Abstract
Thrombotic thrombocytopenic purpura (TTP) either occurs in a congenital form caused by ADAMTS13 gene mutations or it is acquired and most often due to ADAMTS13 inhibitory autoantibodies. In congenital TTP siblings are often affected, while acquired TTP occurs sporadically and familial clustering has not been described so far. We report identical twin sisters suffering from acquired TTP due to immunoglobulin G (IgG) autoantibodies inactivating ADAMTS13, suggesting an important role of hitherto unidentified genetic determinants of ADAMTS13 inhibitor formation. These cases also demonstrate that familial clustering is not sufficient for unambiguously diagnosing hereditary ADAMTS13 deficiency and congenital TTP. (Blood. 2004;103:4195-4197)
Introduction
The metalloprotease ADAMTS13 (Adisintegrin and metalloprotease with thrombospondin type 1 domains-13)1-5 specifically cleaves the von Willebrand factor (VWF) subunit at the peptide bond Tyr842-Met843.6,7 A severely deficient ADAMTS13 activity (< 5% of that in normal plasma) was found to be a strong risk factor for thrombotic thrombocytopenic purpura (TTP).8,9
There are 2 fundamental mechanisms known to cause severe ADAMTS13 deficiency. Homozygous or compound heterozygous mutations of the ADAMTS13 gene lead to hereditary ADAMTS13 deficiency in congenital TTP,3,10-14 often affecting siblings. In contrast, most cases of acquired TTP are caused by autoantibodies inactivating ADAMTS13.8,9,15 Acquired TTP occurs sporadically, and familial clustering has not been described so far. Distinction between both forms is important as their management may differ. While regular infusion of limited amounts of fresh frozen plasma (FFP) usually reverts or prevents disease manifestations in congenital TTP, plasma exchange with FFP replacement, often combined with corticosteroids or other immunosuppressants, is the current therapy of choice in acute acquired TTP.16
We report identical twin sisters suffering from acquired TTP due to severe ADAMTS13 deficiency caused by circulating inhibitory immunoglobulin G (IgG) autoantibodies.
Study design
Patients
Previously healthy identical twin sisters suffered from a first episode of acute TTP at 23 (sister 1) and 24 (sister 2) years of age. Sister 1 presented with fever, neurologic symptoms, thrombocytopenia, and microangiopathic hemolytic anemia. Since therapy was initiated promptly, symptoms were less pronounced in sister 2. These episodes resolved under plasma exchange with FFP replacement and corticosteroids; sister 1 received vincristine, in addition. Follow-up for now 37 (sister 1) and 25 (sister 2) months was uneventful except for a short relapse in sister 1, which occurred 13 months after the initial episode and was treated by plasma exchange. Since then, no plasma has been administered to either of the sisters.
Both sisters are otherwise healthy without indication of another autoimmune disease, especially systemic lupus erythematosus (SLE), and have the same living and working conditions. No pregnancy has so far occurred in either sister. No drugs, including oral contraceptives, were taken in association with these episodes, and no other potential precipitating factors could be identified.
The study was conducted according to the responsible ethics committee's guidelines on research on human subjects (Kantonale Ethische Kommission, Bern, Switzerland). Written consent was obtained from both sisters.
ADAMTS13 activity and inhibitors
ADAMTS13 activity and inhibitory autoantibodies were determined by immunoblotting of purified VWF substrate degraded by BaCl2-activated ADAMTS13 in citrated plasma taken during acute TTP and in remission as described,8 with slight modifications.17
ADAMTS13 activity was estimated by visual comparison of the extent of VWF degradation by 1:20-diluted patient plasma and by dilutions of a normal human plasma (NHP) pool between 1:20 (100% activity) to 1:640 (3%), and buffer control (0%).
For inhibitor detection, patient plasma (heat-inactivated for 30 minutes at 56°C, centrifuged for 15 minutes at 15 000g) or purified IgG was mixed 1:1 (vol/vol) with NHP and incubated for 2 hours at 37°C. This mixture was diluted 1:10, resulting in a final NHP dilution of 1:20. Inhibitor titers were estimated by visual comparison of the residual VWF degradation with the degradation obtained with NHP mixed 1:1 (vol/vol) with heat-inactivated NHP, incubated for 2 hours at 37°C, and diluted to final NHP concentrations of 1:20 (100% activity), 1:40 (50%), 1:80 (25%), and buffer control (0%). One Bethesda unit (BU) of inhibitor reduced the ADAMTS13 activity of an equal volume of NHP by 50%.
Purification of ADAMTS13 inhibitory IgG
Total IgG was purified from plasma taken during acute TTP by adsorption to protein A Sepharose CL-4B (Pharmacia, Uppsala, Sweden), and concentrated to its original plasma level by centrifugation through filter membranes (Millipore, Bedford, MA; molecular-weight cutoff, 30 kDa). The ADAMTS13 inhibitory effect of purified IgG and of IgG-depleted plasma was tested by immunoblotting assay.15,17
Autoimmune serology and HLA typing
Screening for circulating autoantibodies included antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (c-/p-ANCA), and rheumatoid factor (RF) in samples taken during acute TTP and in remission, using routine assays.
HLA types were determined using polymerase chain reaction-based routine assays.
Results and discussion
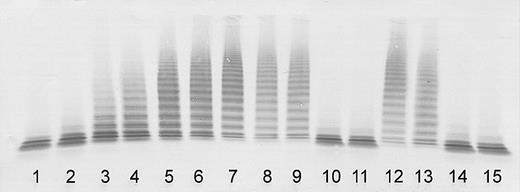
ADAMTS13 activity was severely deficient (< 3% of the normal) in plasma of both sisters taken during the initial episode of their thrombotic microangiopathy, confirming classical TTP (Figure 1).
Determination of ADAMTS13 activity by immunoblotting of VWF substrate degraded by BaCl2-activated ADAMTS13 in patient plasma (diluted 1:20). Lanes 1-7: assay calibration by normal plasma dilutions of 1:20 (100% activity), 1:40 (50%), 1:80 (25%), 1:160 (12.5%), 1:320 (6.25%), 1:640 (3%), and buffer control (0%). Sister 1: acute initial TTP episode (lane 8); clinical remission 17, 35, and 37 months after the initial episode (lanes 9-11). Sister 2: acute initial TTP episode (lane 12); clinical remission 5, 23, and 25 months after the initial episode (lanes 13-15).
Determination of ADAMTS13 activity by immunoblotting of VWF substrate degraded by BaCl2-activated ADAMTS13 in patient plasma (diluted 1:20). Lanes 1-7: assay calibration by normal plasma dilutions of 1:20 (100% activity), 1:40 (50%), 1:80 (25%), 1:160 (12.5%), 1:320 (6.25%), 1:640 (3%), and buffer control (0%). Sister 1: acute initial TTP episode (lane 8); clinical remission 17, 35, and 37 months after the initial episode (lanes 9-11). Sister 2: acute initial TTP episode (lane 12); clinical remission 5, 23, and 25 months after the initial episode (lanes 13-15).
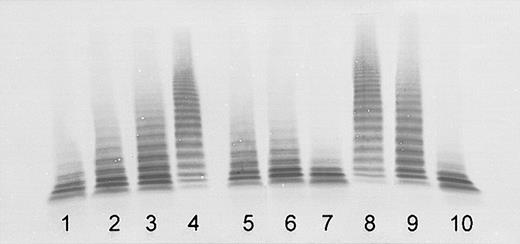
Inhibition of the ADAMTS13 activity in normal plasma by plasma of both sisters indicated the presence of inactivating autoantibodies. The titer was estimated to be approximately 1 BU/mL in sister 1 and more than 2 BU/mL in sister 2 (Figure 2). The purified total IgG had an ADAMTS13 inhibitory effect comparable with that of the original plasma. No inhibition was found for the IgG-depleted plasma (Figure 2), confirming that the observed inhibition was due to IgG autoantibodies.
Detection of ADAMTS13 inhibitory autoantibodies by immunoblotting of VWF substrate degraded by BaCl2-activated ADAMTS13 in mixtures of normal human plasma (NHP) with patient plasma obtained at acute initial TTP, or IgG purified from patient plasma (final NHP dilution, 1:20). Lanes 1-4: assay calibration by NHP dilutions of 1:20 (100% activity), 1:40 (50%, equivalent to an inhibitor titer of 1 BU/mL), 1:80 (25%), and buffer control (0%). Sister 1: mixtures of NHP 1:1 (vol/vol) with patient plasma (lane 5), with IgG purified from patient plasma (lane 6), and with IgG-depleted patient plasma (lane 7). Sister 2: mixtures of NHP 1:1 (vol/vol) with patient plasma (lane 8), with IgG purified from patient plasma (lane 9), and with IgG-depleted patient plasma (lane 10).
Detection of ADAMTS13 inhibitory autoantibodies by immunoblotting of VWF substrate degraded by BaCl2-activated ADAMTS13 in mixtures of normal human plasma (NHP) with patient plasma obtained at acute initial TTP, or IgG purified from patient plasma (final NHP dilution, 1:20). Lanes 1-4: assay calibration by NHP dilutions of 1:20 (100% activity), 1:40 (50%, equivalent to an inhibitor titer of 1 BU/mL), 1:80 (25%), and buffer control (0%). Sister 1: mixtures of NHP 1:1 (vol/vol) with patient plasma (lane 5), with IgG purified from patient plasma (lane 6), and with IgG-depleted patient plasma (lane 7). Sister 2: mixtures of NHP 1:1 (vol/vol) with patient plasma (lane 8), with IgG purified from patient plasma (lane 9), and with IgG-depleted patient plasma (lane 10).
Investigation of follow-up samples taken during clinical remission 17 (sister 1) and 5 (sister 2) months after the initial TTP episodes revealed ADAMTS13 activities of less than 5% and the persistence of inhibitors, though of a lower titer. Concordant with other observations of consistent remission18 or absence of events19 despite prolonged severe ADAMTS13 deficiency, this indicates that additional, as yet unknown triggers may be necessary for the onset of acute TTP episodes, at least in some patients.
In samples taken 35 and 37 months (sister 1) and 23 and 25 months (sister 2) after the initial episodes, ADAMTS13 activity had completely normalized in both sisters (Figure 1), and no inhibitor was detectable although plasma therapy had not been administered for more than a year in either of them.
The TTP episodes described here occurred in identical twin sisters at a similar age and were in both cases associated with severe ADAMTS13 deficiency, a constellation typical for a hereditary cause. However, ADAMTS13 inhibitory IgG autoantibodies were detected in both sisters, and normalization of the severely deficient ADAMTS13 activity, without preceding plasma therapy, was observed during follow-up. These findings ruled out hereditary ADAMTS13 deficiency and confirmed an acquired cause. Consequently, familial clustering alone may not allow classifying patients unambiguously as congenital TTP. Distinction between congenital and acquired TTP should be based on additional investigations such as screening for ADAMTS13 autoantibodies, inhibitory15 as well as noninhibitory20 in a fluid-phase assay, determination of ADAMTS13 kinetics after plasma infusion,21 and investigation of the ADAMTS13 gene in suitable cases.3,10-14
ADAMTS13 inhibitors have been reported in SLE,22 although this association seems to be rare.23 SLE shows a strong familial aggregation and a concordance in identical twins,24 and could therefore explain a familial clustering of ADAMTS13 inhibitors. Apart from the described TTP episodes both sisters are healthy without indication of SLE or other systemic autoimmune disease. Screening for autoantibodies including ANA, ANCA, and RF in samples taken during acute TTP and in remission did not give pathologic results.
Investigation of the sisters' HLA types revealed A*0201/0301, B*3901/5101, Cw*12/1502/07, DRB1*1201/1401, DQB1*0503/0301/04/09, and DPB1*0401/0402. A possible association of ADAMTS13 autoantibodies with HLA types has not been investigated so far. A previous study reported a lower frequency of HLA-DR53 in TTP/hemolytic uremic syndrome (HUS) patients compared with controls, suggesting a protective role of this type.25 However, ADAMTS13 was still unidentified at the time of that study.
Our observations suggest a genetic predisposition and hitherto unidentified genetic determinants for ADAMTS13 autoantibody formation. These may include an association with certain HLA types. This could be an important area of future research, providing a deeper insight into the molecular and immunologic basis of ADAMTS13 autoimmunity.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-11-3888.
Supported by a grant from the Swiss National Foundation for Scientific Research (32-66756.01) (B.L.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Zsuzsanna Beleznay, Institute of Immunology, Inselspital, Bern, for her valuable help with autoimmune serology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal