Abstract
PU.1 is a hematopoietic-specific transcriptional activator that is absolutely required for the differentiation of B lymphocytes and myeloid-lineage cells. Although PU.1 is also expressed by early erythroid progenitor cells, its role in erythropoiesis, if any, is unknown. To investigate the relevance of PU.1 in erythropoiesis, we produced a line of PU.1-deficient mice carrying a green fluorescent protein reporter at this locus. We report here that PU.1 is tightly regulated during differentiation—it is expressed at low levels in erythroid progenitor cells and down-regulated upon terminal differentiation. Strikingly, PU.1-deficient fetal erythroid progenitors lose their self-renewal capacity and undergo proliferation arrest, premature differentiation, and apoptosis. In adult mice lacking one PU.1 allele, similar defects are detected following stress-induced erythropoiesis. These studies identify PU.1 as a novel and critical regulator of erythropoiesis and highlight the versatility of this transcription factor in promoting or preventing differentiation depending on the hematopoietic lineage.
Introduction
Hematopoiesis is the dynamic developmental process in which a pluripotent hematopoietic stem cell differentiates into multiple distinct blood lineages. It is coordinated by sets of transcription factors that influence self-renewal, cell fate choice, and differentiation.1-4 One such factor is PU.1, a member of the ets family that is exclusively expressed by hematopoietic cells. PU.1 is critical for the maturation of B lymphocytes and myeloid-lineage cells, which are arrested at the earliest stages of differentiation in PU.1-null mice.5-7 PU.1 may act as a “master switch” in these lineages, because graded levels of this factor in progenitor cells can influence the cell fate choice between B-cell and macrophage development.8 Moreover, PU.1 is required for mast and dendritic cell differentiation and plays a role in the early stages of T lymphopoiesis,9-13 demonstrating that PU.1 is a potent regulator of many hematopoietic lineages.
A potential role for PU.1 in erythropoiesis remains controversial. PU.1 is expressed in early erythroid lineage cells—it is present at the mRNA level in bipotent erythroid-megakaryocytic progenitors and at the protein level in primary murine erythroblasts.14,15 PU.1 is also a major oncogene targeted by the Friend virus complex in murine erythroleukemia. Insertion of the Friend virus upstream of the PU.1 locus leads to the constitutive up-regulation of PU.1, uncontrolled proliferation, and a subsequent block in the differentiation of the affected erythroblasts.16-18 Indeed, overexpression of PU.1 by transgenesis results in a similar development of transformed erythroblasts, and forced expression of murine PU.1 in avian erythroid progenitor cells has shown that high PU.1 levels inhibit the differentiation and apoptosis of these immature cells.19-21
Previous “knock-out” studies, however, have failed to reveal a compelling role for PU.1 in erythropoiesis. In 2 independently generated PU.1-deficient mouse lines, terminally differentiated erythrocytes were detected in the mutant fetuses, suggesting that PU.1 is not essential for erythropoiesis.5,6 In one of these lines, PU.1–/– fetuses were found to be anemic, a phenotype that appeared to be linked to the genetic background of these animals.5,22 The basis for this anemia remains unclear, because the defect may originate at an earlier pluripotent stage of differentiation rather than an intrinsic alteration within the erythroid lineage.7 Thus, there is currently little direct evidence that PU.1 is involved in erythroid differentiation.
Here, we revisit this issue using an independently generated line of PU.1-deficient mice carrying a green fluorescent protein (GFP) reporter at the PU.1 locus. By exploiting the ability to visualize PU.1-expressing cells, we show that PU.1 is in fact a critical regulator of erythropoiesis.
Materials and methods
Targeting vector and mice
The targeting vector contained 9 kilobase (kb) of sequences of the PU.1 locus (1.5 kb upstream and 7.5 kb downstream of exon 1). Twenty-two base pairs containing the initiation ATG and a second ATG located 7 codons downstream were deleted and replaced with XmaI and MluI sites; an XmaI-MluI cassette was introduced within containing (1) a rabbit β-globin intron flanked by cognate 5′ and 3′ exon sequences (derived from the pSG5 plasmid)23 ; (2) the coding sequence for enhanced GFP (EGFP) (Clontech, Palo Alto, CA); (3) a 150–base pair (bp) EcoRI-XbaI fragment containing the polyadenylation sequence from the SV40 virus (derived from pSG5); and (4) a floxed phosphoglycerate kinase–neomycin (PGK-neo) selection cassette cloned in reverse orientation. This construct was linearized with XhoI and electroporated into P1 embryonic stem (ES) cells. Homologous recombination events were detected by Southern blot analysis on SpeI-digested DNA and an external 5′ probe (probe A). Homologous recombination led to the shortening of the endogenous approximate 15-kb fragment to 10 kb. Germ line transmission of the targeted allele was obtained from 2 ES cell clones, FB7 and FB18 (both gave identical phenotypes). Cre-mediated excision of the floxed PGK-neo cassette was accomplished by crossing PU.1+/neo mice with transgenic cytomegalovirus (CMV)–Cre recombinase animals. Excision was verified using probe B on EcoRI-digested tail DNA. Mice carrying excised alleles were further bred with C57BL/6 (B6) mice to remove the CMV-Cre transgene. Mice used in this study have been backcrossed at least 5 times onto the B6 background. Routine genotyping was performed by polymerase chain reaction (PCR) (primers available upon request).
Methylcellulose colony assays and quantification of erythroid precursors
Fetal liver (FL) cells were obtained from fetuses derived from PU.1+/G crosses between 11.5 to 17.5 days after coitus (dpc). “Mixed” erythroid/nonerythroid and erythroid-only colony-forming cell (CFC-mix/ery) colonies were obtained as follows: Cells were plated in triplicate in 3.5-cm plates containing 1.1 mL of the semisolid methylcellulose medium M3434 (StemCell Technologies, Vancouver, BC) containing erythropoietin (Epo), stem cell factor (SCF), interleukin-3 (IL-3), and IL-6, according to the manufacturer's recommendations, and cultured at 37° C. After 7 days, the cultures were stained with a benzidine solution (2 mg/mL benzidine/0.5% glacial acetic acid/0.5% H2O2), and large colonies containing darkly stained cells were counted as CFC-mixed/ery. Erythroid colony-forming unit (CFU-E) colonies were obtained as follows: Cells were plated in triplicate in 3.5-cm plates containing 1.1 mL of the semisolid methylcellulose medium M3234 (StemCell Technologies) supplemented with 0.3 U/mL Epo (R&D Systems, Minneapolis, MN) and cultured at 37° C. After 3 days, cultures were stained with benzidine, and small dark colonies were counted as CFU-Es. The cell concentrations plated at day 0 in both assays varied (3 × 103 to 30 × 103) according to the stage of FL development and were titrated to obtain statistically relevant numbers of colonies per plate.
Antibodies and flow cytometry
The following primary antibodies were used: phycoerythrin (PE)– or purified TER119, biotin– or fluorescein isothiocyanate (FITC)–anti-CD71, biotin–anti-CD117 (c-Kit), purified anti-CD19, PE–anti-CD43, biotin–anti-Mac1, and PE–anti-Gr1 (all from BD Pharmingen, San Diego, CA). Secondary antibodies include cyanin 5 (Cy5)–anti-rat immunoglobulin G (IgG) and PE-anti-rat IgG (Jackson ImmunoResearch Labs, West Grove, PA), streptavidin–Alexa 594 (Molecular Probes, Eugene, OR), streptavidin-Cy5 (Jackson ImmunoResearch Labs), and streptavidin–peridinin chlorophyll protein (PerCP) (BD Pharmingen). Annexin V staining was performed at room temperature in annexin buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]/NaOH [pH 7.4], 150 mM NaCl, 1 mM MgCl2, 5 mM KCl, 1.8 mM CaCl2) using biotin–annexin V (Caltag Laboratories, Burlingame, CA). Lin depletion was performed using antibodies against B220 (clone RA36B2), CD3 (clone KT3), Gr1 (BD Pharmingen), F4/80, TER119 (BD Pharmingen), and NK1.1 (BD Pharmingen). The human truncated nerve growth factor receptor (NGFR) reporter was detected using an anti-NGFR antibody (Ab) (American Type Culture Collection, Manassas, VA; clone HB-8737). Samples were analyzed or sorted using an Epics Elite (Coulter Electronics, Hialeah, FL) or a DiVa (Becton Dickinson, San Jose, CA). Sort purity was more than 90% for culture assays and more than 95% for RNA experiments.
May-Grünwald staining
Cells were cytospun (about 105 cells per slide) at 45g for 5 minutes onto glass slides and stained with May-Grünwald followed by Giemsa, according to standard protocols.
Erythroid progenitor cultures
Cells were cultured in “erythroid medium” consisting of serum-free stem cell expansion medium (StemPro-34; Gibco, Grand Island, NY) supplemented with 100 ng/mL SCF (R&D Systems), 2 U/mL Epo (R&D Systems), 40 ng/mL insulin growth factor-1 (IGF-1) (Sigma-Aldrich, St Louis, MO), and 1 μM dexamethasone (Dex; Sigma-Aldrich). Cultures were counted daily and maintained at 2 × 106 to 4 × 106/mL, diluted with fresh medium. If no dilution was needed, half of the medium was replaced every 2 days. For CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester) labeling, cultured cells were harvested and resuspended at 107/mL in StemPro-34 (without cytokines) with 20 μg/mL CFSE (Sigma-Aldrich) and incubated at 37° C for 10 minutes. After 3 washes with StemPro-34, cells were replated in erythroid medium and cultured for a further 2 days.
RT-PCR
RNA was isolated from 1× 105 to 3 × 105 cells using the RNeasy Kit (Qiagen, Valencia, CA) and resuspended in 12 μL H2O; 3 μL RNA was used in the reverse transcriptase (RT) reactions (20 μL); and 1 to 2 μL cDNA was used for the PCR. PCRs were performed as follows: 94° C for 5 minutes, followed by n cycles of 94° C for 1 minute, 60° C for 1 minute, and 72° C for 1 minute (n = 26 to 35 depending on the gene). Table 1 lists the primers that were used.
Forward and reverse primers
Gene . | Forward primers: 5′ to 3′ . | Reverse primers: 5′ to 3′ . |
|---|---|---|
| c-Myb | CCC TGA AGC GCC TAA TCA C | ATG GCG GAT CAA GTC TCC TC |
| Bcl-xL | TTG GAC AAT GGA CTG GTT GA | TGA GTG GAC GGT CAG TGT CT |
| SCL | ACC TCA CGG CAA GCT AAG TAA | ACG CCG TTG AGC AGG ACT A |
| EKLF | ACC ACC CTG GGA CAG TTT CT | GAA GGG TCC TCC GAT TTC AG |
| GATA-1 | GGG AGC TGA CTT TCC CAG T | GTC TCC TCT GCC ACA AGG TC |
| GATA-2 | CAA GGA TGG CGT CAA GTA CC | ACA GTA ATG GCG GCA CAA G |
| c-Kit | ACA GGA CCT CGG CTA ACA AA | AAC GAG TCA CGC TTC CTT CT |
| LMO2 | CGG CCA TCG AAA GGA AGA | TTG ATC TTG GTC CAC TCG TAG A |
| β-Globin | CTG CAC TGT GAC AAG CTT CAT | CAT TGG CCA CTC CAA TCA C |
| β-Actin | GTG ACG AGG CCC AGA GCA AGA G | AGG GGC CGG ACT CAT CGT ACT |
| GFP | GTG GAT CGA TCT GAG AAC TT | GCG GAT CTT GAA GTT CAC |
| PU.1: P1 | GGA TCT GAC CAA CCT GGA GC | — |
| PU.1: P2 | GGA AGG GTT TTC CCT CAC C | — |
| PU.1: P3 | AGC GAT GGA GAA AGC CAT AG | — |
| PU.1: P4 | — | AGC ACC TCG CCG CTG AA |
Gene . | Forward primers: 5′ to 3′ . | Reverse primers: 5′ to 3′ . |
|---|---|---|
| c-Myb | CCC TGA AGC GCC TAA TCA C | ATG GCG GAT CAA GTC TCC TC |
| Bcl-xL | TTG GAC AAT GGA CTG GTT GA | TGA GTG GAC GGT CAG TGT CT |
| SCL | ACC TCA CGG CAA GCT AAG TAA | ACG CCG TTG AGC AGG ACT A |
| EKLF | ACC ACC CTG GGA CAG TTT CT | GAA GGG TCC TCC GAT TTC AG |
| GATA-1 | GGG AGC TGA CTT TCC CAG T | GTC TCC TCT GCC ACA AGG TC |
| GATA-2 | CAA GGA TGG CGT CAA GTA CC | ACA GTA ATG GCG GCA CAA G |
| c-Kit | ACA GGA CCT CGG CTA ACA AA | AAC GAG TCA CGC TTC CTT CT |
| LMO2 | CGG CCA TCG AAA GGA AGA | TTG ATC TTG GTC CAC TCG TAG A |
| β-Globin | CTG CAC TGT GAC AAG CTT CAT | CAT TGG CCA CTC CAA TCA C |
| β-Actin | GTG ACG AGG CCC AGA GCA AGA G | AGG GGC CGG ACT CAT CGT ACT |
| GFP | GTG GAT CGA TCT GAG AAC TT | GCG GAT CTT GAA GTT CAC |
| PU.1: P1 | GGA TCT GAC CAA CCT GGA GC | — |
| PU.1: P2 | GGA AGG GTT TTC CCT CAC C | — |
| PU.1: P3 | AGC GAT GGA GAA AGC CAT AG | — |
| PU.1: P4 | — | AGC ACC TCG CCG CTG AA |
— indicates that the same reverse primer was used to amplify the different PU.1 exons (see Figure 1A).
Western blotting
Cultured erythroid cells were depleted of TER119+ cells by magnetic bead selection using the TER119 antibody (BD Pharmingen) and anti-rat IgG coupled to magnetic beads (Dynal, Oslo, Norway). Forty micrograms of nuclear extracts from TER119– cells, total BM cells, and murine erythroleukemia (MEL) cells were separated on a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred onto a nitrocellulose membrane, and incubated sequentially with blocking buffer (10% fat-free milk/phosphate-buffered saline [PBS]), overnight at 4° C with a 1:500 dilution of a rabbit anti-PU.1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), 1.5 hours at room temperature with a 1:104 dilution of a horseradish peroxidase (HRP)–conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Labs). Blots were stripped with 2% SDS, 80 mM β-mercaptoethanol at 65° C for 30 minutes, and reprobed with a 1:1000 dilution of goat anti–β-actin antibody (Santa Cruz Biotechnology) and an HRP-conjugated donkey antigoat antibody. The membranes were revealed by SuperSignal chemiluminescent substrate (Pierce, Rockford, IL). The same protocol was used to detect PU.1 in total FL cells using 30 μg nuclear extract.
Retrovirus production and transduction
The PU.1 cDNA sequence was cloned into the Mig-NGFR vector24 and was designated Mig-PU.1. The resulting vectors were transfected into the Eco-Phoenix packaging cell line to produce high-titer retroviral supernatants. After an initial 2 days in erythroid culture, 2 × 106 to 4 × 106 FL cells were cocultured in 6-well plates with 33.3% (vol/vol) of the appropriate retroviral supernatant, keeping the identical cytokine concentrations in a total volume of 1.5 mL containing 4.4 μg/mL polybrene. The cells were spinfected at 900g for 90 minutes at room temperature. Infection efficiency varied between 35% to 62% depending on the experiment. After 12 hours in culture, 0.5 mL erythroid medium was added to the cultures, and the cells were maintained at the appropriate concentrations thereafter, as described for the erythroid progenitor cultures.
Phenylhydrazine treatment
Mice were injected intraperitoneally with phenylhydrazine (60 mg/kg body weight diluted in PBS), 2 injections 24 hours apart. The first injection is labeled as day 0. Spleens were obtained from treated animals on days 3, 4, and 5. Erythrocyte lysis was performed by resuspending spleen cells in 80 mM ammonium chloride. Spleen cells were then plated in CFU-E assays as described for methylcellulose colony assays; in parallel, cells were stained for TER119 and CD71 expression and analyzed by flow cytometry.
Statistical analyses
The statistical significance of the differences observed between phenylhydrazine-treated PU.1+/G and wild-type (WT) mice was evaluated as follows. For a given day (day 4 or 5) and readout combination (CFU-E or TER119+ cells), independent P values were evaluated for each of the 2 experiments using the Student t test. For each day and each readout, the overall significance of both experiments was calculated as the product of the P values from each experiment. The resulting P values were below 10–6.
Results
The PU.1G mouse line
We produced the PU.1G mutation (G for GFP) by targeting the enhanced GFP cDNA into exon 1 of the PU.1 locus by homologous recombination (Figure 1A). The resulting PU.1G/G mutant mice were null for PU.1 by several criteria. PU.1 mRNA and protein were undetectable in the fetal liver (FL); myeloid and B-lineage cells were absent in the FL (Supplemental Figure S1 at the Blood website; see the Supplemental Data Set link at the top of the online article). PU.1G/G fetuses were produced at mendelian frequency, but neonates died within 1 day of birth. These results are in complete agreement with published data. Importantly, the expression of the GFP reporter correlated well with PU.1 expression. In adult heterozygous PU.1+/G animals, GFP was expressed highly in myeloid cells, moderately in B cells, and at background levels in T cells (Supplementary Figure S1).13 In homozygous PU.1G/G FL cells, GFP was expressed by a fraction of immature cells lacking lineage marker expression (Lin– cells) (J.B., manuscript in preparation).
Erythroid phenotype of PU.1G/G fetuses. (A) Representation of targeted PU.1 allele. Black boxes indicate exons (exon I not to scale). Exon numbers are indicated by roman numerals. RT-PCR primers (P1-P4) are shown at the top. The DNA fragment present in the targeting vector is depicted with a thicker line in the WT allele. A and B are the probes used for Southern blot analyses; E, EcoRI; S, SpeI; IVS, intron from the rabbit β-globin gene. (B) Altered erythropoiesis in PU.1G/G FL. The 12.5- and 16.5-dpc FL cells were analyzed for TER119 expression by flow cytometry. Bar graphs display the average number of TER119+ cells per FL, calculated from 6 mutant and 6 WT fetuses for each stage. (C) Quantification of immature (CFC-mix/ery; left panel) and mature (CFU-E; right panel) erythroid precursors at different stages of PU.1G/G and WT FL development. Means and SDs were obtained using more than 3 fetuses of each genotype from 2 different experiments, per stage of development.
Erythroid phenotype of PU.1G/G fetuses. (A) Representation of targeted PU.1 allele. Black boxes indicate exons (exon I not to scale). Exon numbers are indicated by roman numerals. RT-PCR primers (P1-P4) are shown at the top. The DNA fragment present in the targeting vector is depicted with a thicker line in the WT allele. A and B are the probes used for Southern blot analyses; E, EcoRI; S, SpeI; IVS, intron from the rabbit β-globin gene. (B) Altered erythropoiesis in PU.1G/G FL. The 12.5- and 16.5-dpc FL cells were analyzed for TER119 expression by flow cytometry. Bar graphs display the average number of TER119+ cells per FL, calculated from 6 mutant and 6 WT fetuses for each stage. (C) Quantification of immature (CFC-mix/ery; left panel) and mature (CFU-E; right panel) erythroid precursors at different stages of PU.1G/G and WT FL development. Means and SDs were obtained using more than 3 fetuses of each genotype from 2 different experiments, per stage of development.
Altered erythropoiesis in the PU.1G/G fetal liver
Early phenotypic analyses revealed several anomalies in the liver of PU.1G/G fetuses. The cellularity of the PU.1G/G FLs was approximately half that of wild-type (WT) or PU.1+/G organs at 11.5 to 12.5 dpc, although this difference disappeared by 13.5 dpc (Table 2). Interestingly, 12.5-dpc PU.1G/G FLs contained significantly fewer mature erythroid cells than WT FLs, as defined by the TER119 marker (Figure 1B). From 15.5 dpc onward, however, far higher numbers of TER119+ cells were found in the PU.1G/G FLs. These data suggest that the absence of PU.1 alters erythropoiesis during fetal development.
Fetal liver cellularity of PU.1G/G fetuses during development
. | Fetal liver cellularity, × 104 . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | 11.5 dpc . | 12.5 dpc . | 13.5 dpc . | 15.5 dpc . | 17.5 dpc . | ||||
| +/+ | 15.63 ± 0.35 | 126 ± 48.3 | 388 ± 99 | 2000 ± 300 | 6500 ± 800 | ||||
| G/G | 9.34 ± 1.13 | 43.75 ± 18.2 | 340 ± 169 | 1989 ± 600 | 8000 ± 1000 | ||||
. | Fetal liver cellularity, × 104 . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | 11.5 dpc . | 12.5 dpc . | 13.5 dpc . | 15.5 dpc . | 17.5 dpc . | ||||
| +/+ | 15.63 ± 0.35 | 126 ± 48.3 | 388 ± 99 | 2000 ± 300 | 6500 ± 800 | ||||
| G/G | 9.34 ± 1.13 | 43.75 ± 18.2 | 340 ± 169 | 1989 ± 600 | 8000 ± 1000 | ||||
The FL cellularity is indicated for each stage of development. Means and SDs were averaged from at least 4 WT and PU.1G/G fetuses for each stage except for 11.5 dpc (4 WT and 3 PU.1G/G).
To quantify the numbers of erythroid progenitor cells over time, we performed colony assays using FL cells from WT and PU.1G/G 11.5- to 17.5-dpc fetuses. Two types of precursor populations were evaluated: (1) immature precursors that give rise to “mixed” erythroid/nonerythroid and erythroid-only (erythroid burst-forming unit [BFU-E]) colonies, in response to Epo, SCF, IL-3, and IL-6 (collectively termed colony-forming cells [CFC]–mixed/ery); and (2) more mature precursors (CFU-Es) that grow with Epo alone. Both PU.1G/G and WT FL cells contained low numbers of CFC-mix/ery at 11.5 and 12.5 dpc (Figure 1C, left; these cells were twice as numerous in PU.1G/G FLs compared with WT at 11.5 dpc [inset]). Strikingly, while WT numbers increased, CFC-mix/ery numbers remained constant in PU.1G/G FLs. Given the paucity of these immature PU.1G/G erythroid precursors, we expected a similar defect in the more mature CFU-E population. Surprisingly, this was not the case. Although PU.1G/G CFU-Es numbered less than WT CFU-Es at 11.5 to 12.5 dpc, their numbers rapidly increased and outnumbered WT CFU-Es from 13.5 dpc onward (Figure 1C, right). Thus, erythroid progenitor populations are substantially changed in PU.1G/G FLs: consistently low numbers of immature progenitors appear to give rise to a disproportionately larger population of mature cells. Importantly, these changes occurred early in development (13.5 dpc), when myeloid and lymphoid cells account for only a small percentage of the normal FL. These data suggest a primary and intrinsic erythropoietic defect in PU.1G/G fetuses.
Impaired ex vivo expansion of PU.1-deficient erythroblasts
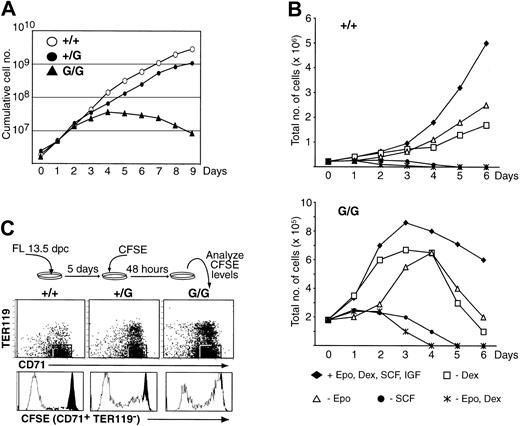
To investigate the mechanism(s) behind these changes, we evaluated the capacity of PU.1G/G erythroid progenitors to proliferate and differentiate ex vivo using a culture system that expands cells in serum-free media containing Epo, SCF, insulin growth factor (IGF), and dexamethasone (defined here as “erythroid medium”).25 Under these conditions, immature erythroid-committed precursors (CD71+TER119–) can be expanded 2- to 3-fold per day for longer than 21 days. When whole, or enriched Lin–, FL cells from WT or PU.1+/G fetuses were cultured, the numbers of cells increased dramatically for both genotypes over a 9-day period (Figure 2A). Intriguingly, PU.1G/G FL cells exhibited a burst of proliferation the first 2 to 3 days, after which their numbers increased more slowly before declining. By day 9, there was a 500-fold difference in the overall yield between PU.1G/G and WT cultures. This failure to expand was not due to the inability of PU.1G/G cells to respond to a specific factor, because selectively omitting factors from culture further reduced their expansion (Figure 2B). Moreover, we consistently observed a slight reduction in the capacity of PU.1+/G FL (Figure 2A), and adult Lin– bone marrow (BM) (Figure 4A), cells to thrive in these cultures. As such “intermediate” phenotypes were also detected in other analyses using PU.1+/G cells (Figures 2C and 3), these data indicate that erythroid precursors are sensitive to changes in PU.1 levels and reinforce the hypothesis that PU.1 functions cell-autonomously in erythroid progenitor cells.
Impaired proliferation of PU.1G/G FL erythroid precursors. (A) Ex vivo expansion of erythroid progenitors. The 13.5-dpc FL cells (2 × 106) were plated in erythroid medium at day 0, and cell numbers were counted each following day. Graph represents 1 of more than 5 experiments with similar results. (B) Optimal proliferation of PU.1G/G FL erythroid precursors requires Epo, SCF, and Dex. The 13.5-dpc FL cells (2.2 × 105 WT, 1.8 × 105 PU.1G/G) were plated in erythroid medium at day 0 in the presence of Epo, SCF, Dex, and IGF or in the absence of the indicated factors. Cell numbers were counted each following day. Graphs represent 1 of 3 independent experiments. (C) Impaired proliferation of PU.1+/G and PU.1G/G erythroid progenitors. The experiment was performed as depicted. Forty-eight hours after CFSE loading, cells were harvested and analyzed for TER119, CD71, and CFSE expression. White histograms represent the CFSE intensities of the gated CD71+TER119– cells; black histograms represent the CFSE intensity of a control population that had not divided.
Impaired proliferation of PU.1G/G FL erythroid precursors. (A) Ex vivo expansion of erythroid progenitors. The 13.5-dpc FL cells (2 × 106) were plated in erythroid medium at day 0, and cell numbers were counted each following day. Graph represents 1 of more than 5 experiments with similar results. (B) Optimal proliferation of PU.1G/G FL erythroid precursors requires Epo, SCF, and Dex. The 13.5-dpc FL cells (2.2 × 105 WT, 1.8 × 105 PU.1G/G) were plated in erythroid medium at day 0 in the presence of Epo, SCF, Dex, and IGF or in the absence of the indicated factors. Cell numbers were counted each following day. Graphs represent 1 of 3 independent experiments. (C) Impaired proliferation of PU.1+/G and PU.1G/G erythroid progenitors. The experiment was performed as depicted. Forty-eight hours after CFSE loading, cells were harvested and analyzed for TER119, CD71, and CFSE expression. White histograms represent the CFSE intensities of the gated CD71+TER119– cells; black histograms represent the CFSE intensity of a control population that had not divided.
PU.1 expression is tightly regulated during FL erythropoiesis. (A) Erythroid precursors are GFP-positive (GFP+). The 13.5-dpc Lin– GFP+ and Lin– GFP– cells were purified and cultured in erythroid medium. Starting from 104 cells, the number of cells in each culture was quantified daily. Lin–: B220–Gr1–TER119–-CD3–F4/80–CD16/32–NK1.1–. (B) GFP expression is lost in culture. The 13.5-dpc PU.1+/G and PU.1G/G FL cells were cultured. GFP expression in the CD71loTER119– and CD71+/hiTER119– populations was measured after indicated times of culture. (C) PU.1 is expressed in “GFP-negative” erythroid progenitors and down-regulated in differentiated erythrocytes. Immature CD71+TER119– and mature TER119+ cells were purified after 7 days of culture (these cells are GFP-negative by flow cytometry) and analyzed for PU.1 and GFP expression by RT-PCR. The residual PU.1 and GFP mRNAs detected in TER119+ cells are probably due to contaminating TER119– cells (about 5%). (D) Erythroid progenitors synthesize PU.1. Western blot of nuclear extracts from FL cells cultured for 7 days, and depleted of TER119+ cells, total BM, and MEL cells using antibodies for PU.1 and β-actin. All experiments were performed in erythroid medium and are representative of more than 3 experiments.
PU.1 expression is tightly regulated during FL erythropoiesis. (A) Erythroid precursors are GFP-positive (GFP+). The 13.5-dpc Lin– GFP+ and Lin– GFP– cells were purified and cultured in erythroid medium. Starting from 104 cells, the number of cells in each culture was quantified daily. Lin–: B220–Gr1–TER119–-CD3–F4/80–CD16/32–NK1.1–. (B) GFP expression is lost in culture. The 13.5-dpc PU.1+/G and PU.1G/G FL cells were cultured. GFP expression in the CD71loTER119– and CD71+/hiTER119– populations was measured after indicated times of culture. (C) PU.1 is expressed in “GFP-negative” erythroid progenitors and down-regulated in differentiated erythrocytes. Immature CD71+TER119– and mature TER119+ cells were purified after 7 days of culture (these cells are GFP-negative by flow cytometry) and analyzed for PU.1 and GFP expression by RT-PCR. The residual PU.1 and GFP mRNAs detected in TER119+ cells are probably due to contaminating TER119– cells (about 5%). (D) Erythroid progenitors synthesize PU.1. Western blot of nuclear extracts from FL cells cultured for 7 days, and depleted of TER119+ cells, total BM, and MEL cells using antibodies for PU.1 and β-actin. All experiments were performed in erythroid medium and are representative of more than 3 experiments.
Increased differentiation and apoptosis of PU.1G/G FL erythroid precursors. (A) Differentiation of erythroid cells. FL cells were analyzed for CD71 and TER119 expression after a 4-day culture. The cells normally amplified in these cultures are CD71+TER119– (red gate). Mature erythroid cells are TER119+ (black gate). Photos show the morphology of cells from day-4 cultures, as evaluated by May-Grünwald-Giemsa staining. Proerythroblasts and erythroblasts are large cells with big nuclei and dark blue cytoplasm; differentiated erythroid cells are small with light cytoplasm. Original magnification, × 400. (B) Increased differentiation of sorted CD71+TER119– PU.1G/G cells. After 3 days of culture, CD71+TER119– cells were purified and reseeded in erythroid medium. Cells were counted after 48 hours and stained for TER119 and CD71 expression. Bar graphs display the total number of cells versus the number of TER119+ cells in each culture. (C) Genes expressed by PU.1G/G erythroid precursors. RNA was extracted from sorted CD71+TER119– cells after 5 days of culture. Expression of the indicated genes was assessed by RT-PCR. “+” or “–” RT indicates the presence or not of reverse transcriptase in the assay. All experiments were performed in erythroid medium. All are representative of more than 3 experiments. (D) Increased apoptosis of PU.1G/G erythroid precursors. The 13.5-dpc FL cells were cultured for 3 days in erythroid medium. Cells were then stained for annexin V positivity. The intensities of annexin V staining are shown for immature CD71+TER119– and mature TER119+ cells. The figure is representative of 4 experiments, analyzing cells from 7 PU.1G/G, 4 PU.1+/G, and 4 WT fetuses.
Increased differentiation and apoptosis of PU.1G/G FL erythroid precursors. (A) Differentiation of erythroid cells. FL cells were analyzed for CD71 and TER119 expression after a 4-day culture. The cells normally amplified in these cultures are CD71+TER119– (red gate). Mature erythroid cells are TER119+ (black gate). Photos show the morphology of cells from day-4 cultures, as evaluated by May-Grünwald-Giemsa staining. Proerythroblasts and erythroblasts are large cells with big nuclei and dark blue cytoplasm; differentiated erythroid cells are small with light cytoplasm. Original magnification, × 400. (B) Increased differentiation of sorted CD71+TER119– PU.1G/G cells. After 3 days of culture, CD71+TER119– cells were purified and reseeded in erythroid medium. Cells were counted after 48 hours and stained for TER119 and CD71 expression. Bar graphs display the total number of cells versus the number of TER119+ cells in each culture. (C) Genes expressed by PU.1G/G erythroid precursors. RNA was extracted from sorted CD71+TER119– cells after 5 days of culture. Expression of the indicated genes was assessed by RT-PCR. “+” or “–” RT indicates the presence or not of reverse transcriptase in the assay. All experiments were performed in erythroid medium. All are representative of more than 3 experiments. (D) Increased apoptosis of PU.1G/G erythroid precursors. The 13.5-dpc FL cells were cultured for 3 days in erythroid medium. Cells were then stained for annexin V positivity. The intensities of annexin V staining are shown for immature CD71+TER119– and mature TER119+ cells. The figure is representative of 4 experiments, analyzing cells from 7 PU.1G/G, 4 PU.1+/G, and 4 WT fetuses.
To examine the proliferative capacity of PU.1G/G erythroid progenitors, FL cells were labeled with the fluorescent dye CFSE after 5 days of culture in erythroid medium and analyzed after 2 additional days of culture. Loss of CFSE reflects cellular division (Figure 2C). (In these experiments, GFP expression did not interfere with the CFSE signal, because GFP was undetectable after 5 days of culture [not shown].) When immature (CD71+TER119–) erythroid precursors were analyzed (Figure 2C), most WT cells expressed background CFSE levels, having undergone significant division. In contrast, while a subpopulation of PU.1G/G cells was CFSE–/lo, most PU.1G/G CD71+TER119– cells were CFSEhi and thus had not significantly divided.
PU.1-deficient erythroblasts differentiate prematurely
Decreased proliferation correlated with increased differentiation. After 4 days in erythroid medium, high numbers of PU.1G/G cells expressed TER119 and were small erythrocytes (normoblasts and macroblasts), while WT cells remained large, immature, and TER119– (Figure 3A). Moreover, the most immature CD71loTER119– population was diminished in PU.1G/G cultures when compared with their WT or PU.1+/G counterparts. To directly investigate the differentiation capacity of immature CD71+TER119– FL cells, we sorted these cells from 3-day WT, PU.1+/G, and PU.1G/G cultures and cultured them for 2 more days. As shown in Figure 3B, PU.1G/G cultures produced significantly more TER119+ cells than PU.1+/G or WT ones. This increased differentiation correlated with a marked reduction in cell number in the PU.1G/G samples (Figure 3B, graph).
We further analyzed the differentiative state of the PU.1G/G CD71+TER119– population. PU.1G/G CD71+TER119– cells were clearly smaller than WT or heterozygous ones, as determined by forward scatter analysis (not shown). At the molecular level, PU.1G/G CD71+TER119– cells expressed more β-globin, stem cell leukemia/T-cell acute lymphoblastic leukemia-1 (SCL/Tal-1), and Bcl-xL, all of which are up-regulated upon differentiation (Figure 3C).26-28 Likewise, c-Kit, c-Myb, and GATA-2 levels, normally down-regulated upon differentiation,28-31 were reduced, suggesting that PU.1G/G CD71+TER119– cells are more mature than PU.1+/G or WT cells. In addition, both PU.1G/G TER119– and (more mature) TER119+ cells were more apoptotic, as measured by positive annexin V staining after 3 days of culture (Figure 3D). Thus, increased cell death may also contribute to the failure of the PU.1G/G progenitors to expand, an observation consistent with the antiapoptotic effect of overexpressed PU.1 in primary cultures of avian erythroblasts.20 Interestingly, the increase in Bcl-xL mRNA levels in PU.1G/G TER119– cells did not appear to protect them from apoptosis, as has been reported for Epo-stimulated cultures26 ; loss of PU.1 might therefore engender apoptosis by a mechanism independent of Bcl-xL.
PU.1 expression in erythroid progenitors
To determine if PU.1 is expressed by the early progenitors that give rise to erythroid-committed cells, we purified and cultured Lin– GFP-positive and GFP-negative cells from 13.5-dpc PU.1+/G and PU.1G/G FLs (Figure 4A). Strikingly, only GFP-positive cells expanded while GFP-negative cells died. GFP expression, however, was transient. In freshly sorted 13.5-dpc Lin– cells at day 0 of culture, both the CD71loTER119– and CD71+TER119– populations contained a significant percentage of GFP-positive cells (Figure 4B); GFP expression decreased with time in both populations and was undetectable by day 7. Differentiated TER119+ cells never expressed GFP. Interestingly, GFP expression disappeared more rapidly in PU.1G/G than PU.1+/G CD71loTER119– cells, supporting the notion that PU.1 is required to maintain this immature population in culture.
Because it was unclear whether the GFP negativity in cultured cells resulted from a lack of PU.1 expression or low reporter expression (ie, below the threshold of detection), we purified CD71+TER119– and TER119+ cells from 7-day FL cultures and analyzed PU.1 and GFP mRNA levels by RT-PCR (Figure 4C). Both GFP (from PU.1+/G- and PU.1G/G-derived RNA) and PU.1 (from WT- and PU.1+/G-derived RNA) were clearly detected in TER119– cells. In contrast, the expression of PU.1 and GFP was much reduced in the mature TER119+ population, demonstrating a sharp regulation of PU.1 mRNA levels during erythrocyte maturation. Correspondingly, TER119– cells from 7-day WT or PU.1+/G cultures exhibited significant amounts of PU.1 protein (Figure 4D, lanes 2-3), albeit at noticeably lower levels than total BM cells (which contain 30% to 50% myeloid-lineage cells; Figure 4D, lane 1). Thus, PU.1 is expressed at low levels in immature erythroid cells, and this expression is extinguished in mature erythrocytes.
Rescue of erythroid progenitor by PU.1 reexpression
To determine if PU.1 directly promotes the self-renewal capacity of erythroid progenitors, we reexpressed PU.1 in these cells using a murine stem cell virus (MSCV)–based retroviral vector to express a bicistronic mRNA encoding the PU.1 protein and an inactive human truncated NGFR as reporter (Mig-PU.1). WT and PU.1G/G FL cells were cultured in erythroid medium for 2 days, transduced with Mig-PU.1 or the Mig-NGFR vehicle (Figure 5A), and analyzed up to 5 days following transduction. Cells that reexpressed PU.1 also expressed NGFR; nontransduced cells were NGFR-negative. While NGFR-negative PU.1G/G cells did not grow in the Mig-PU.1-transduced cultures, NGFR-positive PU.1G/G cells clearly expanded, with kinetics similar to WT FL cells, transduced or not with Mig-PU.1 (Figure 5B). These results demonstrate that PU.1 directly functions at the level of the immature erythroid precursor to maintain its self-renewal capacity, regardless of any previous activity in pluripotent progenitor cells. We examined the effect of forced PU.1 on the differentiation of WT and PU.1G/G erythroid progenitors (Figure 5C). PU.1 expression (Figure 5C, “NGFR+”) consistently slowed the differentiation of WT and PU.1G/G CD71-TER119– cells into the more mature CD71+TER119– and CD71+TER119+ populations. These results show that PU.1 maintains not only the self-renewal capacity of erythroid progenitor cells but also their immature state of differentiation.
Rescue of erythroid progenitor defects by PU.1 reexpression. (A) Diagrams of the retroviral constructs used to transduce erythroid progenitor cells. Mig-NGFR is an MSCV-based retrovirus with an internal ribosomal entry site (IRES) and NGFR sequence insert.24 Mig-PU.1 encodes the entire PU.1 protein. (B) Expansion of transduced erythroid progenitors. The 12.5- or 13.5-dpc FL cells (2 × 106 to 4 × 106) were plated in erythroid medium for 2 days, transduced or not with retrovirus (day 0), and cell numbers counted each following day. The initial burst of proliferation in PU.1G/G FL cultures (Figure 2A) occurred in the first 2 days before transduction and thus is not represented in this graph. (C) PU.1 blocks erythroid differentiation. Two days after transduction, NGFR-negative and NGFR-positive cells from WT and PU.1G/G cultures were analyzed for their expression of TER119 and CD71. The gray histogram indicates background staining of noninfected cells. Numbers denote the percentage of cells in each quadrant. Similar results were obtained 5 days after transduction. (D) PU.1hi-expressing cells rapidly die in culture. NGFR expression was monitored over time in erythroid cultures. The gray histograms represent background staining of noninfected cells. The NGFR mean fluorescence intensity (MFI = x) for each gated population is indicated in the histograms. The MFI of noninfected cells is 0.3 for WT and 1.3 for G/G. These results represent 1 of 3 experiments. ND indicates not done.
Rescue of erythroid progenitor defects by PU.1 reexpression. (A) Diagrams of the retroviral constructs used to transduce erythroid progenitor cells. Mig-NGFR is an MSCV-based retrovirus with an internal ribosomal entry site (IRES) and NGFR sequence insert.24 Mig-PU.1 encodes the entire PU.1 protein. (B) Expansion of transduced erythroid progenitors. The 12.5- or 13.5-dpc FL cells (2 × 106 to 4 × 106) were plated in erythroid medium for 2 days, transduced or not with retrovirus (day 0), and cell numbers counted each following day. The initial burst of proliferation in PU.1G/G FL cultures (Figure 2A) occurred in the first 2 days before transduction and thus is not represented in this graph. (C) PU.1 blocks erythroid differentiation. Two days after transduction, NGFR-negative and NGFR-positive cells from WT and PU.1G/G cultures were analyzed for their expression of TER119 and CD71. The gray histogram indicates background staining of noninfected cells. Numbers denote the percentage of cells in each quadrant. Similar results were obtained 5 days after transduction. (D) PU.1hi-expressing cells rapidly die in culture. NGFR expression was monitored over time in erythroid cultures. The gray histograms represent background staining of noninfected cells. The NGFR mean fluorescence intensity (MFI = x) for each gated population is indicated in the histograms. The MFI of noninfected cells is 0.3 for WT and 1.3 for G/G. These results represent 1 of 3 experiments. ND indicates not done.
Interestingly, NGFRlo cells were selectively maintained in the erythroid cultures over time (Figure 5C). In WT cultures transduced with Mig-NGFR, NGFR-negative and NGFRhi cells grew with similar kinetics up to 5 days following transduction. PU.1G/G cultures transduced with vehicle died rapidly, whether or not the cells expressed NGFR, as expected. In contrast, WT cultures transduced with Mig-PU.1 displayed 2 populations of cells, a NGFR-negative population and a NGFR-positive one that progressively lost NGFR intensity over time. Similarly, PU.1G/G cultures transduced with Mig-PU.1 contained cells that expressed low levels of NGFR that dominated the culture over time, as the nontransduced NGFR- PU.1G/G cells died off. These results are in agreement with the low PU.1 levels detected in WT erythroid progenitors and suggest that cells expressing high PU.1 levels die or are diverted into nonerythroid lineages (such as myeloid cells) that do not expand in these erythroid-specific cultures.32-34
Role of PU.1 in adult erythropoiesis
All of the above results show that PU.1 plays a critical role during fetal erythroid differentiation. An important question is whether PU.1 also regulates adult erythropoiesis. Since heterozygous FL erythroid progenitors consistently differed from WT cells in the assays (Figures 2, 3), we studied erythropoiesis in adult PU.1+/G animals. Little difference was found between WT and PU.1+/G mice in terms of blood erythrocyte, BM BFU-E, CFU-E, or TER119+ cell numbers (not shown). However, we consistently observed an impaired expansion of Lin– PU.1+/G BM cells in erythroid medium cultures (Figure 6A), similar to our results with PU.1+/G FL cells. As steady state erythropoiesis was unaffected in PU.1+/G mice, we asked whether differences could be revealed under stress. WT and PU.1+/G animals were evaluated for their capacity to regenerate erythroid cells following phenylhydrazine treatment, which induces massive erythrocyte lysis and renewed erythropoiesis in the spleen.35 The numbers of splenic CFU-Es and TER119+ cells were quantified 3 to 5 days after treatment (Figure 6B). In 2 experiments, PU.1+/G spleens contained significantly higher numbers of CFU-Es and TER119+ cells at days 4 and 5 (P < 10–6). These results mirror the faster appearance of CFU-Es and TER119+ cells during PUG/G fetal development and demonstrate that PU.1 shapes the adult erythropoietic response to stress.
PU.1 regulates adult erythropoiesis. (A) Expansion of PU.1+/G and WT BM erythroid progenitors cells in erythroid medium. A total of 105 WT or PU.1+/G purified Lin– (B220–Gr1–TER119–CD3–F4/80–CD16/32–NK1.1–) BM cells were seeded in erythroid medium; cell numbers were counted daily. Graph is representative of 3 experiments, 5 mice of each genotype in all. (B) Erythrocyte regeneration following phenylhydrazine-induced stress. PU.1+/G and WT mice were injected with phenylhydrazine (2 injections 24 hours apart) to induce anemia. The number of splenic CFU-E (top) and TER119+ cells (bottom) were quantified between 3 to 5 days after the first injection. Two experiments are shown (means and SDs), 3 mice for each time point.
PU.1 regulates adult erythropoiesis. (A) Expansion of PU.1+/G and WT BM erythroid progenitors cells in erythroid medium. A total of 105 WT or PU.1+/G purified Lin– (B220–Gr1–TER119–CD3–F4/80–CD16/32–NK1.1–) BM cells were seeded in erythroid medium; cell numbers were counted daily. Graph is representative of 3 experiments, 5 mice of each genotype in all. (B) Erythrocyte regeneration following phenylhydrazine-induced stress. PU.1+/G and WT mice were injected with phenylhydrazine (2 injections 24 hours apart) to induce anemia. The number of splenic CFU-E (top) and TER119+ cells (bottom) were quantified between 3 to 5 days after the first injection. Two experiments are shown (means and SDs), 3 mice for each time point.
Discussion
In this report, we reveal a novel function for PU.1 as a regulator of erythropoiesis. In vivo, PU.1 deficiency undermines the capacity to sustain a pool of immature progenitor cells during fetal development, leading to the early appearance of mature progenitor (CFU-E) and differentiated (TER119+) erythroid cells. In adults, higher numbers of CFU-Es and TER119+ cells are also generated following erythrocyte ablation in heterozygous PU.1+/G mice. In vitro, PU.1G/G erythroid precursors cannot maintain an immature phenotype, resulting in proliferative arrest, premature differentiation, and apoptosis. Together, these data indicate that PU.1 is crucial for erythroid homeostasis. Remarkably, this function requires very low levels of PU.1 in erythroid progenitors and is sensitive to small changes in these levels. This feature is particularly evident in the heterozygous phenotype, the inability of transduced erythroid progenitors expressing high levels of PU.1 to expand (Figure 5B), and the modest elevation of PU.1 in Friend erythroleukemia–derived MEL cells where it is sufficient to block differentiation (Figure 4D).33 Our data also provide a physiologic explanation for the selective outgrowth of transformed erythroblasts containing a proviral insertion in the PU.1 locus during Friend erythroleukemia. The constitutive up-regulation of PU.1 leads to an amplification of normal PU.1 function and results in overproliferation and a differentiation block of the affected erythroblasts.
An important issue is whether the phenotypes observed in heterozygous animals or cultures are due to a dose-dependent effect of PU.1 in individual cells or monoallelic expression of the PU.1 locus. The latter possibility would confer a null phenotype to a significant fraction of PU.1+/G cells. Currently, we cannot distinguish between these scenarios. However, there is clearly no mosaic expression of the PU.1 locus in early FL progenitors, because all cells that give rise to erythroid-committed precursors express GFP (Figure 4A). This observation is confirmed by the very low expression of PU.1 in Lin– GFP-negative cells (J.B., unpublished results, data obtained from 5 experiments between May 2001 and April 2002). Thus, monoallelic expression would at best affect only a small percentage of immature hematopoietic cells. A secondary stochastic extinction of one PU.1 allele during culture is also unlikely, because this would, but did not, lead to a quicker loss of GFP-positive cells in the PU.1+/G samples compared with PU.1G/G samples (Figure 4B). Therefore, the most plausible explanation is that both PU.1 alleles are active in immature erythrocytes and that the phenotypes observed in PU.1+/G cells are due to the reduction of PU.1 protein in individual cells.
Scott et al5 have reported the occurrence of anemia in their PU.1-null fetuses, due to a reduction of enucleated reticulocytes and erythrocytes. Although the basis for this anemia was not explored in detail, their observations are at odds with those of our study, as well as those of McKercher et al,6 in which no fetal anemia was detected. Furthermore, the anemia was apparently alleviated on a C57BL/6 background,22 indicating the importance of genetic background on this specific phenotype. The PU.1G line has been continuously backcrossed onto the B6 background for more than 5 generations, and we have thus far detected little difference in our erythroid phenotype between early and late backcross fetuses/animals. As Scott et al7 have shown that PU.1-null cells cannot provide long-term contribution to erythropoiesis, a rapid loss of the hematopoietic stem cell (HSC) pool could explain why the number of early hematopoietic progenitors stagnates over time in PU.1G/G FLs (Figure 1C). Thus, the anemia observed by Scott and coworkers may be due to a severe, genetic background-dependent, depletion of the HSC compartment.
Moreover, Fisher et al36,37 have reported that PU.1–/– FL cells do not respond synergistically to Epo and SCF stimulation in CFU-E and BFU-E assays. Although our respective studies differ in culture and readout assays, their results are broadly consistent with our observation that PU.1G/G erythroid progenitors expand poorly in vitro to Epo, SCF, and dexamethasone. These authors concluded that PU.1-null progenitors could not respond to certain cytokines (SCF in their first study, Epo in their second). Our data, however, strongly suggest that cytokine signaling, particularly Epo signaling, still functions in these cells, because (1) removal of Epo (or SCF) from erythroid cultures clearly reduced the expansion of PU.1G/G FL cells (Figure 2B) and (2) the cells produced in our PU.1G/G cultures were distinctly erythroid in nature, a property that depends critically on Epo signaling. Although we cannot exclude a limited loss in cytokine responses, the reduction in CFU-Es and BFU-Es in the Fisher system may also reflect a skewing of erythroid progenitor cells toward differentiation and apoptosis, issues not addressed in their reports.
Our data further highlight the pleiotropic function of PU.1 in hematopoiesis. While PU.1 inhibits erythroid differentiation, it apparently acts as a positive regulator of myeloid and B-lymphocyte differentiation. These seemingly contradictory functions may in part be explained by the specific interaction of PU.1 with various partners in the different cell types. In myeloid cells, PU.1 has been shown to positively interact with CCAAT/enhancer-binding protein alpha (C/EBPα) or C/EBPϵ,38,39 which in turn activate the c-fms and the granulocyte-macrophage colonystimulating factor (GM-CSF) receptor genes, crucial for myeloid differentiation.40,41 In B cells, PU.1 appears to recruit ets-1, c-Jun, and c-fos to lymphocyte-specific genes,42 such as the IL-7 receptor, the immunoglobulin light and heavy chains, and btk.43-47 In the erythroid lineage, however, overexpression studies have suggested that PU.1 inhibits erythroid differentiation mainly by negatively interacting with GATA-1, Rb, or CPB/p300.28,48,49 If the same scenario holds true for PU.1 in normal erythropoiesis, it would signify a markedly different mode of action for this factor in the erythroid versus the myeloid and lymphoid lineages. The PU.1G allele will clearly be a useful tool to investigate the relevance of these interactions at the molecular and genetic levels. Finally, it has recently been shown that p53 exerts a differentiation promoting activity during erythropoiesis.50 Because PU.1 activation and p53 inactivation frequently cooperate during the pathogenesis of Friend erythroleukemia, it will be interesting to investigate whether the PU.1- and p53-dependent pathways intersect to control normal erythroid maturation.
In conclusion, we propose a revised model for PU.1 function (Figure 7). PU.1 is essential for controlling the balance between self-renewal and differentiation in hematopoietic progenitor cells, but its function is distinct depending on the lineage. While PU.1 is a positive regulator of differentiation in B lymphocytes and myeloid-lineage cells, it acts to maintain self-renewal and prevent differentiation in the erythroid lineage. Thus, PU.1 is a versatile factor that exerts highly pleiotropic functions during hematopoiesis.
Revised model for PU.1 function in hematopoiesis. PU.1 induces differentiation in B-lymphoid and myeloid progenitor cells. Conversely, PU.1 stimulates self-renewal and prevents differentiation in erythroid progenitors. Relative PU.1 levels in the different lineages are indicated.
Revised model for PU.1 function in hematopoiesis. PU.1 induces differentiation in B-lymphoid and myeloid progenitor cells. Conversely, PU.1 stimulates self-renewal and prevents differentiation in erythroid progenitors. Relative PU.1 levels in the different lineages are indicated.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-11-4089.
Supported by institute funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National pour la Recherche Scientifique, and the Hôpital Universitaire de Strasbourg; grants from the Association pour la Recherche sur le Cancer (ARC) (nos. 4399, 4743, and 7646) and the Ligue Régionale contre le Cancer (S.C. and P.K.); and predoctoral fellowships from the Ministère de la Recherche et de la Technologie and ARC (J.B.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Sellars and J. Punt for critical reading of the manuscript; W. Pear for the Mig-NGFR vector and his laboratory for invaluable instruction; G. Nolan for the Eco-Phoenix cell line; S. Dali and A. Mertz for the ES cell work; the IGBMC transgenic facility; C. Waltzinger, J. Barths, and C. Ebel for help with flow cytometry; and F. Memedov and M. Gendron for animal husbandry.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal