Abstract
Donor alloreactive CD4+ T cells are important to the pathogenesis of chronic graft-versus-host disease (cGVHD), but specific subsets of CD4+ T cells responsible for GVHD have not been defined. We hypothesized that cGVHD might be associated with a preponderance of CD4+ effector memory cells (CCR7–/CD62Llow, CD4EM). We analyzed CCR7 and CD62L expression on CD4+ T cells from stem cell transplantation patients, who did or did not develop cGVHD, and healthy donors. Patients with cGVHD had a higher percentage of CD4EM cells (35.5% ± 2.9%) than healthy donors (13.8% ± 0.7%; P < .0001) or patients without cGVHD that received a transplant (21.7% ± 2.1%; P < .01). Using corticosteroid dose as a surrogate marker for cGVHD severity, severe cGVHD was associated with a higher percentage of CD4EM cells. The proportion of CD4EM cells in corticosteroid-dependent patients with systemic lupus erythematosis or Wegener granulomatosis did not differ from patients without cGVHD that received a transplant. This finding implies that overrepresentation of CD4EM cells is a unique feature of cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is the most common late complication of allogeneic stem cell transplantation.1 Donor-derived, alloreactive, postthymic CD4+ T cells play an important role in pathogenesis of cGVHD,2 however, the pathophysiology remains unclear.
Human peripheral blood CD4+ T cells can be divided into 3 populations: naive CD45RA+CCR7+ and 2 memory subsets, CD45RA–CCR7+ (central memory) and CD45RA–CCR7– (effector memory). Chemokine receptor CCR7 is pivotal for migration into secondary lymphoid organs.3 Expression of CD62L (Lselectin), which also guides lymphocytes into lymphoid tissue, is tightly linked to CCR7 expression on memory CD4+ T cells.4 Central memory CD4+ T cells are nonpolarized and retain the capacity to differentiate into either T-helper 1 (Th1) or Th2 when restimulated. Thus, they are effector precursors that regulate immune response. Conversely, effector memory cells are actively involved in inflammation and cytotoxicity.5 When stimulated via T-cell receptor, central memory cells produce only interleukin 2 (IL-2). In contrast, effector memory cells produce high levels of interferon γ (IFN-γ), IL-4, and IL-5 and moderately reduced levels of IL-2 in vitro.4 In healthy individuals, central memory CD4+ T cells are predominant in peripheral blood. An imbalance of the central and effector memory CD4+ T-cell ratio may result in autoimmune or alloimmune disease, but this has not been described.
We examined expression of CCR7 and CD62L on CD4+ T cells in cGVHD patients in order to characterize the balance of central and effector memory cells.
Study design
All cGVHD patients received transplants using myeloablative or nonmyeloablative marrow conditioning from a 6 of 6 or 5 of 6 human leukocyte antigen (HLA)–matched related donor. All patients provided written informed consent for participation in a variety of institutional review board–approved NIH Clinical Center stem cell transplantation protocols.
Fresh peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll separation with lymphocyte separation medium (ICN Biomedicals Inc, Aurora, OH) from 36 adult healthy donors, 25 cGVHD patients, 20 patients after allogeneic stem cell transplantation without cGVHD (> 100 days after transplantation), and 11 patients with autoimmune disorders requiring treatment with corticosteroids (5 systemic lupus erythematosis [SLE] and 6 Wegener granulomatosis patients).
Freshly isolated PBMCs were analyzed using a FACSort flow cytometer (BD Biosciences Immunocytometry Systems, San Jose, CA). Cells were stained with fluorescein isothiocyanate–conjugated antihuman CD4 monoclonal antibody (mAb), phycoerythrin-conjugated antihuman CD45RO mAb, allophycocyanin-conjugated antihuman CD62L mAb, and biotinylated antihuman CCR7 mAb in combination with cychrome-conjugated streptavidin (BD Biosciences Pharmingen, San Diego, CA) following the manufacturer's protocol. Analysis was performed with CellQuest software (BD Biosciences Immunocytometry Systems).
The data are shown as means ± SEM. Two-group comparison was done on the analysis of variance and Student t test. A P value less than .05 was considered statistically significant.
Results and discussion
The central and effector memory T-cell paradigm described by Lanzavecchia and colleagues4 is more firmly established in CD4+ compared with CD8+ T cells. Specifically, recent studies have demonstrated poor correlation between CD8+ effector function and CCR7 and CD62L expression.6-8 It is for this reason that we chose to focus our study on CD4+ T cells.
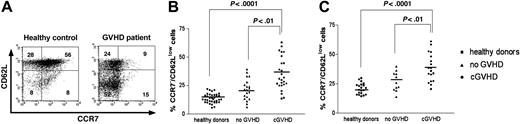
CD62L and CCR7 expression on CD4+ T cells from a representative patient with corticosteroid refractory severe cGVHD and a healthy donor is shown in Figure 1A. The CCR7–/CD62Llow phenotype is characteristic of the effector memory T cell. Chronic GVHD patients had a significantly higher percentage of CCR7–/CD62Llow cells compared with healthy controls (35.5% ± 2.9% vs 13.8% ± 0.7%, respectively; P < .0001) or stem cell transplantation patients without cGVHD (35.5% ± 2.9% vs 21.7% ± 2.1%, respectively; P < .01) in the total CD4+ population (Figure 1B). When the analysis was restricted to the memory (CD45RO+) CD4+ population, cGVHD patients still had a higher percentage of CCR7–/CD62Llow cells compared with healthy controls (38.7% ± 2.8% vs 21.0% ± 1.1%, respectively; P < .0001) or compared with stem cell transplantation patients without cGVHD (38.7% ± 2.8% vs 27.0% ± 2.5%, respectively; P < .01; Figure 1C).
Characterization of CCR7–/CD62Llow CD4+ T-cell populations. (A) Representative dot plot showing CCR7 and CD62L expression on a gated population of CD4+ T cells in a healthy donor and a patient with cGVHD. (B) Pooled data showing the percentage of CCR7–/CD62Llow total CD4+ cells in healthy donors (▪, n = 36), stem cell transplantation patients without cGVHD (▴, n = 20), and stem cell transplantation patients with cGVHD (•, n = 25). (C) Pooled data showing the percentage of CCR7–/CD62Llowmemory (CD45RO+) CD4+ cells in healthy donors (▪, n = 20), stem cell transplantation patients without cGVHD (▴, n = 11), and stem cell transplantation patients with cGVHD (•, n = 18). Horizontal lines indicate the mean values.
Characterization of CCR7–/CD62Llow CD4+ T-cell populations. (A) Representative dot plot showing CCR7 and CD62L expression on a gated population of CD4+ T cells in a healthy donor and a patient with cGVHD. (B) Pooled data showing the percentage of CCR7–/CD62Llow total CD4+ cells in healthy donors (▪, n = 36), stem cell transplantation patients without cGVHD (▴, n = 20), and stem cell transplantation patients with cGVHD (•, n = 25). (C) Pooled data showing the percentage of CCR7–/CD62Llowmemory (CD45RO+) CD4+ cells in healthy donors (▪, n = 20), stem cell transplantation patients without cGVHD (▴, n = 11), and stem cell transplantation patients with cGVHD (•, n = 18). Horizontal lines indicate the mean values.
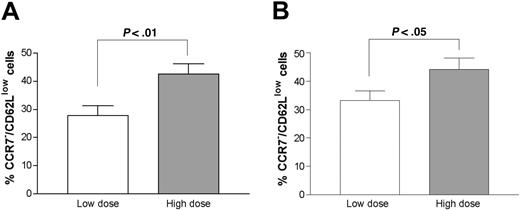
Next we examined the relationship between CD62L and CCR7 expression and severity of cGVHD using corticosteroid dose as a surrogate marker. The median steroid dosage for all GVHD patients was 0.25 mg/kg daily. Patients taking less than 0.25 mg/kg of prednisone daily were designated as the low-dose group and patients taking greater than 0.25 mg/kg daily as the high-dose group. There was no difference in the proportion of CD4+ T cells among all T cells in each patient group (data not shown); however, the CCR7–/CD62Llow subset of total CD4+ T cells or memory CD4+ T cells was statistically significantly higher in the high-dose prednisone group (42.6% ± 3.7% vs 27.8% ± 3.5%, P < .01 in the total CD4 population; 44.2% ± 3.7% vs 33.2% ± 3.4%, P < .05 in the memory CD4 population, respectively; Figure 2).
Comparison of CCR7–/CD62Llow populations. A comparison was made between CCR7–/CD62Llow populations in total CD4+ T cells (A) and memory (CD45RO+) CD4+ T cells (B) in patients with cGVHD on low-dose prednisone (n = 12, 9, respectively) and high-dose prednisone (n = 13, 9, respectively). The error bars indicate SEM.
Comparison of CCR7–/CD62Llow populations. A comparison was made between CCR7–/CD62Llow populations in total CD4+ T cells (A) and memory (CD45RO+) CD4+ T cells (B) in patients with cGVHD on low-dose prednisone (n = 12, 9, respectively) and high-dose prednisone (n = 13, 9, respectively). The error bars indicate SEM.
In order to assess the impact of high-dose corticosteroid therapy on expression of CD62L and CCR7 on CD4+ T cells, we analyzed cells from patients with Wegener granulomatosis and SLE. All of these patients were receiving high-dose (> 0.25 mg/kg/d) prednisone or methylprednisone treatment at the time the blood samples were collected. There was no statistically significant difference in percentage of CCR7–/CD62Llow CD4+ T cells from either SLE or Wegener granulomatosis patients compared with patients without cGVHD that received transplants (15.1% ± 4.3% or 20.9% ± 4.8% vs 21.7% ± 2.1%, respectively; P = .17 or P = .87), but there was a significant difference relative to patients with cGVHD that received transplants (35.5% ± 2.9%; P < .01 or P < .03, respectively; data not shown). We also note a recent report that the distribution of effector memory CD4+ T cells in patients with SLE and rheumatoid arthritis is not abnormal.9 These data suggest that changes in CD62L and CCR7 expression in cGVHD are not a result of corticosteroid administration.
A frequent dilemma in management of cGVHD is the determination of disease activity in patients with advanced sclerodermatoid skin changes and sicca syndrome. Future studies will be needed in order to determine whether the changes in CD4 memory subsets we observed will be a useful marker of disease activity in patients with cGVHD.
Our study does not reveal the mechanism for imbalance of central and effector memory cells. The 8 chronic GVHD patients in our study with the most abnormal ratio of effector to central memory cells (range, 35%-63% effector memory cells) did have decreased numbers of total circulating CD4 cells (343 ± 45/μL), but the absolute number of effector memory cells (165 ± 27/μL) was actually slightly higher than in healthy volunteers. Thus, it appears that the absolute size of the central memory cell pool is depleted rather than there being an expansion of the effector memory pool. Severe cGVHD causes immunologic destruction of thymic tissue, thereby reducing production of naive recent thymic T-cell emigrants.10,11 It is possible that reduction in size of the central memory pool is a consequence of this defect in production of naive T cells, if central memory T cells are proximal in the differentiation pathway from naive T cells to effector memory cells. Thymic destruction is not known to occur with classic autoimmune disorders such as SLE or Wegener granulomatosis where skewing of central and effector memory cell balance was not observed.12,13 However, controversy exists regarding the pathway from naive T cells to central memory T cells. Wherry and colleagues7 have used data from studies of mouse CD8+ T cells to propose an alternate pathway whereby naive T cells first differentiate into effector cells, then effector memory cells, and finally into central memory cells. If this paradigm applies to human CD4+ T cells, then it is possible our observations in cGVHD reflect a block in progression from effector memory to central memory CD4 cells, rather than resulting from defective production of naive T cells.
This is the first report to associate an imbalance of central and effector memory CD4+ T-cell populations with human disease. Prospective analysis of effector and central memory cell changes during treatment of GVHD may provide insight into the significance and mechanism of this imbalance.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-09-3286.
Supported by Japan Society for the Promotion of Science Research Fellowships for Japanese Biomedical and Behavioral Researchers at NIH and in part by Mitsubishi Pharma Research Foundation (K.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Margaret Brown for healthy donor blood samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal