Abstract
The transcription factor PU.1 (Spi-1) is a well-characterized regulator of myeloid and lymphoid development. However, its role in mature functional cells is poorly studied. Here we report the characterization of the novel murine gene pDP4 (PU.1 difference product 4), which is absent from fetal livers of PU.1-deficient mice. pDP4 is transcribed as a single 3.2-kb mRNA with a 1518-base pair open reading frame encoded by 5 exons on chromosome 14. pDP4 expression is strongest in small intestine and bone marrow, in which it is expressed predominately in mature neutrophils. Interestingly, however, pDP4 expression is markedly down-regulated in neutrophils of the peripheral blood and peritoneum. The pDP4 gene encodes a secreted 57-kDa glycoprotein with an olfactomedin-like C-terminus. PU.1 binds to a functional site within the pDP4 promoter, and pDP4 expression in myeloid cells is strictly dependent on PU.1 and the presence of this site. In conclusion, we have identified a novel PU.1-regulated extracellular glycoprotein of the olfactomedin-like family with a possible role in neutrophilic trafficking. (Blood. 2004;103:4294-4301)
Introduction
The ETS-family transcription factor PU.1 is indispensable for both myeloid and lymphoid lineages. The role of PU.1 during hematopoiesis has been extensively studied, and its essential function in triggering critical lineage development decisions is well characterized.1 Mice with a null mutation in the PU.1 gene have no mature myeloid and B-lymphoid cells.2,3 It has been reported that different expression levels of PU.1 in progenitor cells lead to different cell fates in vitro: low PU.1 expression is thought to trigger B-cell development while high levels initiate monocytic differentiation.4 PU.1 is involved in a network of interactions with several other critical hematopoietic transcription factors, such as interferon consensus sequence binding protein (ICSBP) and interferon regulatory factor-4 (IRF-4),5,6 GATA-1 and GATA-2,7-10 as well as CCAAT/enhancer-binding protein alpha (C/EBPα).11 These interactions influence the activity of those factors and thus stimulate or suppress their ability to trigger lineage development decisions.
PU.1 regulates a large subset of myeloid genes (reviewed by Tenen et al12 ). The target genes of PU.1 include CD11b, several granule proteins (myeloperoxidase, neutrophilic elastase, and proteinase-3), as well as the receptors for granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, and M-CSF.13-19 Additionally, several critical lymphoid genes have been implicated as PU.1 targets, such as the interleukin-7 (IL-7) receptor20 and the immunoglobulin light chains.5 Thus, PU.1 largely controls cell lineage decisions by turning on cascades of downstream genes, making it an essential transcription factor in hematopoiesis.
Neutrophils play a critical role in the innate immune response. They are produced in the bone marrow and are released into the peripheral blood as mature cells in a regulated fashion maintaining homeostatic levels of circulating neutrophils.21 Release of neutrophils from the bone marrow is rapidly increased in response to inflammatory or infectious stimuli. Although the mechanisms are largely unknown, granulocytic mobilization from the bone marrow appears to be a highly regulated process. Perturbations in the control of granulocytic mobilization from the bone marrow may contribute to the pathogenesis of leukemias. For example, in chronic myeloid leukemia, expression of the bcr-abl oncogene may, via direct effects on cell adhesion, contribute to the premature release of neutrophils and immature myeloid cells.22
Despite its well-characterized role during hematopoietic development, little is known about the function of PU.1 in mature granulocytes. This is surprising, because expression of PU.1 increases dramatically during maturation of myeloid cells.23,24 Here we characterize a novel member of the olfactomedin-like family of extracellular matrix proteins, pDP4, and establish it as a direct target gene of PU.1 in mature bone marrow neutrophils.
Materials and methods
Mice and cells
Experiments were performed with 2- to 4-month-old mice on a C57BL/6 background. PU.1-deficient mice have been described.25 For in vivo mobilization of neutrophils, mice were injected intraperitoneally with 250 μg human G-CSF per kilogram of body weight twice daily for a total of 4 days. If not mentioned otherwise, all cell lines were cultured in Dulbecco modified Eagle medium (DMEM) or RPMI with 10% fetal bovine serum (FBS). 503 and 503/PU.1 cells were maintained in complete Iscove modified Dulbecco medium (IMDM) supplemented with 5% WEHI-3 and 3% BHK-conditioned cell supernatant as sources of IL-3 and stem cell factor (SCF).
Protein extracts and Western blotting
Cells were lysed on ice in RIPA buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl [pH 7.4], 1% Nonidet P-40 [NP-40], 0.25% sodium deoxycholate, 50 mM NaCl, 10 mM EDTA [ethylenediaminetetraacetic acid]) supplemented with 1 mg/mL antipain, 1 mg/mL aprotinin, 1 mg/mL leupeptin, 1 mg/mL pepstatin, 1 mM Na3(VO)4, and 1 mM phenylmethylsulfonyl fluoride and incubated for 30 minutes on ice. Samples were then centrifuged at 20 000g for 10 minutes, and the supernatant was boiled with 2 × SDS sample buffer. Total cell extracts were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to nitrocellulose membranes, and immunoblotting performed with antibodies as indicated. Secreted proteins were isolated as follows: cells were quantitatively removed from culture medium by centrifugation and sterile filtration. Subsequently, the culture medium was concentrated on Vivaspin concentrator columns (VivaScience, Hannover, Germany) and boiled in SDS sample buffer before loading onto SDS-PAGE gels. Peptide-N-glycosidase F was purchased from New England Biolabs (Beverly, MA) and was used according to the manufacturer's protocol.
Generation of an antiserum against pDP4
The peptide NYDLVFLQTPRQPV corresponding to the residues 492 to 505 at the very C-terminus of murine pDP4 (Figure 1B) was cysteine-conjugated to keyhole limpet hemocyanin (KLH) and injected into rabbits, followed by booster immunizations. Rabbits were bled sequentially, and sera were assayed for specific immunoglobulin (Ig) by Western blot analysis. High-titer serum obtained 14 days after the second boost (designated 4175) was used in a routine immunoblot at a 1:5000 dilution. Rabbit polyclonal antibody against hemagglutinin (HA) tag (HA probe, Y-11) and horseradish peroxidase-conjugated secondary IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
pDP4 is a secreted glycoprotein of the olfactomedin family. (A) Genetic locus of pDP4: The gene is expressed from 5 exons that span about 25 kb on mouse chromosome 14 (CELERA GA_x5J8B7W6JWY and NCBI accession NT_039606, gene LOC239192). Filled boxes symbolize the exons (E). (B) pDP4 is expressed as a peptide with an N-terminal signal peptide (SP) and a C-terminal olfactomedin-like domain (NCBI accession nos. XM_139161 and XP_139161 for the mRNA and peptide, respectively). The lower panel shows the peptide sequence comparison of the olfactomedin-like domains of mouse pDP4, human hGC-1/GW112, and bullfrog olfactomedin. Conserved amino acids are framed. The peptide used for generating the pDP4 antiserum is highlighted in the gray box. (C) Western blot using 60 μg of either total cell extracts or culture medium from mock or pcDNA-pDP4-HA-transfected COS-7 cells. Ngase F treatment of the culture medium extract demonstrates that pDP4 is secreted as a highly glycosylated protein. The membrane was hybridized with an antibody against the HA tag.
pDP4 is a secreted glycoprotein of the olfactomedin family. (A) Genetic locus of pDP4: The gene is expressed from 5 exons that span about 25 kb on mouse chromosome 14 (CELERA GA_x5J8B7W6JWY and NCBI accession NT_039606, gene LOC239192). Filled boxes symbolize the exons (E). (B) pDP4 is expressed as a peptide with an N-terminal signal peptide (SP) and a C-terminal olfactomedin-like domain (NCBI accession nos. XM_139161 and XP_139161 for the mRNA and peptide, respectively). The lower panel shows the peptide sequence comparison of the olfactomedin-like domains of mouse pDP4, human hGC-1/GW112, and bullfrog olfactomedin. Conserved amino acids are framed. The peptide used for generating the pDP4 antiserum is highlighted in the gray box. (C) Western blot using 60 μg of either total cell extracts or culture medium from mock or pcDNA-pDP4-HA-transfected COS-7 cells. Ngase F treatment of the culture medium extract demonstrates that pDP4 is secreted as a highly glycosylated protein. The membrane was hybridized with an antibody against the HA tag.
Immunofluorescence
Bone marrow cytospins were washed in saline-buffered PBS and fixed in 2% paraformaldehyde in PBS for 20 minutes at room temperature. Cells were permeabilized with 0.5% NP-40 for 3 minutes at room temperature and blocked for 30 minutes in 1% bovine seurm albumin (BSA). Primary antibodies (rabbit preimmune serum, anti-pDP4 antiserum, or anti-Gr-1; the latter from BD PharMingen, San Diego, CA) were used at 1:100 dilutions and secondary antibodies (antirabbit Alexa-488 and antimouse Alexa-546; both from Molecular Probes, Eugene, OR) in 1:400 dilutions in 1% BSA. Subsequently, cell nuclei were stained in 1:5000 DAPI (4,6 diamidino-2-phenylindole) (2 μg/mL) in PBS. Finally, slides were washed, air dried, and cells were covered by antifading solution (ProLong Antifade kit, Molecular Probes).
Cell sorting
Single cell suspensions from spleen, thymus, bone marrow, and peritoneal washouts were labeled as indicated by using phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated antibodies against the following surface molecules: Mac-1/CD11b (M1/70), CD3 (KT31.1), B220 (6B2), Gr-1 (8C5), Ter119, CD41 (MWReg30) (all BD PharMingen), and F4/80 (Calbiochem, San Diego, CA). Cell populations were fluorescence-activated cell sorter (FACS)-sorted using a high-speed cell sorter (MoFlo MLS; Cytomation, Fort Collins, CO) according to standard protocols. Alternatively, Gr-1+ granulocytes were isolated from indicated organs using magnetic-analyzed cell sorting (MACS) according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany).
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Trizol-extracted RNA was DNase treated, reverse transcribed, and subsequently amplified using an AbiPrism 7700 Sequence Detector (Applied Biosystems, Foster City, CA) using the following parameters: 48°C (30 minutes), 95°C (10 minutes), followed by 40 cycles of 95°C (15 seconds), 60°C (1 minute). Primers and probe to amplify pDP4 were as follows: sense: 5′-AGTGACCTTGTGCCTGCC-3′, antisense: 5′-CACGCCACCATGACTACA-3′, probe (6-carboxyfluorescein [FAM]-labeled): 5′-ACCCCCTCCTCCTGCTCCT-3′. 18S primers and probe (VIC-labeled) were purchased from Applied Biosystems.
5′ rapid amplification of cDNA ends (RACE) PCR
To identify the 5′ untranslated region of the pDP4 transcript, a Smart RACE cDNA amplification kit (Clontech Laboratories, Palo Alto, CA) was used according to the manufacturer's protocol. Total mouse bone marrow RNA served as a template. The sequence of the gene specific primer in exon 2 was 5′-GTGGCTGAGACACAGTCCCACCTTCTGC-3′. The PCR product was subcloned and sequenced.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays using WEHI-3 cells were performed according to published protocols.26 Normal rabbit IgG, anti-PU.1, and anti-Sp3 antibodies (all from Santa Cruz Biotechnology) were used for immunoprecipitations. To PCR-amplify the 0.5-kb pDP4 promoter, the following primer set was used: sense: 5′-TGGGAAACAGAATTCCTCAAA-3′, antisense: 5′-GCTTCTTCCCAGAGTCTCAG-3′. Primers for thymidine kinase (TK) promoter amplification were as follows: sense: 5′-TGTAGCTCATGGCTGCTGTGCCAAA-3′, antisense: 5′-GCCATGGCCAGATCCGGAGGGTAT-3′. PCR parameters were 95°C (40 seconds), 50°C (40 seconds), 72°C (40 seconds) for 32 cycles.
In vitro translation of PU.1 and gel electrophoretic mobility shift assay
PU.1 was labeled with [35S]methionine (NEN, Boston, MA) and in vitro translated using a pcDNA-based T7 expression vector and the Promega (Madison, WI) TNT T7 coupled transcription/translation system. WEHI-3 nuclear extracts were prepared by lysing cells with a small-gauge syringe as previously described.27,28 The pDP4 promoter oligonucleotide (including base pairs -75 to -78 containing the PU.1-binding site) had the sequence 5′-GTTCCCGTGGCTACTTCCTCTTTCTTCA-3′. The mutated oligonuleotide had the sequence 5′-GTTCCCGTGGCTACgcgCTCTTTCTTCA-3′. For supershift experiments, 2 μL anti-PU.1 antibody (Santa Cruz Biotechnology) was added.
Northern blotting
Total RNA was isolated using Trizol reagent (Molecular Research Center, Cincinnati, OH) according to manufacturer's protocol. Ten micrograms of each RNA sample were resolved by agarose formaldehyde gel electrophoresis and transferred to Biotrans nylon membranes (ICN Biomedicals, Irvine, CA). The blot was hybridized to a radiolabeled 0.5-kb cDNA fragment containing the 3′ end of the pDP4 coding region. To ensure equal loading of RNA samples, the probe was removed and the blot was rehybridized to a labeled 1.3-kb PstI fragment of the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA.
Transfections, luciferase assays, and plasmids
COS-7 cells were transfected with 0.6 μg pXP2 or pXP2-pDP4-prom and pcDNA, pcDNA-PU.1, or pcDNA-ICSBP as indicated by LipofectAMINE reagent (Gibco, Carlsbad, CA). In addition, cells were transfected with 50 pg of the internal control pRL-CMV, and relative light units were standardized relative to the expression of pRL-CMV. Experiments were performed in triplicates. Vectors pRL-CMV and pcDNA were purchased from Promega. The luciferase vector pXP2 has been described previously.29 The 0.4-kb pDP4 promoter (plus 145 bp of exon 1) was isolated by PCR amplification from mouse genomic DNA by the same primers as used in ChIP assays and was subsequently subcloned into the SacI/KpnI sites of pXP2 to create pXP2-pDP4-prom. PU.1 and ICSBP expression vectors were described previously.30,31 WEHI-3 cells were transfected using Fugene 6 (Roche, Basel, Switzerland). Luciferase activities were determined after 24 hours.
Results
pDP4 is a novel extracellular glycoprotein of the olfactomedin-like gene family
We have previously reported the identification of an unknown gene, pDP4 (PU.1 difference product 4), using representational difference analysis (RDA) of PU.1 and C/EBPα knock-out mice.19 Initial Northern blots have shown that pDP4 is expressed as a single transcript of 3.2-kb in size. To elucidate its entire coding sequence, we screened a G-CSF-induced 32D cell expression library using the pDP4 cDNA 3′ end probe that we formerly isolated by RDA. Several clones were found and subsequently sequenced. As a result, we identified 1518 bp as the longest open reading frame within the pDP4 mRNA (data not shown). A basic local alignment sequence tool (BLAST) search screen of the National Center for Biotechnology Information (NCBI) database identified an exact sequence match of pDP4 to a yet not described mouse mRNA (accession no. XM139161) similar to the human GW112 (accession no. AF097021) gene. GW112 has recently been reported as hGC-1, a human member of the olfactomedin-like family of proteins.32 Interestingly, pDP4 and hGC-1/GW112 share 85% cDNA similarity, suggesting that hGC-1/GW112 is the human homolog of murine pDP4.
Screening the CELERA (Rockville, MD) mouse database by using the coding sequence revealed the genomic DNA locus that contains the pDP4 gene on chromosome 14 (CELERA GA_x5J8B7W6JWY). The intron-exon structure was defined by comparing the cDNA to the genomic sequence. As shown in Figure 1A, pDP4 is expressed from 5 exons spanning a range of about 25 kb, with an exon size ranging from 157 bp (exon 2) to 2455 bp (exon 5).
Analysis of the longest open reading frame within the pDP4 mRNA predicted a protein with a calculated molecular weight of 57.3 kDa (Figure 1B). The pDP4 protein has an N-terminal signal sequence and 6 N-linked glycosylation sites. The C-terminal portion of the protein has high similarity (66%) to the olfactomedin-like domain of bullfrog olfactomedin, the founding member of a family of olfactomedin-like proteins that are believed to play a role within the extracellular matrix.33,34 To test whether pDP4 is a secreted protein, we transfected COS-7 cells with an expression plasmid encoding for an HA-tagged pDP4 protein using the longest open reading frame as a coding region. Subsequently, the culture medium was separated from the cells, and both fractions were analyzed by Western blotting using an anti-HA antibody. A specific protein band of about 90 kDa was detected in both total cells extracts and medium (Figure 1C). After treatment of the protein extracts with N-glycosidase, the size of pDP4 decreased to the expected value of 57 kDa. Therefore, pDP4 is a highly glycosylated secreted protein.
Selective expression of pDP4 in bone marrow granulocytes and small intestine
To determine the general expression pattern of pDP4, we performed Northern blot studies with multiple mouse tissues. Strong mRNA expression was exclusively found in small intestine and bone marrow (Figure 2A). However, after exposure of the blotting membrane for several days, very weak expression was also detected in colon and kidney (data not shown). Thus, the pDP4 gene is expressed in a restricted and tissue-specific manner. Differential expression in bone marrow and spleen was also confirmed at the protein level using a novel antiserum raised against the C-terminus of pDP4 in Western blots (Figure 2B). Endogenous pDP4 protein appeared as a glycoprotein of the expected molecular weight of 90 kDa before and 57 kDa after N-glycosidase treatment, thus confirming our initial transfection studies in COS-7 cells. Interestingly, a fraction of pDP4 seemed to be multimerized and incompletely denatured under the SDS-PAGE conditions used. This observation suggests that pDP4 protein can adopt polymeric structures. Moreover, bone marrow cells cultured overnight secreted pDP4 into the medium in a manner similar to that seen for transfected COS-7 cells (Figure 1C and data not shown). Together, our data strongly support the notion that pDP4 is part of an extracellular matrix formed within the bone marrow and small intestine.
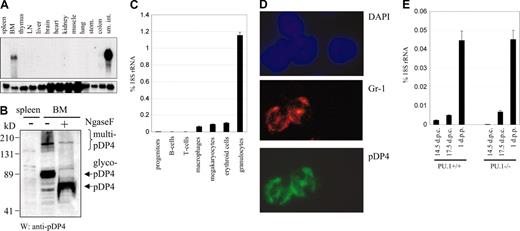
pDP4 is cell type specific. (A) Northern blot with total RNA from various mouse tissues showing that pDP4 is specifically expressed in small intestine and bone marrow (upper panel). The lower panel shows the hybridization of the same membrane with a GAPDH probe as a loading control. (B) pDP4 protein is expressed in bone marrow but not in spleen. Western blot using 60 μg total cell extracts from mouse spleen and bone marrow was incubated with an antiserum directed against the C-terminus of pDP4. Ngase F treatment indicates that pDP4 exists predominately as a glycosylated peptide (glyco-pDP4) in vivo. Multimerized pDP4 (multi-pDP4) can be noted as high molecular fragments. (C) Quantitative real-time RT-PCR of pDP4 transcripts using RNA from defined FACS-sorted hematopoietic cell populations. The following antibodies and mouse organs (in parentheses) were used for the sorting: B220 for B cells (spleen), CD3 for T cells (thymus), F4/80 for macrophages (bone marrow), CD41 for megakaryocytes (bone marrow), Ter119 for erythroid cells (bone marrow), Gr-1/Mac-1 for granulocytes (bone marrow), and a cocktail of all mentioned antibodies for lineage depletion to enrich progenitor cells (bone marrow). pDP4 values of 2 independent experiments are shown as percent expression of 18S rRNA ± standard deviation. (D) Immunofluorescence of a bone marrow cytospin section (original magnification, × 100) costained with anti-Gr-1 (primary)/Alexa-546 red (secondary) and anti-pDP4 (primary)/Alexa-488 green (secondary) antibody combinations as well as DAPI to visualize the nuclei. Only Gr-1+ cells costained with the pDP4 antiserum. (E) pDP4 real-time RT-PCR of intestine RNA from PU.1+/+ and PU.1-/- mice at different embryonic (d.p.c.) and newborn (d.p.p.) stages. pDP4 values of 2 independent experiments are shown as percent 18S rRNA expression ± standard deviation.
pDP4 is cell type specific. (A) Northern blot with total RNA from various mouse tissues showing that pDP4 is specifically expressed in small intestine and bone marrow (upper panel). The lower panel shows the hybridization of the same membrane with a GAPDH probe as a loading control. (B) pDP4 protein is expressed in bone marrow but not in spleen. Western blot using 60 μg total cell extracts from mouse spleen and bone marrow was incubated with an antiserum directed against the C-terminus of pDP4. Ngase F treatment indicates that pDP4 exists predominately as a glycosylated peptide (glyco-pDP4) in vivo. Multimerized pDP4 (multi-pDP4) can be noted as high molecular fragments. (C) Quantitative real-time RT-PCR of pDP4 transcripts using RNA from defined FACS-sorted hematopoietic cell populations. The following antibodies and mouse organs (in parentheses) were used for the sorting: B220 for B cells (spleen), CD3 for T cells (thymus), F4/80 for macrophages (bone marrow), CD41 for megakaryocytes (bone marrow), Ter119 for erythroid cells (bone marrow), Gr-1/Mac-1 for granulocytes (bone marrow), and a cocktail of all mentioned antibodies for lineage depletion to enrich progenitor cells (bone marrow). pDP4 values of 2 independent experiments are shown as percent expression of 18S rRNA ± standard deviation. (D) Immunofluorescence of a bone marrow cytospin section (original magnification, × 100) costained with anti-Gr-1 (primary)/Alexa-546 red (secondary) and anti-pDP4 (primary)/Alexa-488 green (secondary) antibody combinations as well as DAPI to visualize the nuclei. Only Gr-1+ cells costained with the pDP4 antiserum. (E) pDP4 real-time RT-PCR of intestine RNA from PU.1+/+ and PU.1-/- mice at different embryonic (d.p.c.) and newborn (d.p.p.) stages. pDP4 values of 2 independent experiments are shown as percent 18S rRNA expression ± standard deviation.
The presence of pDP4 in bone marrow suggested its expression in hematopoietic cells. We therefore measured pDP4 expression in different primary hematopoietic cell types. We isolated multiple hematopoietic cell populations from adult mice using fluorescent-based cell sorting. Subsequently, RNA was extracted from sorted cells, and real-time PCR assays were performed. pDP4 expression was barely detectable in hematopoietic progenitors (lin-) and B (B220+) and T (CD3+) lymphocytes and was only moderate in megakaryocytic (CD41+), erythroid (Ter119+), and monocytic (F4/80+) cells (Figure 2C). In contrast, high expression was found exclusively in the granulocyte (Gr-1+/Mac-1+) population. Thus, pDP4 mRNA is selectively up-regulated after myeloid commitment toward the granulocytic lineage. High expression in granulocytes was also seen in an immunofluoresence assay after staining bone marrow cells with antibodies against pDP4 and the granulocyte-specific surface protein Gr-1 (Figure 2D). Only cells positive for Gr-1 and with a ring-shaped nucleus typical for mature granulocytes showed positive staining of pDP4. In contrast, Gr-1- cells had no detectable pDP4 protein.
In addition to bone marrow granulocytes, we observed strong pDP4 expression in small intestine as well. We asked whether granulocytes that migrated into intestinal tissue or other small intestinal cell types express pDP4. Therefore, we analyzed pDP4 expression in intestines isolated from PU.1-/- embryos, which are devoid of mature myeloid cells and lack fetal liver pDP4 expression.19 We found that intestines from both PU.1+/+ and PU.1-/- embryos expressed similar levels of pDP4 mRNA, as revealed by real-time PCR (Figure 2E). Interestingly, however, intestines isolated at late embryonic time points or the newborn stage expressed higher amounts of pDP4 transcripts than those from early embryos. Thus, pDP4 is expressed in cells from small intestine and is strongly induced during intestine development.
Down-regulation of pDP4 expression in neutrophils after mobilization into the periphery
We have shown that pDP4 expression in the bone marrow is specific to the granulocytic lineage. Granulocytes mature within the bone marrow and mobilize into secondary tissue after infection or inflammation. To follow pDP4 expression upon maturation of primary granulocytes, we further separated the Gr-1+/Mac-1+ cell population from bone marrow into Gr-1low/Mac-1+ and Gr-1high/Mac-1+ subpopulations (Figure 3A). It was demonstrated before that the Gr-1 surface marker is up-regulated upon granulocyte maturation and differentiation.35 We also FACS-sorted Gr-1high/Mac-1+ cells from thioclycollate-stimulated peritoneal cells (PCs) to analyze pDP4 expression in granulocytes that have been mobilized into peripheral tissue upon induction of acute peritonitis. Subsequently, RNA was extracted from all populations and analyzed by real-time PCR. As demonstrated in Figure 3B, pDP4 expression was still low in the immature Gr-1low/Mac-1+ subpopulation. In contrast, pDP4 mRNA was up-regulated more than 16-fold in mature Gr-1high/Mac-1+ cells. Thus, pDP4 expression increases with granulocytic maturation in the bone marrow, similar to what we found for intestinal cells during embryonic development. Interestingly, however, pDP4 expression profoundly decreased by about 30-fold after activated granulocytes entered the peritoneal cavity, indicating that pDP4 is down-regulated in peripheral granulocytes.
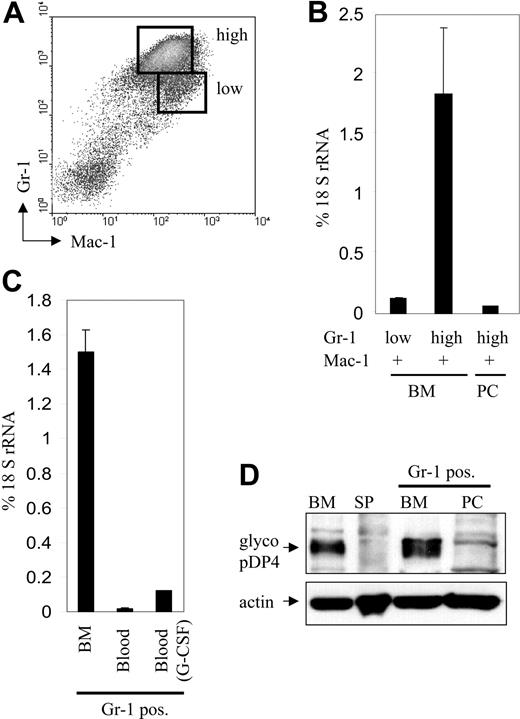
pDP4 expression is induced during granulopoiesis in bone marrow but is down-regulated upon granulocytic mobilization. (A) Gr-1+/Mac-1+ granulocytic cells from bone marrow were further separated into mature Gr-1high/Mac-1+ and immature Gr-1low/Mac-1+ neutrophils using FACS sorting. (B) Quantitative real-time RT-PCR of pDP4 transcripts using RNA from FACS-sorted early (Gr-1low/Mac-1+) or late (Gr-1high/Mac-1+) granulocytes from bone marrow or peritoneum of thioglycollate-induced mice. pDP4 values are shown as percent expression of 18S rRNA ± standard deviation. (C) Gr-1+ granulocytes were isolated from bone marrow and peripheral blood of either uninduced or G-CSF-injected mice using MACS sorting. Real-time RT-PCR values for pDP4 transcripts of 4 mice are shown as percent of 18S rRNA. (D) Western blot with 60 μg total cell extracts from mouse bone marrow and spleen together with MACS-sorted Gr-1+ cells from bone marrow or peritoneum of thioglycollate-treated mice. The membrane was incubated with an antiserum against pDP4 followed by an anti-actin antibody to demonstrate equal protein loading.
pDP4 expression is induced during granulopoiesis in bone marrow but is down-regulated upon granulocytic mobilization. (A) Gr-1+/Mac-1+ granulocytic cells from bone marrow were further separated into mature Gr-1high/Mac-1+ and immature Gr-1low/Mac-1+ neutrophils using FACS sorting. (B) Quantitative real-time RT-PCR of pDP4 transcripts using RNA from FACS-sorted early (Gr-1low/Mac-1+) or late (Gr-1high/Mac-1+) granulocytes from bone marrow or peritoneum of thioglycollate-induced mice. pDP4 values are shown as percent expression of 18S rRNA ± standard deviation. (C) Gr-1+ granulocytes were isolated from bone marrow and peripheral blood of either uninduced or G-CSF-injected mice using MACS sorting. Real-time RT-PCR values for pDP4 transcripts of 4 mice are shown as percent of 18S rRNA. (D) Western blot with 60 μg total cell extracts from mouse bone marrow and spleen together with MACS-sorted Gr-1+ cells from bone marrow or peritoneum of thioglycollate-treated mice. The membrane was incubated with an antiserum against pDP4 followed by an anti-actin antibody to demonstrate equal protein loading.
Granulocyte trafficking is tightly regulated and involves the G-CSF-dependent release of granulocytes from bone marrow into the peripheral blood.21 To address whether pDP4 down-regulation is an early step during granulocyte mobilization, we compared pDP4 expression in Gr-1-sorted cells from the bone marrow to their counterparts from the peripheral blood. We found that granulocytic release into the peripheral blood is accompanied by a more than 13-fold decrease in pDP4 expression (Figure 3C). To confirm that the differential pDP4 expression in mature granulocytes of the bone marrow and periphery also occurred at the protein level, Western blots were performed (Figure 3D). While strong accumulation of pDP4 was seen in Gr-1+ cells sorted from the bone marrow, no protein could be detected in Gr-1+ granulocytes from the peritoneum. Together, our results show that pDP4 expression peaks in mature bone marrow granulocytes but is significantly decreased after these cells leave for the peripheral blood and subsequently into the peritoneum, suggesting that down-regulation of the extracellular matrix protein pDP4 could be an important step for late granulocytes to exit the bone marrow into the periphery.
Transcription factor PU.1 binds to the pDP4 promoter
We have previously cloned pDP4 as a gene expressed in PU.1+/+ but not in PU.1-/- mouse fetal livers.19 However, PU.1-deficient mice lack mature granulocytes. Consequently, we analyzed whether pDP4 expression is directly regulated by PU.1. First, we determined the location of the pDP4 promoter by identifying the exact transcription start site using 5′ RACE PCR with RNA from mouse bone marrow as a template and spleen as a negative control (Figure 4A). Sequencing of the amplified RACE product revealed a 25-bp untranslated region at the 5′ end of the pDP4 transcript. Next, the proximal pDP4 promoter was screened for potential PU.1-binding sites, which were subsequently tested for PU.1 interaction in vitro. While a number of other probes failed to interact with PU.1 in gel shift assays, one oligonucleotide spanning bp -64 to -92 of the pDP4 promoter (Figure 4B) revealed strong and specific binding to both in vitro synthesized PU.1 protein and PU.1 in WEHI-3 nuclear extracts (Figure 4C). The core sequence within this PU.1-binding site was mapped to bp -75 to -78, because mutation of this site (TTCC to GCGA) prevented competition with the native site for interaction with PU.1. Interestingly, we found that the PU.1-binding site is highly conserved between murine pDP4 and its human homolog hGC-1/GW112, further underlining its functional importance for pDP4 transcription in both species (Figure 4B).
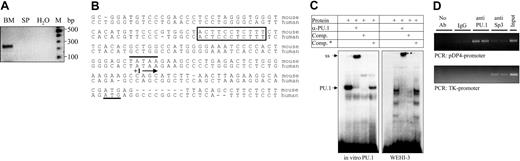
PU.1 binds to the pDP4 promoter in vitro and in vivo. (A) Isolation of the complete 5′ end of the pDP4 transcript by RACE PCR. Using a gene-specific antisense primer, which is located within exon 2 at bp +210, and a 5′ capping primer (for sequence see manufacturer's protocol), a single PCR fragment was amplified from bone marrow (BM) but not spleen (SP) cDNA. Sequencing of the subcloned PCR product revealed a 25-bp-long 5′ untranslated region. (B) Sequence comparison of the murine and human (CELERA database GA_x5J8B7W6JWY and NCBI accession NT_039606) pDP4 proximal promoter region showing a highly conserved PU.1-binding site (boxed), the presence of a TATA box (overlined), the transcription start site (+1 followed by an arrow), and the ATG marking the start of the coding region (underlined). The adenosine residue in the initial methionine codon corresponds to bp 26 196 078 of mouse chromosome 14 (NCBI accession NT_039606). (C) Electromobility shift (EMSA) experiment using reticulocyte lysate synthesized in vitro PU.1 (left panel) or 10 μg nuclear extract from WEHI-3 cells (right panel). The probe spanned base pairs -64 to -92 of the murine pDP4 promoter. A 50-fold excess of the cold probe with (comp.*) or without (comp.) an introduced mutation (TTCC to GCGA) was used to test the specificity of the DNA-protein interactions. ss indicates the PU.1 complex supershifted with specific antisera. (D) Chromatin immunoprecipitation (ChIP) assay to analyze PU.1 binding to the pDP4 promoter in WEHI-3 cells. The upper panel shows a PCR-amplified 0.4-kb pDP4 promoter fragment that was specifically immunoprecipitated by the anti-PU.1 antibody. The lower panel shows a control experiment demonstrating that the anti-PU.1 antibody did not pull down a 0.6-kb thymidine kinase (TK) promoter fragment from the same extracts. The input lane represents a 1:20 dilution of the supernatant from the IgG immunoprecipitation.
PU.1 binds to the pDP4 promoter in vitro and in vivo. (A) Isolation of the complete 5′ end of the pDP4 transcript by RACE PCR. Using a gene-specific antisense primer, which is located within exon 2 at bp +210, and a 5′ capping primer (for sequence see manufacturer's protocol), a single PCR fragment was amplified from bone marrow (BM) but not spleen (SP) cDNA. Sequencing of the subcloned PCR product revealed a 25-bp-long 5′ untranslated region. (B) Sequence comparison of the murine and human (CELERA database GA_x5J8B7W6JWY and NCBI accession NT_039606) pDP4 proximal promoter region showing a highly conserved PU.1-binding site (boxed), the presence of a TATA box (overlined), the transcription start site (+1 followed by an arrow), and the ATG marking the start of the coding region (underlined). The adenosine residue in the initial methionine codon corresponds to bp 26 196 078 of mouse chromosome 14 (NCBI accession NT_039606). (C) Electromobility shift (EMSA) experiment using reticulocyte lysate synthesized in vitro PU.1 (left panel) or 10 μg nuclear extract from WEHI-3 cells (right panel). The probe spanned base pairs -64 to -92 of the murine pDP4 promoter. A 50-fold excess of the cold probe with (comp.*) or without (comp.) an introduced mutation (TTCC to GCGA) was used to test the specificity of the DNA-protein interactions. ss indicates the PU.1 complex supershifted with specific antisera. (D) Chromatin immunoprecipitation (ChIP) assay to analyze PU.1 binding to the pDP4 promoter in WEHI-3 cells. The upper panel shows a PCR-amplified 0.4-kb pDP4 promoter fragment that was specifically immunoprecipitated by the anti-PU.1 antibody. The lower panel shows a control experiment demonstrating that the anti-PU.1 antibody did not pull down a 0.6-kb thymidine kinase (TK) promoter fragment from the same extracts. The input lane represents a 1:20 dilution of the supernatant from the IgG immunoprecipitation.
Next we performed chromatin immunoprecipitation (ChIP) assays to verify whether PU.1 binding to the pDP4 promoter also occurred in vivo. Strong interaction of PU.1 with a genomic DNA region encompassing 0.4 kb directly upstream of the pDP4 transcription start site, which includes the above-described core sequence, was observed (Figure 4D, upper panel). In contrast, no significant binding of the ubiquitous transcription factor Sp3 to this sequence was detected. The specificity of our assay was proven by coprecipitation of the thymidine kinase (TK) promoter with Sp3 but not PU.1 in the same samples (Figure 4D, lower panel). Therefore, a specific site in the pDP4 promoter is a target for PU.1 binding in myeloid cells.
PU.1 is required for pDP4 expression in myeloid cells
To investigate whether PU.1 regulates pDP4 transcription directly, a luciferase reporter construct under the transcriptional control of the pDP4 promoter was transfected into COS-7 cells, a line that lacks endogenous PU.1 expression.36 As shown in Figure 5A, the pDP4 regulatory sequence was transactivated following cotransfection of a PU.1 expression construct. Consequently, PU.1 acts as a positive transcriptional regulator of pDP4. Cotransfection of another lineage-specific transcription factor, ICSBP, along with the pDP4 promoter construct, resulted in decreased reporter activity, thus confirming the specificity of the PU.1-induced transactivation.
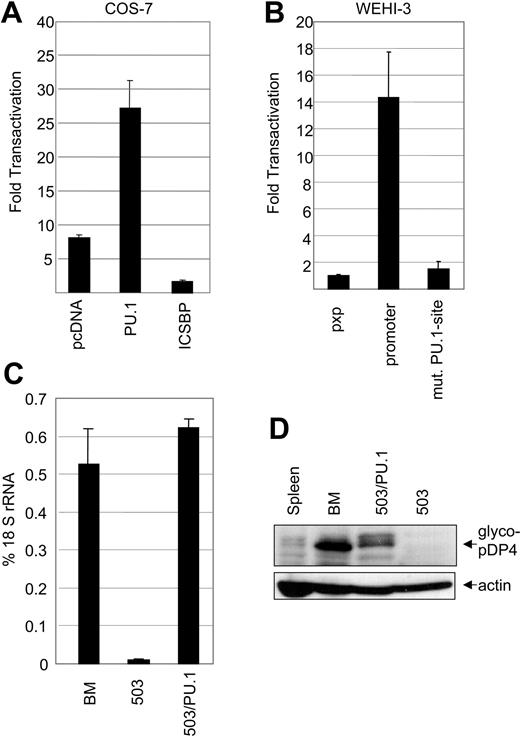
pDP4 promoter activity is regulated by PU.1. (A) COS-7 cells were transiently transfected with pXP2-pDP4-prom (300 ng), a plasmid that expresses a luciferase gene under the transcriptional control of the proximal 0.4-kb pDP4 promoter, along with (200 ng each) pcDNA3, pcDNA3-PU.1, or pcDNA3-ICSBP, and the reference vector pRL-CMV (50 pg). The graph shows a representative experiment of 3 independent assays ± standard deviation. (B) WEHI-3 cells, which express endogenous pDP4, were transiently transfected with (1.9 μg each) either a promoterless pXP2, pXP2-pDP4-prom, or pXP2-pDP4-prom(mut.) carrying a mutated (TTCC to GCGA) PU.1-binding site, along with the control plasmid pRL-TK (100 ng). The graph shows a representative experiment of 2 independent assays. (C) Real-time RT-PCR of pDP4 transcripts from bone marrow (BM), 503, or 503/PU.1 cells. The results were normalized to 18S rRNA values. (D) Western blot of total cell extracts (60 μg) from spleen, bone marrow, the 503 PU.1-/- line, and the 503/PU.1 line showing the absence of pDP4 protein expression in 503 cells before the retroviral restoration with PU.1. The upper panel was immunoblotted with the pDP4 antiserum while the lower panel demonstrates equal protein loading as judged by immunoblotting with an anti-actin antibody.
pDP4 promoter activity is regulated by PU.1. (A) COS-7 cells were transiently transfected with pXP2-pDP4-prom (300 ng), a plasmid that expresses a luciferase gene under the transcriptional control of the proximal 0.4-kb pDP4 promoter, along with (200 ng each) pcDNA3, pcDNA3-PU.1, or pcDNA3-ICSBP, and the reference vector pRL-CMV (50 pg). The graph shows a representative experiment of 3 independent assays ± standard deviation. (B) WEHI-3 cells, which express endogenous pDP4, were transiently transfected with (1.9 μg each) either a promoterless pXP2, pXP2-pDP4-prom, or pXP2-pDP4-prom(mut.) carrying a mutated (TTCC to GCGA) PU.1-binding site, along with the control plasmid pRL-TK (100 ng). The graph shows a representative experiment of 2 independent assays. (C) Real-time RT-PCR of pDP4 transcripts from bone marrow (BM), 503, or 503/PU.1 cells. The results were normalized to 18S rRNA values. (D) Western blot of total cell extracts (60 μg) from spleen, bone marrow, the 503 PU.1-/- line, and the 503/PU.1 line showing the absence of pDP4 protein expression in 503 cells before the retroviral restoration with PU.1. The upper panel was immunoblotted with the pDP4 antiserum while the lower panel demonstrates equal protein loading as judged by immunoblotting with an anti-actin antibody.
Next we analyzed if binding of PU.1 to the core site is required for pDP4 transcription in myeloid cells. Transfection of the pDP4 promoter-driven luciferase construct in WEHI-3 cells led to more than 14-fold increased reporter activity as compared with the promoterless plasmid (Figure 5B). In contrast, mutation of the core PU.1-binding site resulted in a decrease of transactivation capacity of the pDP4 promoter to almost background levels. Thus, PU.1 binding and subsequent transactivation is crucial for pDP4 transcription in myeloid cells.
We further elucidated the role of PU.1 in mediating expression of pDP4 using the PU.1-/- myeloid cell line 503 before and after the reintroduction of PU.1 by retroviral transduction (503/PU.1). 503 cells have previously been described as an IL-3-dependent line with granulocytic characteristics that was isolated from PU.1-deficient neonatal mouse livers.37 To analyze pDP4 expression in these cells, we performed real-time PCR assays. While low levels of pDP4 transcripts could be detected in PU.1-deficient 503 cells, a more than 40-fold increase in expression was measured after restoration of PU.1 in 503 cells (Figure 5C). A similar result was seen on protein levels using Western blots (Figure 5D). Together, our results provide clear evidence that PU.1 is a potent transactivator of the pDP4 promoter and is directly required for pDP4 expression in myeloid cells.
Discussion
Although several studies have demonstrated that PU.1 expression is strongest in mature myeloid cells,23,24 its function and target genes in these terminally differentiated effector cells are poorly understood. Here we report that PU.1 directly regulates the expression of a novel glycoprotein member of the olfactomedin-like gene family. We further define a tightly regulated expression pattern for pDP4, which in the hematopoietic system is predominately expressed in mature bone marrow granulocytes.
The olfactomedin-like gene family is defined by sequence homology to its founding member, bullfrog olfactomedin, which was isolated as a secreted protein from olfactory epithelia.33 The main feature of this growing family is a highly conserved olfactomedin-like protein domain, which usually is located in the carboxyl-terminus.38 Although the biologic functions of olfactomedin and most other members of this gene family are not well understood, they are most likely involved in formation of the extracellular matrix.38 All members are highly glycosylated and carry a small N-terminal signal peptide that directs the secretion of the mature protein forms. Furthermore, based on sequence as well as biochemical and immunohistochemical data, it was suggested that olfactomedin-like proteins multimerize through disulfide bonds to large network structures.33 Accordingly, the bulk of pDP4 protein exists in a glycosylated form, which is secreted and most likely multimerized.
The expression profiles of those olfactomedin-like genes that have been studied are highly selective. For example, olfactomedin is restricted to the brain and nervous system,33 and noelin is limited to the dorsal neuraltube.39 Myocilin, a factor that has been implicated in the development of the eye disease primary open angle glaucoma, is exclusively expressed in the eye, skeletal muscle, heart, brain, and testis.40 We show here that pDP4 expression is also highly tissue specific. Relevant expression is limited to the bone marrow, in which pDP4 is predominately synthesized by mature granulocytes, and small intestine. Similar to its pattern during granulocytic maturation, pDP4 expression also is induced during the development of intestine cells. The probable human homolog of pDP4, hGC-1/GW112, has been reported to have a similar expression profile.32 hGC-1/GW112 was isolated by mRNA differential display from GM-CSF-induced peripheral blood mononuclear cells and is restricted to the bone marrow, small intestine, colon, and prostate.32
Interestingly, pDP4 expression is strongly down-regulated in granulocytes that have left the bone marrow into the peripheral blood and subsequently the peritoneum. Thus, pDP4 could play a role in trafficking of granulocytes from bone marrow into the periphery. However, the exact biologic function of pDP4 remains to be determined. Therefore, we are currently generating mice with a targeted disruption of the pDP4 gene.
Several proteins of the olfactomedin-like family have been implicated in developmental processes. Although the exact molecular mechanism is still unclear, it has recently been reported that tiarin, a protein that is closely related structurally to pDP4, is a pattering molecule secreted by the non-neural head ectoderm that flanks the developing central nervous system (CNS) in Xenopus embryos.41 Tiarin directly stimulates the dorsalization of neural tissues and antagonizes ventralization. Another family member, noelin, has been shown to have a role in chick neural crest development.39 Thus, the mechanisms behind these olfactomedin-related gene functions generally include cellular movement and trafficking.
The restricted expression patterns of the olfactomedin-like genes suggest that they are under tight transcriptional control. However, there is no report so far linking any transcription factor to the regulation of this gene family. Here, we have identified a specific binding site for PU.1 in the proximal promoter of the pDP4 gene, which is conserved in the human gene. Moreover, this site is essential for the transactivation of the pDP4 promoter in WEHI-3 cells, because mutation of its core abolished reporter gene expression. Therefore, pDP4 expression is directly controlled by PU.1 in myeloid cells. Interestingly, the pDP4 promoter contains a TATA box. In general, promoters regulated by PU.1 lack TATA boxes, and PU.1 is believed to recruit the basal transcription machinery via binding to TATAA-binding protein (TBP) to the myeloid promoters.12,42-44 We do not know at this point whether the TATA box within the pDP4 promoter is active in myeloid cells or whether its function is restricted to cells of the small intestine, which also demonstrated strong pDP4 expression.
Similar to pDP4, PU.1 expression profoundly increases in mature granulocytes as compared with hematopoietic precursor cells.23,24 However, PU.1 knock-out mice have no mature myeloid cells, thus preventing elucidation of the biologic function of PU.1 in these cells. The link between PU.1 and pDP4 suggests a role for PU.1 in neutrophilic trafficking and migration. PU.1 has been shown to regulate the gene encoding the G-CSF receptor,18,19 and G-CSF signaling is essential for the release of neutrophils from the bone marrow.21 In addition, one of the first cellular targets identified for PU.1 was the alpha chain of the granulocyte integrin CD11b,13 a surface protein that plays an important role in granulocyte adhesion.45 Therefore, it seems likely that PU.1 provides the transcriptional basis to orchestrate granulocytic mobilization upon inflammation and/or infection.
In summary, pDP4 is a direct PU.1 target gene encoding a novel olfactomedin-related extracellular matrix protein with a highly restricted expression pattern in small intestine and mature granulocytes of the bone marrow, and our findings suggest that pDP4 plays a role in controlling migration of granulocytes.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-08-2688.
Supported by DFG (German Research Foundation) research fellowships RO 2295/1-1 (F.R.) and WA 1584/1-1 (K.W.) and National Institutes of Health (NIH) grant CA41456 (D.G.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank, in particular, Kristin Geary for her excellent help with the mice and Victoria Petkova and Tajhal Dayaram for real-time PCR assays. We are also grateful to Bruce Torbett for 503 and 503/PU.1 cells as well as Steffen Koschmieder for comments on the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal