Abstract
Blood microparticles (MPs) in sickle cell disease (SCD) are reportedly derived only from erythrocytes and platelets. Yet in SCD, endothelial cells and monocytes are activated and abnormally express tissue factor (TF). Thus, sickle blood might contain TF-positive MPs derived from these cells. With the use of flow cytometry to enumerate and characterize MPs, we found total MPs to be elevated in crisis (P = .0001) and steady state (P = .02) in subjects with sickle cell disease versus control subjects. These MPs were derived from erythrocytes, platelets, monocytes, and endothelial cells. Erythrocyte-derived MPs were elevated in sickle crisis (P = .0001) and steady state (P = .02) versus control subjects, as were monocyte-derived MPs (P = .0004 and P = .009, respectively). Endothelial and platelet-derived MPs were elevated in sickle crisis versus control subjects. Total TF-positive MPs were elevated in sickle crisis versus steady state (P = .004) and control subjects (P < .0001) and were derived from both monocytes and endothelial cells. Sickle MPs shortened plasma-clotting time compared with control MPs, and a TF antibody partially inhibited this procoagulant activity. Markers of coagulation were elevated in patients with sickle cell disease versus control subjects and correlated with total MPs and TF-positive MPs (P < .01 for both). These data support the concept that SCD is an inflammatory state with monocyte and endothelial activation and abnormal TF activity. (Blood. 2003;102:2678-2683)
Introduction
Microparticles (MPs) are small, membrane-derived vesicles released by cells on activation or during apoptosis.1,2 Because the process of MP formation entails a loss of normal membrane lipid asymmetry, in which aminophospholipids are confined to the inner leaflet of the cell membrane, MPs abnormally display phosphatidylserine (PS) on their outer leaflets.3 MPs of various cellular origins are present in blood and typically bear cell membrane antigens that reflect their cellular origin.4 MPs from platelets5 and red cells6 have been described in sickle cell disease. Because endothelial cells and monocytes are activated in sickle disease,7-9 we hypothesized that MPs would also arise from these cells.
In addition to being an inflammatory condition,10 sickle cell disease is also believed to be a prothrombotic state.11 Activation of the coagulation system is evidenced by platelet activation, thrombin generation, and fibrinolysis in patients with sickle cell disease11-16 and may play a role in thrombotic complications of sickle cell disease such as stroke.17 Consistent with these findings, in sickle cell disease tissue factor (TF) is abnormally expressed on circulating endothelial cells (CECs),8 and whole blood TF procoagulant activity, believed to be associated with monocytes, is elevated.18 Studies in healthy individuals have demonstrated a blood-derived thrombogenic pool of TF that is not cell associated but apparently MP associated.19 The origin of this circulating MP-associated TF is not clearly defined; however, evidence cited earlier suggests that it could be derived from blood cells (monocytes) or the vessel wall (endothelial cells). Therefore, we hypothesized that endothelial cell- and monocyte-derived MPs in the blood of patients with sickle cell disease would also be TF positive.
Patients, materials, and methods
Reagents and assays
We obtained cell-specific monoclonal antibodies (MoAbs) against CD14 for monocytes (CRIS-6; Biosource International, Camarillo, CA), and against CD144 for endothelial cells (55-7H1), CD41a for platelets (HIP8), and glycophorin A for red blood cells (RBCs; JC159) (BD-Pharmingen, San Diego, CA). Fluorescein isothiocyanate (FITC)-labeled MoAb against TF (CJ4068) was obtained from American Diagnostica (Greenwich, CO), and isotype control mouse immunoglobulin G1 (IgG1) labeled with either FITC or phycoerythrin (PE; 11711.11) was obtained from R&D Systems (Minneapolis, MN). Cy5-labeled Annexin V was obtained from BD-Pharmingen. Fluorescent microbeads of different sizes (0.3-2.0 μm) for gating experiments were obtained from Molecular Probes (Eugene, OR) and Sigma Chemical (St Louis, MO), and 7.2-μm nonfluorescent beads for enumeration of MPs were obtained from Bangs Laboratories (Fishers, IN).
The cell specificity of the MoAbs used in our MP flow cytometric experiments was verified in preliminary experiments using endothelial cells, monocytes, platelets, and RBCs (data not shown). The antibodies were also titrated in preliminary experiments to determine the optimal labeling concentrations for each (range, 0.1-1.25 μg/mL).
For plasma clotting experiments, we used coagulation control plasma-level-1 plasma from Fischer Diagnostics (Middletown, VA), Innovin (recombinant re-lipidated human-TF) from Dade International (Miami, FL), and a polyclonal rabbit antihuman TF-blocking antibody with preimmune IgG control (courtesy of Dr Ron Bach, University of Minnesota).
Study subjects
Twenty-seven adult patients with a diagnosis of sickle cell disease (23 with HbSS or HbSβthalasemia; 4 with HbSC) provided 21 samples in acute crisis and 16 samples in steady state. These included samples from 12 patients who were studied in both crisis and steady state. The subjects included 15 men and 12 women, with a mean age of 26 years (range, 19-45 years). All but one were receiving hydroxyurea. Two patients were started on warfarin (Coumadin) therapy for catheter-related thrombi between attainment of the crisis sample and the follow-up steady-state sample. None of the patients studied were routinely on aspirin therapy, and some of the patients in crisis were febrile.
A “crisis” was defined as a visit to a medical facility for pain that had no evident cause other than sickle cell anemia and for which the patient was hospitalized and treated with parenteral narcotics. Samples obtained from the time of onset of a crisis and throughout hospitalization were considered to be “crisis” samples. We defined “steady state” as any time exclusive of the 4 weeks prior to or after a crisis period. Healthy control subjects (n = 13) were all race matched and were similar in age (mean = 30 years; range, 22-42 years) and sex (M = 6, F = 7) to the patient population. In addition, we studied 3 individuals with sickle trait (HbAS) and 2 individuals with persistent hemolytic anemia (autoimmune hemolytic anemia and pyruvate kinase deficiency) who had previously undergone surgical splenectomy. All study participants signed informed consent forms, and the Institutional Review Boards of the University of Minnesota and Hennepin County Medical Center approved the protocol.
Samples
Blood samples were drawn using a 21-gauge needle into buffered citrate (Becton Dickinson, San Jose, CA) after discarding the first 3 mL. Platelet-free plasma (PFP) was prepared immediately after blood collection using a 2-step centrifugation procedure: initially at 1500g for 10 minutes at 20°C to make platelet-rich plasma and then at 13 000g for 10 minutes at 20°C to make PFP. Samples were used immediately or stored at -80°C for future flow cytometric or enzyme-linked immunosorbent assay (ELISA) experiments. Preliminary experiments showed that subsequent results were not affected by a freeze-thaw cycle.
Preparation of microparticles
MPs were isolated from PFP using a 2-step sequential ultracentrifugation process. Briefly, 250 μL PFP was washed in 10 mL wash buffer (HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) 10 mM, NaCl 140 mM, KCl 4.5 mM, bovine serum albumin [BSA] 1%, Na azide 0.1%, CaCl2 2.5 mM, pH 7.4; 0.2 μm filtered) and ultracentrifuged at 100 000g for 60 minutes 2 times (total wash ratio 1:400). After careful aspiration of all but 1 mL supernatant, pelleted MPs were resuspended in the remainder wash buffer by gentle vortexing and pipetting and used immediately for flow cytometric experiments.
Flow cytometric detection of microparticles
All reagents and buffers were sterile and filtered using a 0.2-μm filter (Pall, Ann Arbor, MI). MP aliquots (90 μL) were incubated for 30 minutes in the dark with either specific antibodies (in concentrations found to be optimal in preliminary experiments) or an equal concentration of isotype control, always done in parallel. Samples were washed by centrifugation in 1 mL wash buffer at 32 000g (Hettich Mikro 22R, Tuttelingen, Germany) for 60 minutes at 20°C. After careful removal of all but 200 μL supernatant, pelleted MPs were resuspended in the remainder wash buffer, and 3 μL annexin V-Cy5 was added. After incubation for 15 minutes in the dark at room temperature, enumeration beads were added, and the samples were ready for fluorescence-activated cell sorting (FACS) study.
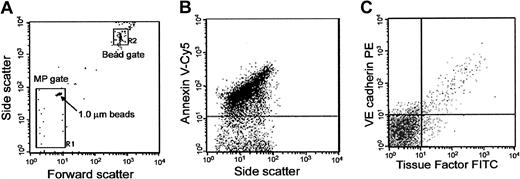
Sample data were acquired using a FACS Calibur flow cytometer with CellQuest pro software (Becton Dickinson). Forward (FSC) and side scatter of light was set in log scale, and threshold was set at the FSC parameter. MP gating was accomplished by preliminary standardization experiments using fluorescent microbeads of different sizes. First, MPs were simulated using varying sizes of standard microbeads (0.3-2.0 μm), and a microparticle gate (MP gate) was determined using these standards. The MP gate included 1.0-μm beads in its upper and outer corner so that it would contain all microparticles 1.0 μm or less (Figure 1A). Events in the MP gate were further assessed for labeling with annexin V-Cy5 to distinguish true events from electronic noise and thereby increase the specificity of MP detection (Figure 1B).
Flow cytometric analysis and quantification of MPs. (A) Determination of forward (FSC) and side-scatter (SSC) characteristics of light by 1.0-μm latex beads in buffer to establish the R-1 or MP gate. The R-2 or bead gate includes 7.2-μm latex beads used for enumerating MPs. (B) Detection of phosphatidylserine (PS)-positive MPs in platelet-free plasma (PFP) by annexin V-Cy5 labeling on the y-axis in relation to SSC on the x-axis. (C) Representative sample of MPs in PFP from a sickle sample triple labeled for annexin V (not shown), anti-TF-FITC on the x-axis, and anti-VE cadherin-PE on the y-axis.
Flow cytometric analysis and quantification of MPs. (A) Determination of forward (FSC) and side-scatter (SSC) characteristics of light by 1.0-μm latex beads in buffer to establish the R-1 or MP gate. The R-2 or bead gate includes 7.2-μm latex beads used for enumerating MPs. (B) Detection of phosphatidylserine (PS)-positive MPs in platelet-free plasma (PFP) by annexin V-Cy5 labeling on the y-axis in relation to SSC on the x-axis. (C) Representative sample of MPs in PFP from a sickle sample triple labeled for annexin V (not shown), anti-TF-FITC on the x-axis, and anti-VE cadherin-PE on the y-axis.
For MP quantification we used a modification of the methods of Combes et al1 in which a known quantity of 7.2-μm beads (250 000) was added to each sample, and acquisition was stopped after 10 000 beads were counted in the R-2 bead gate. The diameter of these beads allowed discrimination from the MP population on light scatter and could be counted using a separate gate (bead-gate; Figure 1A). Total MP number (N in MP per milliliter) was calculated using the following formula: n = C × Beadsadded/Beadscounted × plasma volume (mL) × 12, where C represents the number of positive events with the background signal subtracted and 12 is a dilution factor. For MP quantification, standard deviations for interassay and intra-assay variability were 10% and 4%, respectively.
Data analysis was performed using Flojo software. During data analysis, a “positive event” was defined as one that exhibited greater fluorescent intensity than the isotype control (IgG1 FITC or PE) or EDTA (ethylenediaminetetraacetic acid) buffer in the case of annexin V. Background signal from either isotype control or EDTA buffer accounted for 3% to 5% of the total signal in a typical experiment. Events falling in the R-1 MP gate (based on size) that also were annexin V-positive were defined as MPs, and the background signal (false positive) obtained using buffer containing only annexin V-Cy5 was subtracted. Annexin V-positive events were then further examined for fluorescent labeling with a cell-specific antibody and a TF antibody (Figure 1C).
To investigate the cellular origin of TF on MPs, all samples were triple labeled with Cy5-labeled annexin V, a cell type-specific PE-labeled MoAb against either CD14 or CD144 and a FITC-labeled MoAb against TF (Figure 1C). Although we did not expect platelet- or RBC-derived MPs to express TF, MPs from 6 patients were also triple-labeled with Cy5-labeled annexin V, PE-labeled anti-CD41a, or glycophorin A, and FITC-labeled anti-TF.
Procoagulant activity of MPs
MP aliquots isolated from plasma of study subjects were added as a potential source of TF, to normal citrated plasma. After recalcification, plasma clotting time was determined using an automated prothrombin time analyzer (Diagnostica Stago-ST4, Tokyo, Japan). In parallel, the clotting time of samples preincubated with a polyclonal blocking anti-TF antibody (9 μg/mL final concentration for 60 minutes at room temperature) was also measured. The difference between the 2 clotting times represents inhibition of TF-dependent activity. Clotting time was stopped at 1000 seconds if the samples had not clotted.
ELISA assay for D-dimer, TAT, and PF1.2
Markers of coagulation activation, prothrombin fragment F1.2 (PF1.2), thrombin antithrombin complex (TAT; Dade Behring, Marburg, Germany), D-dimer (Dimertest Gold EIA; American Diagnostica) were measured by ELISA. Upper limits of normal for D-dimer, TAT, and PF1.2 among healthy subjects determined by the manufacturer were 120 ng/mL, 4 μg/L, and 1.1 nM, respectively.
Statistical analysis
Because the data obtained had a non-Gaussian distribution, statistical differences between groups were evaluated using nonparametric methods. The Kruskal-Wallis test was used for an overall comparison between the groups. Significance determined by the Kruskal-Wallis test enabled subsequent intergroup comparisons using the Mann-Whitney U test; P < .05 (2-tailed) was considered significant. Bivariate correlations were done using the Spearman rho test. Paired sample analysis was done using the Wilcoxon signed log-rank test. Statistical analysis was performed using SPSS version 10.0 for Windows (Chicago, IL).
Results
We used a rigorous definition for plasma MPs because we found methods in the literature to be insufficient for our goals. For instance, some reports have simply defined MPs as annexin V-positive events in plasma by flow cytometry, but we find that plasma also contains annexin V binding material that cannot be sedimented by ultracentrifugation (A.S.S., R.P.H., unpublished data, August 10, 2001). Additionally, some groups have defined MPs as events by flow cytometry that are 2 μm or less, but we found this size was large enough to erroneously include platelets (A.S.S., R.P.H., unpublished data, August 10, 2001). Therefore, we defined MPs as vesicles removable from plasma by ultracentrifugation that are 1 μm or less in size and that bind annexin V. Our data for MP number, cellular origin, and tissue factor expression are shown in Figures 2, 3, and the statistical comparisons between the different subject groups are shown in Table 1 (group data) and Table 2 (paired data).
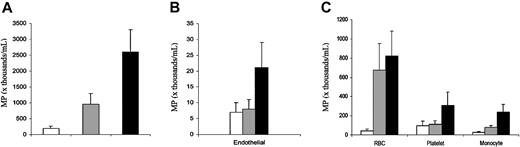
Total blood MPs and cellular origin of MPs. Data are expressed as MP number per milliliter of platelet-free plasma and shown as mean ± SEM, and statistical analysis is presented in Table 1. In each panel, the clear bars (□) show data for healthy state, gray bars (▦) show data for steady state, and black bars (▪) show data for crisis. (A) Total MP number. (B) Endothelial-derived MP number. (C) MP number derived from red blood cells, platelets, and monocytes. Error bars represent SEM.
Total blood MPs and cellular origin of MPs. Data are expressed as MP number per milliliter of platelet-free plasma and shown as mean ± SEM, and statistical analysis is presented in Table 1. In each panel, the clear bars (□) show data for healthy state, gray bars (▦) show data for steady state, and black bars (▪) show data for crisis. (A) Total MP number. (B) Endothelial-derived MP number. (C) MP number derived from red blood cells, platelets, and monocytes. Error bars represent SEM.
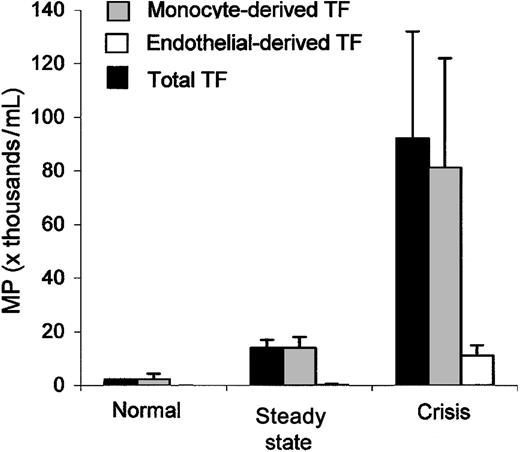
Tissue factor-positive MP. Data are expressed as MP number per milliliter of platelet-free plasma and shown as mean ± SEM, and statistical analysis is shown in Table 1. Total TF-positive microparticles (▪) are elevated among sickle patients in steady state and during crisis and are derived from monocytes (▦) and endothelial cells (□). Error bars represent SEM.
Tissue factor-positive MP. Data are expressed as MP number per milliliter of platelet-free plasma and shown as mean ± SEM, and statistical analysis is shown in Table 1. Total TF-positive microparticles (▪) are elevated among sickle patients in steady state and during crisis and are derived from monocytes (▦) and endothelial cells (□). Error bars represent SEM.
Statistical comparisons of group data among patients with sickle cell disease (crisis, n = 21; steady state, n = 16) and race-matched control subjects (n = 13)
. | P* . | . | . | ||
|---|---|---|---|---|---|
. | Crisis vs steady state . | Steady state vs healthy state . | Crisis vs healthy state . | ||
| Total MPs | .02 | .02 | .0001 | ||
| Red cell MPs | .15 | .02 | .0001 | ||
| Monocyte MPs | .12 | .009 | .0004 | ||
| Total TF+ MPs | .004 | .002 | <.0001 | ||
| Monocyte TF+ MPs | .009 | .004 | <.0001 | ||
| Endothelial TF+ MPs | .02 | .36 | .01 | ||
| D-dimer | .13 | .001 | .0002 | ||
| TAT | .08 | .0002 | .0001 | ||
| PF1.2 | .48 | .008 | .003 | ||
. | P* . | . | . | ||
|---|---|---|---|---|---|
. | Crisis vs steady state . | Steady state vs healthy state . | Crisis vs healthy state . | ||
| Total MPs | .02 | .02 | .0001 | ||
| Red cell MPs | .15 | .02 | .0001 | ||
| Monocyte MPs | .12 | .009 | .0004 | ||
| Total TF+ MPs | .004 | .002 | <.0001 | ||
| Monocyte TF+ MPs | .009 | .004 | <.0001 | ||
| Endothelial TF+ MPs | .02 | .36 | .01 | ||
| D-dimer | .13 | .001 | .0002 | ||
| TAT | .08 | .0002 | .0001 | ||
| PF1.2 | .48 | .008 | .003 | ||
P values determined by Mann-Whitney U test.
Statistical comparison of paired data for patients with sickle cell disease sampled both during crisis and steady state (n = 12)
. | P* . |
|---|---|
| Total MPs | .002 |
| Red blood cell MPs | .04 |
| Monocyte MPs | .01 |
| Total TF+ MPs | .007 |
| Monocyte TF+ MPs | .007 |
| Endothelial TF+ MPs | .06 |
| D-Dimer | .01 |
| TAT | .04 |
| PF1.2 | .06 |
. | P* . |
|---|---|
| Total MPs | .002 |
| Red blood cell MPs | .04 |
| Monocyte MPs | .01 |
| Total TF+ MPs | .007 |
| Monocyte TF+ MPs | .007 |
| Endothelial TF+ MPs | .06 |
| D-Dimer | .01 |
| TAT | .04 |
| PF1.2 | .06 |
P values are calculated from steady state versus crisis and determined by Wilcoxon signed rank test.
Total MPs are elevated among sickle patients
Enumeration revealed race-matched healthy subjects to have total MPs of 192 ± 70 × 103/mL PFP (mean ± SEM). In comparison, total MPs were significantly elevated for both patients with sickle cell disease in steady state (950 ± 346 × 103/mL; P = .02 versus healthy subjects) and in crisis (2592 ± 703 × 103/mL; P = .0001 versus healthy subjects) (Table 1; Figure 2A). For total MP number, both the overall group data of steady state versus crisis donors and the paired data of patients sampled during both steady state and crisis showed a significant difference (P = .02 and P = .003; Tables 1, 2). There was no difference in total MP number linked to age or sex (data not shown). We measured total MPs in 2 individuals who had undergone splenectomy with hemolytic anemia and found numbers similar to those of healthy subjects (338 × 103/mL and 110 × 103/mL), suggesting that absent splenic function per se does not markedly influence MP number. Similarly, samples from 3 individuals with sickle trait were not significantly different from the race-matched healthy subjects (207 ± 195 × 103/mL).
Cell origin of MPs
Using specific markers, we confirmed findings of others5,6 that there are blood MPs in patients with sickle cell disease derived from red blood cells (glycophorin A+) and platelets (CD41a+). However, we also found MPs derived from endothelial cells (VE-cadherin+) and monocytes (CD14+) (Figure 2B-C). Red blood cell- and monocyte-derived MPs were significantly increased in both steady state and crisis compared with healthy subjects. Both red blood cell- and monocyte-derived MPs were also elevated in sickle crisis compared with steady state (Figure 2), but this achieved significance only in the paired data analysis (Table 2). Red blood cell-derived MP number correlated inversely with hemoglobin (r = -0.452; P = .003), and monocyte-derived MP number correlated positively with absolute monocyte count (r = 0.442; P = .001).
Endothelial cell- and platelet-derived MPs were each similar in healthy subjects and patients with sickle cell disease in steady state, but each was elevated during crisis (Figure 2). However, these differences were not statistically significant (Table 1). For endothelial cell-derived MPs this clearly was due to variability among patients, as 10 of 21 sickle samples in crisis had no detectable endothelial MPs. For platelet-derived MPs, the difference approached statistical significance both among crisis versus healthy subjects and crisis versus steady state in the paired data.
Expression of tissue factor on endothelialand monocyte-derived MPs
Testing MPs for TF, we found that a portion were TF positive (Figure 3). Patients in crisis had significantly higher total TF-positive MPs compared with those in steady state or healthy subjects, and subjects in steady state had significantly higher numbers than healthy subjects (Table 1). Group data revealed a significant difference between steady state and crisis for both monocyte- and endothelial-derived tissue factor-positive MPs (Table 1). TF-positive MPs were derived from endothelial cells and monocytes (Figure 3) but not from red blood cells or platelets. Most of the sickle samples in crisis (20 of 21) had TF-positive MPs that were derived from monocytes. A smaller number of samples in crisis (8 of 21) had TF-positive MPs that were derived from endothelial cells, so 13 of 21 samples had no detectable endothelial-derived TF-positive MPs.
MPs without using annexin V binding criterion
To assess both PS-positive and PS-negative MPs, it is possible to relax the criteria for MP definition by eliminating the need for annexin V binding. In doing so, we thus defined MPs as small (≤ 1.0 μm) particles removable by ultracentrifugation that were labeled with cell-specific markers. Total MP number enumerated by this method for healthy subjects was 218 ± 98 (mean ± SEM), and for patients with sickle cell disease was 1312 ± 424 in steady state and 1844 ± 542 in crisis. This discrepancy indicates that there are a number of PS-positive MPs in crisis that do not label with the cell-specific markers that we used. For the MPs of specific cell origin, monocyte- and endothelial-derived MP number was increased by 3-fold and 4-fold, respectively, by using this definition compared with the previous data, which additionally requires annexin V binding. However, the RBC- and platelet-derived MP number did not change. Inclusion of PS-negative MPs, however, did not change the number of patients with sickle cell disease demonstrating endothelial MPs. The percentage of monocyte-derived MPs that were TF+ and PS- was 23% in steady state and 25% in crisis. The percentage of endothelial-derived MPs that were TF+ and PS- was 7% in steady state and 32% in crisis.
Procoagulant activity of sickle MPs
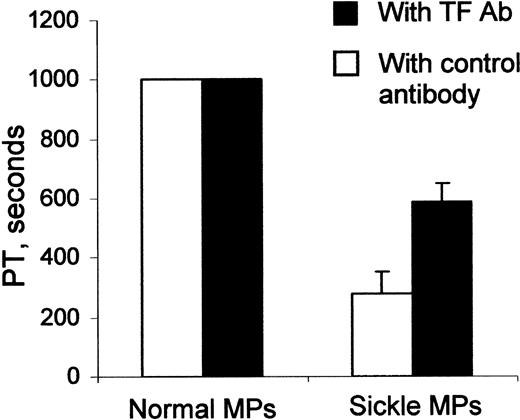
The MP preparation from individual patients with sickle cell disease significantly shortened the plasma clotting time, compared with the MP preparation from healthy control subjects (Figure 4; P = .001). This procoagulant activity of sickle MPs was partially inhibited by a blocking anti-TF antibody (Figure 4; P = .002), providing evidence that sickle MPs possessed TF-dependent procoagulant activity. The anti-TF antibody blocked approximately 55% of the total procoagulant activity, suggesting that it did not completely inhibit TF in this assay. Of note, MPs from healthy subjects were unable to shorten the clotting time in this assay, probably because the normal MP preparation included fewer total MPs (Figure 2) and fewer TF-positive MPs (Figure 3).
Effect of MPs on plasma clotting time. MP aliquots from patients with sickle cell disease could shorten plasma clotting time, whereas similar MP aliquots from healthy subjects could not (P = .001). Parallel samples (▪) show that MP shortening of plasma clotting time could be partially inhibited by a TF antibody, demonstrating TF-dependent procoagulant activity (P = .002). Error bars represent SEM.
Effect of MPs on plasma clotting time. MP aliquots from patients with sickle cell disease could shorten plasma clotting time, whereas similar MP aliquots from healthy subjects could not (P = .001). Parallel samples (▪) show that MP shortening of plasma clotting time could be partially inhibited by a TF antibody, demonstrating TF-dependent procoagulant activity (P = .002). Error bars represent SEM.
Markers of coagulation activation are elevated and correlate with MP numbers
We measured plasma D-dimer (a marker of fibrinolysis), as well as TAT and PF1.2 (markers of thrombin generation) in our study subjects. Each marker of coagulation activation was significantly elevated among patients with sickle cell disease in both steady state and crisis compared with healthy subjects (Table 1). The paired data indicated significant differences between crisis and steady state for D-dimer and TAT (Table 2). All 3 markers correlated modestly but significantly with total MPs, total TF-positive MPs, monocyte-derived TF-positive MPs, and with RBC-derived MPs (Table 3). For endothelial-derived TF-positive MPs, correlations did not achieve significance (not shown), again because only a subgroup of patients had any TF-positive endothelial MPs.
Correlation coefficients (Spearman rho) for all study samples
. | Total MPs . | RBC MPs . | Monocyte MPs . | Total TF+ MPs . | Monocyte TF+ MPs . |
|---|---|---|---|---|---|
| TAT | 0.436 (.002) | 0.506 (.001) | 0.459 (.002) | 0.367 (.01) | 0.406 (.007) |
| D-dimer | 0.387 (.008) | 0.365 (.01) | 0.356 (.01) | 0.454 (.002) | 0.455 (.002) |
| PF1.2 | 0.398 (.008) | 0.307 (.04) | 0.258 (.09) | 0.366 (.01) | 0.380 (.01) |
. | Total MPs . | RBC MPs . | Monocyte MPs . | Total TF+ MPs . | Monocyte TF+ MPs . |
|---|---|---|---|---|---|
| TAT | 0.436 (.002) | 0.506 (.001) | 0.459 (.002) | 0.367 (.01) | 0.406 (.007) |
| D-dimer | 0.387 (.008) | 0.365 (.01) | 0.356 (.01) | 0.454 (.002) | 0.455 (.002) |
| PF1.2 | 0.398 (.008) | 0.307 (.04) | 0.258 (.09) | 0.366 (.01) | 0.380 (.01) |
Data shown as r values, and P values are presented in parentheses.
Discussion
Although blood MPs were first described more than a decade ago,20 there is no consensus as to the definition, pathophysiologic role, and fate of blood-derived MPs. The published literature describes various methods for isolating and defining MPs using flow cytometry.4,5,21 We used a very rigorous definition for MPs: vesicular material that is removable from PFP by ultracentrifugation, with a size of 1.0 μm or less, and surface PS positive as evidenced by annexin V binding. Imposition of these criteria probably sacrifices sensitivity to some degree but probably preserves specificity for true MPs. Although the demonstration of surface PS by annexin V binding adds a level of rigor to the methodology, this level may be unnecessary if markers of cell surface antigens are used instead. The present data indicate that, although all RBC- and platelet-derived MPs are PS positive, this is not true of endothelium and monocyte-derived MPs. Indeed, a substantial portion of endothelial- and monocyte-derived MPs are PS negative. The biologic relevance of this finding is currently uncertain, but clearly PS-positive MPs have more relevance in the setting of coagulation.
The findings in this study led to 5 key observations. (1) Total blood MPs are elevated in sickle crisis compared with steady state. (2) Sickle blood contains MPs derived from endothelial cells and monocytes. (3) A portion of the MPs derived from these 2 cell types is TF positive. (4) MPs in sickle cell disease have procoagulant activity, some of which is tissue factor dependent. And (5), in general, MP and TF-positive MP values for patients with sickle cell disease in steady state were intermediate between those of healthy and crisis subjects. However, the subjects in steady state were not always significantly different from those in crisis.
RBC-derived MPs almost always constitute the majority of blood MPs in patients with sickle cell disease but not among control subjects in which platelet-derived MPs predominate. Accounting for this observation are the findings by Allan et al22 that sickle erythrocytes release vesicles as a result of repetitive sickling and unsickling during hypoxia and reoxygenation. RBC-derived MPs are elevated both during crisis and steady state, supporting these findings. They are also more elevated during crisis compared with steady state. Therefore, the presence of these circulating vesicles might explain previous reports of elevated plasma hemoglobin during sickle crisis.23,24 Indeed, we have observed that 50% ± 32% of PFP hemoglobin can be removed by ultracentrifugation and is, therefore, most likely MP associated (A.S.S. and R.P.H., unpublished data, February 10, 2003).
Monocyte-derived MPs were significantly elevated during steady state and crisis compared with healthy subjects, suggesting that sickle monocytes are activated at all times in sickle disease. Although endothelial-derived MPs were elevated during crisis compared with healthy state, this difference was not significant statistically from healthy subjects. This is due to intergroup variability because only a subgroup of the patients with sickle cell disease (11 of 21) had demonstrable endothelial-derived MPs. Platelet-derived MPs were elevated but not significantly. This elevation is most likely due to our careful avoidance of platelet contamination, our stringent size definition (≤ 1.0 μm, which is much less likely to include platelets than the size previously used ≤ 2.0 μm5), and our use of a platelet-specific antibody (CD41) compared with previously used antibody5 (CD61) that reacts against epitopes shared by platelets and endothelial cells.
Overall, these data indicate that patients with sickle cell disease have elevated MPs both in steady state and crisis, implying that even patients in steady state are fundamentally different from healthy subjects. In general, stimuli causing cellular activation or injury result in membrane perturbation and vesiculation, ie, release of MPs. Examples of these are agents such as tumor necrosis factor α (TNFα) and endotoxin that cause vesiculation from endothelial cells and monocytes, respectively.1,25 In vivo, vesiculation occurs pathologically in conditions characterized by endothelial damage, such as thrombotic thrombocytopenic purpura, sepsis, and disseminated intravascular coagulation.4,21 Thus, the findings of endothelial- and monocyte-derived MPs in sickle blood imply endothelial and monocyte activation in this disease, or possibly apoptosis. Notably, sickle endothelial cells probably manifest an activated phenotype, with abnormal expression of adhesion molecules.7 Similarly, monocytes are in an activated state, demonstrating up-regulation of CD11b and increased intracellular cytokines.9 There is mounting evidence that sickle cell anemia is an inflammatory condition10 associated with endothelial perturbation.26 The present data support this concept and suggest that there is a chronic, on-going activation of blood cells and the vascular wall.
The expression of TF by blood MPs is of great potential relevance to disease pathobiology. TF is the principal initiator of coagulation,27 and its increased in vivo expression promotes thrombotic events in patients with a variety of clinical disorders.28,29 In sickle cell disease, there is abnormal expression of tissue factor by monocytes18 and perhaps endothelium8 and an association with thrombotic manifestations such as stroke17 and pulmonary thrombosis.30 TF-positive MPs could potentially contribute to thrombotic manifestations by activating the clotting system and generating thrombin. We found that TF-positive MPs were more elevated in crisis than in steady state, but even patients in steady state demonstrated significant differences from healthy control subjects, suggesting that hemostatic perturbation is a constant feature of sickle cell disease.
In parallel, markers of coagulation activation were abnormally elevated in both crisis and steady state compared with healthy state, consistent with previous reports.13,16,31 Total MPs and total TF-positive MP levels correlated modestly, but significantly, with all 3 markers of coagulation activation that were studied. Biochemical markers such as D-dimer, TAT, and PF1.2 are measures of global activation of the coagulation system that may be less sensitive as markers of tissue factor exposure, explaining the rather modest correlations obtained.
Recent reports in patients with type 2 diabetes mellitus suggest that platelet-derived MPs are positive for TF, but that study used a platelet marker (CD61) that is shared by endothelial cells.32 Our inability to detect platelet-derived TF-positive MPs could be related to low levels, not detectable by FACS, or a biologic difference between sickle cell disease (SCD) and diabetes. Although RBC- and platelet-derived MPs were not positive for TF, they could still have potential functional significance because they express surface PS and other coagulation factor receptors33 and may be involved in acceleration of coagulation once it is initiated.31
In summary, our data demonstrate that blood MPs in sickle cell disease are derived in part from endothelial cells and monocytes and a portion of these are also TF positive. This microparticle-associated TF is functionally active and could, therefore, contribute to thrombotic occlusive events such as stroke in sickle cell disease.17 In any case, their presence argues for the existence of abnormal monocyte and endothelial activation in patients with sickle cell disease both in steady state and crisis. In aggregate, these data further support the idea that sickle disease is a chronic inflammatory state with abnormal activation of the blood and vessel wall.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-03-0693.
Supported by grants PO1 HL55552 (R.P.H.) and R29 HL55219 (N.S.K.) from the National Institutes of Health. A.S.S. is a scholar of the Sickle Cell Disease Association of America, and the Lillihei Heart Institute, Minneapolis, MN.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ron Bach for providing the anti-TF antibody and helpful discussions and Carol Taubert for secretarial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal