Abstract
Holmium-166 1, 4, 7, 10-tetraazcyclododecane-1, 4, 7, 10-tetramethylenephosphonate (166Ho-DOTMP) is a radiotherapeutic that localizes specifically to the skeleton and can deliver high-dose radiation to the bone and bone marrow. In patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantation two phase 1/2 dose-escalation studies of high-dose 166Ho-DOTMP plus melphalan were conducted. Patients received a 30 mCi (1.110 Gbq) tracer dose of 166Ho-DOTMP to assess skeletal uptake and to calculate a patient-specific therapeutic dose to deliver a nominal radiation dose of 20, 30, or 40 Gy to the bone marrow. A total of 83 patients received a therapeutic dose of 166Ho-DOTMP followed by autologous hematopoietic stem cell transplantation 6 to 10 days later. Of the patients, 81 had rapid and sustained hematologic recovery, and 2 died from infection before day 60. No grades 3 to 4 nonhematologic toxicities were reported within the first 60 days. There were 27 patients who experienced grades 2 to 3 hemorrhagic cystitis, only 1 of whom had received continuous bladder irrigation. There were 7 patients who experienced complications considered to be caused by severe thrombotic microangiopathy (TMA). No cases of severe TMA were reported in patients receiving in 166Ho-DOMTP doses lower than 30 Gy. Approximately 30% of patients experienced grades 2 to 4 renal toxicity, usually at doses targeting more than 40 Gy to the bone marrow. Complete remission was achieved in 29 (35%) of evaluable patients. With a minimum follow-up of 23 months, the median survival had not been reached and the median event-free survival was 22 months. 166Ho-DOTMP is a promising therapy for patients with multiple myeloma and merits further evaluation. (Blood. 2003;102:2684-2691)
Introduction
Multiple myeloma is a malignant plasma cell disorder that affects over 14 000 new patients and causes 10 000 deaths in the United States annually.1 Until recently, standard therapy for patients with multiple myeloma has been the combination of melphalan and prednisone.2 With this regimen, approximately 40% of the patients have a more than 75% reduction in their myeloma protein, with a median survival of 3 years.3 Combination chemotherapy regimens using vincristine, doxorubicin, BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea), cyclophosphamide, and steroids, although generally associated with higher response rates, have not clearly been associated with a survival benefit.4 Resistance to conventional-dose chemotherapy will eventually develop in all patients with myeloma. Dose intensification with or without stem cell support has become a widely explored strategy to overcome myeloma resistance, since the initial observations by McElwain et al5 and Barlogie et al6 demonstrated the validity of this concept in patients with advanced multiple myeloma.
High-dose chemotherapy with autologous stem cell support has been shown in one randomized and multiple nonrandomized trials to improve complete remission rates, progression-free survival, and overall survival in patients with multiple myeloma, and is rapidly becoming the standard of care for younger patients with good performance status.7-9 The most common cause of treatment failure after autologous transplantation is recurrence of the disease. Modifications of the conditioning regimen have failed to improve outcomes, and no particular regimen seems to be significantly more effective than melphalan at 200 mg/m2. The use of tandem transplantation to improve transplantation outcomes has been extensively explored and may be beneficial in a subset of patients.10,11
Myeloma is a radiosensitive malignancy. External beam radiation and total body irradiation (TBI) have been used in many regimens. However, TBI adds considerably to the regimen-related toxicity without adding any demonstrable benefit in long-term disease control.12,13 Alternate methods of delivery of radiotherapy could be used to administer more intense yet less toxic radiation within the preparative regimen. An example of this approach for malignancies largely limited to the bone and bone marrow would be to administer bone-seeking radiopharmaceuticals that accumulate in areas of active bone turnover.14-18 Phosphonate chelates are taken up in the skeleton and could be combined with radioactive isotopes to deliver high levels of radiation to bone and bone marrow while sparing normal tissues. This targeted skeletal radiotherapy approach has been extensively explored in humans for treatment of painful bone metastasis with both strontium-8914,19 and samarium-153-ethylenediamine-tetramethylenephosphonic acid (153Sm-EDTMP).20,21 153Sm-EDTMP has also been investigated as a preparative regimen for stem cell transplantation. Although results from experiments done in dogs suggested that 153Sm-EDTMP might result in incomplete marrow ablation,22 data from small human studies have suggested that 153Sm-EDTMP may in fact be useful in the transplantation setting.23,24
Holmium-166 (166Ho) is potentially a better-suited radionuclide for the purpose of delivering a bone-based dose of radiation therapy. Holmium-166 is primarily a beta emitter with high energy (maximum effect = 1.85 MeV) and a relatively short half-life of 26.8 hours, thereby permitting reinfusion of stem cells within 6 to 8 days. It also has a minor gamma component suitable for imaging and dosimetry. Studies in dogs indicate that administration of a phosphonate chelate, holmium-166-ethylenediamine-tetramethylenephosphonic acid (166Ho-EDTMP) or holmium-166 1, 4, 7, 10-tetraazcyclododecane-1, 4, 7, 10-tetramethylenephosphonate (166Ho-DOTMP), results in selective uptake in the bone with the remainder eliminated in the urine, with little toxicity to other tissues.25
A single-agent dose-escalation phase 1 study of 166Ho-DOTMP, with the objective of delivering targeted radiotherapy to the bone marrow, was conducted at the University of Texas M. D. Anderson Cancer Center (MDACC) in 6 patients with multiple myeloma.26,27 Dosages up to 2100 mCi (77.7 Gbq) of 166Ho-DOTMP were administered, delivering to a red marrow dose up to 41 Gy as determined by MIRDOSE 2 (Medical Internal Radiation Dose 2) determination (corresponding to a dose of 29 Gy by MIRDOSE 3 [Medical Internal Radiation Dose 3] determination, used in the present study). Autologous marrow or blood stem cell transplantation was used in all patients. There was no extramedullary toxicity. Subsequently, 3 additional patients were treated at a higher dose level with up to 2700 mCi (99.9 Gbq) 166Ho-DOTMP administered. Of these 3 patients, 1 had a transient drop in myeloma protein with improvement in bone pain, and a second had a decrease in myeloma protein levels that lasted longer than 8 months. The third patient also received high-dose thiotepa, busulfan, and cyclophosphamide, and achieved a complete response (CR). The duration of response was approximately 5 years with no evidence of late toxicities (R.C., unpublished observations, July 2002).
Based on the results from this phase 1 study, two phase 1/2 studies were conducted at MDACC, Fred Hutchinson Cancer Research Center (FHCRC), and the University of Miami to evaluate the maximum tolerated dose of 166Ho-DOTMP in combination with melphalan as part of a myeloablative preparative regimen for autologous transplantation for multiple myeloma. Melphalan was selected because it is one of the most active agents in multiple myeloma; it is well-tolerated, and it is frequently used either alone or in combination with total body irradiation as a preparative regimen for autologous transplantation. Herein we report the initial results of these phase 1/2 trials, representing the largest series of patients studied to date with skeletal targeted radiotherapy followed by stem cell transplantation.
Patients and methods
Patient eligibility
Patients were eligible if they had a confirmed diagnosis of myeloma, either with chemotherapy-responsive disease or primary refractory disease. Patients were required to have adequate cardiac and pulmonary function as defined by echocardiogram and standard pulmonary function tests. All patients were also required to have a performance status of 2 or greater on the Karnofsky scale,28 bilirubin levels of less than 3.0 mg/dL (176 μM) and creatinine levels of less than 2.0 mg/dL (50 μM). Patients with refractory relapse or who had received more than 30 cGy of radiation to the spinal cord or radiation therapy to more than 20% of bone marrow sites were ineligible. Patients over the age of 55 were ineligible for Protocol 9803, which investigated a TBI-containing regimen; Protocol 9804, investigating a non-TBI-containing regimen, was open to patients between 18 and 65 years of age.
Prior to registration, all patients underwent staging evaluations with bone marrow aspirate, serum protein electrophoresis, 24-hour urine for total protein and urine protein electrophoresis, pulmonary function tests, measurement of cardiac ejection fraction either by echocardiogram or nuclear medicine scan, and baseline chemistries and hematologic evaluation. Stem cell collection occurred prior to study entry using institutional procedures. All patients signed written informed consent according to the institutional review boards of participating institutions.
Study design
The goal of these phase 1/2 trials was to explore the safety and efficacy of an escalated targeted radiation dose to the bone marrow from 166Ho-DOTMP (NeoRx, Seattle, WA), in combination with 1 of 3 preparative regimens: 140 mg/m2 melphalan, 200 mg/m2 melphalan, or 140 mg/m2 melphalan in combination with 800 cGy of TBI given as 4 fractions of 200 cGy daily for 4 days. Each cohort was to receive a dose of 166Ho-DOTMP calculated to deliver an average radiation dose to the marrow of 20, 30, or 40 Gy.
Dosimetric calculations
All patients received a tracer dose of 30 mCi (1.110 Gbq) 166Ho-DOTMP intravenously as a bolus injection. Serial whole body counting of 166Ho-DOTMP to assess 166Ho-DOTMP retention and pharmacokinetics was performed on all enrolled patients. Absorbed dose to the bone marrow was estimated by extrapolating data from the whole body clearance curve after 12 hours back to time of injection to estimate the initial bone uptake and rate of clearance. These values were then used to estimate the bone cumulative activity and residence time on the bone surfaces for calculating the patient-specific dose to the marrow from activity localized in the skeleton (using the general method defined by the Medical Internal Radiation Dose [MIRD] Committee of the Society of Nuclear Medicine). The data from the early time points of the whole body clearance curve were used in the calculation to determine the marrow dose contribution from the remainder of the body and also to estimate dose to all other organs.
The dose of 166Ho (in mCi) necessary to deliver the desired dose to the bone marrow was calculated and prescribed by nuclear medicine personnel. A critical assumption in determining the dose to bone marrow is the relative radiation absorption between trabecular and cortical bone. There were 2 sites that used the International Commission on Radiological Protection (ICRP) 1975 guidelines,29 which allow for a 50:50 distribution of the radioisotope between trabecular and cortical bone, while 1 site used the ICRP 1995 guidelines,30 which assign a 62% distribution of the radioisotope to the trabecular bone and 38% to cortical bone. This difference in calculations resulted in a 20% higher administered dose for the same assigned dose level at sites that used ICRP 1975 guidelines. All data presented in the current manuscript, except data organized by intended marrow dose, were based on recalculating the data using the ICRP 1995 guidelines. The recalculation also took into account the body surface area of each patient, resulting in a greater variability of actual bone marrow dose than the nominal targeted marrow dose of 20, 30, and 40 Gy.
From the patient-specific dose estimates to the marrow in cGy per mCi, the 166Ho-DOTMP therapy dose (mCi) was calculated for each patient using the formula: A = D/M where A indicates 166Ho-DOTMP activity to be administered (mCi); D, desired dose to marrow (cGy); and M, patient-specific marrow dose from 166Ho-DOTMP (cGy/mCi)
Data from the tracer dose administration were also used to calculate doses to other organs, for correlation of toxicities with non-target organ doses. These calculations were also performed using the MIRD formalism.
One week after the tracer dose, patients with a skeletal uptake of 15% or more received the therapeutic dose of 166Ho-DOTMP calculated by nuclear medicine personnel to deliver 20, 30, or 40 Gy to the bone marrow. Patients with less than 15% skeletal uptake did not receive the therapeutic dose of 166Ho-DOTMP but could receive any standard preparative regimen followed by autologous stem cell transplantation at the discretion of the attending physician.
Administration of 166Ho-DOTMP
The therapy dose of 166Ho-DOTMP was generally administered 7 to 10 days prior to the expected transplantation date, as an intravenous injection via a central line. Nuclear medicine physicians infused the agent over 1 to 17 minutes. Patients were treated in shielded rooms and remained shielded until their activity decreased to institutional radiation safety standards. The time in a shielded room generally lasted from 6 to 24 hours depending upon the amount of radioactivity delivered and the safety requirements at each institution.
All patients received some form of prophylaxis for hemorrhagic cystitis. In 2 institutions, prophylaxis consisted of hydration at 200 mL/h of a dextrose 5% and half normal saline solution given intravenously in addition to forced diuresis and frequent (every 2 hours) voiding. In the third institution, continuous bladder irrigation through a 3-way intravesical catheter was instituted at a rate of 200 mL/h prior to and until the day following 166Ho-DOTMP administration.
Administration of the preparative regimen and stem cell infusion
Patients received melphalan on day -3 at a dose of 140 or 200 mg/m2 intravenously over 20 to 30 minutes. Patients registered on Protocol 9803 also received TBI 800 cGy in 4 fractions on days -3, -2, -1, and 0. Stem cells were infused intravenously on day 0 if calculated ongoing marrow radiation was less than 1 cGy/h.
Posttransplantation supportive care
Patients received supportive care according to institutional clinical guidelines for autologous stem cell transplantation. Pneumocystis Carinii prophylaxis consisted of trimethoprim-sulfamethoxazole (TMP-SMZ) twice daily twice a week from engraftment through 6 months after transplantation or 300 mg pentamidine intravenously every 3 to 4 weeks in patients who were TMP-SMZ intolerant. Herpes simplex virus prophylaxis consisted of 5 mg/kg acyclovir intravenously every 8 to 12 hours or 500 mg valacyclovir orally daily as a single dose.
Filgrastim (Neupogen; Amgen, Thousand Oaks, CA) was administered to patients after transplantation at a dose of 5 μg/kg until granulocyte recovery according to institutional guidelines. Administration of bisphosphonates was not allowed in the 28 days preceding the 166Ho-DOTMP diagnostic dose. Bisphosphonates were resumed after transplantation according to institutional guidelines.
Study end points and statistical analysis
The primary end points for these studies were engraftment, safety, and toxicity. Engraftment was defined by both neutrophil and platelet recovery. Neutrophil recovery was defined as the first of 3 consecutive days when the absolute neutrophil count was greater or equal to 0.5 × 109/L. Platelet engraftment was defined as the first of 7 consecutive days with a platelet count of 20.0 × 109/L, independent of platelet transfusions. Early transplantation-related toxicity was scored using the Bearman criteria.31
A subset of 45 patients enrolled at a single center had detailed pharmacokinetic analysis performed on blood and urine samples. Blood samples were collected at approximately 0.1, 0.5, 1, 2, 4, and 24 hours after dosing, and urine samples were taken from pooled samples from the following intervals: 0 to 6 hours, 6 to 12 hours, 12 to 24 hours, and 24 to 48 hours. Radioactivity of plasma and urine samples was analyzed in a well counter designed for use as a dose calibrator for beta emitters.
Response was determined by an independent observer and followed the consensus criteria for response as published by the International Bone Marrow Transplant Registry, North American Bone Marrow Transplant Registry, and the European Bone Marrow Transplant Registry.32 These criteria require 2 consecutive negative immunofixation electrophoreses at least 6 weeks apart for determining a complete remission.
Survival and event-free survival were determined as of September 1, 2002, and were calculated using the methods of Kaplan and Meier.
Results
Patient characteristics and study completion
From July 1998 to April 2000, a total of 88 patients were enrolled and received a tracer dose of 166Ho-DOTMP. Of the patients, 5 were ineligible to receive a therapy dose of 166Ho-DOTMP: 3 because of tracer dose skeletal uptake of less than 15%, 1 because of disease progression, and 1 because of extramedullary uptake of 166Ho-DOTMP. Thus, 83 patients received the therapeutic doses of 166Ho-DOTMP, but 1 patient did not receive the planned melphalan because of an intercurrent infection. All 83 patients who received therapeutic doses of 166Ho-DOTMP were evaluated for safety and 82 patients who received the complete treatment were included in the efficacy analyses.
Of the 83 patients who received 166Ho-DOTMP, 50 were males and the median age was 54 years (range, 36-71 years). Patient characteristics are summarized in Table 1. At the time of transplantation, 60 patients (72%) had chemoresponsive disease and 23 patients (28%) had refractory disease. Induction therapy varied in each participating institution and consisted of intermittent high-dose pulse dexamethasone, melphalan-prednisone, or combination chemotherapy with a variety of regimens. The median number of therapies before transplantation was 2. The number of patients enrolled in each study regimen is shown in Table 2.
Patient characteristics
Variable . | Protocol 9803 . | Protocol 9804 . |
|---|---|---|
| No. of patients registered | 29 | 59 |
| No. of patients who received the entire regimen | 25 | 58 |
| Age, y, median (range) | 51 (36-55) | 56 (35-70) |
| % of male patients | 48 | 65.5 |
| Median no. of prior therapies (range) | 2 (1-5) | 2 (1-6) |
| Disease status at transplantation, n | ||
| First complete remission | 0 | 3 |
| First partial remission consolidation | 13 | 38 |
| Primary refractory | 9 | 13 |
| Relapsed disease | 3 | 3 |
| Unknown | 0 | 1 |
Variable . | Protocol 9803 . | Protocol 9804 . |
|---|---|---|
| No. of patients registered | 29 | 59 |
| No. of patients who received the entire regimen | 25 | 58 |
| Age, y, median (range) | 51 (36-55) | 56 (35-70) |
| % of male patients | 48 | 65.5 |
| Median no. of prior therapies (range) | 2 (1-5) | 2 (1-6) |
| Disease status at transplantation, n | ||
| First complete remission | 0 | 3 |
| First partial remission consolidation | 13 | 38 |
| Primary refractory | 9 | 13 |
| Relapsed disease | 3 | 3 |
| Unknown | 0 | 1 |
Number of patients receiving each therapy regimen
Target marrow dose from 166Ho-DOTMP . | No melphalan . | Melphalan, 140 mg/m2 . | Melphalan, 140 mg/m2 + TBI . | Melphalan, 200 mg/m2 . |
|---|---|---|---|---|
| 20Gy | 0 | 5 | 6 | 4 |
| 30Gy | 0 | 4 | 9 | 7 |
| 40Gy | 1 | 7 | 10 | 30 |
Target marrow dose from 166Ho-DOTMP . | No melphalan . | Melphalan, 140 mg/m2 . | Melphalan, 140 mg/m2 + TBI . | Melphalan, 200 mg/m2 . |
|---|---|---|---|---|
| 20Gy | 0 | 5 | 6 | 4 |
| 30Gy | 0 | 4 | 9 | 7 |
| 40Gy | 1 | 7 | 10 | 30 |
Pharmacokinetics
Samples taken from 45 patients enrolled at a single center underwent complete pharmacokinetic analysis. The mean residence time of 166Ho-DOTMP activity in the total blood volume was 4.89 ± 0.97 hours (range, 3-7 hours) and the terminal elimination half-life was 7.5 ± 14.87 hours (range, 3-10 hours). Renal elimination is a major and rapid pathway for the elimination of 166Ho-DOTMP activity, and had a mean clearance of 2.07 ± 0.63 blood vol/h. More than 79% of the dose injected was recovered in the urine by 24 hours; urinary excretion accounted for 96% of the total excreted amount.
Skeletal and non-target organ uptake of 166Ho-DOTMP
The range of skeletal uptake of the 166Ho-DOTMP tracer dose was 12% to 56%. In patients who received the full therapy regimen (skeletal uptake was required to be > 15%), the median skeletal uptake was 24%. The median total therapy activity injected was 2113 mCi (78.181 GBq) (range, 460-4476 mCi [17.020-165.612 Gbq]). Calculated radiation dose to target and non-target organs, organized according to planned radiation dose to marrow, is summarized in Table 3. The bladder wall was the most heavily exposed non-target organ with a median radiation dose of 4250 cGy for all patients. Median calculated dose to the marrow was 3120 cGy (range, 1340-5810 cGy), to the bone surfaces was 5640 cGy (range, 2050-9110 cGy), to the kidney was 430 cGy (range, 70-980 cGy), and to the lung was 100 cGy (range, 20-250 cGy).
Calculated median radiation absorbed dose (cGy) of target and nontarget organs according to intended 166Ho-DOTMP dose level
Nontarget organ . | Without TBI . | . | . | With TBI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | 20 Gy . | 30 Gy . | 40 Gy . | 20 Gy . | 30 Gy . | 40 Gy . | ||||
| Bone | 3850 | 4950 | 6785 | 4440 | 5250 | 7480 | ||||
| Bone marrow* | 2070 | 2420 | 3850 | 2605 | 3360 | 4875 | ||||
| Bladder wal† | 4110 | 2600 | 3900 | 5110 | 5930 | 9255 | ||||
| Kidney | 260 | 370 | 550 | 1100 | 1170 | 1385 | ||||
| Liver | 60 | 90 | 110 | 880 | 890 | 935 | ||||
| Lung | 60 | 90 | 110 | 880 | 890 | 935 | ||||
Nontarget organ . | Without TBI . | . | . | With TBI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | 20 Gy . | 30 Gy . | 40 Gy . | 20 Gy . | 30 Gy . | 40 Gy . | ||||
| Bone | 3850 | 4950 | 6785 | 4440 | 5250 | 7480 | ||||
| Bone marrow* | 2070 | 2420 | 3850 | 2605 | 3360 | 4875 | ||||
| Bladder wal† | 4110 | 2600 | 3900 | 5110 | 5930 | 9255 | ||||
| Kidney | 260 | 370 | 550 | 1100 | 1170 | 1385 | ||||
| Liver | 60 | 90 | 110 | 880 | 890 | 935 | ||||
| Lung | 60 | 90 | 110 | 880 | 890 | 935 | ||||
These median values include adjustment for patient size for bone marrow dose.
These median values include patients with and without bladder irrigation.
Engraftment and acute toxicity
The primary safety variable for this study was hematopoietic engraftment. Since 166Ho-DOTMP delivers high doses of radiation directly to the marrow, there was the potential that the resulting bone marrow microenvironment irradiation could have a negative effect on hematopoietic recovery. Before day 60, 2 patients died from infection and could not be evaluated for long-term engraftment. The median time to neutrophil recovery was 10 days (range, 8-26 days) after transplantation. By day 28, 3 patients did not achieve platelet engraftment (1 of whom died of respiratory syncital virus pneumonia at day 27) and were considered graft failures; the median time to platelet transfusion independence at a level of 20 × 109/L for the remaining patients was 10 days (range, 4-25 days). There was no significant difference in time to engraftment in any of the 166Ho-DOTMP dose levels, as shown in Table 4. No significant differences in red blood cell or platelet transfusion requirements were seen among the treatment groups.
Hematopoietic recovery after transplantation by treatment regimen
Treatment regimen . | Days to ANC higher than 0.5 × 109/L, median (range) . | Days to platelet count higher than 20.0 × 109/L, median (range)* . |
|---|---|---|
| 20 Gy 166Ho-DOTMP; n = 14 | 10 (8-16) | 10 (7-20) |
| 30 Gy 166Ho-DOTMP; n = 19 | 11 (9-19) | 11 (6-25) |
| 40 Gy 166Ho-DOTMP; n = 47 | 10 (8-26) | 10 (4-22) |
| Overall | 10 (8-26) | 10 (4-25) |
Treatment regimen . | Days to ANC higher than 0.5 × 109/L, median (range) . | Days to platelet count higher than 20.0 × 109/L, median (range)* . |
|---|---|---|
| 20 Gy 166Ho-DOTMP; n = 14 | 10 (8-16) | 10 (7-20) |
| 30 Gy 166Ho-DOTMP; n = 19 | 11 (9-19) | 11 (6-25) |
| 40 Gy 166Ho-DOTMP; n = 47 | 10 (8-26) | 10 (4-22) |
| Overall | 10 (8-26) | 10 (4-25) |
ANC indicates absolute neutrophil count.
By day 28, 3 patients did not achieve platelet engraftment.
No instances of regimen-related toxicity greater than grade 2 (Bearman Scale) were observed. Mild nausea and vomiting, when seen, was easily managed with conventional antiemetics. Bearman grade 2 toxicities are summarized by organ site in Table 5. Nonrelapse mortality at 100 and 365 days was 3.6% and 7.2%, respectively.
Bearman grades 1 to 2 early toxicities (less than 60 days after transplantation) by nominal dose level
Toxicity grade . | 20 Gy, n (%) . | 30 Gy, n (%) . | 40 Gy, n (%) . | All doses, n (%) . |
|---|---|---|---|---|
| Cardiac | ||||
| Grade 1 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Grade 2 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Bladder | ||||
| Grade 1 | 0 | 0 | 3 (6.3) | 3 (3.6) |
| Grade 2 | 0 | 1 (5) | 0 | 1 (1.2) |
| Pulmonary | ||||
| Grade 1 | 0 | 0 | 3 (6.3) | 3 (3.6) |
| Grade 2 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Hepatic | ||||
| Grade 1 | 6 (40) | 6 (30) | 18 (37.5) | 30 (36.1) |
| Grade 2 | 4 (26.7) | 7 (35) | 12 (25) | 23 (27.7) |
| Stomatitis | ||||
| Grade 1 | 13 (86.7) | 9 (45) | 25 (52.1) | 47 (56.6) |
| Grade 2 | 0 | 8 (40) | 15 (31.25) | 23 (27.7) |
| Gastrointestinal | ||||
| Grade 1 | 6 (40) | 8 (40) | 19 (39.6) | 33 (39.7) |
| Grade 2 | 0 | 1 (5) | 2 (4.2) | 3 (3.6) |
| Renal | ||||
| Grade 1 | 0 | 1 (5) | 1 (2.1) | 2 (2.4) |
| Grade 2 | 0 | 2 (10) | 2 (4.2) | 4 (4.8) |
| CNS | ||||
| Grade 1 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Grade 2 | 0 | 1 (5) | 1 (2.1) | 2 (2.4) |
Toxicity grade . | 20 Gy, n (%) . | 30 Gy, n (%) . | 40 Gy, n (%) . | All doses, n (%) . |
|---|---|---|---|---|
| Cardiac | ||||
| Grade 1 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Grade 2 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Bladder | ||||
| Grade 1 | 0 | 0 | 3 (6.3) | 3 (3.6) |
| Grade 2 | 0 | 1 (5) | 0 | 1 (1.2) |
| Pulmonary | ||||
| Grade 1 | 0 | 0 | 3 (6.3) | 3 (3.6) |
| Grade 2 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Hepatic | ||||
| Grade 1 | 6 (40) | 6 (30) | 18 (37.5) | 30 (36.1) |
| Grade 2 | 4 (26.7) | 7 (35) | 12 (25) | 23 (27.7) |
| Stomatitis | ||||
| Grade 1 | 13 (86.7) | 9 (45) | 25 (52.1) | 47 (56.6) |
| Grade 2 | 0 | 8 (40) | 15 (31.25) | 23 (27.7) |
| Gastrointestinal | ||||
| Grade 1 | 6 (40) | 8 (40) | 19 (39.6) | 33 (39.7) |
| Grade 2 | 0 | 1 (5) | 2 (4.2) | 3 (3.6) |
| Renal | ||||
| Grade 1 | 0 | 1 (5) | 1 (2.1) | 2 (2.4) |
| Grade 2 | 0 | 2 (10) | 2 (4.2) | 4 (4.8) |
| CNS | ||||
| Grade 1 | 0 | 0 | 1 (2.1) | 1 (1.2) |
| Grade 2 | 0 | 1 (5) | 1 (2.1) | 2 (2.4) |
Patient populations by toxicity group: 20 Gy, n = 15; 30 Gy, n = 20; 40 Gy, n = 48; and all doses, n = 83.
CNS indicates central nervous system.
Late toxicities
Follow-up of these patients revealed 3 main late toxicities. Most of these occurred at actual doses to the marrow of more than 30 Gy (all patients who received doses to the marrow of 30 Gy or less received injected dosages of 35 mCi/kg [1.295 GBq/kg] or less).
Hemorrhagic cystitis. Of the patients, 31 have developed grade 1 or higher hematuria; 27 of these developed hemorrhagic cystitis (defined by the National Cancer Institute [NCI] Common Toxicity Criteria as greater than grade 2 hematuria with or without associated symptoms) with onset from 1 to 45 months after 166Ho-DOTMP therapy. Of the patients with hematuria, only 2 had received continuous bladder irrigation (CBI), and these patients had either a documented relapse in the bladder or a documented viral infection of the bladder.
Patients who developed hemorrhagic cystitis were treated with CBI together with analgesics and antispasmodics. Of the patients, 11 underwent cystoscopy, with 10 patients showing diffuse erythema (particularly around the ureteral outlets) and 4 patients also showing bladder neck obstruction. At last follow-up, hemorrhagic cystitis was totally resolved in 4 patients and ongoing in 11 patients who were alive, 1 patient was lost to follow-up, and 11 patients who had developed hemorrhagic cystitis had died with or without symptoms of hemorrhagic cystitis.
Thrombotic microangiopathy of the kidney and late renal dysfunction. Renal dysfunction in this study was defined as creatinine levels at least 1.5 times the upper limit of normal (NCI grade 2 or higher) for at least 2 measurements within 30 days of each other, whether or not they were consecutive, at any time after transplantation, including renal dysfunction noted after relapse or during subsequent therapy. Using these criteria, 30 patients (36%) had renal dysfunction; 14 of these patients (17% of the total population) had renal toxicity of grade 3 or higher. A stepwise logistic regression analysis showed that the only factor with a significant effect on both the incidence and time to onset of renal dysfunction was activity injected in mCi/kg (P ≤ .03). For time to onset of renal dysfunction, the effect of age at time of transplantation was significant (P = .032) after adjusting for actual dose injected in mCi/kg. Only 11% of patients treated with a dose to marrow of 30 Gy or less had renal impairment of grade 3 or greater, compared with 22% of those who received more than 30 Gy. Renal toxicity according to dose delivered, broken down by grade, is shown in Table 6.
Incidence of late renal toxicity according to injected dose of 166Ho-DOTMP
. | 25 mCi/kg or less; n = 42 . | Between 25 and 30 mCi/kg; n = 15 . | Between 30 and 35 mCi/kg; n = 7 . | Between 35 and 40 mCi/kg; n = 4 . | More than 40 mCi/kg; n = 15 . |
|---|---|---|---|---|---|
| Patients with renal dysfunction, n (%) | 9 (21) | 6 (40) | 1 (14) | 3 (75) | 11 (73) |
| Patients with grades 3 to 4 renal toxicity, n (%) | 2 (5) | 3 (20) | 0 (0) | 2 (50) | 7 (47) |
. | 25 mCi/kg or less; n = 42 . | Between 25 and 30 mCi/kg; n = 15 . | Between 30 and 35 mCi/kg; n = 7 . | Between 35 and 40 mCi/kg; n = 4 . | More than 40 mCi/kg; n = 15 . |
|---|---|---|---|---|---|
| Patients with renal dysfunction, n (%) | 9 (21) | 6 (40) | 1 (14) | 3 (75) | 11 (73) |
| Patients with grades 3 to 4 renal toxicity, n (%) | 2 (5) | 3 (20) | 0 (0) | 2 (50) | 7 (47) |
There were 8 patients who developed a severe form of sustained renal impairment associated with microangiopathic hemolytic anemia, thrombocytopenia, uncontrolled hypertension, and elevated lactate dehydrogenase. One of these cases occurred following an allogeneic bone marrow transplantation given 24 months after 166Ho-DOTMP therapy and was considered unrelated to study drug; this case is not considered further in this report. The syndrome clinically resembled thrombotic thrombocytopenic purpura or hemolytic uremic syndrome (TTP/HUS). The 7 cases occurred between 6 and 13 months after transplantation. With a minimum follow-up of 23.1 months for all patients alive, no new cases of thrombotic microangiopathy have been seen after month 13.
Although the exact cause of this toxicity is not yet known, it was associated with the highest doses of 166Ho-DOTMP and was possibly caused by a high rate of radiation exposure to the kidney immediately after 166Ho-DOTMP infusion. In kidney biopsies of 5 patients with elevated creatinine levels (1 with the TTP/HUS-like syndrome), thrombotic microangiopathy (TMA) consistent with a radiation nephropathy was observed. All 7 patients with the TTP/HUS-like syndrome received an actual radiation dose to the marrow of more than 32 Gy, and 6 of the 7 patients had a dose of more than 40 Gy.
All patients with this syndrome underwent therapy with steroids, total plasma exchange, and transfusion support as required, as well as aggressive antihypertensive therapy. All 7 patients required dialysis. As of September 1, 2002, 2 patients who developed TMA are alive, and 5 have died, 3 of whom also had progressive multiple myeloma.
Myelodysplastic syndrome. One patient developed a myelodysplastic syndrome 4 months after treatment with 166Ho-DOTMP. This patient had normal cytogenetics prior to transplantation, but the follow-up assessment revealed 2 apparent abnormal clonal cell lines. The relationship between this event and 166Ho-DOTMP is unknown; however, it should be noted that this patient had received extensive prior treatment with alkylating agents, a known risk factor for myelodysplasia.33
Disease response
Of the 82 patients who received the full treatment, 53 had greater than a 50% reduction in myeloma protein corresponding to an objective response rate of 65%. Of these, 29 patients (35%) satisfied the criteria for a complete response (absence of monoclonal protein on 2 consecutive immunoelectrophoreses at least 6 weeks apart), and another 7 patients (9%) had a more than 90% reduction in monoclonal protein, qualifying for a very good partial response. There were 5 patients (6%) who had a single immunoelectrophoresis performed after transplantation and therefore were considered to have insufficient data for full evaluation of disease response. Response data according to preparative regimen have been summarized in Table 7. Of note, there was no significant difference in the percent of patients achieving complete response in the group treated with 140 mg/m2 melphalan and TBI (10 of 25; 40%) and those treated with 200 mg/m2 melphalan without TBI (16 of 41; 39%).
Disease responses according to treatment group
| Treatment . | CR, n (%) . | VGPR* . | PR . | Stable . | PD . | Insufficient data . |
|---|---|---|---|---|---|---|
| 166Ho-DOTMP + Mel 140 | 3 (19) | 0 | 2 | 9 | 1 | 1 |
| 166Ho-DOTMP + Mel 140 + TBI | 10 (40) | 1 | 8 | 3 | 0 | 3 |
| 166Ho-DOTMP + Mel 200 | 16 (39) | 6 | 7 | 10 | 1 | 1 |
| Total, n (%) | 29 (35) | 7 (9) | 17 (21) | 22 (27) | 2 (2) | 5 (6) |
| Treatment . | CR, n (%) . | VGPR* . | PR . | Stable . | PD . | Insufficient data . |
|---|---|---|---|---|---|---|
| 166Ho-DOTMP + Mel 140 | 3 (19) | 0 | 2 | 9 | 1 | 1 |
| 166Ho-DOTMP + Mel 140 + TBI | 10 (40) | 1 | 8 | 3 | 0 | 3 |
| 166Ho-DOTMP + Mel 200 | 16 (39) | 6 | 7 | 10 | 1 | 1 |
| Total, n (%) | 29 (35) | 7 (9) | 17 (21) | 22 (27) | 2 (2) | 5 (6) |
Patient populations: 166Ho-DOTMP + Mel 140, n = 16; 166Ho-DOTMP + Mel 140 + TBI, n = 25; 166Ho-DOTMP + Mel 200, n = 41; and total, n = 82. PR indicates partial remission; VGPR, very good partial remission; and PD, progressive disease.
As defined by Attal et al.8
The conversion rate to complete response was 37% for patients in partial remission prior to transplantation (n = 51) and 23% for patients with primary refractory disease (n = 22). Of the 37 patients who received an actual dose to marrow of 30 Gy or less, 11 (30%) achieved a complete response and 12 (32%) achieved a partial response. Response rates according to disease status prior to transplantation are summarized in Table 8.
Disease responses according to disease status prior to transplantation
Disease status prior to transplantation . | All patients . | 30 Gy or less marrow dose . | More than 30 Gy marrow dose . | P* . |
|---|---|---|---|---|
| Primary refractory, %CR / %PR | 23/45 | 23/31 | 22/67 | NS |
| First remission consolidation, %CR / %PR | 40/22 | 50/33 | 38/19 | NS |
Disease status prior to transplantation . | All patients . | 30 Gy or less marrow dose . | More than 30 Gy marrow dose . | P* . |
|---|---|---|---|---|
| Primary refractory, %CR / %PR | 23/45 | 23/31 | 22/67 | NS |
| First remission consolidation, %CR / %PR | 40/22 | 50/33 | 38/19 | NS |
For comparison of 30 versus more than 30 Gy. NS indicates not significant.
Survival and progression-free survival
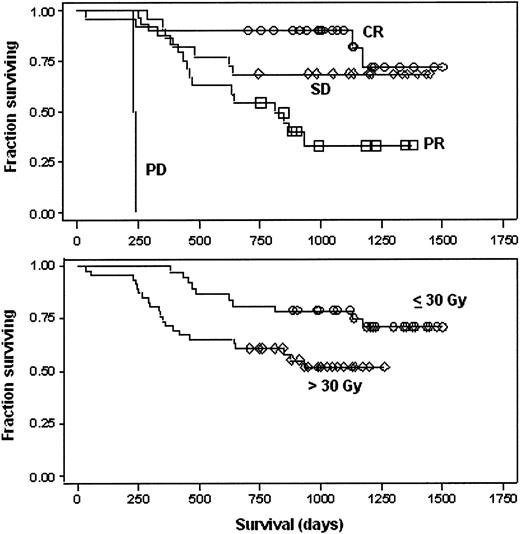
As of September 2002, the median follow-up was 31.4 months (minimum, 23.1 months). The median survival time has not been reached and the actuarial overall survival at 1 and 2 years was 84% and 71%, respectively. Of the patients, 47 have progressed and the median event-free survival is 22 months; the actuarial 1- and 2-year event-free survival was 61% and 40%, respectively. Overall survival according to disease response to transplantation is depicted in Figure 1 (top panel). All 37 patients treated with an actual dose of 30 Gy or less to the marrow were alive at 1 year, and 23 (62%) had survival more than 3 years. Survival of patients according to bone marrow dose delivered (> 30 vs ≤ 30 Gy) is shown in Figure 1 (bottom panel).
Patient survival. Symbols represent time points at which observations were made. Top panel: survival of all patients (n = 82) according to patient response to transplant. ○ indicates complete response; ⋄, stable disease; □, partial response; and no symbols, progressive disease. Bottom panel: survival of patients according to actual dose to the marrow. ○ indicates patients who received an actual dose of 30 Gy or less to the marrow (n = 37); ⋄, patients who received more than 30 Gy to the marrow (n = 45).
Patient survival. Symbols represent time points at which observations were made. Top panel: survival of all patients (n = 82) according to patient response to transplant. ○ indicates complete response; ⋄, stable disease; □, partial response; and no symbols, progressive disease. Bottom panel: survival of patients according to actual dose to the marrow. ○ indicates patients who received an actual dose of 30 Gy or less to the marrow (n = 37); ⋄, patients who received more than 30 Gy to the marrow (n = 45).
Discussion
166Ho-DOTMP is a bone-seeking, beta-emitting radiopharmaceutical that demonstrates enhanced uptake in areas of active bone turnover, similar to a diagnostic bone scan. It can thus deliver large doses of radiation to bone metastases throughout the skeleton. This study suggests that high-dose 166Ho-DOTMP plus melphalan can be an effective preparative regimen in patients with multiple myeloma.
Skeletal uptake of 166Ho-DOTMP was found to vary from 12% to 56% of the injected dose. The low uptake in some patients may be due to the lytic nature of bone lesions in multiple myeloma. In these patients, large doses of radiation (up to 4500 mCi [166.5 Gbq]) were required in order to deliver the highest targeted dose of radiation (40 Gy) to the bone marrow.
Although no engraftment delays were seen even at the highest doses used in this study, higher absolute exposure to critical non-target organs, such as the kidney and the bladder, was observed and likely contributed to the late, nonhematologic toxicities that developed. Late toxicities were limited to the urinary tract, the route of 166Ho-DOTMP excretion, and developed in patients not receiving bladder irrigation and in patients receiving higher doses of 166Ho-DOTMP. In this study the use of different assumptions in regard to the relative distribution of 166Ho-DOTMP between the trabecular and cortical bone resulted in a 20% higher administered dose of 166Ho-DOTMP in 2 of the 3 sites to achieve the same target marrow dose. It is clear that standardization of dosimetry procedures including centralized dosimetry will be essential components for the effective use of this treatment modality.
Hemorrhagic cystitis was a common adverse event in this study. Bladder exposure can be minimized by the use of continuous bladder irrigation. The efficacy of this intervention in preventing hemorrhagic cystitis is evident from the experience in this trial, since only 1 (3%) of 32 patients developed hemorrhagic cystitis if they received continuous bladder irrigation while 26 (55%) of 51 patients who had not received continuous bladder irrigation developed hemorrhagic cystitis. Thus future trials of this agent will require mandatory bladder irrigation to prevent hemorrhagic cystitis.
Dose-limiting toxicity occurred in patients treated at the 40 Gy level. The dose-limiting toxicity was primarily renal and consistent with renal thrombotic microangiopathy. In the setting of progenitor cell transplantation, such dysfunction has taken the name of posttransplantation nephropathy and shares many features of other microangiopathic hemolytic anemias.33-38 While designated by some as posttransplantation TTP/HUS, this syndrome does not show abnormalities of von Willebrand multimers, nor is it associated with deficiencies or abnormalities of the von Willebrand cleaving protein.39
Similar toxicities have been seen as the result of other types of radiotherapy. Renal thrombotic microangiopathy has been described as a consequence of radiation exposure, but usually at higher doses of external beam radiation. External beam radiation has long been known to produce late nephrotoxicity that can present as proteinuria, hypertension, and decreased renal function (including anuria and renal failure), and in many cases is accompanied by evidence of microangiopathic hemolytic anemia.34,35 In addition, renal dysfunction commonly occurs during the clinical course of patients with multiple myeloma, and it may have contributed to some of the late nephrotoxicity in patients with relapsing or refractory disease.33
When external beam radiation is associated with radiation-induced nephropathy the delivered radiotherapy dose has usually been more than 2000 cGy.36 Radiation nephropathy has been shown to occur at lower doses, around 750 cGy, in patients receiving bone marrow transplants,37,38 and the use of cyclophosphamide, common in patients with myeloma, may also be a contributing factor.38 The median dose to the kidney from 166Ho-DOTMP of the 7 patients who experienced severe TMA nephropathy in this study was 710 cGy, suggesting that the observed toxicity may be due to the high initial dose rate immediately after infusion and not the total kidney exposure. No instances of renal thrombotic microangiopathy were seen at doses of 166Ho-DOTMP delivering less than 32 Gy to the marrow, suggesting that this should be the dose level explored in future phase 2 and phase 3 trials. It is also possible that the use of other dosing schedules (for example, split doses) might allow delivery of higher doses of 166Ho-DOTMP with improved patient safety.
The primary end point of this study was engraftment and tolerance. Engraftment kinetics were prompt at all dose levels of 166Ho-DOTMP, and similar to what would be expected after autologous transplantation without targeted skeletal radiotherapy. Thus, problems due to irreversible damage to marrow stroma from exposure to 166Ho-DOTMP do not seem likely. Notwithstanding, only long-term follow-up will determine whether 166Ho-DOTMP exposure could increase the incidence of secondary acute leukemias that are occasionally seen after high-dose chemotherapy programs and autologous stem cell transplantation.
The rate of complete remissions observed was encouraging. For patients receiving autografts as part of an initial remission consolidation the complete remission rate was 37% (95% confidence interval [CI], 24-52); this contrasts favorably to the overall CR rates of 32% and 36% for single or tandem autografts reported by Attal et al.40 Of particular interest is the 23% complete remission rate in patients with primary refractory disease (95% CI, 8%-45%); this compares favorably to a complete response rate of 10% or less in this patient population with either single agent melphalan or other preparative regimens.40-42 Efficacy results did not seem to be diminished in the subset of patients who received an actual dose of 30 Gy or less to the marrow (Figure 1). In this subset of patients, the incidence of long-term toxicities was lower than that seen in patients treated at higher doses, suggesting that this may be the appropriate dose of this radiotherapeutic for futures studies.
In summary, this report demonstrates the feasibility of targeted skeletal radiotherapy with 166Ho-DOTMP as part of a standard preparative regimen with either 200 mg/m2 melphalan or 140 mg/m2 melphalan and 800 cGy of TBI. As TBI has been associated with higher rates of regimen-related toxicity,12,13 future trials will be designed to determine the safety and efficacy of 166Ho-DOTMP without TBI. The data from this study suggest that 166Ho-DOTMP in addition to a standard preparative regimen can result in a high rate of complete remissions in myeloma patients undergoing autografts. Thus, further exploration of targeted skeletal radiotherapy with 166Ho-DOTMP in combination with melphalan is warranted.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-10-3250.
Supported by NeoRx and the Jose Carreras Foundation Against Leukemia.
H.B. and R.G. are employed by NeoRx, whose potential product (166Ho-DOTMP) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to acknowledge the invaluable assistance of the clinical personnel at the sites, including Patricia Williams, RN; Kathy Lilleby, RN; James Hanlon, RN; Larry Durack; and Denise Sackner for their clinical assistance; and Terry Cook for help with manuscript preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal