Abstract
The availability of the relevant mutant mouse lines provided an opportunity to test the doctrine that platelet activation and fibrin formation account for the importance of thrombin for hemostasis. Prothrombin-deficient mice that survive to birth exsanguinate in the perinatal period. By contrast, protease-activated receptor 4 (PAR4)–deficient mice, which have platelets that fail to respond to thrombin, survive to adulthood with only a mild bleeding diathesis, and fibrinogen-deficient mice show perinatal bleeding but those that survive this period can have a relatively normal life expectancy. We now report that mice that lacked both PAR4 and fibrinogen exsanguinated at birth like prothrombin-deficient mice. However, while approximately half of prothrombindeficient embryos die during midgestation, mice lacking both PAR4 and fibrinogen developed normally. At face value, these results suggest that platelet activation and fibrin formation are together sufficient to account for the importance of thrombin for hemostasis but not for its importance for embryonic development.
Introduction
Thrombin is a multifunctional serine protease that plays a critical role in hemostasis.1 Specific thrombin inhibitors such as hirudin have a profound effect on hemostasis,2 and the bleeding phenotypes associated with even incomplete prothrombin deficiency support a key role for thrombin in hemostasis in man.1 The essential role of thrombin in hemostasis has been emphasized by mouse knockout studies; Sun et al3 and Xue et al4 both demonstrated that mice deficient in prothrombin die either at midgestation with an incompletely understood defect in vascular development or shortly after birth from hemorrhage. Thus, absolute thrombin deficiency is incompatible with postnatal hemostasis and survival to adulthood.
Classically, the importance of thrombin for hemostasis has been attributed to its ability to activate platelets and to trigger fibrin formation, but this has not been formally tested. Interestingly, mice that lack either one of these effector pathways have relatively mild phenotypes compared with mice that lack prothrombin.
Protease-activated receptors (PARs) mediate thrombin signaling in a variety of cell types, and mice deficient in PAR4 have platelets that fail to respond to thrombin.5 PAR4-deficient mice are born at the expected mendelian frequency, grow normally, and are fertile.5 They show little spontaneous bleeding and are not anemic, but they do have prolonged tail bleeding times and are protected in models of thrombosis.5 These phenotypes can be conferred to wild-type mice by bone marrow reconstitution with PAR4-deficient marrow and rescued in PAR4 null mice by reconstitution with wild-type marrow (J.R.H. and S.R.C., manuscript in preparation). Moreover, thrombin signaling is preserved in endothelial and smooth muscle cells from PAR4-deficient mice due to the presence of another thrombin receptor, PAR1.6 Thus, impaired hemostasis in PAR4-deficient mice is almost certainly due to ablation of thrombin signaling in platelets and is mild compared with that caused by loss of thrombin itself.
Mice deficient in fibrinogen are born at the expected mendelian frequency but can exhibit perinatal hemorrhage and death at frequencies that vary from 10% to 70%, depending upon genetic background (K. W. Kombrinck and J. L. Degen, personal communication, August 4, 2003). Long-term survival of mice that escape perinatal hemorrhage can approach that of wild-type, again depending upon genetic background.7 Thus, like isolated PAR4 deficiency, isolated fibrinogen deficiency does not recapitulate the full severity of the bleeding phenotype associated with prothrombin deficiency.
The discrepancy between the profound bleeding phenotype associated with prothrombin deficiency and the less severe phenotypes associated with either PAR4 or fibrinogen deficiency raised the question of whether combined PAR4 and fibrinogen deficiency would recapitulate the defective embryonic development and severe hemostatic defect associated with prothrombin deficiency. Our results support the hypothesis that platelet activation and fibrin formation are the key effectors of thrombin in hemostasis. However, they also suggest that functions other than platelet activation and fibrin formation contribute to thrombin's role in embryonic development, consistent with the recent finding that PAR1 signaling in endothelial cells is important for proper development of blood vessels in the embryo.8
Materials and methods
Generation of mice deficient in PAR4 and fibrinogen has been described.5,7 The fibrinogen-deficient mice were generously provided by Dr Jay L. Degen (Children's Hospital, Cincinnati, OH).7 The original PAR4 null allele was generated in a 50% C57BL6/J and 50% 129SvJae strain background. The original fibrinogen null allele was generated in a 50% C57BL6/J and 50% 129SvJ strain background. The PAR4 and fibrinogen mice were both backcrossed 6 generations (N6) into the C57BL6/J strain background to generate the mice used for the present study. Genotyping was performed by Southern analysis of DNA from extraembryonic tissues (E18.5 embryos) or tail biopsies (10- to 14-day-old pups). Statistical significance was tested by chi-square analysis.
Results
We first examined survival of mice derived from Par4+/– intercrosses and Fib+/– intercrosses. Mice were in the same strain background (C57BL/6J, N6), and surviving offspring were genotyped approximately 2 weeks after birth. Par4+/– intercrosses yielded live PAR4 nulls at the expected mendelian frequency, consistent with our previous results in a mixed 129SV/C57BL6 background5 (Table 1). Fib+/– intercrosses yielded live fibrinogen nulls at approximately half of the expected mendelian frequency (Table 1). Those fibrinogen knock-outs that died usually did so within 2 days of birth, and death was often associated with obvious intra-abdominal bleeding. This is consistent with previous results.7
Live offspring at 2 weeks versus genotype
Par4+/- intercross | |||
| Genotype | Par4+/+ | Par4+/- | Par4-/- |
| Observed | 67 | 120 | 59 |
| Expected | 61.5 | 123 | 61.5 |
| Fib+/- intercross | |||
| Genotype | Fib+/+ | Fib+/- | Fib-/- |
| Observed | 35 | 74 | 14* |
| Expected | 31.75 | 61.5 | 31.75 |
| Fib+/-: Par4-/- intercross | |||
| Genotype | Fib+/+: Par4-/- | Fib+/-: Par4-/- | Fib-/-: Par4-/- |
| Observed | 44 | 90 | 1* |
| Expected | 33.75 | 67.5 | 33.75 |
Par4+/- intercross | |||
| Genotype | Par4+/+ | Par4+/- | Par4-/- |
| Observed | 67 | 120 | 59 |
| Expected | 61.5 | 123 | 61.5 |
| Fib+/- intercross | |||
| Genotype | Fib+/+ | Fib+/- | Fib-/- |
| Observed | 35 | 74 | 14* |
| Expected | 31.75 | 61.5 | 31.75 |
| Fib+/-: Par4-/- intercross | |||
| Genotype | Fib+/+: Par4-/- | Fib+/-: Par4-/- | Fib-/-: Par4-/- |
| Observed | 44 | 90 | 1* |
| Expected | 33.75 | 67.5 | 33.75 |
Live offspring from the indicated crosses were genotyped 2 weeks after birth.
Overall distribution is significantly different from expected by chi-square analysis (P < .01).
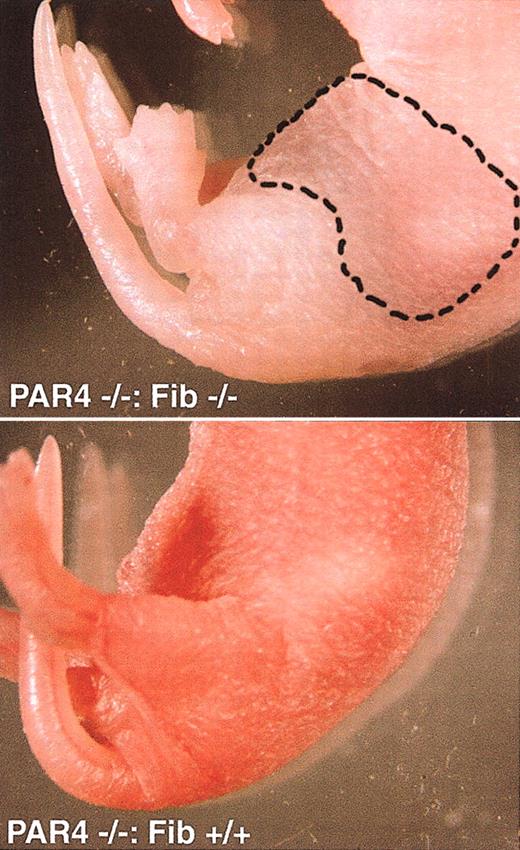
We next examined survival of fibrinogen/PAR4 double knockouts. Because virtually no lethality was observed for PAR4 nulls, we first generated Fib+/– mice on a Par4–/– background then intercrossed these mice to generate the double nulls (Table 1). Mice were in the same strain background (C57BL/6J, N6) as that used for the individual Par4+/– intercrosses and Fib+/– intercrosses, and offspring were again genotyped at 2 weeks of age. Fib+/–: Par4–/– offspring were present at the expected mendelian frequency, but only one Fib–/–: Par4–/– mouse was alive 2 weeks after birth versus the 34 expected. This one mouse died after a tail sample was taken for genotyping, possibly as a result of internal bleeding related to handling. Otherwise, all Fib–/–: Par4–/– pups died spontaneously within 2 days of birth. Affected pups were pale and often had obvious intraperitoneal (Figure 1) or intestinal bleeds. Thus, like prothrombin deficiency, combined deficiency of PAR4 and fibrinogen is associated with a severe and highly penetrant phenotype of perinatal bleeding.
Affected and unaffected pups. Intraperitoneal bleed in a PAR4–/–: Fib–/– neonate (top). Note general pallor compared with a PAR4–/–: Fib+/+ littermate (bottom) as well as discoloration of the abdomen (hatched line). Autopsy confirmed the presence of blood in the peritoneal cavity.
Affected and unaffected pups. Intraperitoneal bleed in a PAR4–/–: Fib–/– neonate (top). Note general pallor compared with a PAR4–/–: Fib+/+ littermate (bottom) as well as discoloration of the abdomen (hatched line). Autopsy confirmed the presence of blood in the peritoneal cavity.
To determine whether fibrinogen/PAR4 double knock-outs also exhibited the embryonic lethality seen in prothrombin nulls, we next collected and genotyped embryos from Fib+/–: Par4–/– intercrosses at E18.5. Live double knockout embryos were present at approximately the expected mendelian frequency at this time (Table 2). Indeed, of 31 double knockout embryos collected at E18.5, only 1 was dead and only 2 showed signs of abdominal bleeding; 28 embryos appeared to be normal and were indistinguishable from their littermates. Thus development of embryos that lack both PAR4 and fibrinogen appears to proceed normally with the exception of occasional bleeding events in late gestation. This phenotype is in striking contrast to that seen in prothrombin-deficient embryos, approximately half of which die between E9.5 and E11.5.3,4
Fib+/-: Par4-/- intercross: no. alive at E18.5
Genotype | Fib+/+: Par4-/- | Fib+/-: Par4-/- | Fib-/-: Par4-/- |
| Observed | 34 | 55 | 30 |
| Expected | 29.75 | 59.5 | 29.75 |
Genotype | Fib+/+: Par4-/- | Fib+/-: Par4-/- | Fib-/-: Par4-/- |
| Observed | 34 | 55 | 30 |
| Expected | 29.75 | 59.5 | 29.75 |
Embryos from the indicated cross were collected at E18.5, scored as alive or dead based on the presence or absence of a heartbeat, and genotyped. Note that 31 Fib-/-: Par4-/- embryos were collected and 30 were alive. Of these 30 embryos, 28 appeared to be normal and were indistinguishable from their littermates. Two embryos were alive but showed intra-abdominal bleeding.
Discussion
Our results show that combined deficiency of PAR4 and fibrinogen recapitulates the profound hemostatic defect associated with prothrombin deficiency. Thrombin does have multiple effectors besides fibrinogen and platelet PAR4 and several may contribute to hemostasis. For example, thrombin-activatable fibrinolysis inhibitor (TAFI) may slow fibrin clearance,9 activation of endothelial cell PARs may help recruit platelets and tissue factor–bearing leukocytes and microparticles to sites of injury,10,11 and activation of smooth muscle PAR1 may promote vasoconstriction.12 Our results certainly do not exclude such contributions. However, the observation that the PAR4:fibrinogen double knock-out recapitulates the bleeding phenotype associated with prothrombin deficiency does imply that other thrombin effector pathways are not sufficient for hemostasis and is consistent with the view that platelet activation and fibrin formation are indeed the major effectors of thrombin in hemostasis.
The contrast between the severity of bleeding seen in mice that lack either PAR4 or fibrinogen alone versus those that lack both implies that fibrinogen is important in the absence of thrombin-induced platelet activation. Fibrinogen might contribute to hemostasis in Par4–/– mice by supporting fibrin formation and/or platelet aggregation in response to agonists other than thrombin, such as adenosine diphosphate and thromboxane A2.
The contrast between the level of bleeding seen in mice that lack either PAR4 or fibrinogen alone versus those that lack both also implies that thrombin-induced platelet activation is important in the absence of fibrinogen. Von Willebrand factor or fibronectin13-15 may support aggregation of thrombin-activated platelets in the absence of fibrinogen. Alternatively, thrombin-activated platelets may contribute to hemostasis via P-selectin–mediated interactions or by other fibrinogen-independent mechanisms.16,17
Our results also demonstrate that combined deficiency of PAR4 and fibrinogen does not recapitulate the midgestational embryonic lethality associated with prothrombin deficiency. We cannot exclude the possibility that transfer of fibrinogen from the circulation of Fib+/– dams to Fib–/– embryos confounds interpretation of this result. (The present study could not be performed in Fib–/– females because they cannot support a pregnancy.7 ) However, fibrinogen is large compared with prothrombin (340 kDa vs 72 kDa), and fibrin(ogen) functions as a structural component of a clot or matrix rather than enzymatically like thrombin. Thus it seems unlikely that transfer of small quantities of maternal fibrinogen might fully suppress a phenotype in Fib–/– embryos given that transfer of maternal prothrombin does not fully suppress the phenotype seen in prothrombin-deficient embryos. Moreover, the embryonic phenotype associated with prothrombin deficiency is grossly similar to that associated with deficiency of PAR1, another thrombin receptor.3,4,8 In both cases, approximately half of embryos die at midgestation, and in both cases death is associated with an apparent defect in vascular integrity. PAR1-deficient embryos were rescued by a transgene in which PAR1 expression was driven by the relatively endothelial-specific Tie2 promoter/enhancer.8 These and other data suggest that sensing of thrombin by endothelial cells may be important for normal development of the embryonic vasculature. At face value, the contrast between death of approximately half of prothrombin-deficient embryos at midgestation and normal development of Par4–/–: Fib–/– embryos is consistent with such an alternative role for thrombin in embryonic development, that is, a role distinct from platelet activation and fibrin formation.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-08-2707.
Supported by National Institutes of Health (NIH) grants HL65590, HL65185, and HL44907 (S.R.C.). E.C. was supported by the Julius H. Comroe fellowship. J.R.H. was supported by a C. J. Martin Fellowship (no. 166904) from the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. L. Degen of Children's Hospital (Cincinnati, OH) for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal