Abstract
To assess whether Fanconi anemia (FA) patients might be at risk for acute graft-versus-host disease (AGvHD) despite using low-intensity conditionings, we retrospectively analyzed the incidence of AGvHD and its impact on outcome in 37 FA patients and 73 patients with acquired aplastic anemia (AAA) that received transplants at Saint Louis Hospital from HLA-genotypic identical siblings with similar conditionings (thoraco-abdominal irradiation plus cyclophosphamide 20 [FA] or 150 mg/kg [AAA]). Despite being younger, FA patients had an increased risk of grades II to IV AGvHD (relative risk [RR], 2.00; P = .021), especially in younger patients (RR, 7.93; P = .014). The risks of requiring systemic corticosteroids to treat AGvHD and experiencing cortico-resistant AGvHD were significantly increased in FA patients. Although non-FA and FA patients had similar 10-year outcomes, acute and chronic GvHD had a biphasic effect on FA patient outcome with an additional cluster of lethal events starting by 5 years after transplantation. This late survival fall, restricted to FA patients, was closely related to head and neck carcinomas (15-year incidence: 53%). FA patients represent a group at risk regarding AGvHD when using irradiation-based conditionings. The impact of AGvHD on survival may not be limited to the early posttransplantation period and may be a major risk factor for head and neck carcinomas and late mortality in FA patients.
Introduction
Fanconi anemia (FA) is an autosomal recessive disorder belonging to the group of chromosomal instability syndromes.1 The natural history of FA is to evolve toward progressive bone marrow failure, which is most often lethal before the end of the second decade of life without treatment.2,3 Allogeneic stem cell transplantation (SCT) is currently the only way to restore normal hematopoiesis in these patients.4 The increased sensitivity of FA cells to alkylating agents5 and ionizing radiations6 have been the main explanations to the poor outcome of FA patients receiving conventional preparative regimens for allogeneic bone marrow transplantation.7 The combination of low-dose cyclophosphamide and thoraco-abdominal irradiation (TAI), as proposed by Gluckman and colleagues, has resulted in a major reduction of early multiorgan failures and severe acute graft-versus-host disease (AGvHD), leading to a dramatic decrease of the early transplantation-related morbidity and mortality in FA patients who received transplants of HLA-identical siblings.4,6,8 Because FA cells display defective DNA repair processes and an increased sensitivity to the proapoptotic effects of tumor necrosis factor-α, γ-interferon, and reactive oxygen species9 (ie, cytokines directly or indirectly involved in AGvHD pathogenesis10 ), we were concerned that FA patients might still have a high propensity to develop severe AGvHD following our low-dose preparative regimen.6 Therefore, we compared the incidence and clinical severity of AGvHD in patients with either FA or acquired aplastic anemia (AAA) who underwent transplantation from HLA-identical donors using a similar combination of cyclophosphamide and TAI as preparative regimen but at higher doses for AAA. In addition, we focused on the impact of AGvHD in these 2 groups of patients with respect to chronic GvHD, secondary malignancies, and overall survival.
Patients, materials, and methods
Patients
Included in the study were 110 patients with a primary diagnosis of AAA (n = 73, non-FA group) or FA (n = 37) who underwent bone marrow transplantation from HLA-genotypic identical sibling donors at Saint Louis Hospital between January 1981 and December 1996. Fanconi anemia and AAA were defined according to standard criteria.2,11,12 The preparative regimens were cyclophosphamide 20 mg/kg total dose plus 5 Gy TAI for FA patients6 and cyclophosphamide 150 mg/kg total dose plus 6 Gy TAI for non-FA patients.13 Thoraco-abdominal irradiation was delivered with a linear accelerator as a single fraction with a dose rate of 12.9 cGy per minute. Both lungs, part of the right liver lobe, and testicles in males were shielded. Cyclosporine (CsA) alone was used in FA patients for GvHD prophylaxis and was started the day before graft infusion at a daily dose of 3 mg/kg given as a continuous infusion over 24 hours. Cyclosporine was given either alone (n = 47) or combined with short-course methotrexate (MTX; n = 26) in non-FA patients, as previously described.14 Rules to manage CsA after graft infusion were the same in all patients and were based on pharmacokinetics data, urea levels, and creatinine levels. All patients were isolated in laminar air flow rooms for at least 30 days after graft infusion and received oral broad-spectrum antibiotics and antifungal drugs to achieve total gut decontamination. Acyclovir was given for prevention of herpes simplex infections. Patients who had evolved to myelodysplastic syndrome or acute leukemia before transplantation and who also received other conditioning regimens, GvHD prophylaxes, cord blood transplantation, granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood progenitor cells, or who did not achieve neutrophil recovery were excluded from this analysis. Main characteristics of the patients according to the underlying disease and GvHD prophylaxis are summarized in Table 1.
Descriptive statistics according to primary disease and GvHD prophylaxis
. | Group A1, n = 47 . | Group A2, n = 26 . | Group FA, n = 37 . |
|---|---|---|---|
| Diseases | AAA TAI | AAA TAI | Fanconi |
| GvHD prophylaxis | CsA | CsA + MTX | CsA |
| Year of transplantation* | 1983/1983/1984 | 1987/1988/1989 | 1984/1989/1992 |
| Range | 1981-1987 | 1986-1990 | 1981-1996 |
| Follow-up time, y* | 11.2/15.1/17.8 | 8.6/12.7/14.9 | 10.6/13.2/15.2 |
| Recipient sex, male, % | 65 (30) | 54 (14) | 62 (23) |
| Recipient age at transplantation, y* | 12/16/22 | 13/26/32 | 7/12/15 |
| Range | 5-46 | 9-46 | 4-26 |
| Diagnosis to BMT, mo* | 2/3/7 | 2/4/12 | 11/22/49 |
| Range | 0-117 | 1-143 | 3-147 |
| Etiology of AAA, % | |||
| Idiopathic | 79 (37) | 54 (14) | — |
| After hepatitis | 13 (06) | 31 (08) | — |
| Others | 8 (04) | 15 (04) | — |
| Treatments before BMT, % | |||
| Androgens | 45 (21) | 27 (07) | 59 (22) |
| ATG | 23 (11) | 31 (08) | 0 (00) |
| At least 20 transfusions | NA | NA | 35 (13) |
| Malformative syndrome, % | |||
| Extensive† | — | — | 27 (10) |
| Kidney + urogenital tract | — | — | 43 (16) |
| Urogenital tract | — | — | 11 (04) |
| Limbs | — | — | 54 (20) |
| Donor sex, male, % | 63 (29) | 54 (14) | 59 (22) |
| Donor/recipient sex match, % | |||
| Female to female | 9 (05) | 27 (07) | 19 (07) |
| Female to male | 28 (13) | 19 (05) | 22 (08) |
| Male to female | 26 (12) | 19 (05) | 19 (07) |
| Male to male | 37 (17) | 35 (09) | 41 (15) |
| Donor/recipient ABO blood groups | |||
| No incompatibility | 79 (37) | 77 (20) | 78 (29) |
| Major incompatibility | 5 (02) | 8 (02) | 11 (04) |
| Recipient CMV serostatus, +, % | 28 (08) | 69 (18) | 52 (14) |
| Nucleated cell dose, 108/kg* | 2.8/3.4/4.8 | 1.9/2.8/3.5 | 2.4/3.6/4.7 |
| Range | 1.0-8.5 | 1.1-5.8 | 0.8-9.8 |
| Neutrophil recovery, d* | 12/16/19 | 14/17/19 | 12/13/18 |
| Range | 8-28 | 8-35 | 10-49 |
. | Group A1, n = 47 . | Group A2, n = 26 . | Group FA, n = 37 . |
|---|---|---|---|
| Diseases | AAA TAI | AAA TAI | Fanconi |
| GvHD prophylaxis | CsA | CsA + MTX | CsA |
| Year of transplantation* | 1983/1983/1984 | 1987/1988/1989 | 1984/1989/1992 |
| Range | 1981-1987 | 1986-1990 | 1981-1996 |
| Follow-up time, y* | 11.2/15.1/17.8 | 8.6/12.7/14.9 | 10.6/13.2/15.2 |
| Recipient sex, male, % | 65 (30) | 54 (14) | 62 (23) |
| Recipient age at transplantation, y* | 12/16/22 | 13/26/32 | 7/12/15 |
| Range | 5-46 | 9-46 | 4-26 |
| Diagnosis to BMT, mo* | 2/3/7 | 2/4/12 | 11/22/49 |
| Range | 0-117 | 1-143 | 3-147 |
| Etiology of AAA, % | |||
| Idiopathic | 79 (37) | 54 (14) | — |
| After hepatitis | 13 (06) | 31 (08) | — |
| Others | 8 (04) | 15 (04) | — |
| Treatments before BMT, % | |||
| Androgens | 45 (21) | 27 (07) | 59 (22) |
| ATG | 23 (11) | 31 (08) | 0 (00) |
| At least 20 transfusions | NA | NA | 35 (13) |
| Malformative syndrome, % | |||
| Extensive† | — | — | 27 (10) |
| Kidney + urogenital tract | — | — | 43 (16) |
| Urogenital tract | — | — | 11 (04) |
| Limbs | — | — | 54 (20) |
| Donor sex, male, % | 63 (29) | 54 (14) | 59 (22) |
| Donor/recipient sex match, % | |||
| Female to female | 9 (05) | 27 (07) | 19 (07) |
| Female to male | 28 (13) | 19 (05) | 22 (08) |
| Male to female | 26 (12) | 19 (05) | 19 (07) |
| Male to male | 37 (17) | 35 (09) | 41 (15) |
| Donor/recipient ABO blood groups | |||
| No incompatibility | 79 (37) | 77 (20) | 78 (29) |
| Major incompatibility | 5 (02) | 8 (02) | 11 (04) |
| Recipient CMV serostatus, +, % | 28 (08) | 69 (18) | 52 (14) |
| Nucleated cell dose, 108/kg* | 2.8/3.4/4.8 | 1.9/2.8/3.5 | 2.4/3.6/4.7 |
| Range | 1.0-8.5 | 1.1-5.8 | 0.8-9.8 |
| Neutrophil recovery, d* | 12/16/19 | 14/17/19 | 12/13/18 |
| Range | 8-28 | 8-35 | 10-49 |
N = 83 for recipient CMV-positive serostatus; N = 103 for neutrophil recovery. N = 110 for all others.
Numbers after percents are frequencies.
AAA indicates acquired aplastic anemia; TAI, thoraco-abdominal irradiation; CsA, cyclosporine A; MTX, methotrexate; N, the number of non-missing values; BMT, bone marrow transplantation; —, not applicable; ATG, antithymocyte globulins; NA, not available; and CMV, cytomegalovirus.
Lower quartile/median/the upper quartile for continuous covariates.
At least 3 anatomic sites involved.24
Definitions and statistical analysis
End points were assessed on the date of last patient contact and were analyzed on April 1, 2002. Acute and chronic GvHD were graded according to standard criteria.16-18 Acute GvHD was considered as cortico-resistant when methyl-prednisolone used at a daily dose of 2 mg/kg was unable to control AGvHD evolution. Therefore, patients who required anti–T-cell monoclonal antibodies, antithymocyte globulins, methyl-prednisolone at a daily dose of more than 2 mg/kg, or any other immunosuppressive drug were considered as having cortico-resistant AGvHD. Analysis of chronic GvHD included patients who survived longer than 90 days from transplantation. Probabilities of grades I to IV, grades II to IV, grades III to IV, and cortico-resistant AGvHD, as well as the probability to receive systemic corticosteroid first-line treatment for AGvHD by day +100 were computed from the time of transplantation using cumulative incidences and considering all death not related to AGvHD as the competing risk factor.19 Cumulative incidences were used to estimate the incidence of secondary malignancies, all causes of death not related to secondary malignancy being considered as the competing risk factor. The Gray test was used to compare cumulative incidences between groups.19 Estimate of the overall survival was computed according to the Kaplan-Meier product-limit method.20 Groups were compared using the 2-tailed log-rank test. P-spline method and penalized Cox model were used to define the most appropriate cut-off value(s) for continuous covariates.21 The following covariates were analyzed in univariate analysis: primary diagnosis (non-FA vs FA), etiology of the AAA (idiopathic vs after hepatitis vs toxic or drug related), year of transplantation (continuous covariate), recipient age at transplantation (continuous covariate, and < 12 years vs ≥ 12 years), recipient sex, use of androgens and/or antithymocyte globulins between diagnosis and transplantation, number of transfusions before transplantation (< 20 vs ≥ 20, data available for FA patients only) nucleated cell dose infused (continuous covariate, and < 3 × 108/kg vs ≥ 3 × 108/kg), donor sex, recipient/donor sex match, recipient/donor ABO blood group compatibility (no vs minor or major incompatibility, major incompatibility vs others), recipient cytomegalovirus serostatus before transplantation, and GvHD prophylaxis (CsA alone vs CsA + MTX). For multivariate analysis, covariates found significant at P < .10 in univariate analysis were introduced in the Cox proportional hazards model and were selected through a stepwise procedure.22 Potential interactions between the significant covariates were tested adding cross-product terms to the model. Departure from the proportional hazards assumption was assessed using methods based on partial residuals and a graphic approach.22 If the proportional hazards assumption did not hold for a covariate, the extended Cox model with time-dependent covariate was used. When groups were compared according to continuous covariates, Mann Whitney U test or Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks test was used for difference in medians. According to the group sizes, Chi-square analysis or Fisher exact test was used to compare categoric covariates. S Plus 2000 Professional 3 (Insightful, Seattle, WA) was used for all statistical analysis.
Results
Acute GvHD incidence, risk factors, and organ involvement
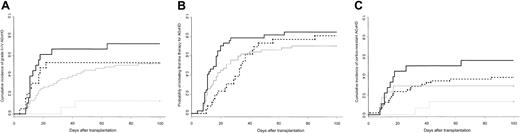
Cumulative incidence of grades II to IV AGvHD in patients with AAA given CsA or CsA plus MTX and in FA patients was 45% (95% confidence interval [CI], 29%-57%), 42% (95% CI, 29%-57%), and 62% (95% CI, 43%-75%), respectively (P = .035). Fanconi anemia as primary diagnosis was the only significant predictor of grades II to IV AGvHD in univariate analysis (RR, 2.00; 95% CI, 1.17-3.43; P = .021). As for grades I to IV AGvHD (data not shown), the increased risk of grades II to IV AGvHD between FA and non-FA patients was mainly observed in recipients younger than 12 years of age at the time of transplantation (Figure 1A). The difference between young FA and non-FA patients remained significant when GvHD prophylaxis was taken into consideration since grades II to IV AGvHD incidence was 9% and 25%, respectively, in young non-FA patients given CsA alone and CsA plus MTX, versus 72% in FA patients (P = .0013). In multivariate analysis, FA as primary diagnosis was significantly associated with grades II to IV AGvHD in FA patients younger than 12 years of age at the time of transplantation (RR, 7.93; 95% CI, 1.52-41.43; P = .014), whereas younger age at transplantation had a protective effect for the non-FA patients (RR, 0.20; 95% CI, 0.05-0.84; P = .028). Fanconi anemia patients were not a population at risk with respect to grades III to IV AGvHD in univariate or multivariate analyses (RR, 1.13; 95% CI, 0.49-2.62). Regarding organ involvement, stages 2 to 4 were more frequently observed in FA than in non-FA patients (skin, 46% vs 36%; gut, 19% vs 16%; liver, 22% vs 16%), but the difference between these 2 groups only reached significance for stages 3 to 4 liver involvement (14% in FA vs 1% in non-FA patients; P = .028).
Cumulative incidences of grades II to IV and cortico-resistant acute GvHD according to primary diagnosis and recipient age at the time of transplantation. (A) Cumulative incidence of grades II to IV AGvHD in patients younger than 12 years of age at the time of transplantation was 72% in FA (solid black line; n = 18) versus 13% in non-FA patients (dashed gray line; n = 15; P = .0012). Cumulative incidence of AGvHD for patients of at least 12 years of age at the time of transplantation was 53% in FA (dashed black line, n = 19) versus 52% in non-FA patients (solid gray line, n = 58; P = NS). (B) Probability of requiring systemic corticosteroid therapy for treatment of AGvHD by day +100 after transplantation was 85% in FA patients (thick black line) versus 70% in non-FA patients given CsA alone (thin black line) and 81% in non-FA patients given CsA plus MTX (dashed line) (P = .045 when all 3 groups compared; probability, 74% for all non-FA patients; P = .013 when FA patients compared with all non-FA patients). (C) Cumulative incidence of cortico-resistant AGvHD in patients younger than 12 years of age at the time of transplantation was 56% in FA (solid black line, n = 18) versus 13% in non-FA patients (dashed gray line, n = 15; P = .008). Cumulative incidence of cortico-resistant AGvHD in patients of at least 12 years of age at the time of transplantation was 29% in FA (solid gray line, n = 19) versus 38% in non-FA patients (dashed black line, n = 58; P = NS).
Cumulative incidences of grades II to IV and cortico-resistant acute GvHD according to primary diagnosis and recipient age at the time of transplantation. (A) Cumulative incidence of grades II to IV AGvHD in patients younger than 12 years of age at the time of transplantation was 72% in FA (solid black line; n = 18) versus 13% in non-FA patients (dashed gray line; n = 15; P = .0012). Cumulative incidence of AGvHD for patients of at least 12 years of age at the time of transplantation was 53% in FA (dashed black line, n = 19) versus 52% in non-FA patients (solid gray line, n = 58; P = NS). (B) Probability of requiring systemic corticosteroid therapy for treatment of AGvHD by day +100 after transplantation was 85% in FA patients (thick black line) versus 70% in non-FA patients given CsA alone (thin black line) and 81% in non-FA patients given CsA plus MTX (dashed line) (P = .045 when all 3 groups compared; probability, 74% for all non-FA patients; P = .013 when FA patients compared with all non-FA patients). (C) Cumulative incidence of cortico-resistant AGvHD in patients younger than 12 years of age at the time of transplantation was 56% in FA (solid black line, n = 18) versus 13% in non-FA patients (dashed gray line, n = 15; P = .008). Cumulative incidence of cortico-resistant AGvHD in patients of at least 12 years of age at the time of transplantation was 29% in FA (solid gray line, n = 19) versus 38% in non-FA patients (dashed black line, n = 58; P = NS).
Acute GvHD treatments and response to first-line therapy
Fanconi anemia patients were more likely to receive corticosteroids as first-line treatment for AGvHD than non-FA patients (Figure 1B). Focusing on patients younger than 12 years of age at the time of transplantation, the probability to receive corticosteroids by day +100 after transplantation was 89% in FApatients (95% CI, 59%-97%) versus 55% in non-FA patients given CsA alone (95% CI, 13%-76%) and 75% in non-FA patients given CsA plus MTX (95% CI, 0%-95%) (P = .039 when all 3 groups compared; probability, 60% for all non-FA patients; P = .011 when FA patients compared with all non-FA patients). In addition, FA patients more frequently required second-line treatments (ie, antithymocyte globulins; cumulative incidences, 27% vs 10%; P = .015) and methyl-prednisolone daily doses more than 2 mg/kg (cumulative incidences, 39% vs 24%; P = .11) to control AGvHD. Focusing on the younger group of patients, FA was associated with an increased risk of experiencing cortico-resistant AGvHD (RR, 6.06; 95% CI, 1.32-27.8; Figure 1C), even if the type of GvHD prophylaxis used in the non-FA group was considered (data not shown).
Predictors of acute GvHD in Fanconi anemia patients
In univariate analysis, urogenital malformations (RR, 4.67; 95% CI, 1.46-14.9; P < .01), donor/recipient ABO blood group incompatibility (RR, 4.40; 95% CI, 1.47-13.1; P < .01), and donor/recipient sex mismatch (RR, 3.31; 95% CI, 1.47-7.47; P < .01) were predictors of grades II to IVAGvHD. The only covariate associated with an increased risk of grades III to IV AGvHD in FA patients was a donor/recipient major ABO blood group incompatibility (RR, 10.5, 95% CI, 3.09-36.0, P < .001). In addition, urogenital malformations (RR, 3.09, 95% CI, 0.86-11.10, P = .08) and donor/recipient major ABO blood group incompatibility (RR, 6.02; 95% CI, 1.66-21.80; P < .01) were associated with an increased risk of cortico-resistant AGvHD. In univariate analysis, year of transplantation, time interval between diagnosis and transplantation, number of pretransplantation transfusions, and the use of androgens before transplantation were not significant predictors of AGvHD, whatever the considered grade was. No multivariate analysis was performed because of the small size of the FA group.
Chronic GvHD and secondary malignancies
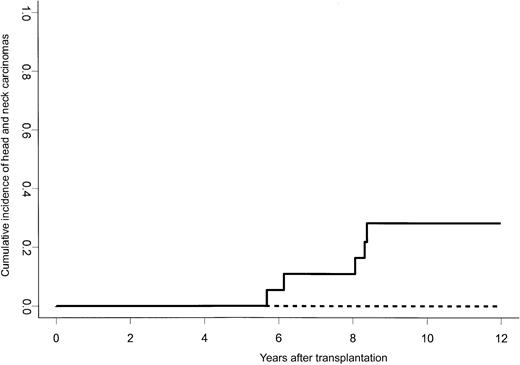
Sixty-one percent of the overall group of patients experienced chronic GvHD, which was limited in 23 cases and extensive in 39 cases (assessable patients, n = 101), respectively. Fanconi anemia as primary diagnosis (odds ratio [OR], 2.69; 95% CI, 1.04-6.94; P = .04) and male recipient were covariates significantly associated with an increased risk of chronic GvHD. However, FA patients were not a population at risk with respect to extensive chronic GvHD (P = .75). Secondary malignancies occurred in 8 FA patients and 3 non-FA patients (head and neck carcinoma, n = 7; breast cancer, n = 1; osteosarcoma, n = 1; post–hepatitis C liver carcinoma, n = 1; squamous cell carcinoma of the skin, n = 1; sex ratio, 9 males/2 females). In addition, one FA patient developed severe oral leukodysplasia. Head and neck carcinomas were exclusively observed in FA patients and were diagnosed at a median of 8.3 years after transplantation (range, 5.3-13.9 years), leading to a cumulative incidence of head and neck carcinoma in FApatients of 20% (95% CI, 9%-44%) and 53% (95% CI, 31%-93%) by 10 and 15 years after transplantation, respectively. By 10 years after transplantation, grades II to IV AGvHD (Figure 2) and chronic GvHD (cumulative incidence if occurred, 23%) were significant predictors of head and neck carcinomas, which were only observed in FA patients experiencing one of these 2 complications.
Cumulative incidence of head and neck carcinomas in Fanconi anemia patients according to the occurrence of grades II to IV AGvHD. The 10-year cumulative incidence of head and neck carcinomas in FA patients experiencing grades II to IV AGvHD was 28% (solid line) versus 0% for those who did not develop this complication (dashed line; P < .001).
Cumulative incidence of head and neck carcinomas in Fanconi anemia patients according to the occurrence of grades II to IV AGvHD. The 10-year cumulative incidence of head and neck carcinomas in FA patients experiencing grades II to IV AGvHD was 28% (solid line) versus 0% for those who did not develop this complication (dashed line; P < .001).
Overall survival
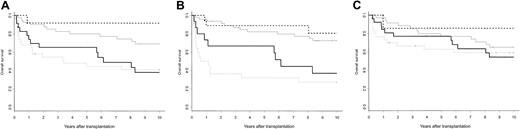
The 10-year posttransplantation estimated survival of FA patients was similar to the one of non-FA patients whatever their GvHD prophylaxis was (Kaplan-Meier estimates: 57% in FApatients; 56% inAAApatients given CsA; 58% in patients given CsA plus MTX; P = NS [not significant]). In addition, 8 deaths occurred more than 10 years from transplantation (patients at risk, n = 43), which were related to secondary malignancies in 6 cases (FA patients, n = 5). In multivariate analysis, a primary diagnosis of FA did not significantly affect the 10-year overall survival (RR, 0.89; 95% CI, 0.35-2.27; P = .80), and no interaction between FA and the other significant covariates (major ABO incompatibility, recipient age at transplantation, interval between diagnosis and transplantation) reached significance. These results were not modified with the introduction of posttransplantation covariates such as grades II to IV, grades III to IV, or cortico-resistant AGvHD. However, the outcome of FA and non-FA patients along the first 10 years after transplantation was different. By 5 years after transplantation, FA patients who experienced grades II to IV AGvHD had an estimated survival of 69% (95%CI, 52%-91%), which was slightly higher than the one of non-FA patients (Kaplan-Meier estimate 44%; 95% CI, 30%-66%; P = .11). However, by 10 years after transplantation while the survival curve remained stable in the non-FA group it has significantly fallen below 40% in the FA group, mainly because of secondary malignancies (Figure 3A). Considering cortico-resistant AGvHD, the impact of this complication was mainly observed during the first 18 months after transplantation in the non-FA group, whereas it was biphasic in the FA group, with a first impact during the first 18 months after transplantation—as for non-FA patients—and a second cluster of events observed after 5 years after transplantation, which was closely related to the occurrence of secondary malignancies (Figure 3B). Considering chronic GvHD, such a 2-step process affecting survival could also be identified (Figure 3C).
Posttransplantation estimated survival according to primary diagnosis and occurrence of grades II to IV, cortico-resistant AGvHD, or chronic GvHD. (A) The 10-year estimated survival of FA patients experiencing grades II to IV AGvHD was 38% (solid black line) versus 41% for non-FA patients (dashed gray line; P = NS). The 10-year estimated survival of FA patients who did not experience grades II to IV AGvHD was 92% (dashed black line) versus 69% for non-FA patients (solid gray line) (P = NS). (B) The 10-year estimated survival of FA patients experiencing cortico-resistant AGvHD was 37% (solid black line) versus 27% for non-FA patients (dashed gray line; P = NS). The 10-year estimated survival of FA patients who did not experience cortico-resistant AGvHD was 81% (dashed black line) versus 73% for non-FA patients (solid gray line; P = NS). (C) The 10-year estimated survival of patients experiencing chronic GvHD was 54% for FA patients (solid black line) and 65% for non-FA patients (solid gray line), respectively (P = NS). The 10-year estimated survival of FA patients who did not experience chronic GvHD was 86% (dashed black line) versus 59% for non-FA patients (dashed gray line; P = NS).
Posttransplantation estimated survival according to primary diagnosis and occurrence of grades II to IV, cortico-resistant AGvHD, or chronic GvHD. (A) The 10-year estimated survival of FA patients experiencing grades II to IV AGvHD was 38% (solid black line) versus 41% for non-FA patients (dashed gray line; P = NS). The 10-year estimated survival of FA patients who did not experience grades II to IV AGvHD was 92% (dashed black line) versus 69% for non-FA patients (solid gray line) (P = NS). (B) The 10-year estimated survival of FA patients experiencing cortico-resistant AGvHD was 37% (solid black line) versus 27% for non-FA patients (dashed gray line; P = NS). The 10-year estimated survival of FA patients who did not experience cortico-resistant AGvHD was 81% (dashed black line) versus 73% for non-FA patients (solid gray line; P = NS). (C) The 10-year estimated survival of patients experiencing chronic GvHD was 54% for FA patients (solid black line) and 65% for non-FA patients (solid gray line), respectively (P = NS). The 10-year estimated survival of FA patients who did not experience chronic GvHD was 86% (dashed black line) versus 59% for non-FA patients (dashed gray line; P = NS).
Discussion
In this retrospective monocentric study about allogeneic bone marrow transplantation using an irradiation-based preparative regimen and HLA-identical siblings for aplastic anemia, we found that FA patients had an increased risk of experiencing AGvHD when compared with patients with AAA. Despite the fact that FA patients were conditioned using a reduced-intensity preparative regimen, a dramatic difference of grades II to IV AGvHD incidence was observed, especially when considering patients younger than 12 years of age, which represent most FA patients undergoing transplantation. Surprisingly, when considering the “older patients” group, no significant difference in terms of AGvHD incidence was observed between FA and non-FA patients. Since these older FA patients had less pronounced FA phenotypes, such as less frequent extensive malformative syndromes (11% vs 44%; P = .02) or renal malformations (22% vs 72%; P = .003) for instance, they might indeed represent a distinct group of FA patients with respect to the characteristics of their intrinsic disease, leading to a different posttransplantation outcome. In that way, it has already been shown that mosaicism could be a risk factor for graft failure in the setting of unrelated donor transplantations in FA.23 Our previous study analyzing results from unrelated transplantations in FA showed that patients with urogenital and/or renal malformations had an increased risk of severe AGvHD in this setting.24 In the present study, we also found that urogenital malformations were associated with an increased risk of grades II to IV and cortico-resistant AGvHD. An Italian study has also found that urogenital malformations were associated with an increased risk of grades II to IV AGvHD in FA patients receiving stem cell transplantation from HLA-matched related donors.25 Taken together, these findings support the hypothesis that the impaired DNA repair process and increased tendency of the cells to undergo apoptosis under stressing conditions might be important factors influencing the occurrence and severity of AGvHD in FA.
The high incidence of AGvHD requiring systemic corticosteroid therapy and the substantial number of FA patients for whom additional immunosuppressive therapies were needed (cumulative incidence of cortico-resistant AGvHD in FA patients, 43%) point out that a more intense GvHD prophylaxis should be proposed. There is no convincing evidence in children that received bone marrow transplantation that combining MTX to CsA would result in a synergistic effect to prevent AGvHD.26 Here in this study, the addition of MTX to CsA was associated with a delayed onset of AGvHD rather than with a decreased incidence of that complication in non-FA patients. These findings suggest that the addition of MTX to CsA in FA patients is unlikely to decrease the overall incidence of AGvHD. Various approaches are currently available to decrease the incidence of AGvHD in FA patients. The use of antithymocyte globulins combined to low-dose corticosteroid during the preparative regimen phase and after graft infusion as an in vivo T-cell–depletion method has been shown to dramatically reduce the incidence of AGvHD in a small set of FA patients that received transplants from HLA-identical siblings after a similar preparative regimen.27 Ex vivo T-cell depletion is currently under investigation in this setting as well. However, these 2 attractive approaches deserve attention with respect to the potential risk of increasing the immune deficiency during the first months after transplantation, leading to higher transplantation-related morbidity and mortality due to opportunistic infections and graft rejections. Mycophenolate mofetil, which exerts a stronger synergistic effect than MTX when used with CsA to prevent AGvHD, could be the immunosuppressive drug of choice to combine with CsA in FA patients.28,29 Our preliminary results with this drug have not been associated with a delayed hematopoietic recovery, a potential side effect that has been observed after prolonged use of this drug. Whether mycophenolate mofetil will be associated with an increased risk of opportunistic infections will require further investigations. Irradiation-free preparative regimens based on an intermediate-dose of cyclophosphamide alone have also given some encouraging results with a 13% AGvHD incidence in a group of 16 FA patients.30 Recent results on a limited number of patients also suggest that fludarabine, which has a more restricted cytotoxicity but a potent immunosuppressive effect, could advantageously replace TAI regarding early transplantation-related complications in FA or AAA patients.15,31-34
It has been shown that FA patients who did not receive a transplant have an increased risk of developing head and neck carcinomas when compared with a control healthy population.35 In this study, acute GvHD, maybe by triggering chronic GvHD, had a dramatic influence on the risk of developing such lethal malignancies in FA patients. Therefore, reducing AGvHD incidence by one of the approaches described above should improve not only the early posttransplantation outcome but also the long-term outcome of FA patients. Whether the high incidence of posttransplantation head and neck carcinomas reported in our FA patients is related to the use of an irradiation-based preparative regimen cannot be solved in this study since we had no control group of FA patients who received transplants in our institution with an irradiation-free preparative regimen during the same time period. However, irradiation-free preparative regimens, by avoiding an additional risk factor interacting with the main biologic defect of FA (ie, DNA repair processes), could be currently the optimal strategy to consider in FA patients undergoing HLA geno-identical transplantation in the absence of clonal evolution.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-06-2146.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal