Abstract
Hematopoietic cytokines such as filgrastim are used extensively to stimulate granulocyte production or to mobilize hematopoietic progenitors into the circulation; however, their effect on more primitive hematopoietic progenitor and stem cells in vivo is unknown, particularly in large animals or humans. In particular, there is concern that chronic therapy with cytokines could result in stem cell exhaustion or clonal dominance; however, direct assessment of the dynamics of individual stem and progenitor cell clones in vivo has not been previously reported. A number of models can be proposed regarding the mechanisms by which the marrow responds to cytokine stimulation, including recruitment of previously quiescent clones, stimulation of proliferation of already active clones, or prevention of apoptosis of more mature progenitors from all clones. Using retroviral marking and comprehensive insertion site tracking of individual stem and progenitor cell clones in 2 rhesus macaques, we analyzed the effect of chronic administration of granulocyte colony-stimulating factor (G-CSF), or a combination of G-CSF plus stem cell factor (SCF). The overall number of contributing clones remained constant, and the relative output from each clone did not change significantly during or following cytokine treatments. These results suggest that individual transduced stem or progenitor cells can contribute to hematopoiesis for prolonged periods, with no evidence for an effect of G-CSF or G-CSF/SCF on the number, the lifespan, or the relative activity of individual stem or progenitor cell clones. These relevant large animal studies are reassuring regarding clinical applications of cytokines and provide new insights into their mechanisms of action.
Introduction
Patients in a number of situations receive chronic or repeated cycles of granulocyte colony-stimulating factor (G-CSF) or other hematopoietic cytokines, including patients with congenital neutropenias, aplastic anemia, or myelodysplasia, and following multiple cycles of cancer chemotherapy. It has been debated whether long-term G-CSF or other cytokine administration can predispose patients to stem or progenitor cell depletion, clonal outgrowth of abnormal precursors, and/or an increased risk of myelodysplasia, leukemia, or marrow failure Increasing use of G-CSF to mobilize peripheral blood stem cells or granulocytes from volunteers has intensified the discussion about long-term risks.1,2 Only limited follow-up information from healthy donors treated with G-CSF are available.3,4
G-CSF is a growth factor that promotes the production and maturation of granulocytes and, in particular, the proliferation and differentiation of granulocyte progenitors.5,6 This agent is one of the most widely used hematopoietic growth factors in clinical practice today. G-CSF can reduce the duration of neutropenia after chemotherapy and allogeneic or autologous transplantation and is also approved for use in patients with severe chronic neutropenia to prevent infectious complications.7-9 Patients in a number of different clinical situations may receive chronic or repeated courses of G-CSF therapy, including patients with congenital neutropenias, aplastic anemia, or myelodysplasia, and following multiple cycles of cancer chemotherapy. Severe chronic neutropenia (SCN) and recurrent serious infections are features of a heterogeneous group of disorders of myelopoiesis, including congenital neutropenia, cyclic neutropenia, and idiopathic neutropenia.10 Almost all patients respond to G-CSF with increased neutrophils, reduced infections, and improved survival.11
However, with longer follow-up it has been found that a significant number of patients with congenital neutropenia treated with chronic G-CSF treatment have developed myelodysplastic syndromes and acute myeloblastic leukemia (MDS/AML), associated with mutations in the G-CSF receptor.12,13 A correlation between duration of therapy with G-CSF and progression of aplastic anemia to MDS has been noted in Japanese children.14 It is unclear whether MDS or AML will occur with increased frequency in other patient populations that receive G-CSF on a chronic basis, because the long-term effects of G-CSF on hematopoiesis are not known. In the previous database study, no statistically significant relationships were found between age at onset of MDS or AML and patient sex, G-CSF dose, or duration of G-CSF therapy.13 However, multistep acquisition of aberrant cellular genetic change in marrow cells from patients who transformed, including activating ras oncogene mutations, clonal cytogenetic abnormalities,15 and G-CSF receptor mutations,16,17 was observed.
It has also been debated whether long-term G-CSF treatment can result in stem cell depletion. Overexpression of G-CSF in transgenic mice results in osteoporosis, and given recent information indicating the crucial role of osteoblasts in the hematopoietic “stem cell niche” it is possible that chronic G-CSF therapy could also affect stem cell dynamics by way of changes in osteoblast activity.18,19 There is also a study reporting a high rate of osteoporosis in children with congenital neutropenia: many were on chronic G-CSF, but of the children studied before G-CSF was begun a significant number also had osteoporosis, indicating that the abnormality in bone turnover may be related to the underlying disease.20
Stem cell factor (SCF) is a growth factor that promotes the proliferation and differentiation of the most primitive hematopoietic progenitor cells into committed progenitor cells.21 The receptor for SCF, c-kit, is found on all cells able to reconstitute long-term hematopoiesis, at least in mice.22 Combined treatment with G-CSF and SCF results in expansion of total body hematopoietic stem cell (HSC) activity in the mouse at least 2-fold, and 2 weeks following discontinuation of the factors a 10-fold increase in HSC activity in mice was seen.23 This finding indicates that combination therapy could have more profound effects on HSC behavior in vivo than G-CSF alone; however there are little data in animals about the effect of extended therapy, and in humans, the combination has been given only for peripheral blood stem cell (PBSC) mobilization for 5 to 7 days.24 A cohort of patients with refractory aplastic anemia has been treated with chronic SCF, with or without additional G-CSF. Results have not yet been published, but sustained responses have occurred, as have progression to monosomy 7, MDS, and acute leukemia (N. Young, personal communication, March 9, 2004). However, patients with refractory aplastic anemia appear to have an inherent risk of progression to clonal hematopoiesis and leukemia; thus, the role of SCF or other cytokines is unclear.
An understanding of the effect of G-CSF or G-CSF in combination with other cytokines on the in vivo behavior of hematopoietic stem and progenitor cells is important both to better assess the long-term risks of cytokine administration and to understand and optimize the use of cytokines for standard and novel applications. G-CSF was initially developed for its ability to stimulate production of committed myeloid precursors and neutrophils. However, G-CSF also has numerous direct and indirect effects on more primitive hematopoietic cells, including increasing the number of repopulating HSCs in mice. Receptors for G-CSF and SCF have been detected on even the most primitive HSCs.25 It is unknown whether G-CSF or G-CSF in combination with SCF act in vivo to recruit previously quiescent cells or whether repeated stimulation of primitive cells results in exhaustion or altered output from HSCs.
Analysis of hematopoietic dynamics in vivo, particularly in large animals and humans, has been difficult because the clonal output of individual stem and progenitor cells could be assessed only indirectly by ways of X-chromosome inactivation analysis in cells from heterozygous females.26,27 Marking of hematopoietic stem and progenitor cells with retroviral vectors is a powerful method to identify and then track clonal output from individual precursor cells.28,29 Retrovirus marking is an ideal tool, because these vectors insert semi-randomly and permanently; thus, the vector-genomic DNA junction serves as a unique marker for the initially transduced stem or progenitor cell and its progeny.30 We have efficiently marked rhesus macaque long-term repopulating cells with retroviral vectors and tracked contributions of individual clones to hematopoiesis for the first time, using the linear amplification mediated polymerase chain reaction (LAM-PCR) technique.31,32 Previously, hematopoiesis from marked cells was shown by us to be highly polyclonal and stable, with individual clones contributing to the myeloid and lymphoid lineages for more than 4 years.32,33 We have now assessed the effect of chronic G-CSF or G-CSF/SCF on contributions from individual marked clones, asking whether the overall pattern of hematopoiesis changes and whether individual clones are either lost or newly recruited with prolonged cytokine administration.
Materials and methods
Rhesus macaque transplantation model
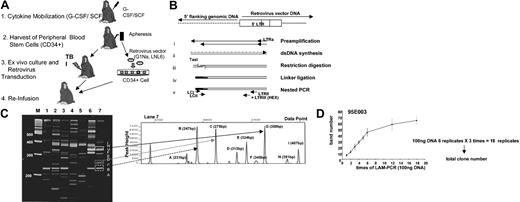
Young rhesus macaques (Macaca mulatta) were housed and handled in accordance with the guidelines set by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHHS publication no. NIH 85-23), and the protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. The collection of stem cell factor (SCF)/granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood cells, purification and retroviral transduction of CD34-enriched mobilized peripheral blood cells has been described in detail previously for the 2 animals included in this study.34,35 Both animals received cells transduced with the retroviral vectors LNL6 and G1Na, containing the neomycin resistance gene, for 4 days in the presence of interleukin-3 (IL-3), IL-6, SCF, Flt-3 ligand (Flt-3L) on fibronectin CH-296 fragment or autologous stroma and were reinfused after 1000 cGy of total body irradiation (Figure 1A).
Clonal tracking analysis. (A) Rhesus macaque model: autologous transplantation of retrovirally transduced CD34+ cells. Animals were given G-CSF + SCF for 5 days, and mobilized PBSCs were collected by leukapheresis. Purified CD34+ cells were transduced with either G1Na or LNL6 standard retroviral marking vectors as previously described.33,35 Transduced CD34+ cells were reinfused into the monkeys after 500 cGy × 2 total body irradiation. Animals were followed without intervention for at least 1 year before entry into the current cytokine treatment studies. (B) Schematic of the LAM-PCR methodology for identification of retroviral insertion sites. (i) Linear PCR by repeated primer extension from a biotinylated oligonucleotide and enrichment of the DNA product by capture with avidin-coated magnetic beads. (ii) Double-stranded DNA synthesis on the primer extension product by random hexanucleotide priming. (iii) DNA restriction digestion with TasI. (iv) Ligation of an asymmetric oligonucleotide ligation cassette (LC) to the overhanging sequence at the TasI digestion site. (v) Nested exponential PCR amplifications using primer pairs LCI-LTRII, LCII-LTRIII. (C) Final PCR amplification using a fluorescent primer allows separation and precise sizing of LAM-PCR products by way of comparison to size standards on an automated sequencer and analysis by using Gene Scan software. (D) Samples (100 ng) of a single DNA preparation from the granulocytes of an animal following transplantation with vector-transduced cells. The absolute number of independent clones detected by performance of each duplicate LAM-PCR procedure is shown. By the time 6 duplicates are run, the absolute clone number present is approached, and the mean clone number is calculated by counting the bands present in 6 duplicates on each sample run independently 3 separate times (for a total of 18 replicates on each sample). Error bars indicate the standard deviation for 3 independent experiments.
Clonal tracking analysis. (A) Rhesus macaque model: autologous transplantation of retrovirally transduced CD34+ cells. Animals were given G-CSF + SCF for 5 days, and mobilized PBSCs were collected by leukapheresis. Purified CD34+ cells were transduced with either G1Na or LNL6 standard retroviral marking vectors as previously described.33,35 Transduced CD34+ cells were reinfused into the monkeys after 500 cGy × 2 total body irradiation. Animals were followed without intervention for at least 1 year before entry into the current cytokine treatment studies. (B) Schematic of the LAM-PCR methodology for identification of retroviral insertion sites. (i) Linear PCR by repeated primer extension from a biotinylated oligonucleotide and enrichment of the DNA product by capture with avidin-coated magnetic beads. (ii) Double-stranded DNA synthesis on the primer extension product by random hexanucleotide priming. (iii) DNA restriction digestion with TasI. (iv) Ligation of an asymmetric oligonucleotide ligation cassette (LC) to the overhanging sequence at the TasI digestion site. (v) Nested exponential PCR amplifications using primer pairs LCI-LTRII, LCII-LTRIII. (C) Final PCR amplification using a fluorescent primer allows separation and precise sizing of LAM-PCR products by way of comparison to size standards on an automated sequencer and analysis by using Gene Scan software. (D) Samples (100 ng) of a single DNA preparation from the granulocytes of an animal following transplantation with vector-transduced cells. The absolute number of independent clones detected by performance of each duplicate LAM-PCR procedure is shown. By the time 6 duplicates are run, the absolute clone number present is approached, and the mean clone number is calculated by counting the bands present in 6 duplicates on each sample run independently 3 separate times (for a total of 18 replicates on each sample). Error bars indicate the standard deviation for 3 independent experiments.
Cytokine treatment
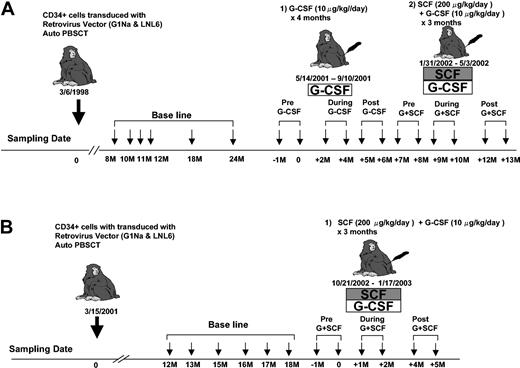
One to 2 years following transplantation with retrovirally transduced CD34+ cells, animal 95E003 was treated with recombinant human (rh) G-CSF 10 μg/kg/d (Amgen, Thousand Oaks, CA) subcutaneously 5 days per week, continuously for 15 weeks. Animals 95E003 and RQ2265 received rhG-CSF 10 μg/kg/d and rh stem cell factor (SCF) 200 μg/kg/d (Amgen) subcutaneously 5 days per week continuously for 3 months (Figure 2).
Experimental design. At least one year following transplantation of autologous retrovirally transduced CD34+ cells, each animal (95E003, panel A; and RQ2265, panel B) had baseline clonal analysis performed for 6 months prior to administration of cytokines. Granulocytes were collected at multiple time points before, during, and after cytokine treatments as shown and used for LAM-PCR analysis.
Experimental design. At least one year following transplantation of autologous retrovirally transduced CD34+ cells, each animal (95E003, panel A; and RQ2265, panel B) had baseline clonal analysis performed for 6 months prior to administration of cytokines. Granulocytes were collected at multiple time points before, during, and after cytokine treatments as shown and used for LAM-PCR analysis.
Linear amplification mediated (LAM)–PCR analysis
Density gradient purification, antibody staining, cell sorting, and DNA extraction were performed as previously described.36 Genomic DNA was extracted by using the QIAmp blood kit (Qiagen, Chatsworth, CA). The LAM-PCR procedure has been previously described in detail32 and is diagramed in Figure 1B. Samples (100 ng) were amplified, and products were separated on a Spreadex high-resolution gel (Elchrom Scientific, Cham, Switzerland) (Figure 1C). Specific DNA bands were excised, reamplified with primers LC III (5′-AGTGGCACAGCAGTTAGG-3′) and LTR IV (5′-CCTTGCAAAATGGCGTTACT-3′) for a total of 45 cycles. PCR products were then sequenced directly or after cloning into the TOPO TA cloning vector (Invitrogen, Carlsbad, CA).
Gene scan analysis
The LTRIII primer in standard LAM-PCR was replaced with a fluorescent primer labeled at the 5′-end with 5-carboxyfluoresein (fluorescein phosphoramidite [FAM] or hexachlorofluorescein [HEX]). PCR product (2 μL) was mixed with 12 μL formamide and 0.5 μL of an internal size standard (Genescan-1000; Applied Biosystems, Foster City, CA) to allow precise determination of the lengths of the amplified bands. After denaturation, the products were loaded on an automated sequencer capillary and analyzed by automatic fluorescence qualification, using the computer program GENESCAN 672 (ABI 373A; Applied Biosystems, Weiterstadt, Germany) (Figure 1C).
Tracking of individual clones
Three individual primer sets were designed to amplify DNA containing the junction between the proviral long terminal repeat (LTR) and specific genomic flanking DNA. DNA (100 ng) from each sample was used in a 35-cycle PCR reaction with an annealing temperature of 56° C and other conditions as described earlier. Four percent of this product was used as a template for the second nested PCR. Except for a 60° C annealing temperature, PCR conditions were identical to the first PCR. Insertion-specific primers as follows were used: 398-I (5′-GAGAACCACCGGTGTAGAATATAC-3′), 157-I (5′-GTGGCACAGCAGTTAGGATT-3′), or 349-I (5′-GCATTGCGTGTGCGTCCCTTC-3′) with LTR-IV (5′-CCTTGCAAAATGGCGTTACT-3′), followed by 398-II (5′-GGTGTTATGTAGACAGCCATAG-3′),157-II (5′-GATGCAGAAAACCGGTATGAT-3′), or 349-II (5′-CTCTGAGCACACCTGTGTGATG-3′) with the LTR-specific internal primer LTR V (5′-CAAACCTACAGGTGGGGTCT-3′) for the nested PCR. Final PCR products were separated on 2% agarose gels.
Statistical analysis
The distribution of clonal production at each visit was described by using means and histograms. To evaluate whether the clonal production was changed by intervention, Wilcoxon signed rank tests were used to compare production before versus during intervention, and during versus after intervention. Additionally, a Poisson model for the number of detections was assumed for each visit. This model allowed different means for intervention and nonintervention periods and was estimated by using generalized estimating equations to accommodate the correlation across visits in clonal production. All tests are 2-sided with a P value less than .05 denoting statistical significance. Rank correlations of the number of detections were calculated for select pairs of visits over all clones. Equality of rank correlations was tested by using the bootstrap.
Results
Study design
Animal 95E003 received transplants with CD34+ PBSCs 3 years prior to initiation of cytokine treatment35 (Figure 1A). The animal had very stable marking levels of approximately 5% in circulating granulocytes, marrow granulocyte-macrophage colony forming unit (CFU-GM), and lymphoid lineages.33,35 We sampled granulocyte DNA for analysis, because granulocytes have a brief life span of less than 3 to 4 days in the blood and rapid maturation from primitive precursor cells; thus, analysis of this compartment best reflects ongoing hematopoiesis, as compared with lymphocytes that may have life spans of months to years in the circulation or lymph nodes following full maturation. Granulocytes are also the lineage most profoundly stimulated by G-CSF or G-CSF in combination with SCF.5,6 Following collection of baseline samples for 6 months, G-CSF at a dose of 10 μg/kg/d 5 times a week was given continuously for 15 weeks (Figure 2A). This dose was chosen on the basis of prior studies in rhesus and baboons, indicating significant stimulation of neutrophil production and evidence for efficient mobilization of PBSCs in our own rhesus model. This dose is similar to or higher than doses given to human patients on chronic cytokine therapy for neutropenia and is also the dose given for 5 days to mobilize PBSCs efficiently.34
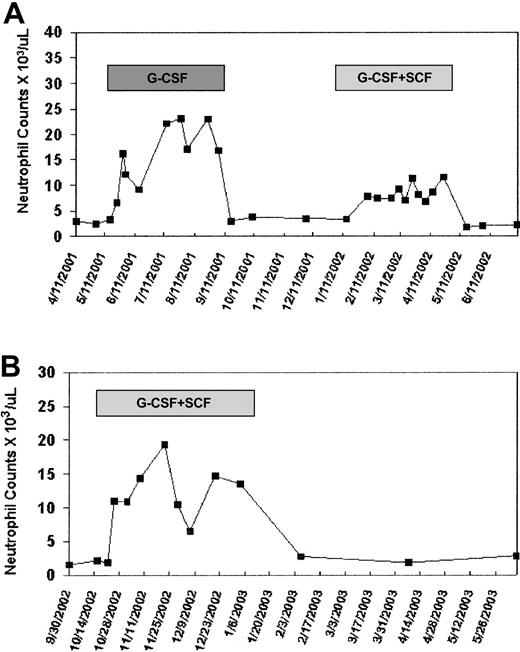
The peripheral blood absolute neutrophil count increased from a baseline of 2.9 ± 0.4 × 109/L (2900 ± 400 cells/μL) to a mean of 16.3 ± 6 × 109/L (16 300 ± 6000 cells/μL) on G-CSF therapy (Figure 3A). Lymphocyte counts did not change significantly from baseline during G-CSF treatment. Samples were collected for molecular analysis monthly. Following a 4-month period without cytokines and normalization of blood counts, the animal was treated with G-CSF (10 μg/kg/d) plus SCF (200 μg/kg/d) 3 times a week for an additional 3 months (Figure 2A). The combination of G-CSF and SCF results in very efficient PBSC mobilization short term and sustained stimulation of myelopoiesis long term without significant side effects.34,37 The peripheral blood absolute neutrophil count on G-CSF plus SCF increased to a mean of 8.6 ± 1.7 × 109/L (8600 ± 1700 cells/μL) during treatment, without change in the absolute lymphocyte count (Figure 3A). The second animal, RQ2265, received a transplant with transduced CD34+ PBSCs 18 months prior to initiation of cytokine treatment.34 The animal had stable marking levels of approximately 1%. Following collection of baseline samples for 6 months, G-CSF plus SCF was given 5 times a week for 3 months (Figure 2B). The peripheral blood neutrophil count in this animal increased from 1.9 ± 0.3 × 109/L (1900 ± 300 cells/μL) at baseline to a mean of 12.6 ± 3.8 × 109/L (12 600 ± 3800 cells/μL) during G-CSF plus SCF treatment (Figure 3B).
Neutrophil counts during study. (A) 93E003. (B) RQ2265. The y-axis shows the absolute neutrophil count per microliter before, during, and following cytokine therapy.
Neutrophil counts during study. (A) 93E003. (B) RQ2265. The y-axis shows the absolute neutrophil count per microliter before, during, and following cytokine therapy.
Analysis of the number of clones contributing to granulocytopoiesis
To accurately quantitate the number of stem and progenitor cell clones contributing to granulocyte production, LAM-PCR amplification of vector insertion sites was performed by using fluorescent primers for the final amplification, and products were run both on high-resolution gels and on an automated sequencer with an internal size standard (Figure 1B-C). The results were analyzed by using Gene Scan software, allowing precise sizing of amplification products. Criteria for identification of true peaks on Gene Scan analysis were set on the basis of reproducible visualization of the same LAM-PCR product bands on Spreadex gel electrophoresis, and confirmation that bands of an area under the curve of more than 10 000 represented true vector insertion sites as shown by sequencing of bands cut out from the gel.
Because the animals have polyclonal hematopoiesis with as many as 50 to 100 different clones contributing to an overall marking level of 5%, sampling of a single 100-ng sample of DNA will not detect all contributing clones, even assuming 100% efficiency of LAM-PCR amplification. Prior analysis has shown that 5 copies of a single vector insertion can be detected within a 100-ng sample containing nontransduced cells or cells with different vector insertions. However, 100 ng DNA corresponds to only 10 000 cells at most, and with a marking level of 5% and 100 clones contributing, 100 ng DNA contains close to the detectable limit of each individual clone, even if each clone contributes equally and amplification is maximally efficient. Increasing the amount of DNA per reaction to more than 100 ng results in interference; thus, replicates of 100-ng samples were tested. We found that by increasing the number of replicates, a more complete representation of all clones present could be obtained, with a maximum number of clones detected after approximately 18 replicates, in animals with marking levels of 0.5% to 10% (Figure 1D). The number of times an individual clone is detected per 18 replicates provides an estimate of the frequency of contribution of this clone to the cell population being studied, and the number of detections of each clone was used for statistical analysis of clonal contributions over time and in relation to cytokine therapy.
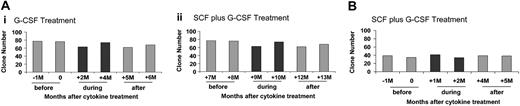
Figure 4 summarizes the total number of individual clones detected at time points before, during, and after G-CSF or G-CSF plus SCF therapy in each animal, analyzing 18 replicates from each time point and scoring a clone as present whether detected one time or more. The animals remained highly polyclonal during cytokine therapy, with no consistent change in the total clone number over time. There was no evidence for progression to monoclonality or oligoclonality. The overall marking level in each animal did not change, remaining 5% during and following first G-CSF and then G-CSF plus SCF therapy in 95E003 and 1% during G-CSF/SCF therapy in RQ2265, suggesting no preferential positive or negative activity of the cytokines on clones that had been successfully transduced.(data not shown) We also examined clone number in sorted B and T cells at a single time point before and then at a second time point at least 3 months after completion of cytokine therapy. With 6 replicates (as compared with 18 because of DNA limitations) the B-cell clone number in 95E003 was 31 both before and following G-CSF and then G-CSF/SCF. The T-cell clone number in 95E003 was 19 following G-CSF and G-CSF/SCF.
Summary of clone numbers. (A) 95E003. (B) RQ2265. Summary of clones contributing to granulocytes before, during, and after cytokine treatments. Eighteen replicates (6 replicates in 3 separate LAM-PCR reactions) were performed as described, and the number of unique clones was determined for each set of 18 replicates. For each animal, samples were obtained for at least 6 monthly time points prior to cytokine therapy, at 2 time points during cytokine therapy, and at least 2 monthly time points after cytokine therapy.
Summary of clone numbers. (A) 95E003. (B) RQ2265. Summary of clones contributing to granulocytes before, during, and after cytokine treatments. Eighteen replicates (6 replicates in 3 separate LAM-PCR reactions) were performed as described, and the number of unique clones was determined for each set of 18 replicates. For each animal, samples were obtained for at least 6 monthly time points prior to cytokine therapy, at 2 time points during cytokine therapy, and at least 2 monthly time points after cytokine therapy.
We confirmed the presence or absence of representative individual clones scored by using LAM-PCR and Gene Scan analysis by conventional nested PCR, using clone-specific primers designed from sequencing of the actual insertion site, together with an LTR primer for 3 individual clones (example shown in Figure 5). In all cases Gene Scan analysis and scoring of clones as being present or absent in a given DNA sample was validated by these clone-specific PCR reactions.
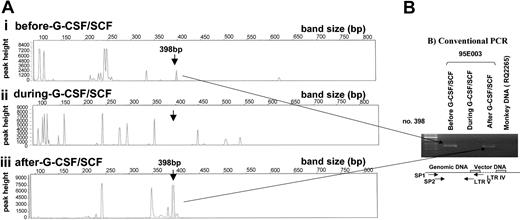
Tracking of individual clones by way of LAM-PCR and conventional PCR. (A) Gene Scan results of LAM-PCR. DNA (100 ng) isolated from 95E003 granulocytes before, during, and after G-CSF plus SCF treatment show the presence and the absence of clone no. 398. (B) Conventional PCR for clone no. 398 using clone-specific primers. DNA (100 ng) was used for nested PCR analysis using no. 398 specific primers (SP1, SP2) with an LTR primer. Note the presence of no. 398 clone before and after cytokine treatment. DNA from an animal (RQ2265) that received transduced cells with the same vector was used as a negative control.
Tracking of individual clones by way of LAM-PCR and conventional PCR. (A) Gene Scan results of LAM-PCR. DNA (100 ng) isolated from 95E003 granulocytes before, during, and after G-CSF plus SCF treatment show the presence and the absence of clone no. 398. (B) Conventional PCR for clone no. 398 using clone-specific primers. DNA (100 ng) was used for nested PCR analysis using no. 398 specific primers (SP1, SP2) with an LTR primer. Note the presence of no. 398 clone before and after cytokine treatment. DNA from an animal (RQ2265) that received transduced cells with the same vector was used as a negative control.
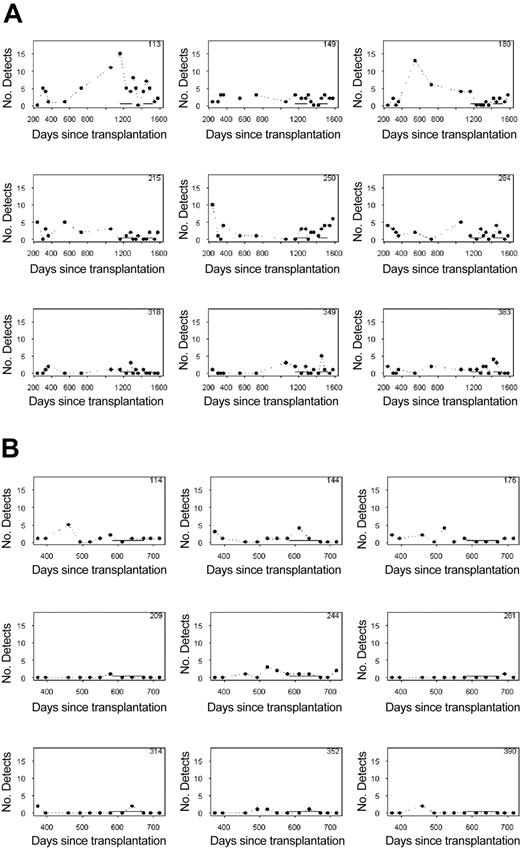
To assess the effect of cytokine therapy on the output from individual clones, histograms summarizing the number of detections (between 0 and 18) for each clone were constructed. Histograms for 9 randomly selected clones from each animal followed over the course of the experiment are shown in Figure 6. Solid lines near the x-axis denote the intervention periods, first with G-CSF, and then with G-CSF plus SCF in 95E003, and G-CSF plus SCF in animal RQ2265. At each time point the number of detections (of a maximum of 18 possible) for the clone is shown. Although stochastic variations in the frequency of detections for each clone occur, as predicted from prior models of large-animal hematopoiesis, there is no clear effect of cytokine administration on these patterns. To get a more global picture of the effect of cytokine intervention on clonal contributions, Figure 7 gives histograms summarizing the number of clones with various detection frequencies for the 2 monkeys at each time point before, during, and following treatment. These numbers would be expected to change with intervention if the cytokines either resulted in marked depletion or recruitment of individual clones. For both monkeys, the summary histograms appear similar before, during, and following cytokine therapy, with no effect of the cytokine interventions apparent in terms of the mean clonal contributions. For 95E003, the mean number of detections for all 90 total clones ranged from 1.3 to 3.0 over the 18 visits. Averaging these means by intervention periods reveals that the average means are 2.4, 2.1, and 2.3 during GCSF, SCF + G-CSF, and nonintervention periods, respectively. Neither intervention mean differs significantly from the nonintervention mean (P > .10). Using Wilcoxon signed rank tests, the mean number of detections was unchanged following the introduction of G-CSF (P > .10) and also following the cessation of G-CSF (P > .50). A similar result obtained for the G-CSF + SCF intervention, (P = .17 and .50, respectively). For RQ2265, the mean number of detections of the total of 88 clones at a visit ranged from 0.7 to 1.2 over the 12 visits. Averaging these means by intervention/nonintervention periods reveals that the average mean is 0.9 for the 2 SCF plus G-CSF samples and 1.0 for the 10 samples collected before or after treatment. These means did not differ significantly (P > .50). With the use of Wilcoxon signed rank tests, the mean number of detections did not change following the introduction of G-CSF plus SCF (P > .50). The same was true following the cessation of G-CSF plus SCF (P > .50).
Frequency of detections for randomly selected clones. (A) For 9 randomly selected representative clones from animal 95E003, the number of times the clone was detected of a total of 18 LAM-PCR reactions (6 replicates in 3 independent experiments) for each time point is shown. Each panel shows detections for an individual clone, and the clone designation (size of LAM-PCR product encompassing the shared LTR sequence and the genomic insertion through the next Tas1 site, in base pairs) is given in the right-hand upper corner of each panel. The periods of cytokine administration are indicated by the solid horizontal lines, first G-CSF alone, then G-CSF plus SCF. These typical patterns show the stochastic variation seen in the relative level of contributions from each clone and the lack of significant effect of either G-CSF alone or G-CSF plus SCF on these patterns. Clones such as 318 that contribute at a relatively low level (infrequent detections) continue in this pattern during and following cytokines, whereas clones with more frequent detections overall (clone 149) continue to contribute at this level during and following cytokine administration. (B) Frequency of detection of 9 randomly selected clones from animal RQ2265. Overall, the level of marking and frequency of detection of clones in this animal were lower, but the cytokines (G-CSF and SCF, administration shown as solid horizontal bar) again did not change the overall pattern of the frequency of clone detection.
Frequency of detections for randomly selected clones. (A) For 9 randomly selected representative clones from animal 95E003, the number of times the clone was detected of a total of 18 LAM-PCR reactions (6 replicates in 3 independent experiments) for each time point is shown. Each panel shows detections for an individual clone, and the clone designation (size of LAM-PCR product encompassing the shared LTR sequence and the genomic insertion through the next Tas1 site, in base pairs) is given in the right-hand upper corner of each panel. The periods of cytokine administration are indicated by the solid horizontal lines, first G-CSF alone, then G-CSF plus SCF. These typical patterns show the stochastic variation seen in the relative level of contributions from each clone and the lack of significant effect of either G-CSF alone or G-CSF plus SCF on these patterns. Clones such as 318 that contribute at a relatively low level (infrequent detections) continue in this pattern during and following cytokines, whereas clones with more frequent detections overall (clone 149) continue to contribute at this level during and following cytokine administration. (B) Frequency of detection of 9 randomly selected clones from animal RQ2265. Overall, the level of marking and frequency of detection of clones in this animal were lower, but the cytokines (G-CSF and SCF, administration shown as solid horizontal bar) again did not change the overall pattern of the frequency of clone detection.
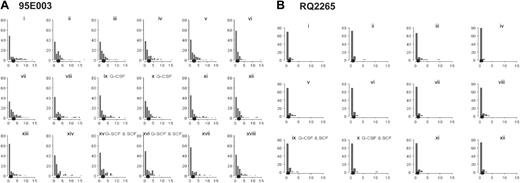
Histograms of the number of detections. (A) For each timed granulocyte DNA sample analyzed by LAM-PCR and Gene Scan from animal 95E003, a histogram is depicted showing the number of detections (of a maximum of 18 possible) on the x-axis and the number of clones with that frequency of detection on the y-axis, including all clones detected (n = 90) at any time during the pretreatment baseline samples (histograms i-viii), during G-CSF (histograms ix and x), following G-CSF (histograms xi-xiv), during G-CSF plus SCF (histograms xv and xvi), and following G-CSF plus SCF (histograms xvii and xviii). The mean number of detections per clone is shown as the solid dot. After 2 months of G-CSF, for instance, histogram x shows that 33 clones were detected zero times, 21 clones were detected 1 time, 15 clones were detected 2 times, 6 clones were detected 3 times, and so forth. (B) The same analysis for animal RQ2265, with histograms ix and x obtained on G-CSF and SCF.
Histograms of the number of detections. (A) For each timed granulocyte DNA sample analyzed by LAM-PCR and Gene Scan from animal 95E003, a histogram is depicted showing the number of detections (of a maximum of 18 possible) on the x-axis and the number of clones with that frequency of detection on the y-axis, including all clones detected (n = 90) at any time during the pretreatment baseline samples (histograms i-viii), during G-CSF (histograms ix and x), following G-CSF (histograms xi-xiv), during G-CSF plus SCF (histograms xv and xvi), and following G-CSF plus SCF (histograms xvii and xviii). The mean number of detections per clone is shown as the solid dot. After 2 months of G-CSF, for instance, histogram x shows that 33 clones were detected zero times, 21 clones were detected 1 time, 15 clones were detected 2 times, 6 clones were detected 3 times, and so forth. (B) The same analysis for animal RQ2265, with histograms ix and x obtained on G-CSF and SCF.
Another way of examining stability of clonal production over intervention periods is by the use of rank correlation between the numbers of detections for each clone at 2 time points. If clonal production were not stable, one might expect the rank correlation to change with the cytokine intervention. To examine this we calculated 4 correlations for pairs of samples visits: both samples before intervention, before intervention and during intervention, during intervention and after intervention, and both after intervention. The nonintervention pair of visits provides a control, and we test whether the nonintervention correlation differs from the intervention correlation (Table 1).
Rank correlation between pairs of visits for number of clonal detections
Monkey . | Intervention . | Corr pre/pre . | Corr pre/int . | P . | Corr int/post . | Corr post/post . | P . |
|---|---|---|---|---|---|---|---|
| 95E003 | GCSF | .46 | .39 | > .10 | .33 | .43 | > .10 |
| 95E003 | SCF + GCSF | .32 | .36 | > .10 | .29 | .35 | > .10 |
| RQ2265 | SCF + GCSF | .19 | .45 | .05 | .38 | .26 | > .10 |
Monkey . | Intervention . | Corr pre/pre . | Corr pre/int . | P . | Corr int/post . | Corr post/post . | P . |
|---|---|---|---|---|---|---|---|
| 95E003 | GCSF | .46 | .39 | > .10 | .33 | .43 | > .10 |
| 95E003 | SCF + GCSF | .32 | .36 | > .10 | .29 | .35 | > .10 |
| RQ2265 | SCF + GCSF | .19 | .45 | .05 | .38 | .26 | > .10 |
Corr indicates correlation; pre, before intervention; int, during intervention; post, after intervention.
For monkey 95E003 there was little effect of intervention on the correlation; for RQ2265, the correlation increases following the intervention but remained stable thereafter. Overall, the production from individual clones does not seem to be consistently affected by chronic cytokine administration. No more clones appeared or disappeared during or after cytokine administration than during a similar time period before treatment. Clonal “exhaustion” or preferential clonal stimulation was not evident.
Discussion
Understanding the in vivo composition and dynamics of the hematopoietic process is important for optimization of clinical applications, such as gene therapy, ex vivo expansion, or stem cell transplantation, and may provide insights into the pathogenesis of disorders such as myelodysplasia, paroxysmal nocturnal hemoglobinuria (PNH), and aplastic anemia. Retroviral marking allows tracking of the contributions to hematopoiesis from individual stem and progenitor cell clones, and we have recently reported the first comprehensive analysis of clonal contributions to hematopoiesis in a large animal model, using a newly described PCR technique. During hematopoietic reconstitution, we have shown that shortterm repopulating clones are responsible for hematopoiesis for several months following transplantation and are then replaced by highly polyclonal contributions from long-term repopulating cell clones that contribute stably when followed for at least 4 years.32 After complete steady-state characterization of hematopoiesis in these animals, we have now begun to investigate the effect of clinically relevant interventions such as cytokine therapy (in this report), chemotherapy, and radiation (K.K. et al, manuscript in preparation; and M. Laukkanen et al, manuscript in preparation). The recent report of the development of leukemia in 2 children with severe combined immunodeficiency treated soon after birth with CD34+ cells transduced with a corrective vector expressing the common gamma cytokine receptor has resulted in concern that retroviral integration can transform transduced stem or progenitor cells.38 However, in our very long-term analysis of 46 macaques that received transplants with CD34+ cells transduced with vectors containing marker genes, no animals have progressed to clonal dominance or oligoclonality and all remain hematologically healthy.39 Thus, we believe that at least with marking vectors, an individual insertional “hit” is unlikely to transform or change the behavior of the cell or its progeny and that tracking studies such as ours using the integration sites as tracking devices are valid.
In the present study, we found that there was no evidence for a significant effect of chronic G-CSF or G-CSF plus SCF treatment on clonal stem and progenitor cell dynamics. The 2 animals included in this study had been previously fully ablated with total body irradiation and then received transplants with ex vivo cultured and transduced CD34+ cell grafts. It is possible that the marrow microenvironment was damaged by the irradiation, and it is likely that total stem cell reserve was decreased by the transplantation procedure. Both animals had approximately 50% of the usual neutrophil response seen with G-CSF or G-CSF plus SCF therapy, indicating potentially less robust functional stem cell reserve; however, individual variability may also have affected the degree of leukocytosis. Following the initial 5 days of either G-CSF or G-CSF plus SCF treatment, both animals mobilized CD34+ cells into the peripheral blood, as expected. However, with continued therapy the concentration of CD34+ cells fell to baseline, even by the second week (data not shown). The events leading to mobilization are not yet fully understood but may be independent from the proliferative effects of the cytokines. Both animals had sustained leukocytosis with the chronic therapy; thus, there was no dimunition of the effects on proliferation or survival of myeloid cells and no evidence for development of anticytokine antibodies. The number of clones detected did not change significantly during G-CSF or G-CSF + SCF therapy, and there was no evidence for clonal exhaustion or recruitment as common events, as demonstrated by statistical analysis of the number of detections of individual clones in replicate analyses of individual samples. It is possible that the duration of cytokine therapy was too short to result in perturbations. But, if patients on chronic cytokine therapy have developed full-blown leukemia in 2 to 3 years or less, it might be expected that a pattern of clonal dominance, restriction of clonal diversity, or some other perturbation would begin within several months, because full manifestations of hematopoietic transformation is expected to require multiple steps, beginning with dominance of an individual clone.
In murine models, it has been estimated that HSCs cycle quite rapidly even in steady-state marrow, with a median time to cell division of only 6 days, and entry into cell cycle of more than 90% of HSCs by 1 month, as assessed by 5-bromodeoxyuridine (BrdUrd) uptake and detection in purified HSCs following BrdUrd feeding.40 Treatment with a combination of cyclophosphamide followed by G-CSF results in an increase in the percentage of HSCs in cycle and mobilization of primitive cells that have just transited cell cycle. However, one study in baboons found a much lower fraction of primate HSCs traversing the cell cycle during steady state, using similar BrdUrd methodology.41 With G-CSF treatment for 5 days, the fraction of phenotypically primitive cells sharply increased, suggesting that G-CSF does have significant effects on the proliferation of primitive hematopoietic precursors, at least short term in the context of mobilization regimens. There are much fewer data on the effect of chronic therapy on these parameters. The fact that cytokine therapy appeared to stimulate all clones more or less equally in our study may be based on the relatively rapid cycling induced by the cytokines, with all HSC clones coming into cycle soon after beginning cytokine treatment. Lack of clonal exhaustion despite increased cycling may be related to expression of telomerase at high levels in primitive hematopoietic cells, or little effect of chronic cytokine therapy on cycling of the most primitive stem cell compartment.42
On the basis of data from murine and feline models, HSCs dividing in the marrow must be eliminated in some manner by way of apoptosis or differentiation to maintain homeostasis, as the number of HSCs appears to be very tightly controlled.27,43,44 The egress of primitive cells from the marrow at a low level even during steady state may represent a death pathway, but cytokine therapy allows circulating primitive cells to retain viability and compete again for marrow niches.45
We have described a novel system to explore the clonal dynamics of in vivo hematopoiesis and analyzed the effect of chronic cytokine administration in nonhuman primates using the newly described LAM-PCR technique of retroviral insertion site analysis. Neither chronic G-CSF alone nor G-CSF + SCF therapies had significant effects on the number, relative output, or life span of individual transduced primitive stem or progenitor cell clones. This study also showed that individual transduced stem or progenitor cell clones originally derived from PBSC grafts can contribute to hematopoiesis for prolonged periods in the primate model, both in steady state and even following a proliferative stress such as prolonged cytokine treatment. This stability is reassuring in terms of predicting longevity of allogeneic PBSC grafts and prolonged effect of successfully transduced HSCs in gene therapy applications.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-08-2934.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Amgen for supplying rhuG-CSF and rhuSCF for these studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal