large Philadelphia pedigree is now lost to follow-up (P. G. Gallagher, written personal communication, July 2001) and has not been studied, but further study of this family could be key to understanding many of these points, and would also offer an invaluable mapping opportunity.
In the Western blotting studies, the tests identified in normal cells at high exposure (Figure 2A-B) a principal band at 32 kDa (ie, stomatin) and a series of bands at higher molecular weight. These higher bands were not present in the patient, and it seems likely that these higher bands represent oligomers of stomatin itself rather than other proteins, which would be expected to be seen in the patient as well. The polyclonal antibody did not bind to SLP1 or SLP2 (Figure 2C). These data are in accordance with the results of Snyers et al56 showing that structure of stomatin in a human epithelial cell line of amniotic origin is oligomeric.
This work extends understanding of the distribution of stomatin in the peripheral blood cells. Strong stomatin immunoreactivity was seen in both platelets and neutrophils, and there was no difference between patients and controls. In both healthy controls and patients, peripheral lymphocytes fell into stomatin-positive and -negative categories, and this was not simply correlated to B- and T-cell populations in the spleen, a major-blood filtering organ, on which we focused in this study.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2002-06-1705.
Supported by The Wellcome Trust, Action Research, The Sir Jules Thorn Trust, Telethon (prog GP0202Y02), and by the University of Foggia to A.I.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients for their generous cooperation and hospitality; Dr Karl Kadler for laboratory facilities; Prof N. M. Hooper and Dr E. T. Parkin (University of Leeds) for assistance with raft studies; Mr Keith Miller at the Department of Histopathology, University College Hospital, for assistance with bone marrow immunocytochemistry; Drs J. R. Trounce, S. M. Jowitt, and A. M. Will for permission to report their patients; Dr M. Harvey and colleagues for useful discussions; Katja Rumpf and Luzie Augustinowski for technical assistance; Dr Jürgen Nowotny, Haematological Department of the University Essen and Prof Klaus-Michael Müller, Department of Pathology at the Ruhr-University for donation of control tissue samples; and Huw Jones, University College London, for help with photography.

![Figure 2. Western blots of different cell and tissue preparations. (A) Normal and OHSt red cell membranes. Blots 1A, IB, and II were probed with rabbit polyclonal serum, and blot III was probed with mouse monoclonal. Blots IA, IB, and III show comparison of patient A-II-1 with healthy control, whereas blot II shows patient B-II-1. At the shorter ECL exposure time (blot IB), no signal can be seen in the “patient” lane (P), but at the longer exposure time (IA, II), further bands at higher molecular weight are evident in the “healthy” lane (N), which most likely represent oligomers of stomatin itself, since they are not evident in the patient lane. In blot III, the monoclonal antibody identifies further bands at slower migration (*), which probably represent nonspecific binding since the bands are present in both samples. (B) Probing of dilution series of normal red cell membranes with polyclonal antistomatin antibody. Serial dilutions of normal red cell membranes were run on a 12% polyacrylamide gel, blotted to nitrocellulose, and probed with the rabbit polyclonal antibody using a long ECL exposure time. At low dilutions, bands at 55 to 65 kDa are evident, as in blots IA and II seen in panel C. (C) Test of binding of polyclonal antibody to SLP1 and SLP2, in the form of glutathione-s-transferase (GST) fusion proteins expressed in Escherichia coli. Upper panel, Coomassie stain; lower panel, antistomatin antibody Western blot of an identical gel. Lanes 1 to 4 in both panels: E coli lysates containing isopropyl β-D-thiogalactopyranoside (IPTG)–induced fusion proteins (lane 1, GST-SLP1; lane 2, GST-SLP2; lane 3, GST alone; lane 4, GST-stomatin), (aa 144-288; lane 5, molecular weight markers; lane 6, normal red cell membranes; lanes 8-9, 1:50 and 1:100 dilutions of GST-stomatin [aa 144-288] sample used in lane 4). Letters indicate positions of protein bands of interest as follows: a, GST-SLP1; b, GST-SLP2; c, GST alone; d, GST-stomatin (aa 140-288); e, stomatin in normal red cell membranes; f, 1 in 50 dilution of stomatin GST (aa 140-288); and g, 1 in 100 dilution of same. The antibody binds only to GST-stomatin (aa 140-288) (bands d, f, g) and native stomatin in red cells (band e), and not to SLP1 or SLP2. (D) Subcellular fractionation of nonerythrocyte circulating cells from normal blood, probed with polyclonal antibody. Platelets, neutrophils, lymphocytes, and monocytes were isolated as described in “Patients, materials, and methods.” Purities of more than 90% were achieved for each cell type, with less than 0.1% contaminating erythrocytes. Subcellular fractions were isolated by centrifugation on a sucrose step gradient, as described in “Patients, materials, and methods.” In each cell type, all fractions except cytosol show a positive band at 32 kDa. (E) Shown are 4 samples of normal and 1 sample of OHSt liver (patient A-I-2) probed with polyclonal antibody. A predominant single band at 32 kDa is evident. (F) Association of stomatin protein with buoyant, cholesterol + sphingomyelin–rich, Triton-insoluble material purified from normal and OHSt red cell membranes. Red cell membranes were treated with cold Triton X-100 and subjected to sucrose gradient ultracentrifugation.36 Fractions were aspirated and run on 12% SDS polyacrylamide gels and blotted to nitrocellulose. Left-most lanes reflect the lower part of the gradient (45% sucrose wt/vol), whereas the right-most reflect the upper part of the gradient (0% sucrose). The blots were probed with polyclonal antistomatin antibody (upper panels) and antiflotillin antibody (lower panels). The upper panels show that about half of the stomatin was associated with this buoyant raft material, while the remainder was not. The lower panels confirm, first, that flotillin is present in both normal and abnormal red cells, second, that flotillin is exclusively associated with rafts in both cell types, and third, that even in the presence of a deficiency of stomatin, rafts still exist in these abnormal cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/6/10.1182_blood-2002-06-1705/6/m_h81834927002.jpeg?Expires=1767967991&Signature=DWqS9CjDSysK5FzLu0x7u6H0oZYy7eWRHm2CpyArfvGb1ZC0G8a0H2kqj5JKh3XXi2ThnrBB-eFHQGg4aK9CO~UN~wRmG7vSy6CkzM60P4Ro6NrtaqBJ6-ntgM7x93xfaIR8Q0Z1K2qPlHL43DJQYunjnfGOZBu0pCGcKgg2cKQGFPcO2ds3DX97Enk8A2nMDaBoYhnwRdIthDYO0v5IX0DAdS1mmsWptle5svhF1TTOhJcymTfRJ6PZHBhjY7vymsGS3d7fvWCBHPnBciXl6PcE80QLhkZAEDdXYT9K7WtqObMF8oEoa7msRSmO5d0hDtZ9Rzwr3lMpcOMtZqqDOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal