Abstract
There were 21 patients with rapidly progressive multiple sclerosis (MS) treated on a phase 1/2 study of intense immune suppressive therapy and autologous hematopoietic stem cell (HSC) support with no 1-year mortality. Following transplantation, one patient had a confirmed acute attack of MS. Neurologic progression defined by the expanded disability status scale (EDSS) did not increase in disability by 1.0 or more steps in any of 9 patients with a pretransplantation EDSS of 6.0 or less. In 8 of 12 patients with high pretransplantation disability scores (EDSS > 6.0), progressive neurologic disability as defined by at least a 1-point increase in the EDSS has occurred and was manifested as gradual neurologic deterioration. There were 2 patients with a pretransplantation EDSS of 7.0 and 8.0 who died from complications of progressive disease at 13 and 18 months following treatment. Our experience suggests that intense immune suppression using a total body irradiation (TBI)-based regimen and hematopoietic stem cell transplantation (HSCT) are not effective for patients with progressive disease and high pretransplantation disability scores. Further studies are necessary to determine the role of intense immune suppressive therapy and HSC support in ambulatory patients with less accumulated disability and more inflammatory disease activity. Specifically, more patients and longer follow-up would be required in patients with an EDSS of 6.0 or less before drawing conclusions on this subgroup. (Blood. 2003;102:2373-2378)
Introduction
Although a disease with low mortality, multiple sclerosis (MS) has significant long-term morbidity for which no current treatment is curative.1 Most patients with MS present with intermittent symptomatic events termed “relapsing-remitting disease.” Over time most such patients eventually develop secondary progressive disease manifested as gradual neurologic impairment often progressing without acute relapses, although acute relapses may persist.2-6 Natural history studies show that by 15 years following disease onset, at least 50%, and by 30 years at least 80%, of subjects lose the ability to ambulate without support.6-9
Neurologic disability is defined at 0.5-step increments by the expanded disability status scale (EDSS) from zero (no disability) to 10.0 (death related to neurologic progression). At an EDSS of 4.0, a person has moderate disability but is able to ambulate without difficulty. At an EDSS of 6.0, a person requires support to ambulate, while at an EDSS of 7.0, they are no longer ambulatory for more than a few steps. The initial rate of disease progression is highly variable since the time that lapses between no disability (EDSS 0) to moderate disability (EDSS 4.0) varies from 1 to more than 30 years.10 The time that lapses from an EDSS of 4.0 to 7.0 is more predictable and generally varies from 9 to 12 years.
Immune-based therapies have moderate efficacy in reducing the relapse rate, but have not been proved efficacious at stopping disease progression once the relapsing-remitting phase has converted to a secondary progressive course. The current interpretation is that during the relapsing-remitting phase MS is mainly influenced by inflammatory demyelination and, consequently, responds to immune modulation.10 In contrast, progressive disability results from cumulative destructive damage to axonal nerve fibers. Despite common practice as a salvage therapy, there is no proven role for immune modulation in progressive disease. MS, therefore, appears to involve at least 2 pathophysiologic alterations, an immune-mediated demyelinating and an axonal degenerative process.11-14 Autoimmune demyelination dominates in early relapsing-remitting disease, while axonal degeneration predominates in progressive disease.
Hematopoietic stem cell transplantation (HSCT) is thought to have immunosuppressive and immune modulatory effects that may suppress immune-mediated demyelination.15 Due to the unknown risks of this therapy in patients with multiple sclerosis, most initial candidates in this study had progressive disease with high neurologic disability scores. While from ethical considerations, it was necessary to first treat only patients with high EDSS scores, it is likely that HSCT, a therapy that is meant to abrogate immune-mediated demyelination,16-26 may not be useful in altering the course of late progressive disease. Even if not beneficial, the intense immune suppression that accompanies HSCT may help to clarify the role, if any, for immune-based therapies that are currently used as salvage regimens for progressive MS.
Patients, materials, and methods
Subject selection
Subjects were considered for inclusion if they met Poser criteria27 for a diagnosis of clinically definite MS and had documented neurologic progression within the year prior to screening evaluation of at least 1.5 steps on the expanded disability status scale (EDSS)28 if their grade at entry was 6.0 or less, or at least 1.0 step if their EDSS at screening was 6.5 or greater. Baseline EDSS levels had to be sustained for at least 3 months, and be unresponsive to at least 6 months of interferon and steroid therapy in doses of at least 500 mg/day for 3 or more days. Initially candidates had to have an EDSS of 5.5 to 8.0 at time of enrollment. As the study progressed, these criteria were changed to an EDSS of 3.0 to 7.0. Candidates had to be younger than 55 years and have normal cardiac, renal, and hepatic function. The study was approved by the institutional review board of Northwestern University and the US Food and Drug Administration under IDE 6440.
Hematopoietic stem cell procurement
Hematopoietic stem cells (HSCs) in the first 2 subjects were collected from iliac crest bone marrow. Due to low yield, supplemental collection from peripheral blood was used to obtain the minimum of 2 × 106 CD34+ cells/kg body weight. Collections of HSCs from subsequent subjects were obtained solely from peripheral blood. Initially, HSCs were mobilized by administering granulocyte colony-stimulating factor (G-CSF, 10 μg/kg per day) for 4 to 5 days. Leukopheresis was performed on day 4, and if necessary to generate sufficient HSCs on day 5, using a continuous flow blood cell separator (either Fenwall CS3000; Baxter, Deerfield, IL, or Cobe Spectra, Lakewood, CO). Due to flare of disease activity in subject 4, while receiving G-CSF alone for mobilization, subsequent HSCs were collected by infusion of cyclophosphamide (2.0 g/m2) followed by daily G-CSF (5 μg/kg per day) beginning 72 hours after cyclophosphamide infusion. Leukopheresis was initiated when the white blood count (WBC) rebounded to more than 1 × 109/L (1000/μL) (usually 10 days after cyclophosphamide). The peripheral blood cells were enriched for CD34+ cells by passage through a CEPRATE (CellPro, Bothell, WA) or Isolex (Baxter, Chicago, IL) stem cell concentrator.
Conditioning regimen
Immune suppression was achieved over 6 days by administration of 60 mg/kg cyclophosphamide per day, intravenously for 2 days, followed by total body irradiation (TBI) in doses of 150 cGy, twice daily, for 4 consecutive days yielding a total dosage of 1200 cGy to the midplane at the level of the umbilicus. TBI was administered in the anteroposterior/posteroanterior (AP/PA) position with 50% dose attenuation to the lungs, 20% to the right lobe of the liver, and 30% to the kidneys. Radiation was delivered using 10 mV photons, at a dose rate of approximately 10 cGy/minute. In vivo dosimetry was performed on each subject to confirm the accuracy of radiation doses delivered to multiple body points. Methylprednisolone (1 g) was administered intravenously on each of the 4 days of TBI. HSCs were infused on the day following completion of TBI (day 0).
Supportive care
Subjects were treated in a high-efficiency particulate air (HEPA)-filtered medical unit or National Institutes of Health (NIH)-funded General Clinical Research Center. They were fed a low microbial diet and treated prophylactically with 400 mg/day fluconazole and 500 mg/day valacyclovir. During the period of intense myelosuppression, irradiated, leukocyte-depleted, and cytomegalovirus (CMV)-safe packed red blood cells and single-donor platelets were administered to keep hemoglobin levels higher than 8.0 mg/dL and platelet counts higher than 30 000/μL. Ciprofloxacin (750 mg orally twice a day [bid]) was given from admission until the absolute neutrophil count (ANC) was less than 0.5 × 109/L (500/μL), at which time intravenous piperacillin/tazobactam or cefipime was administered until the ANC rebounded to more than 0.5 × 109/L (500/μL). Fluconazole and valacyclovir were administered daily and trimethoprimsulfamethoxazole 3 days weekly for 6 months following HSC transfusion.
Outcome measures
Clinical outcomes. Standardized neurologic evaluations were performed by the neurologist-investigators at study visits scheduled at approximately 3, 6, and 12 months, and then yearly for 5 years. A patient's neurologic disability was rated using the Kurtzke EDSS by 0.5-step increments from 0 (normal neurologic exam) to 10 (dead).28 Worsening or improvement from baseline neurologic impairment was defined by a 1-step or greater change on the EDSS. An MS flare is defined as an acute neurologic deterioration lasting more than 24 hours occurring in the absence of fever or other intercurrent illness and manifesting objective neurologic changes on examination.
Magnetic resonance imaging (MRI) outcome. MRI scans of the brain were obtained at screening using proton density and T2-weighted turbo spin-echo, fluid attenuated inversion recovery (FLAIR), and pre- and postgadolinium-enhanced T1 spin-echo pulse sequences. Subsequent MRI scans of the brain were also obtained at or around the time of follow-up study visits. Change in lesion number was obtained by blindly presenting the base line and the most recent MRIs in a random order to a neuroradiologist. Measurements of third ventricular volume were calculated using the method of Simon.29
Results
Subjects ranged from 21 to 52 years of age and the duration of their MS from 9 months to 15 years at entry, with a duration of progressive neurologic disease of 0 to 15 years (Table 1). Mean posttransplantation follow-up for patients with an EDSS score of more than 6.0 was 2.6 years (range, 6 months to 5 years), for patients with an EDSS of 6.0 or less, posttransplantation follow-up 1.0 year (range, 1-2 years) (Table 2).
Patient baseline characteristics
Patient . | Age, at time of transplantation, y/sex . | Pretransplantation EDSS . | Type of MS . | Duration MS from diagnosis, y/duration progressive MS, y . |
|---|---|---|---|---|
| 1 | 43/F | 8.0 | SPMS | 3.5/2.5 |
| 2 | 34/F | 8.5 | SPMS | 14/8 |
| 3 | 35/F | 7.0 | PRMS | 15/15 |
| 4 | 40/M | 8.0 | PRMS | 4/4 |
| 5 | 44/F | 8.0 | SPMS | 4/2 |
| 6 | 21/F | 8.0 | SPMS | 3/* |
| 7 | 34/F | 6.5 | SPMS | 5/* |
| 8 | 47/M | 7.0 | SPMS | 7/* |
| 9 | 52/M | 7.5 | SPMS | 11/4 |
| 10 | 44/F | 3.0 | PRMS | 2/1 |
| 11 | 24/M | 3.5 | PRMS | 2/2 |
| 12 | 40/M | 7.0 | SPMS | 7/2 |
| 13 | 40/M | 7.0 | SPMS | 7/3 |
| 14 | 47/F | 5.5 | SPMS | 16/3 |
| 15 | 52/M | 6.0 | SPMS | 18/4 |
| 16 | 29/M | 3.5 | RRMS | 9 months |
| 17 | 28/M | 5.5 | PRMS | 5/1 |
| 18 | 28/M | 7.0 | PRMS | 10/* |
| 19 | 45/F | 6.0 | SPMS | 14/3 |
| 20 | 38/M | 5.0 | SPMS | 4/* |
| 21 | 51/F | 6.0 | SPMS | 12/* |
Patient . | Age, at time of transplantation, y/sex . | Pretransplantation EDSS . | Type of MS . | Duration MS from diagnosis, y/duration progressive MS, y . |
|---|---|---|---|---|
| 1 | 43/F | 8.0 | SPMS | 3.5/2.5 |
| 2 | 34/F | 8.5 | SPMS | 14/8 |
| 3 | 35/F | 7.0 | PRMS | 15/15 |
| 4 | 40/M | 8.0 | PRMS | 4/4 |
| 5 | 44/F | 8.0 | SPMS | 4/2 |
| 6 | 21/F | 8.0 | SPMS | 3/* |
| 7 | 34/F | 6.5 | SPMS | 5/* |
| 8 | 47/M | 7.0 | SPMS | 7/* |
| 9 | 52/M | 7.5 | SPMS | 11/4 |
| 10 | 44/F | 3.0 | PRMS | 2/1 |
| 11 | 24/M | 3.5 | PRMS | 2/2 |
| 12 | 40/M | 7.0 | SPMS | 7/2 |
| 13 | 40/M | 7.0 | SPMS | 7/3 |
| 14 | 47/F | 5.5 | SPMS | 16/3 |
| 15 | 52/M | 6.0 | SPMS | 18/4 |
| 16 | 29/M | 3.5 | RRMS | 9 months |
| 17 | 28/M | 5.5 | PRMS | 5/1 |
| 18 | 28/M | 7.0 | PRMS | 10/* |
| 19 | 45/F | 6.0 | SPMS | 14/3 |
| 20 | 38/M | 5.0 | SPMS | 4/* |
| 21 | 51/F | 6.0 | SPMS | 12/* |
Patients listed by order of enrollment on study.
y indicates years; MS, multiple sclerosis; SPMS, secondary progressive multiple sclerosis; PRMS, progressive relapsing multiple sclerosis; and RRMS, relapsing-remitting multiple sclerosis.
Duration of progressive disease could not be accurately determined from medical records.
Complications of hematopoietic stem cell transplantation
Patient . | Last follow-up, years after HSCT . | Change in EDSS from before HSCT to last follow-up . | Infections . | Other events . |
|---|---|---|---|---|
| 1 | 5 | 8.0 to 9.0 | Dermatomal zoster | Engraftment syndrome.* Transient asymptomatic hypotension |
| 2 | 5 | 8.5 to 9.0 | None | None |
| 3 | 3 | 7.0 to 8.5 | Urine Klebsiella, dermatomal zoster, sinusitis | Asymptomatic subdural hematoma |
| 4 | 4 | 8.0 to 8.0 | Blood Pseudomonas aeruginosa bacteremia | G-CSF-associated disease flare during mobilization |
| 5 | 4 | 8.0 to 9.0 | None | None |
| 6 | Died at 18 mo | 8.0 to 10 | None | Traumatic intracerebral hemorrhage. Died 18 months after HSCT from complications of progressive disease |
| 7 | 2 | 6.5 to 7.5 | None | Failure to thrive requiring enteral feeding |
| 8 | 2 | 7.0 to 7.0 | None | None |
| 9 | 6 mo | 7.5 to 9.0 | None | Engraftment syndrome* |
| 10 | 2 | 3.0 to 3.5 | None | None |
| 11 | 1 | 3.5 to 3.5 | None | None |
| 12 | 2 | 7.0 to 8.0 | Disseminated varicella zoster at 20 mo | Acute flare of MS at 14 months |
| 13 | Died 13 mo | 7.0 to 10 | None | Engraftment syndrome.* Transient asymptomatic hypotension. Died 13 months after HSCT from complications of progressive disease |
| 14 | 1 | 5.5 to 6.0 | None | None |
| 15 | 1 | 6.0 to 6.5 | None | Engraftment syndrome* |
| 16 | 2 | 3.5 to 1.0 | None | Atrial fibrillation from central line |
| 17 | 1 | 5.5 to 6.0 | None | Mild hemorrhagic cystitis |
| 18 | 1 | 7.0 to 7.5 | Clostridium difficule diarrhea | Engraftment syndrome,* transient asymptomatic hypotension |
| 19 | 1 | 6.0 to 6.0 | None | Peritransplantation vaginal bleeding, vaginal bleeding engraftment syndrome |
| 20 | 1 | 5.0 to 5.5 | None | None |
| 21 | 1 | 6.0 to 6.5 | None | Deep venous thrombosis and pulmonary emboli 2 mo after HSCT |
Patient . | Last follow-up, years after HSCT . | Change in EDSS from before HSCT to last follow-up . | Infections . | Other events . |
|---|---|---|---|---|
| 1 | 5 | 8.0 to 9.0 | Dermatomal zoster | Engraftment syndrome.* Transient asymptomatic hypotension |
| 2 | 5 | 8.5 to 9.0 | None | None |
| 3 | 3 | 7.0 to 8.5 | Urine Klebsiella, dermatomal zoster, sinusitis | Asymptomatic subdural hematoma |
| 4 | 4 | 8.0 to 8.0 | Blood Pseudomonas aeruginosa bacteremia | G-CSF-associated disease flare during mobilization |
| 5 | 4 | 8.0 to 9.0 | None | None |
| 6 | Died at 18 mo | 8.0 to 10 | None | Traumatic intracerebral hemorrhage. Died 18 months after HSCT from complications of progressive disease |
| 7 | 2 | 6.5 to 7.5 | None | Failure to thrive requiring enteral feeding |
| 8 | 2 | 7.0 to 7.0 | None | None |
| 9 | 6 mo | 7.5 to 9.0 | None | Engraftment syndrome* |
| 10 | 2 | 3.0 to 3.5 | None | None |
| 11 | 1 | 3.5 to 3.5 | None | None |
| 12 | 2 | 7.0 to 8.0 | Disseminated varicella zoster at 20 mo | Acute flare of MS at 14 months |
| 13 | Died 13 mo | 7.0 to 10 | None | Engraftment syndrome.* Transient asymptomatic hypotension. Died 13 months after HSCT from complications of progressive disease |
| 14 | 1 | 5.5 to 6.0 | None | None |
| 15 | 1 | 6.0 to 6.5 | None | Engraftment syndrome* |
| 16 | 2 | 3.5 to 1.0 | None | Atrial fibrillation from central line |
| 17 | 1 | 5.5 to 6.0 | None | Mild hemorrhagic cystitis |
| 18 | 1 | 7.0 to 7.5 | Clostridium difficule diarrhea | Engraftment syndrome,* transient asymptomatic hypotension |
| 19 | 1 | 6.0 to 6.0 | None | Peritransplantation vaginal bleeding, vaginal bleeding engraftment syndrome |
| 20 | 1 | 5.0 to 5.5 | None | None |
| 21 | 1 | 6.0 to 6.5 | None | Deep venous thrombosis and pulmonary emboli 2 mo after HSCT |
—indicates not applicable.
Engraftment syndrome is rash, fever, and fatigue.30 Patients listed by order of enrollment on study.
Safety
All 21 patients completed immune suppression and stem cell transplantation. Duration of inpatient hospitalization ranged from 3 to 4 weeks. Time to WBC higher than 10 ×109/L (1000/μL) varied from 7 to 14 days. Time to platelet count transfusion independence varied from 12 to 18 days, except 1 patient who required intermittent platelet transfusions for 2 months. The potentially only serious infection during hospitalization for HSCT was in one patient who developed fever and Pseudomonas bacteremia while neutropenic (Table 2). Late opportunistic infections were dermatomal zoster occurring in 2 patients and disseminated zoster at 20 months after HSCT requiring hospitalization in a third patient (Table 2).
One subject was found to have a small asymptomatic subdural hematoma on the 1-month follow-up MRI, which resolved spontaneously. Another subject was slow to engraft platelets, and continued to require intermittent platelet transfusions for 2 months following discharge. This subject fell while transferring from a wheelchair and struck her head on a cement curb resulting in a frontal intracerebral hemorrhage. She suffered additional neurologic impairment as a result of this traumatic hemorrhage confounding further assessment of any treatment. Her platelet count at the time of the injury was 88 000/mm3. This patient went on to develop progressive neurologic deterioration and died 18 months following the procedure.
A transient and reversible posttransplantation syndrome of rash, fever, and fatigue occurred in 5 patients (Table 2) and has been attributed to an engraftment syndrome that we have previously reported.30 Symptoms resolved either spontaneously or within 2 weeks of starting corticosteroids.
No mortality occurred within the first 100 days or within 1 year after HSCT. There were 2 patients with pre-HSCT EDSS scores of 7.0 and 8.0, 1 of whom was the patient who suffered head trauma, who died of complications related to progressive neurologic impairment at 18 and 13 months following HSCT, respectively. Both patients entered nursing homes within 6 months of HSCT and suffered neurologic decline eventually becoming unable to use their hands or upper extremities along with difficulty in swallowing. Autopsies were not performed and the final event(s) leading to death was undetermined.
Clinical neurologic outcomes
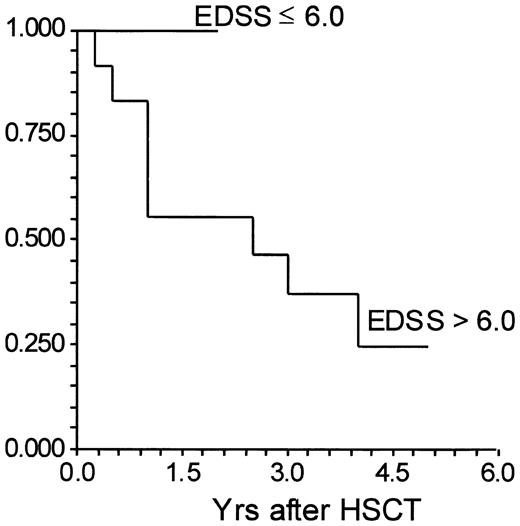
There was no correlation between disease progression and patient's age, type of MS, or disease duration, although the sample size was small. For 9 patients with pretransplantation EDSS of 6.0 or less, no patient has progressed by 1.0, 6 have progressed by 0.5, 2 patients remain unchanged, and 1 has improved by 2.5 EDSS steps (Tables 2, 3). All patients are alive. No patient has had a documented relapse. For 12 patients with pretransplantation EDSS of more than 6.0, the EDSS increased by 1.0 or more steps in 8 subjects (Table 3). Another 2 subjects with pretransplantation EDSS equal or greater then 6.5 have progressed by 0.5 steps on the EDSS; 2 subjects are unchanged. With pretransplantation EDSS scores of 7.0 and 8.0, 2 patients have died of complications related to neurologic deterioration (Table 2). Relapse was confirmed in one patient 14 months following transplantation (Table 2). The difference in progression by 1.0 or more EDSS steps between a pre-HSCT EDSS of 6.0 or less and an EDSS more than 6.0 approaches significance (P = .07) (Figure 1).
Change in EDSS following hematopoietic stem cell transplantation
Pre-HSCT EDSS . | No. of patients . | No progression . | Increase in EDSS by 0.5 point . | Increase by 1.0 EDSS point . | Increase by 1.5 EDSS points . | Increase by 2.0 or more EDSS points . |
|---|---|---|---|---|---|---|
| 3.0 to 6.0 | 9 | 3* | 6 | 0 | 0 | 0 |
| 6.5 to 8.5 | 12 | 2 | 2 | 4 | 2 | 2 |
Pre-HSCT EDSS . | No. of patients . | No progression . | Increase in EDSS by 0.5 point . | Increase by 1.0 EDSS point . | Increase by 1.5 EDSS points . | Increase by 2.0 or more EDSS points . |
|---|---|---|---|---|---|---|
| 3.0 to 6.0 | 9 | 3* | 6 | 0 | 0 | 0 |
| 6.5 to 8.5 | 12 | 2 | 2 | 4 | 2 | 2 |
One patient improved by 2.5 EDSS steps.
Time to progression by 1.0 EDSS point between 10 patients with a pretransplantation extended disability status scale (EDSS) of 6.0 or less and 18 patients with a pretransplantation EDSS of more than 6.0. HSCT indicates hematopoietic stem cell transplantation. Y-axis is percent failing by at least 1-point increase in the EDSS.
Time to progression by 1.0 EDSS point between 10 patients with a pretransplantation extended disability status scale (EDSS) of 6.0 or less and 18 patients with a pretransplantation EDSS of more than 6.0. HSCT indicates hematopoietic stem cell transplantation. Y-axis is percent failing by at least 1-point increase in the EDSS.
Magnetic resonance imaging
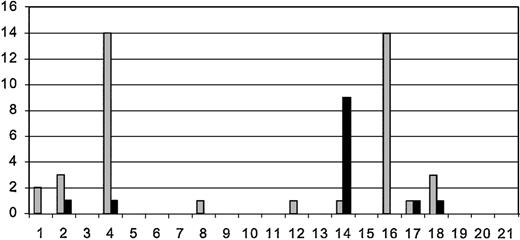
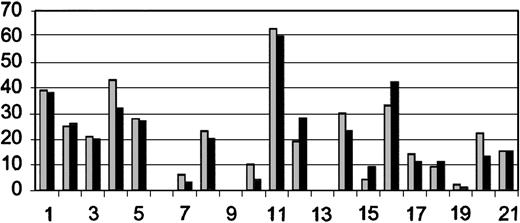
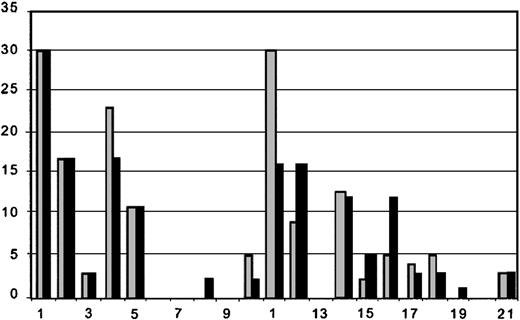
Pretransplantation MRIs were performed in all patients. Posttransplantation MRIs were performed on 18 of 21 patients. There were 2 patients who suffered neurologic decline and died and 1 who worsened who could not be transported for follow-up MRIs. Comparison between the pretransplantation and most recent post-transplantation MRIs suggests a trend toward reduction in numbers of gadolinium-enhancing lesions, T2 and T1 lesions. Although the number of gadolinium-enhancing lesions was generally reduced between pre- and most recent posttransplantation MRIs, 5 patients (3 with pretransplantation EDSS > 6.0 and 2 with pretransplantation EDSS ≤ 6.0) had small enhancing lesions on the posttransplantation MRI (Figure 2), suggesting persistent inflammatory disease activity. The number of T2 hyperintense lesions (Figure 3), which represents either acute (inflammation, edema, demyelination) or chronic (gliosis and axonal degeneration) central nervous system pathology, was reduced in 12 posttransplantation MRIs (6 with pretransplantation EDSS > 6.0 and 6 with pretransplantation EDSS ≤ 6.0) and unchanged in one patient. T2 lesion numbers were increased in 5 patients' posttransplantation MRIs (3 with pretransplantation EDSS > 6.0 and 2 with pretransplantation EDSS ≤ 6.0). The number of “black holes” or T1-weighted lesions (Figure 4) were reduced in 5 patients (2 with pretransplantation EDSS > 6.0 and 3 with pretransplantation EDSS ≤ 6.0), unchanged in 7 patients (5 with pretransplantation EDSS > 6.0, 2 with pretransplantation EDSS ≤ 6.0), and increased in 5 patients (2 with pretransplantation EDSS > 6.0 and 3 with pretransplantation EDSS ≤ 6.0). Third-ventricle diameter, which is a measure of brain atrophy, generally increased in posttransplantation MRIs. Third-ventricle dimensions (Figure 5) increased in 8 posttransplantation MRIs (3 with a pretransplantation EDSS > 6.0 and 5 with pretransplantation EDSS ≤ 6.0). Third-ventricle diameter decreased in only 2 patients (both with pretransplantation EDSS > 6.0) and was unchanged in the rest.
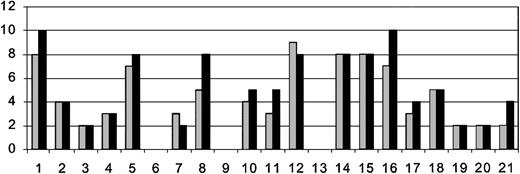
Number of MRI gadolinium lesions on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is the number of gadolinium-enhancing lesions.
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is the number of gadolinium-enhancing lesions.
Number of MRI gadolinium lesions on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is the number of gadolinium-enhancing lesions.
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is the number of gadolinium-enhancing lesions.
Number of T2 lesions on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T2-enhancing lesions.
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T2-enhancing lesions.
Number of T2 lesions on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T2-enhancing lesions.
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T2-enhancing lesions.
Number of T1 lesions on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T1 lesions.
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T1 lesions.
Number of T1 lesions on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T1 lesions.
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is number of T1 lesions.
Third-ventricle diameter on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is third-ventricle width (mm).
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is third-ventricle width (mm).
Third-ventricle diameter on pretransplantation and most recent posttransplantation MRI. Patients are listed on x-axis in order of enrollment. There were 3 patients who did not have posttransplantation MRIs for comparison. Order of patients and time between pre- ( ) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is third-ventricle width (mm).
) and posttransplantation (▪) MRIs are as listed in Table 2. The y-axis is third-ventricle width (mm).
Discussion
While no patients died within 1 year after HSCT, disease progression occurred in a significant number of patients. The EDSS score is a standard measure of MS-related neurologic disability, but it is not a linear scale and does not change at a constant rate. The average number of years a patient stays at each level before progressing has been reported to vary by the level at initial observation. The median time at an EDSS of between 4 and 6 or 7 and 8 before progressing to the next level in one large study is 4 years.31
It could, therefore, be anticipated that patients with an EDSS of between 6 and 8 would require on average 3 to 5 years to progress 1.0 EDSS step. However, in this study, 75% of patients with a pretransplantation EDSS more than 6.0 progressed in an average of 2.6 years. The effect of selecting patients with a recent worsening of EDSS on their expected time to subsequent progression might have opposing influences. It is possible that having just moved to a new step, they may constitute a group more likely to remain at the new level for a longer period of time (regression to the mean). Alternatively, patients selected for rapidly deteriorating progressive disability may have resulted in a study cohort with a more aggressive and rapidly declining natural history.
Although we cannot determine with certainty the natural rate of progression for an individual patient, the short-term progression seen in most patients with an EDSS of more than 6.0 suggests caution in performing HSCTs in this type of patient. The study also suggests that intense immune suppressive therapies may not be beneficial in late progressive MS. When the intensity of immune suppression in this study is considered, it seems unlikely that more modest immune suppressive agents would be more effective in advanced MS. Therefore, one conclusion from this study is that any immune suppressive therapies in MS patients without active inflammatory disease manifested by clinical relapses or enhancing lesions on MRI should be used with caution when performed outside of a clinical trial. Alternatively, it could be questioned whether at least one of the conditioning agents, despite being immune suppressive, contributed to axonal injury and accelerated the rate of axonal degeneration and neurologic disability. This concern arises because axonal degeneration becomes a more prominent feature in progressive when compared with relapsingremitting disease, and disability appears to correlate better with axonal degeneration than acute demyelination.32
Cyclophosphamide in doses lower than used in this study has been used in MS and has not been reported to result in neurotoxicity.33 However, there are limited data on high-dose cyclophosphamide as used in this clinical protocol. Craniospinal irradiation has been tested as therapy for MS patients and was not found to be beneficial.34-42 Tumoricidal doses of cranial radiation (> 4000 cGy) have been reported to be associated with both progression and exacerbation of MS in small numbers of patients.36,37 Our ability to extrapolate these observations to the lower dose of radiation (1200 cGy) in our conditioning regimen is limited by the small number of patients and the difficulty in discriminating between radiation-induced neurologic damage and functional decline due to the primary disease process.
Recently it has been reported in a rat model that cranial irradiation adversely affects neural stem cells and mechanisms of brain repair though apoptosis, alteration in cell cycle progression, or destruction of a favorable neural mileu by invasion of macrophages and microglia.43 The radiation dose studied was 10 cGy, a dose lower than the 12 cGy used in this study. We, therefore, cannot exclude the possibility that some patients including the 2 subjects who progressed rapidly to death may have experienced radiation-related neuronal toxicity from this intervention.
While a high rate of progression occurred in patients with an EDSS of more than 6.0, it is too early to comment on the lack of disease progression in patients with an EDSS of less than 6.0, who entered the study later and consequently have not yet been followed as long. The only subject to demonstrate improvement in disability by at least 1.0 EDSS step following treatment was also the only patient with relapsing-remitting disease. In this patient, the EDSS decreased from 3.5 at transplantation to 1.0 at the 2-year follow-up after HSCT. We are continuing to follow patients with a pretransplantation EDSS of less than 6.0 to determine whether and for how long their current stability will endure.
The rate of disease progression in this protocol demonstrates the importance of long-term follow-up before inferring efficacy in a disease such as MS, and suggests caution in selecting patients for a TBI-based HSCT regimen who have progressive disease and EDSS scores of more than 6.0. It is unknown if, in the same patient cohort, a non-TBI conditioning regimen would have resulted in the same rate of progressive disability since the contribution of radiation to later disease progression remains to be determined.
In conclusion, future MS HSCT trials should include measures of axonal integrity and should focus on patients with active inflammatory disease and lower disability scores. These trials should incorporate controlled and blinded observations of clinical data, and modern automated techniques of MRI analysis. Trial design should give consideration to avoiding conditioning agents that, while immune suppressive, could contribute to axonal injury or hinder central nervous system repair from neural or oligodendrocyte progenitor cells. These trials should be of sufficient length, and incorporate sufficiently frequent clinical and radiographic monitoring, to accurately determine the influence of this therapy on both progressive neurologic impairment and recurrent inflammatory activity in MS.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-03-0877.
William H. Burns died August 13, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal