Abstract
Relapse is a major problem after transplantation in children with acute B-lineage leukemias, and new therapies are needed to increase graft-versus-leukemia (GvL) effects without inducing graft-versus-host disease (GvHD). Here, we studied the ability of effector cells recovered from patients after transplantation with positive-selected stem cells from alternative donors to induce antibody-dependent cellular cytotoxicity (ADCC). For this purpose, a chimeric CD19 antibody, CD19-4G7chim, was generated. This antibody efficiently mediated ADCC against primary acute lymphoblastic leukemia (ALL) blasts by using purified natural killer (NK) cells from healthy donors or mononuclear cells from patients as effector cells. Increased lysis was obtained after stimulation of effector cells with interleukin-2 (IL-2). ADCC was not prevented by inhibitory effects mediated by HLA class I. We propose that treatment with chimeric CD19 antibodies leading to ADCC by donor-derived NK cells may become a therapeutic option for the post-transplantation treatment of minimal residual B-lineage ALLs.
Introduction
Transplantation of T-cell–depleted peripheral stem cells from unrelated and mismatched related donors has become an option for the treatment of children with high-risk acute lymphoblastic leukemias (ALLs).1-4 Graft-versus-host disease (GvHD) has been reduced significantly by positive selection of CD34+ progenitors5,6 and other T-cell–depletion techniques, but relapses still remain a major problem.7 Recovery of T cells has been reported to be delayed because of graft manipulation.8 This was also observed in our patients who received transplants with CD34+-selected stem cells.2,5 Therefore, we investigated the feasibility of recruiting the more rapidly regenerating donor-derived natural killer (NK) cells for antileukemic effects.
Natural killer cells are capable of lysing several types of malignant cells, including lymphomas and leukemias, but high expression of HLA class I antigens on some B-lineage ALLs may impair NK cell lysis.9-11 Moreover, NK cell alloreactivity because of killer cell immunoglobulin-like receptor (KIR) ligand incompatibility has been reported in acute myeloid leukemia (AML) but not in ALL.11,12 However, both high HLA class I expression and resistance of ALL cells to alloreactive NK lysis may be overcome by deploying antibody-dependent cellular cytotoxicity (ADCC).9,13
ADCC is generally accepted to be an important mechanism for tumor cell elimination by therapeutic antibodies.14,15 However, to our knowledge, no systematic trials addressing the usefulness of antibody therapy for the elimination of minimal residual disease (MRD) after allogeneic stem cell transplantation have been reported. Consequently, we investigated whether NK cells of patients who received transplants were capable of exerting ADCC against B-lineage ALL blasts in combination with a chimeric CD19 antibody produced in insect cells.
Study design
Patients
ADCC was carried out with immunomagnetically selected NK cells (CD56+ microbeads; Miltenyi-Biotec, Gladbach, Germany) from 7 healthy donors and with mononuclear cells (MNCs) from 16 pediatric patients with ALL, AML/MDS (myelodysplastic syndrome) and others after transplantation. The patients received positive-selected CD34+ peripheral stem cells (CLINIMACS; MiltenyiBiotec) from unrelated or mismatched related donors with a median number of only 7000 residual T cells/kg body weight. No posttransplantation immunosuppression was administered to patients with completely depleted grafts. Six patients received T-cell add-backs and a short course of cyclosporin A. This study was approved by the ethical committee of the University of Tuebingen. Informed consent was provided according to the Declaration of Helsinki.
Generation of the CD19-specific chimeric (immunoglobulin G1 [IgG1] kappa) antibody CD19-4G7chim
Variable heavy- and variable light-chain sequences were amplified by polymerase chain reaction (PCR) from the DNA of CD19-reactive scFvs previously generated from the hybridoma 4G716 by the phage display technique using standard procedures.17,18 Cleavage sites for restriction enzymes were introduced and subsequently used for the insertion of these fragments into the baculoviral expression vector pAc-K-CH3.19 Sf21 insect cells were cotransfected with the baculoviral expression construct and Sapphire Baculovirus DNA (Orbigen, San Diego, CA). Purification of the secreted recombinant protein from culture supernatants was performed by protein A agarose affinity chromatography.
Cytotoxicity assay
Effector cells were stored overnight, either without or with interleukin-2 (IL-2) at 40 or 1000 international units (IU)/mL. The MHH4 cell line (DSMZ, Braunschweig, Germany) and cryopreserved primary B-lineage ALL blasts from 4 patients were used as target cells in a 2-hour BATDA europium release assay (Wallac, Turku, Finland). The blasts expressed CD10, CD19, and CD34 (> 90%), but all were CD20–. MHH4 cells expressed both CD19 and CD20 (> 95%). Cytolytic assays were performed as published9 by using antibody concentrations of 0.15 to 0.4 μg/mL (CD19-4G7chim or chimeric CD20 IgG1 antibody; Mabthera; Roche, Basel, Switzerland). For blocking experiments the murine IgG2a antibody W6/32 (Dako, Hamburg, Germany), specific for human HLA class I, or W6/32 Fab fragments were used.20,21 Only those assays were evaluated for which the spontaneous release was less than 25% (MHH4) or less than 30% (blasts).
Statistical analysis
Differences in specific lysis of targets were analyzed by the paired Wilcoxon rank sum test.
Results and discussion
Childhood B-lineage ALLs consistently express CD19.22 In our experience, only 12% of pro-B, pre-B, and common ALLs expressed CD20, but all leukemic cells were uniformly positive for CD19. Therefore, a high-affinity chimeric CD19 antibody (CD19-4G7chim; KD, 5.5 ± 1.7 × 10–9 M) was constructed and expressed in Sf21 insect cells.
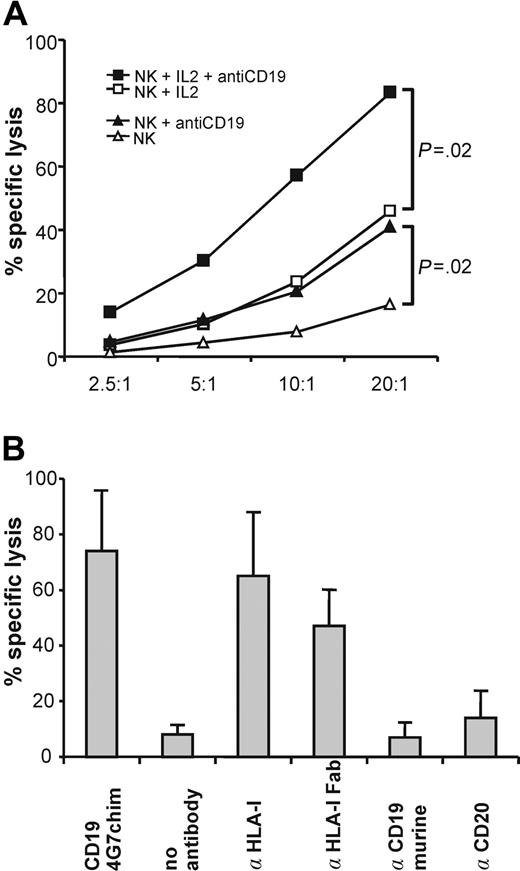
CD19-4G7chim mediated specific lysis of cryopreserved primary pre-B/common ALL blasts with enriched NK cells from 7 healthy donors (Figure 1A). NK cells from each donor showed significantly enhanced lysis after addition of CD19-4G7chim. Preincubation of the effector cells with IL-2 at 40 IU/mL increased specific lysis to its maximum value. Thus, both these IL-2 and antibody concentrations fall into a range, which may be reached in clinical applications.23 As expected, the murine 4G7 antibody did not induce ADCC. Specificity of CD19-4G7chim was shown by addition of the chimeric CD20 IgG1 antibody, which failed to raise specific lysis. Blocking of HLA class I antigens on target cells relevant for NK cell inhibitory receptors20,21 with complete W6/32 antibody or Fab fragments increased specific lysis comparable to CD19-4G7chim (Figure 1B). This suggests that masking of HLA class I on ALL blasts may abolish possible HLA class I–mediated inhibition of NK cell lysis. However, these inhibitory effects did not prevent ADCC by CD19-4G7chim.
ADCC with enriched NK cells from healthy donors. (A) NK cells efficiently lyse cryopreserved primary B-lineage ALL blasts in the presence of CD19-4G7chim. Mean specific lysis induced by CD19-4G7chim, averaged over 10 experiments with enriched NK cells (> 90% CD16+CD56+) from 7 healthy donors and cryopreserved primary CD19+CD20– preB/cALL blasts from 3 different patients at several effector-target (E/T) ratios. Open symbols indicate the absence of antibody; closed symbols, with CD19-4G7chim; triangles, NK cells not stimulated with IL-2; squares, NK cells stimulated with IL-2 at 40 IU/mL. Although the donor-target pairs each produced different levels of spontaneous lysis, NK cells from each donor showed significantly enhanced lysis after addition of CD19-4G7chim (P = .02, paired Wilcoxon rank sum test). (B) ADCC with CD19-4G7chim or blocking of HLA class I result in similar lysis of B-lineage ALL blasts. In 3 additional experiments, blocking of HLA class I by incubation with the murine IgG2a antibody W6/32 (α HLA-I, which binds to conformation-dependent epitopes, relevant for NK cell inhibitory receptors) resulted in strongly increased lysis. Blocking with W6/32 Fab fragments, used to exclude induction of ADCC by W6/32 itself, resulted in comparable lysis. These results suggest that interactions between HLA class I on cALL blasts and NK cells inhibit lysis. However, enhanced lysis of target cells is possible by ADCC with CD19-4G7chim. Underlying mechanisms remain to be investigated. A chimeric CD20 antibody of the same isotype as CD19-4G7chim (IgG1) did not increase specific lysis. Thus, ADCC mediated by CD19-4G7chim was specific. Furthermore, the parental murine 4G7 antibody did not induce enhanced lysis, demonstrating that a human Fc portion was needed for ADCC. Error bars represent SDs of all experiments.
ADCC with enriched NK cells from healthy donors. (A) NK cells efficiently lyse cryopreserved primary B-lineage ALL blasts in the presence of CD19-4G7chim. Mean specific lysis induced by CD19-4G7chim, averaged over 10 experiments with enriched NK cells (> 90% CD16+CD56+) from 7 healthy donors and cryopreserved primary CD19+CD20– preB/cALL blasts from 3 different patients at several effector-target (E/T) ratios. Open symbols indicate the absence of antibody; closed symbols, with CD19-4G7chim; triangles, NK cells not stimulated with IL-2; squares, NK cells stimulated with IL-2 at 40 IU/mL. Although the donor-target pairs each produced different levels of spontaneous lysis, NK cells from each donor showed significantly enhanced lysis after addition of CD19-4G7chim (P = .02, paired Wilcoxon rank sum test). (B) ADCC with CD19-4G7chim or blocking of HLA class I result in similar lysis of B-lineage ALL blasts. In 3 additional experiments, blocking of HLA class I by incubation with the murine IgG2a antibody W6/32 (α HLA-I, which binds to conformation-dependent epitopes, relevant for NK cell inhibitory receptors) resulted in strongly increased lysis. Blocking with W6/32 Fab fragments, used to exclude induction of ADCC by W6/32 itself, resulted in comparable lysis. These results suggest that interactions between HLA class I on cALL blasts and NK cells inhibit lysis. However, enhanced lysis of target cells is possible by ADCC with CD19-4G7chim. Underlying mechanisms remain to be investigated. A chimeric CD20 antibody of the same isotype as CD19-4G7chim (IgG1) did not increase specific lysis. Thus, ADCC mediated by CD19-4G7chim was specific. Furthermore, the parental murine 4G7 antibody did not induce enhanced lysis, demonstrating that a human Fc portion was needed for ADCC. Error bars represent SDs of all experiments.
ADCC by CD19-4G7chim produced in insect cells was more effective compared with ADCC by the same antibody produced in mammalian cells (K.B., unpublished data, April 2003). This may be due to different glycosylation, which has been shown to be critical for antibody-mediated effector functions. In particular, fucosylation of the Asn297-linked IgG1 carbohydrate, which plays a critical role in enhancement of ADCC, may have contributed to these results.24,25
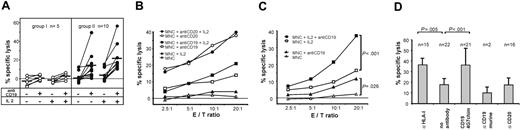
Next, we investigated whether patient-derived effector cells were also capable of exerting ADCC. MNCs from the majority of patients (66%) produced substantial lysis of MHH4 cells in combination with CD19-4G7chim. Lysis was enhanced by incubation of effector cells with IL-2 (Figure 2A, right panel). Mabthera was more effective than CD19-4G7chim against CD19+CD20+ targets (Figure 2B). However, for the treatment of pediatric B-lineage leukemias, often lacking CD20 expression, a CD19-directed antibody therapy may be more appropriate. Effector cells from the other one third of patients produced specific lysis below 10%, although the percentage of NK cells among MNCs was not significantly different for both groups (Figure 2A, left panel). This may be attributable to a polymorphism of FcγRIIIa, which has been reported to influence therapeutic response to rituximab.26 Therefore, genotype determinations of this receptor on effector cells should be included in future experiments.
ADCC with donor-derived MNCs, obtained from patients after transplantation. (A) ADCC against MHH4 target cells mediated by CD19-4G7chim. Effector cells from the majority of patients produced enhanced specific lysis of MHH4 target cells, whereas a minor fraction did not. For greater clarity, the patients (n = 15) have been separated into 2 groups: patients with effector cells producing more than 10% (n = 10, group II) or less than 10% (n = 5, group I) specific lysis with CD19-4G7chim and IL-2 stimulation (E/T ratio = 20:1). The mechanism underlying this differential response is still unknown. In group II, both unstimulated and IL-2–stimulated effector cells elicited significantly enhanced lysis after addition of CD19-4G7chim (P < .01 for E/T ratio = 20:1 in both cases, paired Wilcoxon rank sum test). Each patient is represented by connected data points (without or with antibody). Median values are shown as black bars. Effector cells from patients in group I produced no substantial ADCC lysis (< 10%). Specific lysis was measured at varying E/T ratios. Only values obtained at E/T = 20:1 are shown. (B) ADCC against MHH4: comparison of CD19-4G7chim and anti-CD20–mediated effects. Specific lysis by effector cells from patients in group II (A) at various E/T ratios. Data points are mean values of specific lysis averaged over experiments with MNCs from 5 (Mabthera) or all 10 patients (CD19-4G7chim). Antibodies were used in equivalent concentrations. (C) ADCC against cryopreserved primary B-lineage ALL blasts with CD19-4G7chim. ADCC with MNCs from 7 patients against allogeneic (CD19+CD20–) cALL blasts (obtained from 2 other patients) and with MNCs from 1 patient against autologous cALL blasts. To increase reliability of the results, 2 to 4 independent experiments were performed with effector cells from each patient. Mean values of 22 experiments are shown. MNCs of each patient produced significantly enhanced lysis after addition of CD19-4G7chim (P = .026 unstimulated, P < .001 stimulated; paired Wilcoxon rank sum test). (D) Specificity controls and blocking of HLA class I on primary B-lineage ALL blasts. Data points are mean values obtained with IL-2–stimulated MNCs at E/T = 20:1, and bars 2 and 3 represent the same data given in panel C for E/T = 20:1. n = number of experiments used to calculate mean values. Blocking of HLA class I on leukemic blasts with antibody W6/32 (α HLA-I) increased lysis to values reached with CD19-4G7chim, suggesting that CD19-4G7chim mediated ADCC despite the possible presence of HLA class I–mediated inhibitory signals. Differences between MNC and (MNC + anti-CD19) or (MNC + anti–HLA I) were highly significant with P < .001 and P < .005, respectively (paired Wilcoxon rank sum test). As expected, neither the parental murine 4G7 antibody nor the chimeric anti-CD20 IgG1, used as specificity control, induced enhanced target cell lysis.
ADCC with donor-derived MNCs, obtained from patients after transplantation. (A) ADCC against MHH4 target cells mediated by CD19-4G7chim. Effector cells from the majority of patients produced enhanced specific lysis of MHH4 target cells, whereas a minor fraction did not. For greater clarity, the patients (n = 15) have been separated into 2 groups: patients with effector cells producing more than 10% (n = 10, group II) or less than 10% (n = 5, group I) specific lysis with CD19-4G7chim and IL-2 stimulation (E/T ratio = 20:1). The mechanism underlying this differential response is still unknown. In group II, both unstimulated and IL-2–stimulated effector cells elicited significantly enhanced lysis after addition of CD19-4G7chim (P < .01 for E/T ratio = 20:1 in both cases, paired Wilcoxon rank sum test). Each patient is represented by connected data points (without or with antibody). Median values are shown as black bars. Effector cells from patients in group I produced no substantial ADCC lysis (< 10%). Specific lysis was measured at varying E/T ratios. Only values obtained at E/T = 20:1 are shown. (B) ADCC against MHH4: comparison of CD19-4G7chim and anti-CD20–mediated effects. Specific lysis by effector cells from patients in group II (A) at various E/T ratios. Data points are mean values of specific lysis averaged over experiments with MNCs from 5 (Mabthera) or all 10 patients (CD19-4G7chim). Antibodies were used in equivalent concentrations. (C) ADCC against cryopreserved primary B-lineage ALL blasts with CD19-4G7chim. ADCC with MNCs from 7 patients against allogeneic (CD19+CD20–) cALL blasts (obtained from 2 other patients) and with MNCs from 1 patient against autologous cALL blasts. To increase reliability of the results, 2 to 4 independent experiments were performed with effector cells from each patient. Mean values of 22 experiments are shown. MNCs of each patient produced significantly enhanced lysis after addition of CD19-4G7chim (P = .026 unstimulated, P < .001 stimulated; paired Wilcoxon rank sum test). (D) Specificity controls and blocking of HLA class I on primary B-lineage ALL blasts. Data points are mean values obtained with IL-2–stimulated MNCs at E/T = 20:1, and bars 2 and 3 represent the same data given in panel C for E/T = 20:1. n = number of experiments used to calculate mean values. Blocking of HLA class I on leukemic blasts with antibody W6/32 (α HLA-I) increased lysis to values reached with CD19-4G7chim, suggesting that CD19-4G7chim mediated ADCC despite the possible presence of HLA class I–mediated inhibitory signals. Differences between MNC and (MNC + anti-CD19) or (MNC + anti–HLA I) were highly significant with P < .001 and P < .005, respectively (paired Wilcoxon rank sum test). As expected, neither the parental murine 4G7 antibody nor the chimeric anti-CD20 IgG1, used as specificity control, induced enhanced target cell lysis.
In contrast to MHH4 cells, cryopreserved primary B-lineage blasts were more susceptible to NK lysis, although leukemic blasts from a patient with refractory disease were predominantly used as targets. In repeated experiments with effector cells from 7 patients against these allogeneic blasts and from 1 patient for whom autologous blasts were available, a consistent pattern emerged: specific lysis by unstimulated MNCs was less extensive than specific lysis by unstimulated MNCs plus CD19-4G7chim. IL-2 stimulation of effector cells increased specific lysis. Combination of antibody and IL-2 stimulation significantly enhanced specific lysis to a maximum (Figure 2C). As expected, both the parental murine 4G7 antibody and Mabthera, used as controls, failed to raise specific lysis, consistent with the fact that these target cells were CD20–. Blocking of HLA class I with W6/32 antibody raised specific lysis to the same extent as treatment with CD19-4G7chim (Figure 2D), suggesting that NK cells were the major effectors and that CD19-4G7chim mediated ADCC also in the posttransplantation setting, despite possible HLA class I–induced inhibition of effector cells. ALL patients in particular may benefit from such antibody-augmented GVL effects, because NK cell alloreactivity alone was shown to have no effect on adult ALL even in mismatched transplantations.11,12
Additional advantages of the use of the antibody in a posttransplantation situation include the following. First, the leukemia cell load is reduced to a minimum. Thus, an early-acting immunotherapy based on the recruitment of rapidly regenerating donor-derived NK cells can be focused on MRD, for which high effector–target cell ratios can be expected. Second, although CD19 antibodies may also lead to a deficiency of donor-derived nonmalignant B cells, additional infusions of donor stem cells after immunotherapy are expected to compensate for this lack.
In summary, our data encourage clinical trials with chimeric CD19 antibodies for patients with B-lineage ALL in an attempt to control MRD after transplantation.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-05-1735.
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (SFB 510 and Fe 140/5-2) (P.L. and G.H.F.), the Reinhold Beitlich Stiftung (P.L.), the Wilhelm Sander Stiftung (grants no. 2001.048.1 and 1996.047.3) (S.J.Z. and G.H.F.) and by a stipend through the Research Training Grant GK 592 from the DFG (K.B.).
P.L. and K.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs M. Liang and S. Dübel for the baculoviral expression vector pAc-K-CH3, Dr R. Levy for the 4G7 hybridoma, Dr G. Jung for providing monoclonal antibodies, and D. Saul and A. Babarin-Dorner for excellent technical assistance. Constructive criticism of the manuscript by Drs T. Allen, G. Jung and B. Emmerich is gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal