Abstract
Defining the relative importance of protease-activated receptors (PARs) for thrombin signaling in mouse endothelial cells is critical for a basic understanding of thrombin signaling in these cells and for the rational use of knockout mice to probe the roles of thrombin's actions on endothelial cells in vivo. We examined thrombin- and PAR agonist–induced increases in cytoplasmic calcium, phosphoinositide hydrolysis, extracellular signal-regulated kinase (ERK) phosphorylation, and gene expression in endothelial cells from wild-type and PAR-deficient mice. PAR1 and PAR4 agonists triggered responses in wild-type but not in Par1–/– and Par4–/– endothelial cells, respectively. Calcium imaging confirmed that a substantial fraction of individual endothelial cells responded to both agonists. Compared with wild-type cells, Par1–/– endothelial cells showed markedly decreased responses to low concentrations of thrombin, and cells that lacked both PAR1 and PAR4 showed no responses to even high concentrations of thrombin. Similar results were obtained when endothelial-dependent vasorelaxation of freshly isolated mouse aorta was used as an index of signaling in native endothelial cells. Thus PAR1 is the major thrombin receptor in mouse endothelial cells, but PAR4 also contributes. These receptors serve at least partially redundant roles in endothelial cells in vitro and in vivo and together are necessary for the thrombin responses measured.
Introduction
Thrombin triggers a host of responses in endothelial cells that may contribute to hemostasis, inflammation, and development of embryonic blood vessels.1-3 For example, thrombin causes the release of von Willebrand factor, the mobilization of P-selectin to the endothelial surface,4 and the production of platelet-activating factor5,6 and chemokines7,8 —events likely to be involved in recruiting platelets and leukocytes to sites of vascular injury.9-12 Thrombin triggers changes in the junctional complexes between endothelial cells and cell rounding, and it increases the permeability of endothelial monolayers.13-16 Thrombin also stimulates endothelial cell migration and the production of growth factors and their receptors, cytokines, and matrix proteins—events that may be involved in the proper formation and maintenance of blood vessels during embryonic development.3,17-20 Identifying the thrombin receptors that mediate endothelial cell activation is a necessary step toward defining the relative importance of endothelial cell responses to thrombin in vivo.
Thrombin triggers cellular responses at least in part through G-protein–coupled protease-activated receptors (PARs).1 In the mouse, PAR1 and PAR4 can each mediate thrombin responses; PAR2 is not activated by thrombin, and PAR3 does not itself mediate transmembrane signaling but instead functions as a cofactor that promotes PAR4 activation by thrombin in mouse platelets.21,22 PARs are activated by the proteolytic unmasking of a tethered peptide ligand that resides in the receptor's N-terminal exodomain, and synthetic peptides that mimic this sequence function as agonists that activate PARs independent of receptor cleavage.23,24 Activation of PAR1 with such a peptide is sufficient to trigger most, if not all, of the known endothelial responses to thrombin. However, it is unknown whether PAR1 accounts for all thrombin signaling in endothelial cells. Indeed, recent studies with PAR1 and factor V knockout mice suggest that other targets of thrombin—perhaps other endothelial PARs—are important for embryonic development and possibly vascular diseases.3
We now report studies that address the relative importance of different PARs for thrombin signaling in vascular endothelial cells and whether PARs account for thrombin signaling in these cells.
Materials and methods
Materials
The peptides TFLLRN, YAPGKF, AYPGKF, and SLIGRL were synthesized as carboxyl terminal amides and purified by high-pressure liquid chromatography (Anaspec, San Jose, CA). Cell culture reagents were from the University of California San Francisco Cell Culture Facility unless otherwise specified. Human α-thrombin was from Enzyme Research Laboratories (South Bend, IN). Mouse vascular endothelial growth factor 164 (VEGF164), [3H]-myoinositol, porcine skin gelatin, and human fibronectin were from R&D Systems (Minneapolis, MN), Amersham (Piscataway, NJ), Sigma (St Louis, MO), and Roche (Indianapolis, IN), respectively.
Mouse endothelial cell isolation and culture
Par1–/– and Par4–/– mice25,26 were bred to generate double heterozygotes and then double knockouts. Phenotypes of these mice will be reported separately. In most experiments involving wild-type, Par1–/–, and Par4–/– mice, the strain background was greater than 97% C57BL6. In experiments involving mice lacking both PAR1 and PAR4, the background was 50% C57BL6/129Sv; wild-type endothelial cells from mice of this background responded to thrombin as did those from C57BL6 (not shown). Eight to 10 “neonatal” mice (2-6 days old) were used for each endothelial cell preparation. After euthanasia, skin was removed along the natural cleavage plane together with subcutaneous tissue, decontaminated in 10% Hibiclens (Zeneca, Wilmington, DE), and washed once in 70% ethanol and twice with phosphate-buffered saline (PBS). Lungs were dissected free and dipped in 70% ethanol for 10 seconds and washed with PBS. Tissue was minced into 1- or 2-mm pieces and collected in Dulbecco modified Eagle medium (DMEM) with 1% bovine serum albumin (BSA), and incubated in DMEM with 1% BSA containing Collagenase B (Roche) (1.5-2.0 mg/mL) and Dispase I (0.125-0.25 mg/mL) or Dispase II (Roche) (3-5 mg/mL) at 37°C for 60 minutes with shaking. Digested tissue was dispersed by pipetting 8 times through a 14-gauge laboratory canula (VWR, West Chester, PA) and filtered through stainless mesh (100 mesh/140 μm) (Bellco, Vineland, NJ). Cells were collected by centrifugation and plated in DMEM/F12 with 20% fetal bovine serum (Hyclone, Logan, UT), 100 μg/mL heparin (Sigma), penicillin-streptomycin, and 50 μg/mL endothelial cell growth supplement (BTI, Stoughton, MA) in 0.5% gelatin-coated Primaria tissue culture plates (Falcon, Franklin Lakes, NJ). After 2 days, endothelial cells were immunopurified by a modification of published methods.27 Goat antirat immunoglobulin G (IgG) Dynabeads (Dynal, Oslo, Norway) were incubated with antimouse intracellular adhesion molecule-2 (ICAM-2) antibody (PharMingen, San Diego, CA) overnight, then washed twice by DMEM/0.1% BSA to remove unbound antibody. The ICAM-2 antibody–coated beads were added to the culture plate and incubated for 40 to 60 minutes with gentle shaking at room temperature. Culture plates were washed with PBS, trypsinized, and applied to a magnetic separator (Dynal). Bead-bound cells were washed 4 to 5 times by DMEM/0.1% BSA, suspended in growth media, and cultured on gelatin-coated plates. Four or 5 days later, a second round of immunopurification was performed; twice-immunopurified cells were used for experiments within 8 to 11 days of the initial tissue digestion.
Characterization of cultured mouse endothelial cells
The purity of endothelial cell cultures was examined by direct immunostaining of cultured cells, flow cytometry, and LacZ staining of cultures from Tie2-LacZ mice.28 For immunostaining, cells were fixed with ice-cold methanol for 5 minutes, washed with PBS, blocked in PBS with 2% instant milk, and incubated with rat primary antibodies against the mouse endothelial cell markers, platelet endothelial cell adhesion molecule (PECAM) (MEC13.3; 1:250), and ICAM-2 (3C4; 1:250; PharMingen) or with nonimmune rat IgG as a control. Cells were washed, incubated with fluorescein isothiocyanate (FITC)–mouse antirat IgG (1:100; Zymed, South San Francisco, CA) or with horseradish peroxidase-conjugated rabbit antirat IgG (1:200; Biosource, Camarillo, CA) followed by the chromogenic substrate diaminobenzidine. Staining was then visualized by fluorescence or light microscopy. For flow cytometry cells were detached by incubation with PBS–5 mM ethylenediaminetetraacetic acid (EDTA) and then incubated with biotinylated antibodies to PECAM or ICAM-2 or with isotype-matched biotinylated control antibodies (PharMingen) in Hanks/1% BSA solution. After incubation with streptavidin-phycoerythrin (Sigma), cells were analyzed using FACScalibur (Becton, San Jose, CA).
Human endothelial cells
Human umblical vein endothelial cells (HUVECs), human dermal microvascular endothelial cells (HDMVECs), and human lung microvascular endothelial cells (HLMVECs) were obtained from Clonetics (San Diego, CA) and cultured in EGM-2 or EGM-MV-2 media kit (Clonetics, San Diego, CA) on fibronectin (Roche)–coated plates according to the supplier's instructions. More than 95% of the human cells in these cultures expressed the endothelial marker PECAM-1 by immunonstaining using antihuman PECAM antibody (clone P2B1) (Chemicon, Temecula, CA).
Phosphoinositide hydrolysis and ERK phosphorylation
Endothelial cells were plated on gelatin/fibronectin-coated 24-well plates (Costar 3524; Costar, Cambridge, MA) at 90% to 100% confluence and incubated overnight. Cells were then washed 3 times with serum-free DMEM and labeled with [3H]-myoinositol (2 μCi/mL [0.074 MBq]) in inositol-free DMEM/0.5% BSA for 14 hours. After labeling, cells were washed once with DMEM/0.1% BSA and incubated for an additional 2 hours in 200 μL DMEM/0.1% BSA per well. Then 50 μL DMEM/0.1% BSA with 100 mM LiCl without or with agonists was added (final concentrations: TFLLRN, 100 μM; YAPGKF and AYPGKF, 500 μM; thrombin, 10 nM; SLIGRL, 100 μM; and LiCl, 20 mM), and cells were incubated for 2 hours at 37°C. Phosphoinositide hydrolysis was measured as described.29
ERK phosphorylation was assayed using mitogen-activated protein kinase (MAPK) antibody kit (Cell Signaling Technology, Beverly, MA) as recommended by the manufacturer. In brief, endothelial cells in 12- or 24-well plates were incubated for 12 hours in DMEM/F12/1% BSA. Cells were incubated in fresh DMEM/F12/1% BSA for another 2 hours and then stimulated by adding agonists at 5 times their final concentration in DMEM/F12/1% BSA. Whole-cell lysates prepared in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 10 mM dithiothreitol (DTT) and 1 mM sodium orthovanadate were analyzed by immunoblot according to the manufacturer's protocol.
Single-cell calcium imaging
Single-cell calcium assays were performed essentially as described.30 Eight- or 4-well Lab-Tek chambered coverslips (Nunc, Rochester, NY) were coated sequentially with 5 mg/mL poly-l-ornithine (Sigma), 1% gelatin (Sigma), and 1 mg/mL fibronectin (Roche) before plating cells. For cells from Par1–/–;Par4–/– mice, ACLAR film (Ted Pella, Redding, CA) coated with poly-L-lysine, type 1 collagen (Becton), and fibronectin was used to enhance cell attachment and yield; these cells still responded normally to VEGF and other control agonists. Cells were plated at 50% to 70% confluence and incubated overnight. Growth media were replaced with DMEM/0.1% BSA for 1 hour, and cells were then loaded with 5 μM fura-2 (Molecular Probes, Eugene, OR), 0.02% pluronic acid (Molecular Probes) in CIB buffer30 (130 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 0.6 mM MgCl2, 1.2 mM NaHCO3, 10 mM glucose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.4]) for 1 hour at room temperature, washed 3 times by CIB buffer, and stimulated with agonists. Calcium imaging was performed using a Nikon Diaphot fluorescence microscope equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and a charge-coupled device (CCD) camera (Hamamatsu, Middlesex, NJ). Dual images (340 and 380 nm excitation, 510 nm emission) were collected every 4 seconds, and pseudocolor radiometric images were monitored during the experiment (Metafluor software; Universal Imaging, Media, PA). Concentrations of agonists used were 10 μM PAR1 agonist TFLLRN, 10 nM thrombin, 500 μM PAR4 agonist AYPGKF, and 50 ng/mL VEGF.
Northern blot analysis
Endothelial cells isolated from wild-type, Par1–/–, or Par4–/– mice were grown to more than 90% confluence in 100-mm culture dish, serum starved in DMEM/F12/1% BSA overnight, and incubated for 45 minutes with vehicle, 10 μM PAR1 agonist TFLLRN, 10 nM α-thrombin, or 500 μM PAR4 agonist AYPGKF. Egr-1 probe was a 300–base pair EcoRI-NotI fragment from IMAGE clone 576070 (ATCC 734478). c-Fos probe corresponding to the nucleotide sequence 257-1189 from GenBank accession number BC029814 was polymerase chain reaction (PCR)–amplified from the neonatal lung/heart cDNA. Total RNA was isolated by Trizol (Invitrogen, Carlsbad, CA), and 20 μg from each sample was separated in 1% agarose transferred to Hybond-N+ membrane (Amersham) and hybridized with probes for mouse Egr-1, c-Fos, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) generated using Prime-IT II (Stratagene, San Diego, CA). After hybridization for 3 hours in Perfect-HybR solution (Sigma), the membrane was washed 5 times at room temperature (1 × SSC, 0.1% SDS, 10 minutes) and twice at 50°C (0.1 × SSC, 0.1% SDS, 15 minutes), then exposed to film. Northern blot signals were quantitated using a Molecular Dynamics Storm 860 beta scanner (Amersham Biosciences, Piscataway, NJ).
Vasorelaxaton in freshly isolated mouse aorta
The vasorelaxation response in mouse thoracic aorta was analyzed essentially as described.31,32 Thoracic aortae were dissected from adult wild-type, Par1–/–, Par4–/–, and Par1–/–; Par4–/– mice. Aortic ring segments of approximately 3 mm in length were mounted between 2 parallel stainless steel wire hooks and equilibrated for 20 minutes in 37°C baths containing Krebs solution (composition in mmol/L: 144 Na+, 128.7 Cl–, 25
Results
PAR1 and PAR4 are the 2 protease-activated receptors known to be capable of mediating transmembrane signaling in response to thrombin in the mouse.25,33 To determine the relative contributions of PAR1 and PAR4 to thrombin responses in endothelial cells and whether these 2 receptors together account for thrombin signaling in these cells, we compared thrombin responses in endothelial cells immunopurified from skin and lung from wild-type mice and from mice lacking PAR1 or PAR4 or both. Endothelial cultures were used 8 to 11 days after isolation. More than 95% of the cells in these cultures expressed the endothelial cell markers PECAM-1 and ICAM-2, as assessed by flow cytometric analysis (Figure 1) or immunostaining (not shown). Similarly, when endothelial cells were isolated from mice bearing an endothelial-specific Tie2-lacZ transgene,28 more than 95% of the cultured cells stained for β-galactosidase (not shown).
Characterization of endothelial cell preparations from neonatal mouse skin. Cells were stained with biotinylated IgGs (isotype-matched control [left], anti-PECAM [middle], or anti–ICAM-2 [right]) followed by streptavidin-phycoerythrin (PE). Bound PE was quantitated by flow cytometry. Similar results were obtained with lung endothelial cell preparations.
Characterization of endothelial cell preparations from neonatal mouse skin. Cells were stained with biotinylated IgGs (isotype-matched control [left], anti-PECAM [middle], or anti–ICAM-2 [right]) followed by streptavidin-phycoerythrin (PE). Bound PE was quantitated by flow cytometry. Similar results were obtained with lung endothelial cell preparations.
Phosphoinositide hydrolysis in wild-type and PAR-deficient endothelial cells
PAR1 is known to be expressed in endothelial cells.23,34 Accordingly, we first compared thrombin responses in endothelial cultures from wild-type and Par1–/– mice. Phosphoinositide hydrolysis was used as a convenient marker of thrombin signaling. Thrombin increased phosphoinositide hydrolysis to approximately 2.5 times that seen in unstimulated dermal endothelial cell cultures from wild-type mouse skin (P < .001) but only approximately 1.3 times that seen in basal cells from Par1–/– mice (P < .001) (Figure 2A). Responses to the PAR2 agonist SLIGRL were not different in wild-type compared with Par1–/– endothelial cells (not shown). Thus PAR1 was a major mediator of thrombin-induced phosphoinositide hydrolysis in endothelial cells, but the persistence of some thrombin-induced phosphoinositide hydrolysis in Par1–/– endothelial cells indicated the existence of a PAR1-independent pathway.
Phosphoinositide hydrolysis in endothelial cells from neonatal skin and lung. Endothelial cells were isolated from mice of the indicated genotype, labeled with [3H]-myoinositol, then incubated with vehicle, scrambled PAR4 agonist (YAPGKF; 500 μM), PAR4 agonist (AYPGKF; 500 μM), or α-thrombin (10 nM) for 2 hours. Data shown are mean fold increases (± SD) in [3H]-inositol phosphate accumulation normalized to vehicle control (n = 4). Each experiment was reproduced at least 3 times except for that using Par1–/–;Par4–/– lung endothelial cells (Par1,4–/–), which was performed only once because of the difficulty of generating adequate numbers of Par1,4–/– mice. Responses to the PAR2 agonist SLIGRL (100 μM) were also measured in each of the experiments shown; all endothelial cell preparations, regardless of genotype, showed robust responses (A) 10- to 20-fold in endothelial cell preparations from skin and (B) 8- to 10-fold in preparations from lung.
Phosphoinositide hydrolysis in endothelial cells from neonatal skin and lung. Endothelial cells were isolated from mice of the indicated genotype, labeled with [3H]-myoinositol, then incubated with vehicle, scrambled PAR4 agonist (YAPGKF; 500 μM), PAR4 agonist (AYPGKF; 500 μM), or α-thrombin (10 nM) for 2 hours. Data shown are mean fold increases (± SD) in [3H]-inositol phosphate accumulation normalized to vehicle control (n = 4). Each experiment was reproduced at least 3 times except for that using Par1–/–;Par4–/– lung endothelial cells (Par1,4–/–), which was performed only once because of the difficulty of generating adequate numbers of Par1,4–/– mice. Responses to the PAR2 agonist SLIGRL (100 μM) were also measured in each of the experiments shown; all endothelial cell preparations, regardless of genotype, showed robust responses (A) 10- to 20-fold in endothelial cell preparations from skin and (B) 8- to 10-fold in preparations from lung.
PAR4 mRNA was detectable in mouse endothelial cell cultures by reverse transcription–PCR (RT-PCR), but Northern blot analysis suggested that PAR4 was expressed at lower levels than PAR1 (not shown). To test the possibility that low-level expression of PAR4 might account for the residual thrombin signaling in Par1–/– endothelial cells, we examined signaling in response to the PAR4 agonist AYPGKF. AYPGKF increased phosphoinositide hydrolysis to approximately 1.5 times that of basal cells in wild-type dermal endothelial cell cultures (P < .001). The scrambled peptide YAPGKF did not elicit a response in wild-type cultures, and AYPGKF did not elicit a response in cells from Par4–/– mice. PAR4 is functionally expressed in wild-type endothelial cell preparations. Thrombin itself triggered approximately 1.9 times basal phosphoinositide hydrolysis in Par4–/– dermal endothelial cells compared with approximately 2.5 times basal in wild-type cells. Similar results were obtained with endothelial cultures derived from lung instead of skin (Figure 2B). Taken together, these results suggest that both PAR1 and PAR4 contribute to thrombin-triggered phosphoinositide hydrolysis in mouse microvascular endothelial cell preparations.
To determine whether signaling through PAR1 and PAR4 accounts for thrombin-triggered phosphoinositide hydrolysis in endothelial cell cultures, we examined thrombin signaling in cultures derived from mice deficient in both receptors. No thrombin-triggered phosphoinositide hydrolysis was detected in such cultures (Figure 2A), but the response to the PAR2 agonist SLIGRL was unchanged from that seen in wild-type cultures (not shown; see Figure 3). Similar results were obtained with endothelial cells from lung (Figure 2B). PAR1 and PAR4 are necessary for thrombin-triggered phosphoinositide hydrolysis in these endothelial preparations. As an aside, previous studies demonstrated that PAR2 expressed in heterologous systems cannot mediate thrombin signaling,35 and the lack of thrombin signaling in Par2+/+, Par1–/–, Par4–/– endothelial cells confirms this observation in the native context of an endothelial cell.
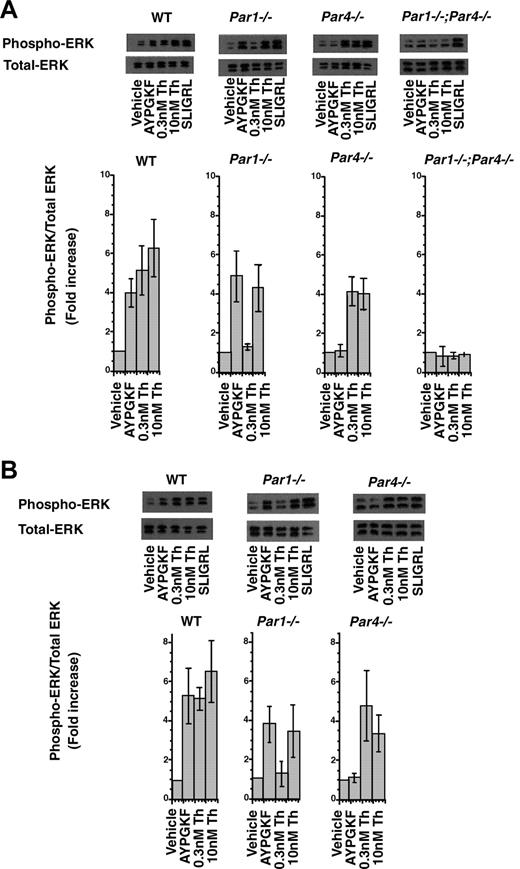
PAR-triggered ERK phosphorylation in dermal (A) and lung (B) endothelial cells. Cultures were stimulated with buffer alone, PAR4 agonist (AYPGKF; 500 μM), α-thrombin (Th; 0.3 nM and 10 nM), and PAR2 agonist (SLIGRL; 100 μM) for 5 minutes. Whole-cell lysates were analyzed by immunoblot for phosphorylated ERK1/2 and total ERK1/2. Representative immunoblots are shown. Bar graphs show the mean (± SE) fold increase in ERK phosphorylation over control from 3 independent experiments. Blots were scanned and quantitated by NIH image, and data are expressed as the ratio of phosphorylated to total ERK1/2 normalized to that of control. In all experiments, the PAR2 agonist SLIGRL triggered comparable increases in ERK phosphorylation (average, approximately 5-fold) regardless of genotype.
PAR-triggered ERK phosphorylation in dermal (A) and lung (B) endothelial cells. Cultures were stimulated with buffer alone, PAR4 agonist (AYPGKF; 500 μM), α-thrombin (Th; 0.3 nM and 10 nM), and PAR2 agonist (SLIGRL; 100 μM) for 5 minutes. Whole-cell lysates were analyzed by immunoblot for phosphorylated ERK1/2 and total ERK1/2. Representative immunoblots are shown. Bar graphs show the mean (± SE) fold increase in ERK phosphorylation over control from 3 independent experiments. Blots were scanned and quantitated by NIH image, and data are expressed as the ratio of phosphorylated to total ERK1/2 normalized to that of control. In all experiments, the PAR2 agonist SLIGRL triggered comparable increases in ERK phosphorylation (average, approximately 5-fold) regardless of genotype.
ERK phosphorylation in wild-type and PAR-deficient endothelial cells
We next examined a second thrombin-induced signaling event, ERK phosphorylation36 (Figure 3A). Endothelial cells were stimulated with agonists for 5 minutes, the time of peak response established in preliminary studies. The PAR4 agonist AYPGKF triggered approximately 4-fold increases in ERK phosphorylation in wild-type and PAR1-deficient endothelial cells but not in PAR4-deficient cells; hence, PAR4 activation can indeed trigger ERK phosphorylation in microvascular endothelial cell preparations. ERK phosphorylation in response to 0.3 nM thrombin was ablated in cells from Par1–/– mice, but 10 nM thrombin triggered relatively robust responses in these cells. In endothelial cell cultures from mice deficient in both PAR1 and PAR4, even 10 nM thrombin did not trigger ERK phosphorylation, but responsiveness to the PAR2 agonist SLIGRL was preserved. Similar data were obtained using endothelial cell preparations from lung (Figure 3B). These results suggest that PAR1 is required for ERK activation in response to low concentrations of thrombin, but in the absence of PAR1 function, PAR4 can mediate endothelial ERK activation in response to high concentrations of thrombin. As with phospoinositide hydrolysis, PAR1 and PAR4 together appear to account for thrombin-induced ERK activation in mouse endothelial cell preparations.
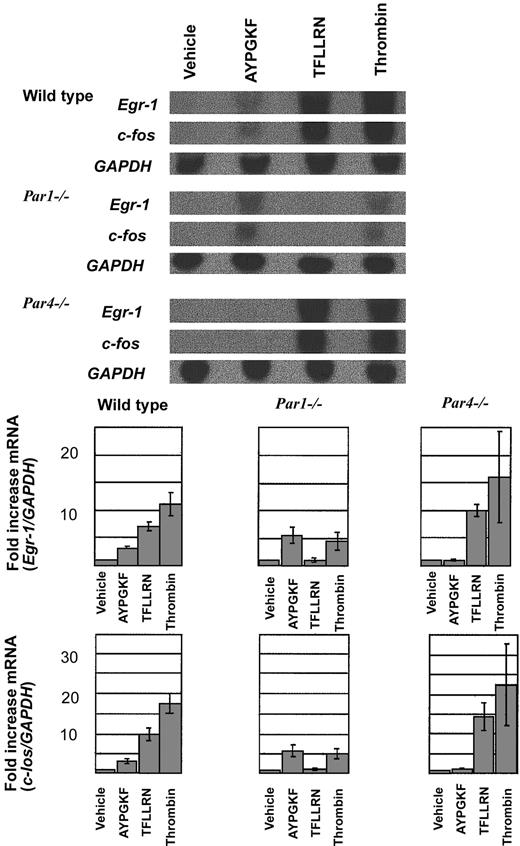
Gene induction in endothelial cells in response to PAR4
To complement our studies of upstream signaling pathways and to provide an additional biologically interesting end point for thrombin signaling, we examined the expression of Egr-1 and c-Fos mRNA by Northern blot analysis (Figure 4). These immediate-early genes were chosen because their induction may contribute to inflammation and tissue remodeling in response to injury. In particular, Fos can induce interleukin-1 (IL-1) and tumor necrosis factor (TNF), and Egr-1 can trigger expression of platelet-derived growth factor and tissue factor.37-40 Thrombin and the PAR1 agonist TFLLRN triggered marked increases in c-Fos and Egr-1 expression in wild-type endothelial cells, and the PAR4 agonist AYPGKF triggered smaller but still clear-cut increases. Responses to the PAR1 and PAR4 agonists were absent in endothelial cells from the cognate knockout mice. PAR1 and PAR4 can each trigger c-Fos and Egr-1 induction, but the PAR1 response is more robust than that mediated by PAR4. c-Fos and Egr-1 induction in response to 10 nM thrombin was substantially decreased but not absent in Par1–/– endothelial cells; little or no decrease in signaling was seen in Par4–/– cells. PAR1 is the major mediator of immediate-early gene induction by thrombin in mouse endothelial cells, but PAR4 can contribute.
Northern blot analysis of Egr-1 and c-Fos induction in mouse endothelial cells. (top) Endothelial cells isolated from wild type, Par1–/–, and Par4–/– mice were grown to confluence, serum-starved overnight (12 hours), and stimulated with vehicle, the PAR4 agonist AYPGKF (500 μM), the PAR1 agonist TFLLRN (10 μM), or thrombin (10 nM) for 45 minutes. Total RNA was isolated and analyzed by Northern blot analysis. Hybridization for GAPDH mRNA was used to control for lane loading. This experiment was replicated 3 times. Quantitation of the results is shown (bottom) as the ratio of c-fos/GAPDH signal intensity normalized to control (mean ± SE; n = 3). Note the decreased response to thrombin in Par1–/– endothelial cells compared with wild-type and the similar magnitude or Egr-1 and c-Fos induction by thrombin and PAR4 agonist in Par1–/– cells.
Northern blot analysis of Egr-1 and c-Fos induction in mouse endothelial cells. (top) Endothelial cells isolated from wild type, Par1–/–, and Par4–/– mice were grown to confluence, serum-starved overnight (12 hours), and stimulated with vehicle, the PAR4 agonist AYPGKF (500 μM), the PAR1 agonist TFLLRN (10 μM), or thrombin (10 nM) for 45 minutes. Total RNA was isolated and analyzed by Northern blot analysis. Hybridization for GAPDH mRNA was used to control for lane loading. This experiment was replicated 3 times. Quantitation of the results is shown (bottom) as the ratio of c-fos/GAPDH signal intensity normalized to control (mean ± SE; n = 3). Note the decreased response to thrombin in Par1–/– endothelial cells compared with wild-type and the similar magnitude or Egr-1 and c-Fos induction by thrombin and PAR4 agonist in Par1–/– cells.
Calcium imaging in wild-type and PAR-deficient endothelial cells
The results described thus far do not distinguish between PAR1 and PAR4 playing partially redundant roles in the same endothelial cells compared with PAR1 mediating thrombin signaling in some endothelial cells and PAR4 mediating signaling in others. To address this question, we examined thrombin- and PAR-activating peptide-induced increases in cytoplasmic calcium in individual cells by fluorometric imaging (Figure 5). In wild-type and Par4–/– dermal endothelial cell cultures, both thrombin (Figure 5A) and the PAR1 agonist TFLLRN (Figure 5B) triggered increases in cytoplasmic calcium in approximately 90% of cells. No response to PAR1 agonist TFLLRN was observed in cultures from Par1–/– mice (not shown), but approximately 30% of Par1–/– cells did respond to 10 nM thrombin (Figure 5A). The PAR4 agonist AYPGKF triggered calcium increases in 32% ± 4% (n = 3) of wild-type cells (Figure 5B) and in a similar fraction of Par1–/– cells but not in Par4–/– cells (not shown). These results suggest that PAR1 and PAR4 are coexpressed in approximately 30% of the endothelial cells in these preparations. Regardless of the genotype, endothelial cells responded almost equally to VEGF stimulation (not shown).
Ratiometric fluorescence calcium imaging of mouse dermal endothelial cells. Fura-2–loaded cells were stimulated with the agonists indicated. Images (original magnification, × 200) at peak increase in [Ca]i are shown. Pseudocolor scales used to indicate the fura-2 340:380 fluorescence ratio are at right. Ratios greater than 20 are shown as white. (A) Thrombin (10 nM) triggered responses in more than 90% of wild-type endothelial cells and Par4–/– endothelial cells. Smaller responses were noted in approximately 30% of Par1–/– endothelial cells. Thrombin (20 nM) triggered virtually no responses in endothelial cells lacking PAR1 and PAR4 (bottom panels). (B) (top) Radiometric images of a single culture responding to sequential addition of PAR1 agonist (TFLLRN; 10 μM), PAR4 agonist (AYPGKF; 500 μM), and VEGF (50 ng/mL). Snapshots 1, 2, 3, and 4 were acquired at the time of peak responses for each agonist, as indicated in the bottom panel (white arrows). Par1–/– cells did not respond to PAR1 agonist (TFLLRN; 10 μM), and Par4–/– cells did not respond to PAR4 agonist (AYPGKF; 500 μM) (not shown). Note that 20% to 30% of the wild-type endothelial cells that responded to TFLLRN responded to subsequent stimulation with AYPGKF. (bottom) Fura-2 fluorescence ratios as a function of time for 3 individual cells (cells a, b, and c, indicated in the top panels). Note that each individual cell responded to all 3 agonists.
Ratiometric fluorescence calcium imaging of mouse dermal endothelial cells. Fura-2–loaded cells were stimulated with the agonists indicated. Images (original magnification, × 200) at peak increase in [Ca]i are shown. Pseudocolor scales used to indicate the fura-2 340:380 fluorescence ratio are at right. Ratios greater than 20 are shown as white. (A) Thrombin (10 nM) triggered responses in more than 90% of wild-type endothelial cells and Par4–/– endothelial cells. Smaller responses were noted in approximately 30% of Par1–/– endothelial cells. Thrombin (20 nM) triggered virtually no responses in endothelial cells lacking PAR1 and PAR4 (bottom panels). (B) (top) Radiometric images of a single culture responding to sequential addition of PAR1 agonist (TFLLRN; 10 μM), PAR4 agonist (AYPGKF; 500 μM), and VEGF (50 ng/mL). Snapshots 1, 2, 3, and 4 were acquired at the time of peak responses for each agonist, as indicated in the bottom panel (white arrows). Par1–/– cells did not respond to PAR1 agonist (TFLLRN; 10 μM), and Par4–/– cells did not respond to PAR4 agonist (AYPGKF; 500 μM) (not shown). Note that 20% to 30% of the wild-type endothelial cells that responded to TFLLRN responded to subsequent stimulation with AYPGKF. (bottom) Fura-2 fluorescence ratios as a function of time for 3 individual cells (cells a, b, and c, indicated in the top panels). Note that each individual cell responded to all 3 agonists.
To further test whether PAR1 and PAR4 function in the same or different cells, wild-type cultures were stimulated sequentially with TFLLRN, then AYPGKF, then VEGF—the latter to confirm that the responding cells were indeed endothelial cells (Figure 5B). Calcium levels, as assessed by radiometric fluorescence imaging, were allowed to return to baseline between agonist additions (Figure 5B, bottom panel). PAR1 agonist TFLLRN triggered responses in approximately 90% of cells. Of these, approximately 30% responded to subsequent additions of AYPGKF; these cells also responded to VEGF (Figure 5B). Similar results were obtained when AYPGKF was added before TFLLRN and when endothelial cells were prepared from lung instead of skin. PECAM staining of cells after calcium imaging confirmed that more than 90% of cells in these experiments expressed this endothelial marker (not shown). Overall, our data suggest that PAR1 and PAR4 are coexpressed and have at least partially redundant roles in at least 30% of endothelial cells derived from mouse skin and lung. The magnitude of increases in cytoplasmic calcium attributable to PAR4 activation was generally less than that associated with PAR1 activation. This technique may underestimate the number of endothelial cells that express both receptors.
PAR4 response in human endothelial cells
In contrast to our findings with mouse microvascular endothelial cells, others and we have been unable to show a significant role for PAR4 signaling in cultured human endothelial cells (O'Brien et al41 and T. Faruqi, S. R. C., unpublished results, June 1999). We again examined phosphoinositide hydrolysis and ERK phosphorylation in HUVECs, HDMVECs, and HLMVECs in response to a high concentration (500 μM) of the PAR4 agonist AYPGKF. AYPGKF triggered no significant increase in phosphoinositide hydrolysis or ERK phosphorylation in HUVECs or HLMVECs. In some HDMVEC preparations, AYPGKF did trigger approximately 25% increases in phosphoinositide generation and 1.5- to 2.0-fold activation in ERK phosphorylation, but such responses were absent in other preparations (data not shown). We have no convincing data supporting a role for PAR4 in thrombin signaling in cultured human endothelial cells. It has been reported that PAR4 was induced by TNF and IL-1α treatment of human coronary vessels and that such vessels showed endothelial-dependent vasorelaxation in response to PAR4 agonist.31 Treatment of cultured human endothelial cells with TNF or IL-1β did not render them responsive to PAR4 agonists as measured by phosphoinositide hydrolysis or ERK phosphorylation (not shown). Defining the extent to which PAR4 is used by human vascular endothelium will require additional study.
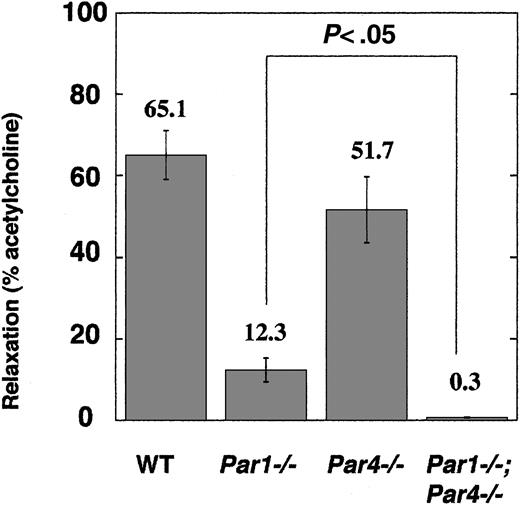
Vasorelaxation response in mouse aorta
Our studies of cultured endothelial cells from neonatal mice beg the question of whether PAR4 is expressed by vascular endothelial cells in adult mice in vivo. In situ hybridization of adult mouse tissues for PAR4 mRNA revealed clear-cut expression in megakaryocytes but not for endothelial cells.33 It was, however, entirely possible that PAR4 is expressed at a level sufficient to support signaling but not sufficient for detection by this technique. Accordingly, we sought to detect endothelial PAR function in freshly isolated vascular tissue by examining endothelial-dependent vasorelaxation in response to thrombin and PAR agonists in thoracic aorta rings from wild type, Par1–/–, Par4–/–, and Par1–/–;Par4–/–mice (Figure 6). Rings from wild-type mice showed a clear vasorelaxation response to 100 nM thrombin (62.1% of maximum relaxation to acetylcholine). Par1–/– mice showed remarkably reduced thrombin-induced vasorelaxation (12.3% of maximum), but this residual thrombin response was seen reliably. By contrast, no vasorelaxation to thrombin was seen in rings from mice lacking both PAR1 and PAR4 (Figure 6). The PAR4 agonist AYPGKF (300 μM) triggered a small (10.6% of maximum) relaxation response in wild-type rings, comparable in magnitude to that triggered in Par1–/– rings by thrombin. Relaxation to AYPGKF was not seen in rings from Par4–/– mice (not shown). All vasorelaxation responses were ablated by mechanical removal of the endothelium. These results strongly suggest that both PAR1 and PAR4 are functionally expressed by aortic endothelium in vivo. Again PAR1 appears to be the main mediator of thrombin signaling, but PAR4 can contribute, and together PAR1 and PAR4 appear to account for thrombin signaling in mouse endothelial cells at the receptor level.
Endothelial-dependent vasorelaxation in freshly isolated aortic rings. Rings from the thoracic aorta of wild-type (n = 5), Par1–/– (n = 5), Par4–/– (n = 4), Par1–/–;Par4–/– (n = 4) mice were tested for vasorelaxation in response to 100 nM thrombin (“Materials and methods”). Data are means ± SE expressed as percentage of relaxation to 100 μM acetylcholine.
Endothelial-dependent vasorelaxation in freshly isolated aortic rings. Rings from the thoracic aorta of wild-type (n = 5), Par1–/– (n = 5), Par4–/– (n = 4), Par1–/–;Par4–/– (n = 4) mice were tested for vasorelaxation in response to 100 nM thrombin (“Materials and methods”). Data are means ± SE expressed as percentage of relaxation to 100 μM acetylcholine.
Discussion
In this study we have illustrated 3 points. First, in addition to PAR1, PAR4 contributes to thrombin-stimulated phosphoinositide hydrolysis, increases in cytoplasmic calcium, ERK phosphorylation, and immediate-early gene induction in mouse microvascular endothelial cells and to endothelial-dependent vasorelaxation of freshly isolated mouse aorta. PAR1 appears to be the more important of the 2 receptors in endothelial cells in that responses to PAR1 activation were generally greater than those seen with PAR4 activation, and isolated PAR1 deficiency, but not isolated PAR4 deficiency, substantially decreased thrombin responsiveness, especially at low thrombin concentrations. Second, PAR1 and PAR4 are both expressed in at least a fraction of individual endothelial cells and serve at least partially redundant signaling functions in these cells. Third, the simultaneous knockout of PAR1 and PAR4 ablates thrombin signaling in endothelial cells as assessed by multiple different measures. PAR1 is the major thrombin receptor in mouse endothelial cells, and PAR1 and PAR4 together appear to account for thrombin signaling in these cells at the receptor level.
For completeness, it is worth briefly discussing the apparent lack of a significant role for PAR3 in thrombin signaling in mouse endothelial cells. Available data suggest that the mouse homolog of human PAR3 is incapable of functioning as a bona fide transmembrane signaling receptor. Instead, mouse PAR3 functions as a cofactor that binds and localizes thrombin to the cell surface, thereby promoting activation of PAR4.21 This arrangement allows mouse platelets to respond to low concentrations of thrombin even though they lack PAR1.26 In accordance with this model, knockout of PAR1 had no effect on mouse platelet responsiveness to thrombin, knockout of PAR3 decreased platelet responsiveness to low concentrations of thrombin (presumably because of decreased PAR4 activation), and knockout of PAR4 ablated platelet responsiveness to thrombin.25,26,33 In contrast to the case in mouse platelets, the current study showed that knockout of PAR1 markedly decreased endothelial cell responsiveness to low concentrations of thrombin (Figure 3), consistent with the model that PAR1-deficient mouse endothelial cells rely on PAR4 to mediate thrombin signaling without the benefit of the PAR3 cofactor function. Indeed, mPAR3 and mPAR4 mRNAs were both barely detectable by Northern blot analysis of mouse dermal endothelial cells, whereas PAR1 and PAR2 mRNAs were readily detected (not shown). Low-level PAR4 expression is sufficient to support some thrombin signaling in mouse endothelial cells, but low-level PAR3 expression appears to be insufficient to promote PAR4 signaling at low thrombin concentrations in this setting.
Recent knockout mouse studies suggest that endothelial signaling by PAR1 is important for hemostasis and vascular integrity in the developing mouse embryo but that other targets of coagulation proteases are also important for normal embryonic development.3 The finding that PAR4 contributes to endothelial responsiveness to thrombin suggests the testable hypothesis that PAR4 is the missing target, and partial redundancy of PAR1 and PAR4 in endothelial cells may explain, in part, the partial penetrance of the phenotype associated with PAR1 deficiency.3 PAR4 agonist peptide was recently reported to induce endothelial cell-dependent relaxation of rat aorta42 and of human coronary vessels treated with cytokines.32 A surprisingly small dose of PAR4 agonist has also been reported to promote leukocyte accumulation in rat peritoneal cavity.43 Our results provide strong genetic support for a role of PAR4 in mouse endothelial cells. The partial redundancy of PAR1 and PAR4 in microvascular endothelial cells suggests that studies designed to address the importance of endothelial activation by thrombin in inflammation, sepsis, and other in vivo processes would best be performed in mice deficient in both receptors. It also raises the question of whether PAR4 simply provides redundancy in an important system in endothelial cells or provides unique capabilities not provided by PAR1. Human PAR4 can mediate signaling to the neutrophil granzyme cathepsin G44 ; therefore, endothelial PAR4 might provide a mechanism for endothelial activation by leukocyte products. Moreover, PAR4 is shut off and internalized poorly compared with PAR1, and PAR4 generates more prolonged signaling than PAR1 in some assays.45 Whether these differences are important in vivo is unknown and is testable, in principle, using knockout mice. Lastly, it is worth noting that responses to PAR2 activation were generally greater than those associated with activation of either PAR1 or PAR4 in our microvascular endothelial cell cultures (Figures 2 and 3). Consistent with this observation, PAR2 is expressed by at least a subset of microvascular endothelial cells in vivo,46,47 and PAR2 agonists cause somewhat greater vasodilation responses than do PAR1 agonists in mice.48 PAR2 can be activated by factor Xa,49,50 raising the possibility that endothelial PAR2 might provide yet a third partially redundant system by which endothelial cells can sense activation of the coagulation cascade.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-04-1130.
Supported by National Institutes of Health grants HL59202, HL44907, HL65590, and HL65185. E.C. and H.K. were supported by postdoctoral fellowships from The American Heart Association and the Japanese Heart Foundation, respectively. J.R.H. was supported by a C.J. Martin Fellowship (no. 166904) from the National Health and Medical Research Council of Australia. D.D.M. was supported by an Arthritis Foundation Postdoctoral Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mette Johansen, Eric Brown, and Mary Gerritsen for advice regarding endothelial cell isolation, Tom Sato for the Tie2-lacZ mouse, Richard Sievers for loaning equipment to assess endothelial-dependent relaxation, and Rommel Advincula for technical support.

![Figure 1. Characterization of endothelial cell preparations from neonatal mouse skin. Cells were stained with biotinylated IgGs (isotype-matched control [left], anti-PECAM [middle], or anti–ICAM-2 [right]) followed by streptavidin-phycoerythrin (PE). Bound PE was quantitated by flow cytometry. Similar results were obtained with lung endothelial cell preparations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-04-1130/6/m_h82135163001.jpeg?Expires=1769264584&Signature=TdABjhldxWJbW~msoiKqMnqkFexeYD0PgC6GGL2RbEtEbUr~NQ6txF8ZFSTjDTAOlyrx4qQS6HtJ1YMfbaOA0wKIqhTuIry78d8TNHN71XlBbLQOKayA8vmyp~jXAsIkTjoUFmNqj7XEb0GelvzB61qPfsFGR3b92Dnqay9-ZvOF4y0VAWAqGxvJ7VGrlEHM81iK7YhWQ-ZD6qVWgfCi0fPudBx7fZNZDxlhawX9QpcHgkcTZBAbh1rZsyPvlWeFxCGWjpY6lKhbeGVe2AjxB~koHfP219FXLqFcVNw-NHjL4ECCKN-5Sf7A-PeCajUMGXx5tRqhxYeOSm49jvQmfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Phosphoinositide hydrolysis in endothelial cells from neonatal skin and lung. Endothelial cells were isolated from mice of the indicated genotype, labeled with [3H]-myoinositol, then incubated with vehicle, scrambled PAR4 agonist (YAPGKF; 500 μM), PAR4 agonist (AYPGKF; 500 μM), or α-thrombin (10 nM) for 2 hours. Data shown are mean fold increases (± SD) in [3H]-inositol phosphate accumulation normalized to vehicle control (n = 4). Each experiment was reproduced at least 3 times except for that using Par1–/–;Par4–/– lung endothelial cells (Par1,4–/–), which was performed only once because of the difficulty of generating adequate numbers of Par1,4–/– mice. Responses to the PAR2 agonist SLIGRL (100 μM) were also measured in each of the experiments shown; all endothelial cell preparations, regardless of genotype, showed robust responses (A) 10- to 20-fold in endothelial cell preparations from skin and (B) 8- to 10-fold in preparations from lung.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-04-1130/6/m_h82135163002.jpeg?Expires=1769264584&Signature=qR60voWmmh1ZpEewpLQpaHQuCDQRfPX75UwwprJSPfSnqIwrW2CAj0k8spsI26QWz2OSkpGUwxPV20ffLPvrNbAzoxVD6OiIntxQadqlKQOB6-g8u9mujpdEoKctLTlQneboIrWKYGWLD3X22h~igMbGo9o5YwnN0MCTwHrZSIfMG6~YMjJWDkVW97AprMeOe04RXopjtqMpcNVP5srRMLiWh~klFHMMaoEnelihudRtvk6hQEmkmp~bewqzGUd0j2eediSlTOVs1WXstWcB4uOEpo63I5FIdRjNwEaVftUAFM3-8vqw4o5IrYdbelT0USmEhaLHO6nCSeF-8~xrJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Ratiometric fluorescence calcium imaging of mouse dermal endothelial cells. Fura-2–loaded cells were stimulated with the agonists indicated. Images (original magnification, × 200) at peak increase in [Ca]i are shown. Pseudocolor scales used to indicate the fura-2 340:380 fluorescence ratio are at right. Ratios greater than 20 are shown as white. (A) Thrombin (10 nM) triggered responses in more than 90% of wild-type endothelial cells and Par4–/– endothelial cells. Smaller responses were noted in approximately 30% of Par1–/– endothelial cells. Thrombin (20 nM) triggered virtually no responses in endothelial cells lacking PAR1 and PAR4 (bottom panels). (B) (top) Radiometric images of a single culture responding to sequential addition of PAR1 agonist (TFLLRN; 10 μM), PAR4 agonist (AYPGKF; 500 μM), and VEGF (50 ng/mL). Snapshots 1, 2, 3, and 4 were acquired at the time of peak responses for each agonist, as indicated in the bottom panel (white arrows). Par1–/– cells did not respond to PAR1 agonist (TFLLRN; 10 μM), and Par4–/– cells did not respond to PAR4 agonist (AYPGKF; 500 μM) (not shown). Note that 20% to 30% of the wild-type endothelial cells that responded to TFLLRN responded to subsequent stimulation with AYPGKF. (bottom) Fura-2 fluorescence ratios as a function of time for 3 individual cells (cells a, b, and c, indicated in the top panels). Note that each individual cell responded to all 3 agonists.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-04-1130/6/m_h82135163005.jpeg?Expires=1769264584&Signature=POofce6ja50Blu5X2zqt86M45V8naRNfsjeztSFziGvbL3eHXiclYyrjnvftfCzyns0dNftrazXy9hLItry-f4NqmiDaStNPHdPEay5XMKOglSpLgz~-m3gSjBU2LtoOyj~pWomPImnHIr5eQxft2vUsWTsginuq3snq3u~r7XOOn05foNb0snqelzNC~eJMaJAKXjaazwglKVs83tXfUJBBOBJzwMXS1443YUmpSQlJ~76~hsi-gTqgLGkWf9AQQkQ8J2LEf3mqNcTV-h-4Xe2VCg-22~dWc-D0zUT1wLwvaMnO~Ktc26vlXvPkwpcU74QqhLNDV9yD7JGpi9JioA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal