Abstract
Apoptosis is an essential process in embryonic tissue remodeling and adult tissue homeostasis. Within the adult hematopoietic system, it allows for tight regulation of hematopoietic cell subsets. Previously, it was shown that B-cell leukemia 2 (Bcl-2) overexpression in the adult increases the viability and activity of hematopoietic cells under normal and/or stressful conditions. However, a role for apoptosis in the embryonic hematopoietic system has not yet been established. Since the first hematopoietic stem cells (HSCs) are generated within the aortagonad-mesonephros (AGM; an actively remodeling tissue) region beginning at embryonic day 10.5, we examined this tissue for expression of apoptosis-related genes and ongoing apoptosis. Here, we show expression of several proapoptotic and antiapoptotic genes in the AGM. We also generated transgenic mice overexpressing Bcl-2 under the control of the transcriptional regulatory elements of the HSC marker stem cell antigen-1 (Sca-1), to test for the role of cell survival in the regulation of AGM HSCs. We provide evidence for increased numbers and viability of Sca-1+ cells in the AGM and subdissected midgestation aortas, the site where HSCs are localized. Most important, our in vivo transplantation data show that Bcl-2 overexpression increases AGM and fetal liver HSC activity, strongly suggesting that apoptosis plays a role in HSC development.
Introduction
The hematopoietic system of adult mammals has its foundation in rare hematopoietic stem cells (HSCs) harbored in the bone marrow (BM). These cells are highly potent, contributing to the billions of mature hematopoietic cells in the blood and hematopoietic tissues throughout the lifetime of the individual.1,2 In the mouse embryo, the first HSCs are autonomously generated in the aorta-gonadmesonephros (AGM)3 region. The onset of HSC activity occurs at embryonic day 10.5 (E10.5) in the AGM, and HSC activity increases from E11 until E14 in the liver.3-7 Thereafter, the number of HSCs generally remains constant throughout life.8,9
The signals and processes by which HSCs are generated, expanded, and/or maintained in embryonic, fetal, and adult stages are largely unknown. The fact that HSC numbers increase significantly in the midgestation mouse, while remaining constant in the adult, suggests that there are differences in the processes controlling HSC numbers at different times in ontogeny.3,5 Although temporally limited hematopoietic cell fate determination processes and HSC proliferation may be primarily responsible for increasing numbers of HSCs in the midgestation embryo, it is possible that the balance between programmed cell death and survival plays an additional role in the quantitative increase in HSCs.
Programmed cell death, or apoptosis, is a highly conserved process involved in adult tissue homeostasis (as reviewed Evan and Littlewood10 and Meier et al11 ) and, within embryos in the remodeling of the limbs, kidney, and other structures.11,12 Important regulators of apoptosis are the B-cell leukemia 2 (Bcl-2) family of proteins; the proapoptotic Bcl-2-associated X (Bax) and Bcl-2 homology 3 (BH3) subfamilies (which includes Bcl-2 interacting mediator of cell death [Bim]), and the antiapoptotic Bcl-2 subfamily (including Bcl-2 and Bcl-x), which promotes cell survival.13 It is thought that proapoptotic and antiapoptotic proteins counteract each other's function via protein-protein interactions (reviewed in Bouillet et al14 and Strasser et al15 ). Hence, appropriately controlled temporal and spatial expression of proapoptotic and antiapoptotic proteins is a prerequisite for normal tissue development.11,16-18
Within the adult hematopoietic system, Bcl-2 and Bcl-x are expressed in immature hematopoietic and lymphoid cells (especially mature thymocytes).19-23 Overexpression of Bcl-2 in T cells increases cell survival and decreases negative selection,24-26 suggesting a role for Bcl-2 in the thymocyte selection process. Apart from the lymphoid system, the role of apoptosis in the regulation of hematopoietic progenitor and stem cells is far less understood. A number of studies have investigated the role of apoptosis in adult BM HSCs using transgenic mouse models in which Bcl-2 overexpression is directed with the use of the hematopoietic specific vav promoter27 or by use of the more general/ubiquitous H-2K promoter.9,28 In both cases, overexpression of Bcl-2 within the HSC/hematopoietic progenitor compartment resulted in enhanced cell survival and radio-resistance of these cells.9,27,28 However, these studies were limited to the analysis of adult and fetal hematopoietic cells and did not study the effects of Bcl-2 overexpression in AGM HSCs.
Since extensive tissue remodeling is ongoing in the AGM region at the time when HSCs first emerge within the midgestation mouse embryo, we set out to specifically analyze apoptosis in AGM HSCs. We show here, within the midgestation AGM and more specifically the aorta, that both proapoptotic and antiapoptotic genes are normally expressed as the AGM is generating HSCs. To further investigate the role of apoptosis in HSC development, we specifically directed overexpression of Bcl-2 to HSCs in the AGM using the transcriptional control elements of the commonly used stem cell antigen-1 (Sca-1) HSC marker.29,30 We have previously demonstrated that these Sca-1 (Ly-6E/A) control elements faithfully express reporter genes in the first functional repopulating HSCs from the midgestation dorsal aorta (the specific AGM site of the first emerging HSCs).31-35 Here we show that Ly-6E/A-directed Bcl-2 overexpression results in increased numbers of Sca-1+ and c-kit+ cells in the AGM, in increased survival of AGM HSCs, and, most importantly, in increased AGM HSC activity. These data strongly suggest that apoptosis plays an important role in the regulation of HSCs beginning at the earliest stages of HSC development in the AGM.
Materials and methods
Generation of transgenic mice
An 865-bp murine Bcl-2 cDNA fragment36 was inserted in the Ly-6E expression cassette.31,37,38 The 14.9-kb NotI linearized fragment was microinjected into (CBA × C57BL/10)F1 oocytes. Out of 17 founder mice born, 6 transmitted the transgene, and 2 lines were used for further studies. Bcl-2 transgenic mice appeared normal, and the total body weight was unchanged from that of the wild-type littermates (Table 3). Matings were set up between Bcl-2 transgenic males and (CBA × C57BL/10)F1 females. The day of the vaginal plug was counted as day 0. Pregnant mice were killed by cervical dislocation, and embryos were collected in phosphate-buffered saline (PBS)/10% fetal calf serum (FCS).Animals were housed according to institutional guidelines (Experimental Dieren Centrum, Erasmus University Medical Center), and animal procedures carried out in compliance with the Standards for Humane Care and Use of Laboratory Animals.
Lymphoid tissue and cell subset analysis of nontransgenic and Bcl-2 transgenic adults
. | Nontransgenic . | Ln479 . | Nontransgenic . | Ln2 . |
|---|---|---|---|---|
| Body weight, g | 29.4 ± 5.7 | 29.4 ± 5.8 | 31.4 ± 4.0 | 32.0 ± 4.7 |
| Absolute cell numbers, × 107 | ||||
| Thymus | 7.7 ± 1.4 | 9.4 ± 1.1 | 10.8 ± 4.3 | 11.1 ± 3.6 |
| Spleen | 14.7 ± 1.6 | 31.9 ± 6.4 | 11.4 ± 1.1 | 37.3 ± 1.9 |
| Thymus cell subsets, % | ||||
| CD4-CD8- | 3.7 ± 1.0 | 4.6 ± 1.7 | 2.0, 3.8* | 5.1 ± 1.7 |
| CD4+CD8+ | 83.5 ± 3.6 | 75.6 ± 4.2 | 87.8, 83.3* | 70.9 ± 5.2 |
| CD4+CD8- | 9.9 ± 3.1 | 13.7 ± 2.0 | 11.9, 9.2* | 18.8 ± 2.4 |
| CD4-CD8+ | 3.0 ± 0.6 | 6.1 ± 0.7 | 1.0, 0.9* | 5.2 ± 1.4 |
| Spleen cell subsets, × 107 | ||||
| CD4+ | 3.1 ± 0.6 | 8.5 ± 1.1 | 2.8, 1.1* | 7.4 ± 1.9 |
| CD8+ | 1.6 ± 0.5 | 4.0 ± 0.3 | 1.0, 0.5* | 2.8 ± 1.6 |
| B220+ | 10.0 ± 0.7 | 21.4 ± 5.1 | 8.0, 2.9* | 18.6 ± 2.7 |
. | Nontransgenic . | Ln479 . | Nontransgenic . | Ln2 . |
|---|---|---|---|---|
| Body weight, g | 29.4 ± 5.7 | 29.4 ± 5.8 | 31.4 ± 4.0 | 32.0 ± 4.7 |
| Absolute cell numbers, × 107 | ||||
| Thymus | 7.7 ± 1.4 | 9.4 ± 1.1 | 10.8 ± 4.3 | 11.1 ± 3.6 |
| Spleen | 14.7 ± 1.6 | 31.9 ± 6.4 | 11.4 ± 1.1 | 37.3 ± 1.9 |
| Thymus cell subsets, % | ||||
| CD4-CD8- | 3.7 ± 1.0 | 4.6 ± 1.7 | 2.0, 3.8* | 5.1 ± 1.7 |
| CD4+CD8+ | 83.5 ± 3.6 | 75.6 ± 4.2 | 87.8, 83.3* | 70.9 ± 5.2 |
| CD4+CD8- | 9.9 ± 3.1 | 13.7 ± 2.0 | 11.9, 9.2* | 18.8 ± 2.4 |
| CD4-CD8+ | 3.0 ± 0.6 | 6.1 ± 0.7 | 1.0, 0.9* | 5.2 ± 1.4 |
| Spleen cell subsets, × 107 | ||||
| CD4+ | 3.1 ± 0.6 | 8.5 ± 1.1 | 2.8, 1.1* | 7.4 ± 1.9 |
| CD8+ | 1.6 ± 0.5 | 4.0 ± 0.3 | 1.0, 0.5* | 2.8 ± 1.6 |
| B220+ | 10.0 ± 0.7 | 21.4 ± 5.1 | 8.0, 2.9* | 18.6 ± 2.7 |
Tissues were isolated from mice ranging in age from 10 to 20 weeks. Both male and female mice are included in each of the groups. For the total thymus and spleen cell counts, sample numbers are as follows: Ln 479 mice, n = 6; their nontransgenic littermates, n = 6; Ln 2 mice, n = 11; their nontransgenic littermates, n = 7. For the spleen and thymus subset cell determinations, n = 3 except for nontransgenic Ln 2 control group. Values are given as mean ± SEM.
Data represent 2 values (n = 2)
Genotyping, copy number determination, and expression analysis
For Bcl-2 and YMT genotyping (myogenin [Myo] control), polymerase chain reaction (PCR) conditions were as follows: 100 to 200 ng DNA; 100 ng each primer (Table 1); 1 × buffer; 0.2 mM deoxynucleoside triphosphate (dNTP); and 1 U AmpliTaq (Applied Biosystems, Foster City, CA), with cycling for 5 minutes at 92°C, 30 × (40 seconds at 92°C, 40 seconds at 60°C, 1 minute at 72°C), and 7 minutes at 72°C.
Primer sequences
Primer . | Primer sequence, 5′ → 3′ . | Sequence or reference on which this was based . | PCR fragment length, bp . |
|---|---|---|---|
| BC2PE | GTGCAGCTGACTGGACATCTCTGC | Miles et al31 ; Negrini et al39 | 450 |
| PE5 | ACTCTGCCTGCAACCTTGTCTGAG | Miles et al31 ; Negrini et al39 | 450 |
| YMT2-1 | CTGGAGCTCTACAGTGATGA | Medvinsky and Dzierzak3 | 342 |
| YMT2-2 | CAGTTACCAATCAACACATCAC | Medvinsky and Dzierzak3 | 342 |
| Myo-1 | TTACGTCCATCGTGGACAGC | Müller et al4 | 245 |
| Myo-2 | TGGGCTGGGTGTTAGTCTTA | Müller et al4 | 245 |
| mBcl-2 forward | GCACAGATGTCCAGTCAGCTG | NM_009741 | 268 |
| mBcl-2 reverse | GCCATATAGTTCCACAAAGGC | NM_009741 | 268 |
| mBcl-x forward | GGCGATGAGTTTGAACTGCG | U51278 | 915 |
| mBcl-x reverse | CCTCACTCAATGGCTCTTGG | U51278 | 915 |
| mBim forward | GAGAAGGTGGACAATTGCAG | AF032461 | 290, 380 |
| mBim reverse | GCCTTCTCCATACCAGACGG | AF032461 | 290, 380 |
| beta-actin forward | CCTGAACCCTAAGGCCAACCG | X03672 | 398 |
| beta-actin reverse | GCTCATAGCTCTTCTCCAGGG | X03672 | 398 |
Primer . | Primer sequence, 5′ → 3′ . | Sequence or reference on which this was based . | PCR fragment length, bp . |
|---|---|---|---|
| BC2PE | GTGCAGCTGACTGGACATCTCTGC | Miles et al31 ; Negrini et al39 | 450 |
| PE5 | ACTCTGCCTGCAACCTTGTCTGAG | Miles et al31 ; Negrini et al39 | 450 |
| YMT2-1 | CTGGAGCTCTACAGTGATGA | Medvinsky and Dzierzak3 | 342 |
| YMT2-2 | CAGTTACCAATCAACACATCAC | Medvinsky and Dzierzak3 | 342 |
| Myo-1 | TTACGTCCATCGTGGACAGC | Müller et al4 | 245 |
| Myo-2 | TGGGCTGGGTGTTAGTCTTA | Müller et al4 | 245 |
| mBcl-2 forward | GCACAGATGTCCAGTCAGCTG | NM_009741 | 268 |
| mBcl-2 reverse | GCCATATAGTTCCACAAAGGC | NM_009741 | 268 |
| mBcl-x forward | GGCGATGAGTTTGAACTGCG | U51278 | 915 |
| mBcl-x reverse | CCTCACTCAATGGCTCTTGG | U51278 | 915 |
| mBim forward | GAGAAGGTGGACAATTGCAG | AF032461 | 290, 380 |
| mBim reverse | GCCTTCTCCATACCAGACGG | AF032461 | 290, 380 |
| beta-actin forward | CCTGAACCCTAAGGCCAACCG | X03672 | 398 |
| beta-actin reverse | GCTCATAGCTCTTCTCCAGGG | X03672 | 398 |
For copy number analysis, a Southern blot with 10 to 20 μg HindIII-digested genomic DNA was probed with a nick-translated (Amersham Pharmacia Biotech, Piscataway, NJ) 32P-labeled 865-bp Bcl-2 fragment. Analysis was performed by phosphorimaging (Molecular Dynamics, Piscataway, NJ) and ImageQuant software (Amersham Pharmacia Biotech).
For Northern blot analysis, 20 μg RNA isolated from several tissues with TRIZOL (Invitrogen/Life Technologies, Carlsbad, CA) was blotted against Genescreen membrane (NEN Life Science Products, Boston, MA). The blot was probed with a 32P-labeled 865-bp Bcl-2 cDNA fragment. Ly6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes are as described previously.31
For reverse-transcription PCR (RT-PCR) analysis, embryos were (sub) dissected and tissues stored in RNAlater (Ambion, Austin, TX) at 4°C. Embryos were genotyped, and tissues from similar genotypes were pooled and homogenized in TRIZOL. RNA was isolated according to manufacturers' instructions. We used 1 to 5 μg DNase-treated RNA (RQ1 RNase free DNase; Promega, Madison, WI) for cDNA synthesis with Superscript II Reverse Transcriptase (Invitrogen/Life Technologies). RT-PCR was performed in a 50-μL vol with 100 ng each primer (Table 1), 0.2 mM dNTP, AmpliTag, and buffer (Perkin Elmer, Shelton, CT), together with a 40 to 100 ng RNA equivalent of cDNA. Cycling conditions were as follows: 5 minutes at 92°C; 28 to 30 × (40 seconds at 92°C, 40 seconds at 58°C, 1 minute at 72°C); and 7 minutes at 72°C. PCR products were run on 1.2% agarose/1 × Tris-borate-EDTA (tris(hydroxymethyl)aminomethane-borate-ethylenediaminetetraacetic acid) (TBE) gels with ethidium bromide (EtBr) and scanned on a Typhoon scanner (Molecular Dynamics).
For Western blot analysis, protein extracts from several adult tissues were made in Laemmli buffer, separated on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel, and blotted to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). Blots were blocked and incubated with an anti-Bcl-2 antibody (Alexis Biochemicals, San Diego CA) in 2% milk/Tris-buffered saline-Tween (TBS-T) followed by an antirabbit-horseradish peroxidase (HRP) antibody (DAKO, Carpinteria, CA) and enhanced chemiluminescence (ECL) detection.
Survival and cell viability assays
Healthy transgenic and nontransgenic littermates mice of 7 to 13 (Ln 2) and 8 to 22 (Ln 479) weeks of age were randomly divided into 3 pools, which received equal split doses of 10.5, 9.5, or 8.5 Gy γ-irradiation. Mice received antibiotics and were checked daily for survival.
Whole BM cells from Bcl-2 transgenic and nontransgenic littermates were collected in PBS/10% FCS, suspended as single cells, and counted (Beckman Coulter, Fullerton, FL). BM cells were seeded at a cell density of 2 × 105/mL in Dulbecco Modified Eagle medium (DMEM)/10% FCS/ penicillin (Pen)/streptomycin (Strep) at 37°C, 5% CO2.At 1, 2, 4, 8, and11 days, viable cell counts were determined by trypan blue exclusion.
FACS analysis, immunostaining, and TUNEL staining
AGM and liver tissues were dissected, and explant cultures for 3 to 4 days were performed as described previously.3,5 During the culture period, embryos were genotyped, and tissues with similar genotypes pooled. Before staining, tissues were collagenase treated as described. BM cells were made directly into a single-cell suspension. Cell staining was done in PBS/10% FCS with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies against Sca-1, c-kit, CD4, CD8, CD11b (macrophage antigen-1 [Mac-1]), Ly-6G(Gr1), B220, and annexin V (Pharmingen, Franklin Lanes, NJ), and fluorescence was measured on a FACScan or FACSVantage (Becton Dickinson, Franklin Lanes, NJ). Dead cells were excluded by 7-amino-actinomycin D (7AAD) or Hoechst 33258 (Molecular Probes, Eugene, OR) staining.
For immunofluorescence, single-cell suspensions from AGM cells from Ly6A-green fluorescent protein (GFP) embryos were made. Cytospin cells were fixed (2% paraformaldehyde/PBS) and stained with Bcl-2 antibody and α-rabbit-Texas Red or cyanine 3.18 (Cy3) secondary antibody.
Embryos were frozen in Tissue-Tek (Sakura Finetek USA, Torrance, CA) and cryosectioned (7-8 μM). transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining was performed with the in situ cell death detection kit/peroxidase (POD) (Roche, Indianapolis, IN) on the basis of the manufacturer's instructions. Staining was visualized with peroxidase substrate AEC (3-amino-9-ethylcarbazde), and sections were dehydrated and embedded in Entallan (Merck, West Point, PA).
In vivo repopulation assays for HSCs
For all transplantations, male donor cells were injected intravenously into (CBA × C57BL/10)F1 females irradiated with a 9.5-Gy split dose of γ-irradiation. Repopulation was assayed at 1 and 4 months after transplantation by a Y-chromosome (YMT)-specific PCR on peripheral blood DNA. Multilineage repopulation was analyzed on several recipients either by PCR on DNA from hematopoietic tissues or as described previously.40
Donor male Bcl-2 transgenic and nontransgenic littermates (10 weeks old) were killed, and BM cells collected and injected into recipients at several cell doses in combination with 105 female competitor BM cells. For repopulation assays on E11 and E12 AGM and liver cells, tissues were dissected and collagenase treated (0.125%) in PBS/10% FCS. Then, 0.3 embryo equivalents (ee) AGM cells (approximately 2 × 105/ee for E11; approximately 3 × 105/ee for E12) or 0.03 to 0.003 ee liver cells (approximately 3 × 106/ee for E12) were injected with 2 × 105 female spleen cells into recipients. For secondary transplantations, BM was collected from reconstituted primary recipients, and 105 cells were injected into secondary recipients. Secondary recipients receiving BM from primary recipients injected with E12 AGM or liver were coinjected with 105 female BM cells.
Results
Expression of proapoptotic and antiapoptotic regulators in the midgestation aorta
To date, little is known concerning the expression of proapoptotic and antiapoptotic regulators in the midgestation AGM region and particularly HSCs. To examine whether apoptosis normally occurs in the AGM, TUNEL analysis was performed on transverse sections through the truncal regions of midgestation embryos. Several TUNEL+ foci are found scattered throughout the representative E11 truncal section shown in Figure 1A. In the AGM region, many highly positive foci are found in the mesonephic tubules of the urogenital ridges (UGRs), while only one strongly positive focus was found a short distance from the dorsal side of the dorsal aorta (Figure 1B). However, many diffuse individually weak TUNEL+ cells are also found surrounding the dorsal aorta, suggesting that apoptotic processes are occurring throughout the AGM region during midgestation.
Apoptotic foci and apoptosis-related gene expression in the E11 AGM. TUNEL staining was performed on transverse sections from the truncal region of an E11 embryo to localize apoptotic cells in the AGM region. (A-B) A representative section is shown at 10 × magnification (A) and at 40 × magnification (B). TUNEL+ (black/gray) foci and diffuse scattered individual cells are observed. DA indicates lumen of dorsal aorta; UGR, urogenital ridge; M, mesentery. TUNEL staining was performed 3 times on 2 wild-type and 2 Bcl-2 transgenic embryos. No obvious difference in the TUNEL staining pattern was observed when sections from Bcl-2 transgenic embryos were compared to sections from nontransgenic embryos. (C) Expression of the antiapoptotic genes Bcl-2 and Bcl-x and the proapoptotic Bim gene in the E11 AGM, aorta, UGRs, and liver region of a nontransgenic embryo. RT-PCR analysis was performed on cDNAs made from the indicated midgestation tissues. Signal intensity from the actin PCR fragment was used as a normalization control. Note that, as expected, the Bim primers amplify 2 different transcript isoforms (splice variants). (D) RT-PCR analysis of a Bcl-2 transgenic embryo reveals altered levels of Bcl-2, Bcl-x, and Bim gene expression in the E11 aorta and UGR. RT-PCR analysis was performed 3 times and confirmed on 3 independent sets of cDNAs.
Apoptotic foci and apoptosis-related gene expression in the E11 AGM. TUNEL staining was performed on transverse sections from the truncal region of an E11 embryo to localize apoptotic cells in the AGM region. (A-B) A representative section is shown at 10 × magnification (A) and at 40 × magnification (B). TUNEL+ (black/gray) foci and diffuse scattered individual cells are observed. DA indicates lumen of dorsal aorta; UGR, urogenital ridge; M, mesentery. TUNEL staining was performed 3 times on 2 wild-type and 2 Bcl-2 transgenic embryos. No obvious difference in the TUNEL staining pattern was observed when sections from Bcl-2 transgenic embryos were compared to sections from nontransgenic embryos. (C) Expression of the antiapoptotic genes Bcl-2 and Bcl-x and the proapoptotic Bim gene in the E11 AGM, aorta, UGRs, and liver region of a nontransgenic embryo. RT-PCR analysis was performed on cDNAs made from the indicated midgestation tissues. Signal intensity from the actin PCR fragment was used as a normalization control. Note that, as expected, the Bim primers amplify 2 different transcript isoforms (splice variants). (D) RT-PCR analysis of a Bcl-2 transgenic embryo reveals altered levels of Bcl-2, Bcl-x, and Bim gene expression in the E11 aorta and UGR. RT-PCR analysis was performed 3 times and confirmed on 3 independent sets of cDNAs.
The expression of antiapoptotic genes Bcl-2 and Bcl-x and the proapoptotic gene Bim in the whole AGM, aorta, UGRs, and liver was examined by semiquantitative RT-PCR. As shown in Figure 1C, Bcl-2 expression is approximately 2-fold higher in the E11 AGM as compared with the E11 liver. Similar results were obtained with E12 tissues (not shown). In the E11 AGM, Bcl-2 is expressed in both the aorta and the UGR, with a higher level of expression in the UGR. Bcl-x and Bim expression levels are comparable in the E11 AGM, and liver, with Bim expression appearing to be slightly higher in the UGR than in aorta. Thus, both antiapoptotic and proapoptotic genes are expressed in the embryonic sites (aorta and liver) harboring the earliest HSCs.
To determine if Bcl-2 is normally expressed in AGM HSCs, immunostaining was performed on cytospins of E12 Ly-6A (Sca-1) GFP transgenic AGM cells. Previous studies in such transgenic embryos31-35 have shown that all AGM HSCs are GFP+. When we performed Bcl-2-specific antibody staining on such cytospins, we observed Bcl-2 expression in many AGM cells (Figure 2C), among which are GFP+ cells (Figure 2B). AGM GFP+ cells include aortic HSCs and endothelial and mesonephric tubule cells.34 Overlay of the 2 fluorescent signals shows that the GFP+ cells coexpress the Bcl-2 protein (Figure 2D). To exclude the GFP+ mesonephric cells, cytospins from subdissected E11 aortas were immunostained with the Bcl-2 antibody. Figure 2F-H shows expression of Bcl-2 in all GFP+ cells, which include only HSCs and endothelial cells. Bcl-2 expression in such cells was confirmed by RT-PCR analysis on sorted c-kit+ AGM cells (c-kit is a marker for HSCs but not mesonephric cells)41 (not shown). Taken together, these data indicate that apoptosis is taking place within the AGM and that Bcl-2 is expressed in aortic and AGM HSCs and/or endothelial cells.
Expression of Bcl-2 by Sca-1+ AGM cells. Immunostaining was performed on Ly-6A (Sca-1) GFP transgenic E12 AGM and E11 aortic cells. AGM and aorta cells were isolated as a single-cell suspension and deposited on microscope slides by cytospin (original magnification, × 100). Panels A, E, and I show 4′,6-diamidino-2-phenylindole (DAPI) staining; panels B, F, and J show Ly-6A (Sca-1) GFP fluorescence; panel C shows anti-Bcl-2 Texas Red fluorescence; and panel G shows anti-Bcl-2 Cy3 fluorescence. Panel K was incubated with no primary antibody. Panels D, H, and L are an overlay of GFP and Texas Red/Cy3 fluorescence. Panels A-D are a cytospin of E12 AGM cells stained with anti-Bcl-2 antibody conjugated with Texas Red. Panels E-H show a cytospin of E11 aorta cells stained with anti-Bcl-2 antibody conjugated with Cy3. Panels I-L show a cytospin of E11 AGM cells incubated with no primary antibody.
Expression of Bcl-2 by Sca-1+ AGM cells. Immunostaining was performed on Ly-6A (Sca-1) GFP transgenic E12 AGM and E11 aortic cells. AGM and aorta cells were isolated as a single-cell suspension and deposited on microscope slides by cytospin (original magnification, × 100). Panels A, E, and I show 4′,6-diamidino-2-phenylindole (DAPI) staining; panels B, F, and J show Ly-6A (Sca-1) GFP fluorescence; panel C shows anti-Bcl-2 Texas Red fluorescence; and panel G shows anti-Bcl-2 Cy3 fluorescence. Panel K was incubated with no primary antibody. Panels D, H, and L are an overlay of GFP and Texas Red/Cy3 fluorescence. Panels A-D are a cytospin of E12 AGM cells stained with anti-Bcl-2 antibody conjugated with Texas Red. Panels E-H show a cytospin of E11 aorta cells stained with anti-Bcl-2 antibody conjugated with Cy3. Panels I-L show a cytospin of E11 AGM cells incubated with no primary antibody.
Sca-1 Bcl-2 transgenic mice overexpress Bcl-2 in hematopoietic cells
To study whether apoptotic/cell survival processes are functional within the embryonic hematopoietic system, we generated transgenic mice in which overexpression of the antiapoptosis gene Bcl-2 was directed by the Sca-1 (Ly-6E) transcriptional control elements. A Bcl-2 cDNA fragment36 was inserted into the first untranslated exon of the 14-kb Ly-6E genomic fragment31,38 (Figure 3A), and 2 transgenic lines (Ln 2 and Ln 479) were analyzed in detail. The copy number was determined by Southern blot analysis, comparing signal intensity of the endogenous 1.4-kb Bcl-2 gene fragment to the 4.7-kb transgenic Bcl-2 band (Figure 3B). The transgene copy numbers of Ln 2 and Ln 479 are 7 and 20, respectively. Northern blot analysis demonstrated overexpression of the Bcl-2 transgene in kidney, liver (Figure 3C), thymus, and spleen (data not shown). Western blotting revealed that Bcl-2 protein expression was also elevated in transgenic animals (Figure 3D). Only low or undetectable levels of endogenous Bcl-2 protein were found in wild-type littermates, consistent with low mRNA expression in the wild type. Thus, the Sca-1 transcriptional regulatory elements direct Bcl-2 expression at high levels in several hematopoietic and nonhematopoietic tissues in the transgenic animals, and this expression pattern correlates with the Ly-6E/A expression pattern as previously reported.31-35
Overexpression of Bcl-2 in hematopoietic tissues of Sca-1 Bcl-2 transgenic mice. (A) Transgene construct. An 865-bp murine Bcl-2 cDNA fragment was inserted into exon one of a 14-kb Sca-1 (Ly-6E) expression cassette. H indicates HindIII. (B) Transgene copy number determination. A Southern blot containing HindIII-digested DNA was probed with a Bcl-2 fragment to reveal the 1.4-kb endogenous gene and 4.7-kb transgene. Ln 479 contains 20 and Ln 2 contains 7 copies of the transgene. (C) Northern blot analysis for Bcl-2 transgene expression in several adult tissues. Bcl-2 is expressed in liver and kidney in Ln 479 and Ln 2 Bcl-2 transgenic (tg) animals. (D) Western blot analysis of Bcl-2 protein expression in Ln 2 and Ln 479 transgenic animals. Bcl-2 is overexpressed in several transgenic tissues, including kidney, thymus, spleen, and bone marrow, as compared with nontransgenic (wild-type [wt]) littermates. In general, no obvious abnormalities were found in the transgenic adults. Blood smears showed no morphologic differences or leukemic cells, and hematocrits did not differ between the transgenic and nontransgenic adults (data not shown).
Overexpression of Bcl-2 in hematopoietic tissues of Sca-1 Bcl-2 transgenic mice. (A) Transgene construct. An 865-bp murine Bcl-2 cDNA fragment was inserted into exon one of a 14-kb Sca-1 (Ly-6E) expression cassette. H indicates HindIII. (B) Transgene copy number determination. A Southern blot containing HindIII-digested DNA was probed with a Bcl-2 fragment to reveal the 1.4-kb endogenous gene and 4.7-kb transgene. Ln 479 contains 20 and Ln 2 contains 7 copies of the transgene. (C) Northern blot analysis for Bcl-2 transgene expression in several adult tissues. Bcl-2 is expressed in liver and kidney in Ln 479 and Ln 2 Bcl-2 transgenic (tg) animals. (D) Western blot analysis of Bcl-2 protein expression in Ln 2 and Ln 479 transgenic animals. Bcl-2 is overexpressed in several transgenic tissues, including kidney, thymus, spleen, and bone marrow, as compared with nontransgenic (wild-type [wt]) littermates. In general, no obvious abnormalities were found in the transgenic adults. Blood smears showed no morphologic differences or leukemic cells, and hematocrits did not differ between the transgenic and nontransgenic adults (data not shown).
Bcl-2 overexpression results in radio-protection and increased hematopoietic cell viability in the adult
To test the validity of our transgenic model, we examined the effects of Bcl-2 overexpression in the adult hematopoietic system. We performed survival experiments to assess whether Sca-1-directed Bcl-2 overexpression could protect cells from radiation-induced apoptosis in vivo. Transgenic animals and normal littermates were challenged with different doses of total body γ-irradiation, and the number of viable animals was determined daily for up to 30 days. At doses of 10.5 (Figure 4A), 9.5, and 8.5 Gy (not shown), both Ln 479 and Ln 2 (not shown) transgenic animals showed higher survival rates at day 30 in comparison with their wild-type littermates.
Effects of Bcl-2 overexpression on adult mice and cells. (A) In vivo survival curves of Bcl-2 transgenic mice and nontransgenic littermates. Adult mice of 7 to 22 weeks of age were subjected to a split dose of γ-irradiation of 10.5 Gy. Survival of both Ln 479 (shown) and Ln 2 (not shown) transgenic mice and littermates was checked daily over 30 days. Results are derived from 2 separate experiments for each transgenic line. The number of mice irradiated at 10.5 Gy was n = 10 for Ln 479 tg and n = 12 for wt littermates. For other irradiation doses, 10 to 15 mice were used per group (not shown). (B) Viable cell numbers of BM cells of Ln 479 transgenic (Bcl-2) and a wild-type littermate (non-tg). Bcl-2 overexpression results in higher viable cell numbers in vitro over a period of 11 days. Note that the scale on the y-axis is logarithmic. These graphs show a representative experiment out of a total of 5 experiments (7 Bcl-2 tg and 7 wt littermates ranging 10 to 24 weeks of age). Error bars indicate SEM.
Effects of Bcl-2 overexpression on adult mice and cells. (A) In vivo survival curves of Bcl-2 transgenic mice and nontransgenic littermates. Adult mice of 7 to 22 weeks of age were subjected to a split dose of γ-irradiation of 10.5 Gy. Survival of both Ln 479 (shown) and Ln 2 (not shown) transgenic mice and littermates was checked daily over 30 days. Results are derived from 2 separate experiments for each transgenic line. The number of mice irradiated at 10.5 Gy was n = 10 for Ln 479 tg and n = 12 for wt littermates. For other irradiation doses, 10 to 15 mice were used per group (not shown). (B) Viable cell numbers of BM cells of Ln 479 transgenic (Bcl-2) and a wild-type littermate (non-tg). Bcl-2 overexpression results in higher viable cell numbers in vitro over a period of 11 days. Note that the scale on the y-axis is logarithmic. These graphs show a representative experiment out of a total of 5 experiments (7 Bcl-2 tg and 7 wt littermates ranging 10 to 24 weeks of age). Error bars indicate SEM.
To test whether this increased survival could be related to protection of cells of the hematopoietic system from apoptosis, we performed in vitro experiments using BM (Figure 4B) cells from Bcl-2 transgenic and wild-type littermates. Over a period of 11 days, in the absence of added hematopoietic growth factors, the survival of nonadherent cells was determined. The number of viable cells derived from Bcl-2 transgenic BM and spleen (not shown) were much greater than those derived from wild-type littermates. Hence, the increased survival rate of the Bcl-2 transgenic mice is due, at least in part, to increased viability of hematopoietic cells.
We next examined whether the increased number of viable cells in these cultures was due to decreased apoptosis by flow cytometric analysis with annexin V (an early marker of apoptosis) and 7AAD staining42 (Table 2). Nontransgenic cells cultured under the stressful hematopoietic growth factor-free conditions showed the expected pattern of cell death, with 31% of cells in the nonviable (7AAD+) quadrants. In contrast, Bcl-2 transgenic cultures showed only 4% 7AAD+ cells, with a much higher percentage of cells in a viable state (91% annexin-7AAD-). The percentage of cells in the early stages of apoptosis (annexin+7AAD-) was almost 3-fold higher in the nontransgenic cultures as compared with the Bcl-2 transgenic cultures. Thus, Bcl-2 overexpression decreases the number of BM cells entering into apoptosis in growth factor-free hematopoietic cultures, resulting in increased numbers of viable cells.
Percentages of cells obtained after annexin V and 7AAD FACS analysis of BM from transgenic and nontransgenic mice
. | Annexin-7AAD- . | Annexin+7AAD- . | 7AAD+ . |
|---|---|---|---|
| Nontransgenic | 58.3 ± 4.7 | 10.5 ± 0.1 | 30.9 ± 4.7 |
| Bcl-2 | 91.0 ± 2.5 | 3.7 ± 0.3 | 4.4 ± 1.3 |
. | Annexin-7AAD- . | Annexin+7AAD- . | 7AAD+ . |
|---|---|---|---|
| Nontransgenic | 58.3 ± 4.7 | 10.5 ± 0.1 | 30.9 ± 4.7 |
| Bcl-2 | 91.0 ± 2.5 | 3.7 ± 0.3 | 4.4 ± 1.3 |
Three transgenic mice (n = 2 Ln 2 and n = 1 Ln 479; mice aged 9 to 23 weeks) and 3 nontransgenic littermates were used. Differences observed between the transgenic and nontransgenic in the annexin-7AAD-, annexin+7AAD-, and 7AAD+ subsets are statistically significant as determined by Student t test. Data are presented as mean ± SEM.
To investigate whether lower apoptosis/higher cell maintenance was occurring in Bcl-2 transgenic hematopoietic cell subsets that normally express Sca-1,43 we examined T- and B-lymphoid cells in the thymus and spleen (Table 3). Increases in the percentages of CD4-CD8-, CD4+, and CD8+ thymocytes and a concomitant large decrease in CD4+CD8+ thymocytes are observed in transgenic adults (Table 3). Also, the absolute number of mature CD4+ and CD8+ T cells in the spleen are increased (2.5- to 4-fold), and a 2- to 3-fold increase in the absolute number of B220+ splenocytes is observed. Taken together, the increases in the absolute number of spleen cells (approximately 17-25 × 107) can be accounted for by the increase in the mature lymphocyte CD4+, CD8+, and B220+ subsets and may be attributed to increased cell survival by overexpression of Bcl-2. Thus, our transgenic animal model results in Bcl-2-induced changes within the expected Sca-1-expressing T- and B-lymphoid subsets of the adult hematopoietic system.
Bcl-2 overexpression results in increased numbers and viability of Sca-1+ and c-kit+ cells in the AGM
An overall examination of cell survival/death within the E11 AGM region of Bcl-2 transgenic embryos by TUNEL staining revealed no obvious changes as compared with wild-type sections. Hence, to determine if Bcl-2 was indeed overexpressed in the AGM, RT-PCR was performed on subdissected E11 AGM Bcl2 trangenic tissues. Gene expression of Bcl-2, as well as antiapoptotic Bcl-x and proapoptotic Bim, was tested. Figure 1D shows representative data from one of several experiments. A 2- to 10-fold increase in Bcl-2 gene expression was consistently observed in the E11 transgenic aorta, as compared with the nontransgenic aorta (Figure 1C). Also, Bim expression is increased (approximately 1.5- to 4-fold), while Bcl-x expression remained as in the nontransgenic tissue. Thus, Bcl-2 is overexpressed within the transgenic E11 aorta (the subregion in which the first HSCs are found).
To determine whether the overexpression of Bcl-2 disrupts the normal balance of apoptosis and survival of phenotypically defined populations of cells containing HSCs, fluorescence-activated cell sorter (FACS) analysis with HSC surface markers c-kit and Sca-1 was performed on cells from E11 control and transgenic AGM explant cultures. As shown in a representative profile of c-kit-stained AGM cells (Figure 5A) (gated on nongranular cells), the percentages of c-kit+ cells are increased by a factor of 1.5 in the Bcl-2 transgenic tissues. This was confirmed in several independent experiments, and the data are shown as fold change in percentage, since the absolute number of cells in transgenic and nontransgenic AGMs was comparable. The increase in percentage of c-kit+ and Sca-1+ cells in Bcl-2 E11 AGM explants ranged from 1.5- to 1.9-fold and 1.5- to 2.2-fold, respectively (Figure 2C). To confirm that the increase in percentage of Sca-1+ cells can in part be due to HSCs and not only to mesonephric cells, FACS analysis was performed on subdissected E11 aorta explants. The percentage of Sca-1+ aorta cells increases 1.9-fold in the Bcl-2 transgenic aortas as compared with nontransgenic aortas, strongly suggesting the increase of HSCs. Moreover, similar increases in the percentages of c-kit+ and Sca-1+ cells in E11 transgenic liver explants were observed (Figure 5A), strongly suggesting an increase in liver HSCs in transgenic tissues. Thus, as shown with 2 independent HSC markers, c-kit and Sca-1, overexpression of Bcl-2 results in increased numbers of E11 AGM, aorta, and liver cell populations containing phenotypically defined HSCs.
Increased numbers and viability of c-kit+ and Sca-1+ cells in Bcl-2-overexpressing AGM and liver. E11 AGM, aorta, and liver tissues from Bcl-2 transgenic and nontransgenic embryos were dissected and cultured as explants for 3 to 4 days, during which time the embryos were genotyped. Tissues from embryos with a similar genotype were pooled, and single-cell suspensions were stained with antibodies specific for c-kit or Sca-1 and with annexin V and 7AAD. (A) Representative FACS plots for c-kit- and Sca-1-stained cells (top and bottom rows, respectively) within the 7AAD- fraction of wild-type (WT) and Bcl-2-overexpressing AGM and aorta tissues, respectively. Side scatter (SSC) is shown on the x-axis and fluorescent antibody staining on the y-axis. Percentages of c-kit+ and Sca-1+ cells are shown for the gated region (HSCs are found within the nongranular side scatter). (B) Within the c-kit+ and Sca-1+ fractions of AGM and aorta tissues, the percentage of annexin V+ cells was determined (indicating the percentage of cells entering the apoptotic pathway). Representative histogram plots are shown with indicated percentages of annexin V+ cells in the c-kit+ (top row) or Sca-1+ (bottom row) cell fractions, respectively, in the AGM or subdissected aorta. Bcl-2-overexpressing tissues (right panels) contain less annexin V+ cells within the c-kit+ and Sca-1+ fraction. (C) Overview of the fold changes (increases) in percentages of c-kit+ and Sca-1+ cells and (D) fold changes (decreases) in percentages of annexin V+ cells within the c-kit+ and Sca-1+ fractions of Bcl-2 transgenic E11 AGM, aorta, or liver cells as compared with wild-type cells. Percentages were determined as indicated in panels A and B. Results are shown for 3 experiments with Sca-1 and annexin V, 3 experiments with c-kit and annexin V, and 2 experiments with E11 aorta cells. Each bar in the graph represents an independent experiment containing embryos from 1 to 3 litters. From each experiment, several samples (each contained a pool of cells from 1 to 3 tissues) were measured, and the average of these samples is shown. Total number of events analyzed ranges from 8 × 104 to 2 × 105.
Increased numbers and viability of c-kit+ and Sca-1+ cells in Bcl-2-overexpressing AGM and liver. E11 AGM, aorta, and liver tissues from Bcl-2 transgenic and nontransgenic embryos were dissected and cultured as explants for 3 to 4 days, during which time the embryos were genotyped. Tissues from embryos with a similar genotype were pooled, and single-cell suspensions were stained with antibodies specific for c-kit or Sca-1 and with annexin V and 7AAD. (A) Representative FACS plots for c-kit- and Sca-1-stained cells (top and bottom rows, respectively) within the 7AAD- fraction of wild-type (WT) and Bcl-2-overexpressing AGM and aorta tissues, respectively. Side scatter (SSC) is shown on the x-axis and fluorescent antibody staining on the y-axis. Percentages of c-kit+ and Sca-1+ cells are shown for the gated region (HSCs are found within the nongranular side scatter). (B) Within the c-kit+ and Sca-1+ fractions of AGM and aorta tissues, the percentage of annexin V+ cells was determined (indicating the percentage of cells entering the apoptotic pathway). Representative histogram plots are shown with indicated percentages of annexin V+ cells in the c-kit+ (top row) or Sca-1+ (bottom row) cell fractions, respectively, in the AGM or subdissected aorta. Bcl-2-overexpressing tissues (right panels) contain less annexin V+ cells within the c-kit+ and Sca-1+ fraction. (C) Overview of the fold changes (increases) in percentages of c-kit+ and Sca-1+ cells and (D) fold changes (decreases) in percentages of annexin V+ cells within the c-kit+ and Sca-1+ fractions of Bcl-2 transgenic E11 AGM, aorta, or liver cells as compared with wild-type cells. Percentages were determined as indicated in panels A and B. Results are shown for 3 experiments with Sca-1 and annexin V, 3 experiments with c-kit and annexin V, and 2 experiments with E11 aorta cells. Each bar in the graph represents an independent experiment containing embryos from 1 to 3 litters. From each experiment, several samples (each contained a pool of cells from 1 to 3 tissues) were measured, and the average of these samples is shown. Total number of events analyzed ranges from 8 × 104 to 2 × 105.
To examine whether the increase in c-kit+ and Sca-1+ cells is related to decreased apoptosis, we costained with annexin V and performed cytometric analysis on the gated c-kit+ and Sca-1+ fractions of 7AAD- AGM, aorta, and liver cells. As shown in Figure 5B, the percentage of annexin V+ cells in the c-kit+ fraction of a Bcl-2 transgenic AGM explant is decreased 1.9-fold as compared with wild-type AGM explants. This decrease ranged from 1.9- to 2.6-fold in Bcl-2 AGM explants and from 1.1- to 1.2-fold in liver explants (Figure 5D). Furthermore, a decrease in the percentage of annexin V+ cells (1.2- to 1.6-fold) was found in the Sca-1+ fraction of aorta explants (although this decrease is less apparent in the histogram, owing to the isolation of fewer cells). Thus, Bcl-2 overexpression leads to a quantitative increase in cell populations containing phenotypically defined HSCs by decreasing the number of these cells entering the apoptotic pathway in midgestation hematopoietic tissues.
Hematopoietic stem cell activity is increased in the AGM of Bcl-2-overexpressing embryos
To directly test whether Bcl-2 overexpression promotes the viability/survival and increases the numbers of HSCs, in vivo hematopoietic repopulation analyses were performed, both on adult BM cells and on midgestation AGM and liver cells. Cells obtained from male Bcl-2 transgenic and nontransgenic adults and E11 and E12 embryos were injected in limiting numbers in vivo into irradiated adult female recipients. Recipients were analyzed for donor cell Y chromosome contribution by peripheral blood DNA PCR at 4 months after injection. At a limiting dose of 104 cells, more HSC activity was found in Bcl-2 transgenic BM (Figure 6A) than in wild-type littermate controls. Frequency analysis was performed (Table 4), and HSCs were found to be increased 2.6-fold (P < .03) in the Bcl-2-overexpressing mice. Furthermore, by flow cytometry on Bcl-2 transgenic BM (n = 3), we found a 1.6- to 3.7-fold (2.6 ± 1.1) increase in the number of c-kit+ cells as compared with nontransgenic BM (not shown). Thus, Bcl-2 overexpression increases the number of BM HSCs.
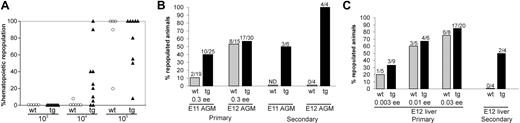
Increased HSC activity in the bone marrow, AGM, and liver of Bcl-2-overexpressing transgenic animals. (A) HSC activity in the BM of Bcl-2-overexpressing mice. Whole BM from a male Ln 479 transgenic (tg) and a nontrangenic (wt) littermate was injected at different limiting doses (103, 104, and 105 cells) together with 105 female BM cells into irradiated female recipients. At 4 months after transplantation, the peripheral blood of the recipient was analyzed in a semiquantitative manner for male donor hematopoietic cells. Black triangles represent individual recipients engrafted with Bcl-2 BM, and gray circles indicate individual recipients engrafted with wt BM. Results of competitive repopulation experiments for Ln 479 transgenic and nontransgenic littermates are shown. A greater than 10% engraftment level in the peripheral blood was used as the criterion for positive HSC repopulation. Ln2 transgenic BM gave similar results (not shown). (B-C) Transplantations were performed with E11 AGM, E12 AGM Ln 479 transgenic (▪; panel B), and E12 liver cells (▪; panel C), as well as nontransgenic control cells (▦). The combined results of 2 independent transplantation experiments show the percentage repopulated recipients (y-axis) and the number of mice positive/the number of total mice injected for each group. ee indicates embryo equivalents injected; and ND, not done. All recipients considered positive at more than 4 months after transplantation showed donor-derived cell engraftment levels exceeding 10% by semiquantitative PCR. In general, a higher percentage of repopulated recipients is seen with Bcl-2 transgenic midgestation AGM and liver cells than with wild-type littermate cells. Results of secondary transplantations are shown on the right. BM cells from primary recipients injected with E11 AGM, E12 AGM, and E12 liver were transplanted in limiting dilutions into irradiated adult secondary recipients. The percentage of repopulated recipients and the number of mice positive/the number of total mice injected are shown for each group. The BM cells of the primary recipients injected with E11 AGM, E12 AGM, and E12 liver cells that were used for secondary transplantation were 75% to 100% repopulated by donor-derived cells. Results were obtained at more than 4 months after transplantation by PCR analysis of peripheral blood DNA. Again, a higher percentage of repopulated recipients is seen with Bcl-2 transgenic as compared with wild-type littermate cells.
Increased HSC activity in the bone marrow, AGM, and liver of Bcl-2-overexpressing transgenic animals. (A) HSC activity in the BM of Bcl-2-overexpressing mice. Whole BM from a male Ln 479 transgenic (tg) and a nontrangenic (wt) littermate was injected at different limiting doses (103, 104, and 105 cells) together with 105 female BM cells into irradiated female recipients. At 4 months after transplantation, the peripheral blood of the recipient was analyzed in a semiquantitative manner for male donor hematopoietic cells. Black triangles represent individual recipients engrafted with Bcl-2 BM, and gray circles indicate individual recipients engrafted with wt BM. Results of competitive repopulation experiments for Ln 479 transgenic and nontransgenic littermates are shown. A greater than 10% engraftment level in the peripheral blood was used as the criterion for positive HSC repopulation. Ln2 transgenic BM gave similar results (not shown). (B-C) Transplantations were performed with E11 AGM, E12 AGM Ln 479 transgenic (▪; panel B), and E12 liver cells (▪; panel C), as well as nontransgenic control cells (▦). The combined results of 2 independent transplantation experiments show the percentage repopulated recipients (y-axis) and the number of mice positive/the number of total mice injected for each group. ee indicates embryo equivalents injected; and ND, not done. All recipients considered positive at more than 4 months after transplantation showed donor-derived cell engraftment levels exceeding 10% by semiquantitative PCR. In general, a higher percentage of repopulated recipients is seen with Bcl-2 transgenic midgestation AGM and liver cells than with wild-type littermate cells. Results of secondary transplantations are shown on the right. BM cells from primary recipients injected with E11 AGM, E12 AGM, and E12 liver were transplanted in limiting dilutions into irradiated adult secondary recipients. The percentage of repopulated recipients and the number of mice positive/the number of total mice injected are shown for each group. The BM cells of the primary recipients injected with E11 AGM, E12 AGM, and E12 liver cells that were used for secondary transplantation were 75% to 100% repopulated by donor-derived cells. Results were obtained at more than 4 months after transplantation by PCR analysis of peripheral blood DNA. Again, a higher percentage of repopulated recipients is seen with Bcl-2 transgenic as compared with wild-type littermate cells.
Frequency analysis of HSCs in embryonic and adult hematopoietic tissues
Tissue transplanted . | Nontransgenic . | . | Bcl-2 trangenic . | . | ||
|---|---|---|---|---|---|---|
| . | HSC frequency . | HSC/ee . | HSC frequency . | HSC/ee . | ||
| E11 AGM | 1/2.7 × 105 | 0.37 | 1/0.6 × 105 | 1.70* | ||
| E12 AGM | 1/0.4 × 105 | 2.54 | 1/0.4 × 105 | 2.78 | ||
| E12 liver | 1/0.017 × 105 | 58.8 | 1/0.013 × 105 | 76.9 | ||
| Bone marrow | 1/6.28 × 104 | NA | 1/2.31 × 104 | NA | ||
Tissue transplanted . | Nontransgenic . | . | Bcl-2 trangenic . | . | ||
|---|---|---|---|---|---|---|
| . | HSC frequency . | HSC/ee . | HSC frequency . | HSC/ee . | ||
| E11 AGM | 1/2.7 × 105 | 0.37 | 1/0.6 × 105 | 1.70* | ||
| E12 AGM | 1/0.4 × 105 | 2.54 | 1/0.4 × 105 | 2.78 | ||
| E12 liver | 1/0.017 × 105 | 58.8 | 1/0.013 × 105 | 76.9 | ||
| Bone marrow | 1/6.28 × 104 | NA | 1/2.31 × 104 | NA | ||
HSC frequencies were determined from the primary transplantation data in Figure 6 and were calculated by means of L-Calc software (StemCell Technologies, Vancouver, BC, Canada). ee indicates embryo equivalent; and NA, not applicable.
Statistical significance; P < .02
Limiting dilution in vivo transplantation experiments with AGM and liver cells from Bcl-2 transgenic and wild-type male embryos revealed that the HSC activity of E11 Bcl-2 transgenic AGM cells (Figure 6B) was higher (10 repopulated of 25 recipients) than in transgenic littermate controls (only 2 of 19 recipients repopulated). Engraftment was high level (up to 100% donor derived), long term, and multilineage (not shown). By E12, HSC activity in transgenic AGMs was similar to that of the E12 nontransgenic AGM, but this may be due to saturating numbers of HSCs in the injected dose. Bcl-2 transgenic E12 liver also showed some increases in HSC activity when compared with nontransgenic liver at limiting injection doses (Figure 6C). Frequency analysis (Table 4) revealed that HSC numbers were significantly increased in the E11 AGM by a factor of 4.5 (P < .02). Thus, Bcl-2 overexpression increases the number of functionally repopulating AGM HSCs.
To further check the potency of these midgestation HSCs, limiting doses of BM cells from the primary Bcl-2 transgenic and nontransgenic AGM- and liver-engrafted recipients were injected into secondary recipients. Bcl-2 transgenic secondary transplanted AGM cells showed potent HSC activity (Figure 6B, right side of panel), while nontransgenic cells provided no engraftment (combined data of transgenic secondary transplants: 7 repopulated of 10 transplanted as compared with nontransgenic transplants, 0 of 4). Similarly, Bcl-2 transgenic secondary transplanted liver cells showed HSC activity, while nontransgenic liver did not give rise to donor cell engraftment (Figure 6C, right side of panel). Thus, these transplantation data demonstrate that Bcl-2-overexpressing AGM and liver tissues possess higher HSC activity and self-renewal ability than nontransgenic tissues, strongly suggesting that apoptosis plays a role in the regulation of HSCs in the midgestation mouse.
Discussion
It has been widely accepted that a tight quantitative control of HSCs is essential in maintaining the appropriate and stable representation of progenitors and differentiated cells in the adult hematopoietic hierarchy. Because the blood system is highly dynamic, responds rapidly to trauma, and also constantly replenishes itself, cell survival and apoptosis play an important role in regulation of this tissue system. Our results show that, during development of the hematopoietic system within the embryo, these processes also appear to play a role.
It can be implied from the observed HSC increases in our Ly-6E (Sca-1) Bcl-2 transgenic mice that components of the Bcl-2 pathway leading to cell survival are intact and functionally relevant in these cells. Until our investigation, it was not known whether Bcl-2 is normally expressed in this region. Our immunostainings have shown that Bcl-2 protein is expressed in the midgestation AGM and, furthermore, by TUNEL analysis, that apoptosis is ongoing in this region. Bcl-2 transcripts are found in both the aorta and the UGRs. While TUNEL+ foci are found in the mesonephric tubules (indicating apoptosis), it appears that the apoptosis occurring in the region surrounding the dorsal aorta is limited to single cells. These data suggest that there is a tight regulation of apoptosis in this region, and we postulate that, in addition to Bcl-2, other apoptotic regulators play a role in the balance between cell survival and apoptosis in the AGM.
We have found expression of the Bcl-x antiapoptotic regulator and the Bim proapoptotic regulator in the midgestation aorta and UGRs. Since the results of other researchers in adult hematopoietic cell subsets and tissues21 show only slight overlap in Bcl-2 and Bcl-x in vivo expression patterns, these molecules may be expressed in different AGM cell subsets. Indeed, gene-targeted mutant mice show different phenotypes.12,22,44 Bcl-2-deficient mice complete embryonic development and show increased apoptosis in lymphocyte populations, and Bcl-x-deficient mice die around E13 with extensive apoptosis of hematopoietic cells in the fetal liver. Interestingly, recent data show that concomitant loss of the proapoptotic Bim gene and the antiapoptotic Bcl-2 gene allows normal physiologic functions, and thus, Bim counterbalances Bcl-2 activity.45 Moreover, Bim expression has been found in several hematopoietic cell lines,46,47 and Bim up-regulation correlates with increased apoptosis induced in E17 fetal liver cells upon hematopoietic growth factor withdrawal.48 Therefore, there are important roles for Bcl-2, Bcl-x, and Bim in regulating apoptosis in hematopoietic cells, and the intracellular ratios of these and other proapoptotic and antiapoptotic proteins are expected to play a critical role in setting the balance between cellular life or death. Semiquantitative transcriptional analysis of these 3 genes in the normal and Bcl-2-overexpressing AGM, aorta, UGRs, and liver suggests that the balance of proapoptotic and antiapoptotic molecules is also important during midgestation hematopoiesis. When Bcl-2 is overexpressed in the aorta in transgenic embryos, a concomitant increase in Bim, but not Bcl-x, is observed. How this balance is achieved at the transcriptional level is unknown. Further studies should determine whether these 3 molecules (or other combinations) are expressed in the same population/subset of AGM cells and the precise balance between these proapoptotic and antiapoptotic molecules that leads to increased HSC activity in the mouse embryo.
We have shown here that Bcl-2 overexpression increases the numbers of c-kit+ and Sca-1+ cells in the AGM, decreases their entry into the apoptotic pathway, and, more important, increases the numbers of functional HSCs beginning at midgestation in the AGM. HSC activity was significantly increased in the E11 AGMs from Sca-1 Bcl-2 transgenic embryos (4.5-fold increase; P < .02). Such an increase was less apparent in transgenic E12 AGM and liver. However, when secondary transplantations were performed to confirm the self-renewal ability of AGM and liver HSCs, increased HSC activity was observed in the Bcl-2 transgenic primary BM as compared with the nontransgenic recipients, suggesting that saturating numbers of HSCs were present in the primary Bcl-2 engrafted recipient. Thus, Bcl-2 overexpression affects the repopulating activity of the first AGM and liver HSCs. Whether Bcl-2 overexpression has a direct effect only on HSC number or has several related effects, such as enhancing engraftment through survival and/or influencing the cell cycle status of HSCs (as for adult BM HSCs), is yet to be determined. Importantly, whatever the effect of Bcl-2 overexpression on HSCs, these cells are normal, renew themselves, and contribute to long-term, multilineage repopulation. The fact that increased HSC numbers are found in the AGM, liver, and adult BM suggests the intriguing possibility that HSCs undergo apoptosis more often and much earlier than was previously appreciated.
Finally, in examining the validity of our Bcl-2 overexpression transgenic mouse model in the adult hematopoietic system, we found that Bcl-2 overexpression occurs in the tissues and hematopoietic cells that are normally expected to express Sca-1.31-35 Sca-1-directed Bcl-2 overexpression affects adult hematopoietic cells under both normal and stressful conditions, most likely by preventing them from entering the apoptotic pathway. As expected from the previous results of others,24,28 Sca-1-directed Bcl-2 overexpression results in radio-protection and in increases of BM HSC activity (as determined by in vivo transplantation). Frequency analysis revealed that BM HSC numbers were significantly increased in Bcl-2 transgenic adults by a factor of 2.7. This increased BM HSC activity may be related to a lower susceptibility of the transgenic HSCs to entering into apoptosis during the engraftment of the recipient. Several studies have reported that Bcl-2 influences the cell cycle by blocking the transition from G1 to S-G2-M.49,50 Since there is a correlation between the cell cycle status of HSCs and their ability to home to the recipient BM (see Jetmore et al51 and references therein), it is possible that altered cell cycle profiles could result in the facilitated engraftment we observed for Sca-1 Bcl-2-overexpressing HSCs. Nonetheless, the Bcl-2-related increase in HSC activity is also due to an increase in the absolute number of HSCs, since we have found more c-kit+ cells in Bcl-2 transgenic BM (1.6- to 3.7-fold increase). This is in accordance with the findings of Domen et al9 in H2K-Bcl2 mice in which the c-kit+ lineage-negative (c-kit+lin-) HSCs are increased 2.4-fold as compared with wild-type mice. Given the notion that Bcl-2-overexpressing HSCs enter into apoptotic stages at a lower frequency and remain viable for longer periods of time, it may now be possible to isolate cell lines of this difficult-to-culture Sca-1+ hematopoietic subset from the AGM and adult BM. Such cell lines would greatly facilitate molecular analyses of developmentally specific HSC genetic programs.
Taken together, our studies show that antiapoptotic and proapoptotic processes are intact, active, and functioning in the earliest expanding HSCs in the AGM region of the mouse embryo and that apoptosis/cell survival plays an important role in these first HSCs.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-06-1827.
Supported by the Medical Research Council (MRC) (National Institute for Medical Research [NIMR], London, United Kingdom); HFSP (RG00345/1999M); Erasmus Breedtestrategie Program; the National Institutes of Health (NIH) (R01 DK51077); and Nederlandse Kankerbestrijding (2001-2442).
C.O. and K.N.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the members of laboratory for helpful discussions and technical assistance, especially Drs S. Mendes, M. Peeters, and M. de Bruijn. We especially thank Dr C. Robin for her critical review of the manuscript and her expert contributions to the flow cytometric analysis.



![Figure 3. Overexpression of Bcl-2 in hematopoietic tissues of Sca-1 Bcl-2 transgenic mice. (A) Transgene construct. An 865-bp murine Bcl-2 cDNA fragment was inserted into exon one of a 14-kb Sca-1 (Ly-6E) expression cassette. H indicates HindIII. (B) Transgene copy number determination. A Southern blot containing HindIII-digested DNA was probed with a Bcl-2 fragment to reveal the 1.4-kb endogenous gene and 4.7-kb transgene. Ln 479 contains 20 and Ln 2 contains 7 copies of the transgene. (C) Northern blot analysis for Bcl-2 transgene expression in several adult tissues. Bcl-2 is expressed in liver and kidney in Ln 479 and Ln 2 Bcl-2 transgenic (tg) animals. (D) Western blot analysis of Bcl-2 protein expression in Ln 2 and Ln 479 transgenic animals. Bcl-2 is overexpressed in several transgenic tissues, including kidney, thymus, spleen, and bone marrow, as compared with nontransgenic (wild-type [wt]) littermates. In general, no obvious abnormalities were found in the transgenic adults. Blood smears showed no morphologic differences or leukemic cells, and hematocrits did not differ between the transgenic and nontransgenic adults (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-06-1827/6/m_zh80110462160003.jpeg?Expires=1767757610&Signature=H4kQUpgVsj2DaK3K0pkTGs1B1hp7AHsA9Z-p7OKbPvuLVdsNzGzaLSJpyhAzS4RTMo6hul048ACAFhxHG~0sdulaJE7BCyruoNX8Lx0Y~03W1hl2BWQ0-TdKPwU18hZ1CsGjalaTbB2Dz-z9xovsZe6JhaNY~FEA6vkkW6jb7jnEXD0vcSPATH44xMWJmJgqw9SV4LGrcXFD1IYLmUFl4CLJZZcsz1ppIQSztLWSSLjUoLYMOV2zm1AuiQPNQoJcbeaeQleJGbA1WSnSqdptE~Euq6XrW4w8hrGuTeEi2mbHIdsWhPCWVDKR7yvvyCXiOw2LxHPiHMFrdOD1qwNvTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal